Abstract
The journey of a prosthetic user is characterized by the opportunities and the limitations of a device that should enable activities of daily living (ADL). In particular, experiencing a bionic hand as a functional (and, advantageously, embodied) limb constitutes the premise for promoting the practice in using the device, mitigating the risk of its abandonment. In order to achieve such a result, different aspects need to be considered for making the artificial limb an effective solution to accomplish ADL. According to such a perspective, this review aims at presenting the current issues and at envisioning the upcoming breakthroughs in upper limb prosthetic devices. We first define the sources of input and feedback involved in the system control (at user-level and device-level), alongside the related algorithms used in signal analysis. Moreover, the paper focuses on the user-centered design challenges and strategies that guide the implementation of novel solutions in this area in terms of technology acceptance, embodiment, and, in general, human-machine integration based on co-adaptive processes. We here provide the readers (belonging to the target communities of researchers, designers, developers, clinicians, industrial stakeholders, and end-users) with an overview of the state-of-the-art and the potential innovations in bionic hands features, hopefully promoting interdisciplinary efforts for solving current issues of upper limb prostheses. The integration of different perspectives should be the premise to a transdisciplinary intertwining leading to a truly holistic comprehension and improvement of the bionic hands design. Overall, this paper aims to move the boundaries in prosthetic innovation beyond the development of a tool and toward the engineering of human-centered artificial limbs.
Export citation and abstract BibTeX RIS
1. Introduction
Over the past 20 years, poly-articulated upper limb prostheses (ULPs) have undertaken several technological and scientific developments to satisfy the different needs of the upper limb amputee community. Nonetheless, in a recent study, Salminger et al (2020) observed overall abandonment rates of ULPs of about 44% in a population of mainly (92%) myoelectric prostheses users. They also highlighted how the past decade of developments still presents technological limiting factors that did not permit the restoration of the full functionalities of a missing limb, hence leading to a substantial increased rate of prosthesis abandonment. The main cause of such ineffectiveness mainly resides in a non-sufficiently patient-tailored design process (Salminger et al 2020).
According to the American Orthotic and Prosthetic Association (Aopa 2016), partial amputations, i.e. finger amputations, represent the majority of upper-limb losses (75.6%), while trans-radial and trans-humeral amputations constitute a percentage oscillating between 5% and 6%. Despite this, the level of impairment caused by trans-radial and trans-humeral amputations is greater than for partial amputations.
Without tracing back all the evolution of ULPs—the reader might find useful the reviews of Trent et al (2019) and Ribeiro et al (2019). Trent et al (2019) work focuses on a classification of the upper-limb prostheses architectures based on the type of adopted actuation, e.g. passive, body-powered or active. On the other hand, Ribeiro et al (2019)'s research investigates the most relevant control signals used for the man-machine interface.
This work focuses on trans-radial and trans-humeral devices, excluding partial amputations, and it details the latest and most technologically advanced solutions, namely poly-articulated myoelectric prostheses. Moreover, this review aims at presenting and analyzing the key elements of state-of-the-art ULPs in a user-centered and human-in-the-loop fashion and to provide guidelines for the development of such prostheses and the relative control algorithms, to possibly achieve solutions capable of promoting the systems use and overcoming the elevated abandonment rates observed so far. Overall, the reader could take advantage of this review as an analytical collection of solutions constituting a premise to provide the user with a seamless control experience.
2. Upper limb prosthetics classification: a twofold perspective
An ULP system can be observed from two main points of views: its mechatronics, namely the combination of the mechanical and electronic components necessary for its operation, and the control strategies and algorithms implemented to orchestrate its functions. Research groups have therefore attempted to solve the prostheses abandonment problem by addressing different technological and scientific challenges, either focusing on mechatronic design, or on control strategies aimed at increasing the human-machine interaction and, in some cases, introducing feedback sources, as detailed in the next sections.
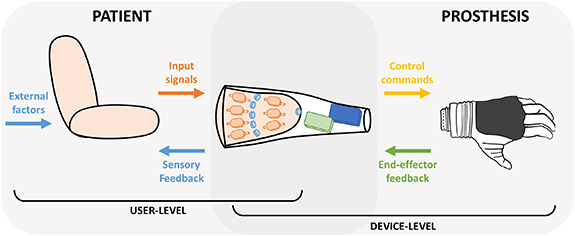
ULP control can be divided into two synergistically interacting sub-systems: the user-level and the device-level, as depicted in figure 1. The user-level includes the patients and the most proximal device component interacting with the user (i.e. the socket), while the device-level extends from the socket to the ULP device. These two sub-systems overlap at the socket level, which is involved in a bidirectional flow of information. On one hand, it receives inputs from the user (i.e. movement intentions) and translates them into movement commands for the device; on the other hand, it receives information (both from the device and the environment) and communicates it to the user through sensory feedback (figure 1). Importantly, the socket itself severely limits the user comfort, and together with the prosthetic weight highly contributes to the prosthetic abandonment.
Figure 1. Graphical representation of a ULP system and its elements. The user level (left panel) includes: input data sent from subject to the prosthesis (input signals), artificial sensory feedback information delivered from the prosthesis to the user (sensory feedback), and external sources of interaction (external factors), such as actuation coming from the unimpaired limb or environmental/accidental sources of feedback such as vision and sound. The device-level (right panel) includes the control commands used to drive the prosthesis and the feedback information collected by the end-effector. The user-device interface is characterized by a bidirectional exchange of information (overlap of the two panels).
Download figure:
Standard image High-resolution imageEven if the state-of-the-art in prosthetic research encompasses studies based on psychological processes too, commercial ULP systems have focused on restoring functional capabilities by capitalizing on the device-level only, therefore on mechatronic, and several solutions can be found on the market for trans-radial level of amputations. Commercially available systems merge basic functionalities and aesthetic requirements, targeting the clinical needs given by a certain kind of amputation, rather than focusing on each patient's specific needs.
Commercial solutions range from tri-digital hands, e.g. VaryPlus Speed, SensorHand Speed by Ottobock (Ottobock 2020c) and Motion Control Hand by Fillauer (Fillauer 2021); through polyarticulated hand under-actuated, e.g. Michelangelo by Ottobock (Ottobock 2020b); to fully actuated polyarticulated hand, e.g. BeBionic by Ottobock (Ottobock 2020a), i-Limb by Ossur (Ossur 2020b), Vincent Hand by Vincent Systems (Systems 2020), TASKA hand by Taska Prosthetics (Taska 2022), BrainRobotics Hand by BrainRobotics (Brainrobotics 2022) and Ability Hand by Psyonic (Psyonic 2022).
In the last decades, many research groups have focused on the mechatronic development of ULP devices, entrusting the intelligence of the device to the embedded mechanics in a very thorough design, structuring the development of the concept of under-actuation, such as the Vanderbilt Multigrasp Hand (Bennett et al 2014), the MIA Hand (Controzzi et al 2016), the SoftHand Pro (Godfrey et al 2018), the KIT Hand (Weiner et al 2018), and the Hannes Hand (Laffranchi et al 2020).
On the other hand, there is a family of very dexterous devices, not yet market-ready, that mimic the complexity of the human hand, implementing a fully-actuated multi-degrees of freedom mechatronics, e.g. the University of Bologna Hand (Meattini et al 2019) or the Shadow Hand (Company 2020).
High level of amputations, as the trans-humeral ones, require prosthetic elbows, such as the Dynamic Arm (Ottobock 2022a), the Dynamic Arm Plus (Ottobock 2022b), and the ErgoArm (Ottobock 2022c) from Ottobock; the Espire Elbow (Classic, Classic Plus, Pro and Hybrid,) from Steeper Inc. (Steeper 2022); and the Fillauer Motion E2 Elbow (Fillauer 2022a) and the Utah Arm 3 (Fillauer 2022b) from Fillauer. In the research context, full robotic arms include the DLR hand system (Grebenstein et al 2011), the APL modular prosthetic limb (Johannes et al 2011), the LUKE Arm (Bionics 2022), the Rehabilitation Institute of Chicago arm (Lenzi et al 2016), and Edinburgh Modular Arm System (Gow et al 2001).
However, this great variety of products does not match with the elevated abandonment rates, demonstrating the lack of satisfaction of the patients' needs from a mechatronic perspective. In particular, structural and supporting part lack of adjustability of user size, allow limited kinematic and motion possibilities and more advanced systems present limited operational time (Harte et al 2017). This leads to limited satisfaction and feeling of security. Moreover, these systems generally present poor personal and social acceptance because of limited anthropomorphism, high weight and presence of acoustic disturbances during use (Harte et al 2017), This suggests that ULP development should not only focus on the device level, but improvements at the user level could play a key role for truly meeting the user requirements and consequently obtain device acceptance. Motivated by this, in this review, we analyze all the possible approaches that could potentially address the user needs in terms of device controllability, robustness and hence embodiment and user experience. To this end, it is fundamental not only to focus on the functionality restoration but also on the sensory information recovery, which are fundamental to effectively control the device. All the described approaches range from improvements in decoding user intentions, hence analyzing all possible input sources and their related control strategies, to inclusion of additional sources of feedback capable to restore the sensory information. These approaches tackle the issues related to poor device control because of lack of intuitiveness and sensory feedback.
Therefore, in this review we present current and emerging methods in ULP development, detailing various sources of input and feedback signals, as well as control strategies. We also highlight current challenges and open issues in the field, specifically focusing on the importance of user experience and involvement in the design and development process. This is fundamental to promote patient-tailored approaches leading to the development of truly personalized devices, which are currently lacking. We finally provide an overview of the most promising approaches that if followed, may one day provide upper limb amputees with a true substitute of their missing arm.
3. Input and feedback signals for prosthetic control
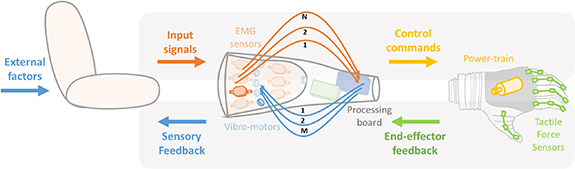
Prosthetic control is regulated by a flow of signals, as depicted in figure 2. Input signal runs from the user to the device and they are often of biological or electrophysiological nature, in which case are called biosignals. Signals flowing in the opposite direction convey information from the device to the user and are therefore defined as sensory feedback signals. Moreover, some external factors convey to the user additional source of feedback (i.e. incidental feedback), such as visual or auditory information that can be used to estimate the prosthesis state (Wilke et al 2019, Sensinger and Dosen 2020, Gonzalez et al 2021).
Figure 2. Graphical representation of information flow of a possible ULP architecture. Input flow (top panel): from user (input signals i.e. from EMG sensors) to prosthesis (control commands i.e. through power train). Feedback flow (bottom panel): from prosthesis (end-effector feedback i.e. from tactile force sensors) to user (sensory feedback i.e. through vibrotactile motors).
Download figure:
Standard image High-resolution imageInput signals include all the sources of information that can be taken from the amputee and translated into motor commands for driving the prosthesis (e.g. electromyography—EMG), see section 3.1. Instead, sensory feedback information encompasses different prosthetic sensing solutions acquired either from the prosthetic device or from the environment, see section 3.2 that can be translated into sensory stimuli for the amputee (e.g. vibrotactile stimulation, see section 3.3). All types of signals can be classified according to their level of invasiveness, with consequent advantages and drawbacks.
3.1. Input signals
In recent years, many research activities have focused on the extraction of useful information from the biological signals in order to suitably control ULPs. Traditionally, the surface EMG (sEMG) is the most widespread signal for prosthesis control but its use still faces many drawbacks (Kyranou et al 2018). In the following, we describe various methods to employ EMG as input signal for ULP control and we also explain how other input sources can be exploited to obtain more dexterous prosthetic behavior, overcoming the limitations of current ULP systems.
Figure 3 collects input signals for ULP control that will be described in the following subsections, ranging from those used by commercial systems, up to those currently under investigation.
Figure 3. Input sources for ULP.
Download figure:
Standard image High-resolution image3.1.1. Biosignals
The term biosignal indicates every possible signal that can be detected and measured from biological beings, humans—in our case. Usually, the term is used for signals of electric nature (i.e. EMG), but actually every signal collected from the activity of different tissues or organs belonging to the human body, can be considered as a biosignal.
We here adopt this latter definition to group input sources that are described next. Given its large use both in research and commercial ULP devices, EMG deserves a dedicated subsection, while other biosignals are grouped together. We also dedicate a whole subsection to brain-derived signals, which are especially used in brain-machine and brain-computer interfaces (BMIs, BCIs), but that are also showing potential use for ULP applications. Table 1 summarizes biosignals for ULP control that will be described in the following subsections.
Table 1. Biosignals used as input sources in prosthetic applications.
| Measured Property | Sensors' placement | PROs | CONs | Sensor Fusion | Examples | ||
|---|---|---|---|---|---|---|---|
| Electromyography (EMG) | Surface EMG | Muscle electric potentials | On the skin over targeted muscles 2–32, up to 192 sensors | Non-invasive, long-term use, a large number of people | Sweating, electrodes shift, muscle fatigue, electromagnetic noise | NIRS, IMU, FMG, SMG, MMG | (Merletti et al 2010) up to 27 gestures |
| Invasive EMG | Underneath the skin, on or inside targeted muscles 4–8 sensors | High signal/noise ratio, directly on the nerve, no shift with respect to the source | Invasive, infections | (Cipriani et al 2014, Ortiz-catalan et al 2020) | |||
| Force-myography (FMG) | Change of muscle morphology measured on the skin surface | Over targeted muscle, over related tendons 8, up to 126 sensors | Physiologic, small size, high signal/noise ratio, flexible | Muscle fatigue, sensors shift, pre-load force, small spatial resolution, crosstalk | EMG | (Xiao and Menon 2019) up to 8 gestures | |
| Mechano-myography (MMG) | Muscle fiber oscillations using microphone or accelerometers | Over targeted muscle 6–20 sensors | Low cost, no pre-amplification, no precise positioning, no skin impedance or sweat influence | Ambient acoustic noise, Adjacent muscle crosstalk, Sensor displacement | EMG, IMU | (Wilson and Vaidyanathan 2017, Guo et al 2017a, Castillo et al 2020) up to 5 gestures | |
| Sono-myography (SMG) | Change of muscle morphology | Over targeted muscle, over related tendons transducers of different shapes | Deep and superficial muscles, some models are cheap and energy-efficient | Probe shift, tissue impedance, no wireless, some models expensive and bulky | EMG | (Dhawan et al 2019) up to 15 gestures | |
| Near-Infrared Spectroscopy (NIRS) | Tissue oxygenation through the amount of scattered light | Over targeted muscle 2–4 sensors | Deep and superficial muscles, high spatial resolution, no electronic interference | Ambient light, Muscle fatigue, tissues heating | EMG, IMU | (Paleari et al 2017) up to 9 gestures | |
| Electrical Impedance Tomography (EIT) | Tissue impedance | Over targeted muscle, over related tendons 8, up to 64 sensors | No need precise positioning | Low time resolution, sweating, electromagnetic noise, high consumption | — | (Zhang et al 2016, Wu et al 2018) up to 8 gestures | |
| Capacitance sensing | Tissue capacitance | Over targeted muscle, over related tendons 3 receiver sensors | Non-invasive, low cost, deep and superficial muscles | Sweating, Electromagnetic noise, displacement, ambient temperature | — | (Cheng et al 2013, Truong et al 2018) up to 2 gestures | |
| Magneto-myography | Magnetic fields generated by muscle | Over/inside targeted muscle 7 sensors | Not sensitive to sensor's shift and sweat | Magnetic interference, can be invasive, movement artifacts | — | (Zuo et al 2020) concept | |
| Peripheral neural interfaces (PNIs) | Electrical activity of the nerves | Microelectrode arrays placed on different fascicles within the median and ulnar nerves | Intuitive, direct maps of complex movements, high accuracy, robust | Invasive, difficult to separate EMG and PNI components, recording channels really closed each other | — | (Nguyen et al 2020) up to 15 DoFs | |
| Intracortical neural signals | Intracortical neural signals from the brain, action potentials of individual neuron | 16–192 high-density channels electrodes inserted into the motor cortex tissue | Accurate and capable of collecting the most information-rich data, high spatial resolution | Very invasive, influenced by tissue reactions | — | (Hochberg et al 2006, Hochberg et al 2012, Collinger et al 2013, Wodlinger et al 2014) 7–10 DoFs | |
| Electrocorticography (ECoG) | Electrical activity of brain's surface | 32–128 high-density channels on sensorimotor regions | Less attenuated than EEG, good spatial resolution and wide frequency content | Surgical procedure and lack to measure single cell activity | — | (Wang et al 2013, Fifer et al 2013, Bleichner et al 2016, Hotson et al 2016) 4 gesture recognition and wrist movements | |
| Electroencephalography (EEG) | Electrical activity of the brain | 6–32 channels headsets | Not invasive, low cost, portable, stable, and very easy to use | Signal attenuated by the dura, the skull, and the scalp, loss of important information | — | (Mcfarland et al 2010, Yang et al 2012, Edelman et al 2019, Fuentes-Gonzalez et al, 2021) single Degree of Freedom (DoF) | |
| Functional near-infrared spectroscopy (fNIRS) | Activity-related brain oxygenation, near-infrared led, and a photodetector measure the amount of IR light absorbed by the hemoglobin in the brain | 10–200 channels of optodes | Non-invasive, simultaneous detection information under the skin, low cost | Few centimeters penetration of cortical tissue, not great accuracy, and system too cumbersome | — | (Syed et al 2020) 3 DoFs trans-humeral amputees | |
3.1.1.1. EMG
While cosmetics, electronic components and computational efforts have undergone a significant improvement, the control strategies currently used in prosthetic applications have not changed since their first appearance in the 1960s (Schmidl 1965). The EMG has been one of the major sources to control ULPs (Merletti and Farina 2016). These signals carry information about neuromuscular activity, and they are used to retrieve human intention. EMG is indeed a technique for studying the activation of the skeletal muscles through the recording of electrical potentials produced by muscle contraction (Hudgins et al 1993). The theory behind the sEMG electrodes is that they form a chemical equilibrium between the detecting surface of the electrode and the skin of the body through electrolytic conduction, so that the current can flow into the electrode.
Multiple methods have been used to obtain the intended gesture from the processed EMG signals, all of which exploit the fact that the amputees can still generate different and repeatable muscular patterns related to each forearm movement with residual muscles of the stump. Low-density EMG is commonly used in prosthetic application, both in research and commercial context. Noteworthy, EMG signals can also be collected with invasive methods. The sEMG can be thus classified according to the level of resolution and density of the sensors. In the following, we provide an overview of the different types of EMG-based biosignals.
3.1.1.1.1. sEMG
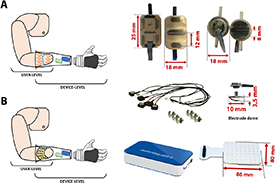
The sEMG can be classified according to the number of electrodes used (figure 4). Low-density EMG generally refers to the use of a small (<10) number of EMG bipolar sensors, that can be either wet, i.e. contain an electrolytic substance that serves as interface between skin and electrodes, or dry (Jamal 2012). Conversely, high-density EMG is typically composed by wet monopolar sensors spread on a planar patch, around 1 cm apart, and with the ground reference generally placed on the wrist or on the elbow (Drost et al 2006). Importantly, sEMG electrodes also differ in their electronic configuration, as they can be either preamplified or not (Zheng et al 2021). Merletti and Muceli (2019) provided a guide with the best practice to acquire and manipulate EMG data according with the different aims, from signal analysis to motion prediction.
Figure 4. sEMG electrodes. (A) Bipolar dry sensors, Ottobock and IIT/INAIL (Marinelli et al 2021) respectively. (B) High-density wet sensors (OT Bioelettronica).
Download figure:
Standard image High-resolution imageProsthetic control with low-density EMG is generally obtained by using two bipolar electrodes placed on antagonist muscles. This configuration allows the control of the prosthetic system in a robust and simple way (Hudgins et al 1993). However, the detection of complex and simultaneous movements of the phantom limb can be improved by using an array of EMG electrodes placed on the superficial skin of the residual forearm (Coapt 2017, Dellacasa Bellingegni et al 2017, Ottobock 2019, Marinelli et al 2020), The use of sEMG in prosthetic applications has become the most widespread source of information about voluntary movement (Schmidl 1965) because of the direct correlation between EMG activity and subjects' intentions.
Differently from the low-density, the high-density sEMG (HD-sEMG) is based on a higher number of electrodes placed on a small portion of the body. Recently, a growing number of researchers has focused on the use of these electrodes aiming to increase the amount of collected data, although at the cost of a greater computational burden. HD-sEMG sensors have been used to discriminate muscular patterns related to different gestures. Their signals can be handled in various ways to retrieve unique and repeatable information, as described in section 4. These sensors have to be positioned according to the distribution of the underlining muscle fibers and this configuration provides a low resolution map of the synergistic activation of the muscles during movement production (Winters 1990, Sartori et al 2018). For example, from contraction of the muscles under the acquisition grids, it is possible to extract bi-dimensional images, in which the EMG amplitude is mapped to a color scale. These maps can be thus handled by complex algorithms, as the ones used for objects detection in robotic navigation (Chen et al 2020). The main limitation of the HD-sEMG, which currently bounds its application to a laboratory scenario, is the skin-electrode contact since it requires conductive gel to reduce the interface impedance. The wet area is mainly needed to reduce artifacts in the EMG signals since it is generally acquired in monopolar configuration. Another disadvantage of this technique consists in the fact that computation is time-consuming.
Overall, the main drawback of sEMG-based approaches is constituted by the influence that skin impedance, sweat, and electrode shift have on the stability of the input signals (De Luca 1997). Additionally, muscle crosstalk and the difficulty to reach deep muscles further limit the quality of the collected signal. In the context of ULP, the use of sEMG can be further complicated by the fact that the amputation strongly affects muscles strength and organization and therefore signal quality, as discussed in section 6.4.
3.1.1.1.2. Invasive EMG and surgical procedures
The invasive approach has been exploited to explore the activity related to the production of movement for many years (Adrian and Bronk 1929) and it is still investigated by many groups. However, the main drawback of this approach is constituted by the surgery and by the technological barriers still faced by the available equipment. On the other hand, invasive electromyography (iEMG) allows to measure single motor unit action potentials, enabling a higher selectivity and a better accuracy of the input signal, overcoming the limitations imposed by sEMG. There are several examples of iEMG, which vary in the type of electrodes and level of invasiveness, as detailed hereafter.
EMG can be invasively detected by inserting electrodes into the internal surface of muscles (Merletti and Farina 2009). This invasive technique exploits two different percutaneous electrodes: needles and fine wires (Jamal 2012, Rubin 2019). The most used are needle electrodes. These electrodes are concentric, and their bare hollow needles contain an insulated fine wire into their cannula, which is exposed on the beveled tip, which is the active recording site. Wire electrodes are typically made of non-oxidizing and stiff materials with insulation, they can be implanted more easily and are usually less painful than needle electrodes.
Since both these sensors are percutaneous, i.e. passing through unbroken skin and leaving an open passage between the internal structures of the body and the external world, the risk of infection is quite probable. For this reason, and because of their intrinsic discomfort due to the percutaneous wire that can easily break, their usage is limited to laboratory research (Hargrove et al 2007, Cloutier and Yang 2013a). A detailed description of invasive electrodes both to record biological signals and to deliver electrical stimulation can be found in Raspopovic et al (2021a).
In the last decades, growing attention has been paid to the development of intramuscular electrodes that could be implanted under the skin of the subject to achieve the advantages of invasive sensors and simultaneously avoid the risks and inconvenience of percutaneous instruments. For example, Weir et al (2008) developed an implantable myoelectric sensor (IMES), a system able to receive and process up to 32 implanted sensors with wireless telemetry. A transcutaneous magnetic link between the implanted electrodes and the external coil allows reverse telemetry, which transfer data from the sensors to the controller, commanding the control of the prosthesis, and forward telemetry to supply power and configuration settings to the electrodes. These sensors are designed for permanent long-term implantation without any kind of servicing requirement and have been tested on animals. Four months after the implantation of IMESs in the legs of three cats, the sensors were still functioning (Weir et al 2008). Intramuscular electrodes have been used in prosthetic application to decode 12 different hand gestures from 4 healthy subjects (Cipriani et al 2014). Moreover, it has been shown that the application of this invasive approach enhances the simultaneous control of multi-DoFs system (Smith et al 2014).
Recently, the group of Ortiz-Catalan showed an invasive procedure for ULP control. They positioned EMG electrodes under the skin of amputated subjects and sutured them directly on the external surface of the muscles (Ortiz-Catalan et al 2020). More precisely, sensors were sewn onto the epimysium of the two heads of the biceps' muscles and the long and lateral heads of the triceps muscles. These invasive electrodes were used in combination with an osseointegrated prosthesis, i.e. a system obtained following a very invasive surgical procedure, which allows to anchor the prosthesis to the remaining limb's bone (Ortiz-Catalan et al 2020). In the context of ULP, osseointegration is offered for trans-humeral amputees, and the prosthesis is anchored to the humerus with two mechanical elements: the fixture, a screw made of titanium placed inside a hole made in the bone that becomes osseointegrated, and the abutment, placed within the fixture and extending outside of the body in a percutaneous way, onto which the prosthesis is connected. This technique was tested on four osseointegrated patients.
This latter example indicates that also surgical approaches can be taken to improve the quality of the collected EMG. A promising surgical technique that is performed in case of high-level amputation is targeted muscle reinnervation (TMR). This method was developed by the group of Kuiken in the early 2000s and consists in transferring residual arm nerves to alternative muscle sites. Following reinnervation, these target muscles are able to produce EMG that can be collected and used to control prosthetic arms (Kuiken et al 2009). This strategy works at the condition that each reinnervated muscle produces an EMG signal in response to only one transferred nerve, with the consequence that native nerves innervating the target muscle has to be cut during the surgical procedure to avoid unwanted EMG signals (Kuiken et al 2017). In the last 15 years, TMR has allowed intuitive control of ULP to several subjects with high-level amputation for whom standard ULP devices allowed a poor restoration of motor functions (Kuiken et al 2017). Importantly, given that it is performed on complex amputations, this technique is strongly tailored to each patient's physical and clinical status (Cheesborough et al 2015, Mereu et al 2021).
Recently, a new surgical method for improving EMG-based control has emerged: the regenerative peripheral nerve interface (RNPI) (Vu et al 2020a). Just as TMR, its goal is to turn a muscle into a biological amplifier of the motor command, in order to improve the quality of the EMG signal recorded, processed and used to drive the prosthesis. To this end, RNPI exploits the regeneration capabilities of nerves and muscles, to implant a transected nerve into a free muscle graft. Following regeneration, revascularization and reinnervation by the transected nerve, the muscle graft effectively becomes a stable peripheral nerve bioamplifier, able to produce high-amplitude EMG signals (Urbanchek et al 2012). The potential of this novel interface has been tested by Vu et al (2020b): they used EMG signals collected by intramuscular bipolar electrodes implanted into RNPIs obtained in amputated individuals, who could successfully perform real-time control of an artificial hand. Surprisingly, subjects were able to control the device with a high level of accuracy even 300 d post-implantation, without recalibration of the control algorithm.
Another surgical technique, not directly related to EMG signals but worth mentioning, is cineplasty, an old method revived in the last years with a new and more modern approach. This method was introduced for the first time by Vanghetti in 1899 and then replicated by Sauerbruch ten years later (Tropea et al 2017). It consisted of the direct mechanical linking of residual muscles and/or residual tendons of the affected limb to the prosthesis through external cables (i.e. Bowden cables). In 2001, Heckathorne and Childress (2001) implemented an evolution of this surgical solution for the control of one DoF ULP by exploiting exteriorized tendons directly linked to a force sensor.
3.1.1.2. Other biosignals
The limitations imposed by the use of EMG (either invasive or non-invasive), have led researchers to study new approaches, aiming at increasing algorithms robustness and accuracy. Some may be soon used in commercial prosthetic systems, while others represent promising research scenarios, but still far from real-life applications. We here describe some of these peripheral signals, both non-invasive and invasive.
For example, forcemyography (FMG) has been widely investigated in the past 20 years (Xiao and Menon 2019) (table 1). This approach is based on force sensors able to record muscle stiffness around the forearm during different movements. The muscle deformation of the stump can be measured with various types of sensors, such as: force sensing resistors (Prakash et al 2020), optical fiber transducers (Fujiwara et al 2018), capacitance-based deformation sensors (Truong et al 2018), Hall-effect based deformation sensors (Kenney et al 1999), barometric sensors (Shull et al 2019), thin arrays of adhesive stretchable deformation sensors (Jiang et al 2019), or high density myo-pneumatic sensors for topographic maps of pressures and residual kinetic images of the stump (Phillips and Craelius 2005, Radmand et al 2016). The accuracy of the sensors may limit the robustness of FMG-based control. Therefore, FMG is often fused with other input sources, such as inertial measurement unit (IMU) (Ferigo et al 2017) or EMG (Nowak et al 2020). FMG is indeed complementary to EMG due to its capability to get information about extrinsic hand muscles placed in several layers underneath the skin, and therefore difficult to be detected with the EMG sensors. Moreover, with respect to EMG-based control strategies, FMG is not influenced by electrode shifting.
Another technique is mechanomyography (MMG), which measures the lateral oscillations, detected as low-frequency vibrations (in the range of 1–100 Hz), generated by deformation in muscle fibers actively involved in the contraction (table 1). This approach can be considered as the mechanical counterpart of EMG and it is also known as acousticmyography, phonomyography or vibromyography, depending on the type of sensor used. It can actually be based on different types of sensors, such as: low mass accelerometers (Farina et al 2008, Youn and Kim 2010), microphones (Castillo et al 2020, Meagher et al 2020), piezoelectric contact (Orizio et al 2008, Tanaka et al 2011), force sensing resistors (Esposito et al 2018), and laser distance sensors (Scalise et al 2013). With respect to EMG, this technique shows some advantages: it is low cost, it does not require pre-amplification or precise positioning, and signals are not influenced by skin impedance or sweat. However, it is very susceptible to environmental noise and motion. Artifact removal can be implemented with the integration of an IMU, as proposed by (Wilson and Vaidyanathan 2017) and (Woodward et al 2017). MMG has also been used in combination with EMG signals (Guo et al 2017a), achieving better control performance and robustness.
The sonomyography (SMG) measures muscle volume changes and thickness using reflected ultrasound waves (table 1). Wave amplitude depends on the acoustic impedance of the tissue, and it can be detected using ultrasound transducers. Currently, no portable prosthetic systems based on SMG have been developed, but the results obtained using this technique are very promising. For example, Dhawan et al (2019) were able to detect 11 different movements in real-time placing the sensor on the stump of a trans-radial amputee, obtaining better results than using EMG signals alone. This non-invasive approach allows a faster user training and the detection of both superficial and deep muscles, but even a small shift of the sensor can change the cross-section view and bring to the failure of the control algorithm. SMG signals have been used in combination with EMG signals, leading, to improved performances with respect to EMG alone (Xia et al 2019, Engdahl et al 2020a).
Near-infrared spectroscopy (NIRS) is a non-invasive technique measuring the level of oxygenation of active muscles under contraction (table 1). The detection unit consists of a near-infrared led emitter and a photodetector, placed on the skin surface. The emitted infrared (IR) light is partly absorbed by the tissue, mostly by hemoglobin, and partly scattered back to the skin surface and detected by the photodetector. NIRS thus detects changes in the amount of IR light scattered back due to muscle contraction (Schneider et al 2003). This technique has a high spatial resolution and is immune to electronic interference. However, tissue heating may take place after prolonged use. Recently, Paleari et al (2017) developed a wireless NIRS unit for hand gesture recognition, indicating the potentiality of this technique for ULP control. NIRS has indeed been used in this context in conjunction with EMG (Guo et al 2017b) and IMU (Zhao et al 2019).
The electrical impedance tomography (EIT) measures the internal electrical impedance of the tissues in the cross-section plane covered by specific surface electrodes (table 1), which may range from 8 to 64 (Padilha Leitzke and Zangl 2020). The measurement is executed by exciting a sine wave of electrical current (amplitudes ranging from 10 µA to 10 mA and frequencies from 10 kHz to 1 MHz (Grushko et al 2020)) and by recording the voltages collected by surface electrodes. The detected changes in phase and amplitude represent the distribution changes of internal conductivity within the affected area, identifying patterns of movement. Wearable systems for ULP control have been developed, such as the ones proposed by Zhang et al (2016) capable to recognize hand gestures, and by Wu et al (2018), who also tested an EIT-based hand prosthesis control system on healthy people, achieving an accuracy of 98.5% with a grouping of three gestures and an accuracy of 94.4% with two sets of five gestures. This non-invasive method does not require a precise positioning of the electrodes, it only needs changes in impedance to be large enough. On the other hand, the current available systems have slow measurement and long processing time, leading to a high-power consumption. Moreover, the technique is affected by surface electrodes issues, namely skin contact conditions, electromagnetic interference, etc.
Capacitance sensing measures capacitance variations between two or more conductors (table 1). A capacitance exists when the two conductors are separated by a given distance d. In ULP context, electrodes may be placed on the prosthetic fingers, which work as capacitor plates. When a user performs a gesture, the skin deformation will cause a change in distance (d) between the conductors. This technique was used for hand gesture prediction in (Cheng et al 2013) and in (Truong et al 2018), using wearable systems. This technique is low cost, non-invasive, and it is capable to detect deep and complex signals, but it owns the standard disadvantages affecting surface electrodes, and it is susceptible to ambient temperature changes.
Magnetomyography is a promising approach aimed at measuring the magnetic fields produced by electrical currents propagating through muscles during contraction (table 1). This technique foresees the placement of magnetometers on the muscle, either non-invasively or beneath the skin, following a surgical procedure. The magnetometers convert the magnetic fields into measurable quantities, such as currents or voltages that can be used for the control of the prosthesis. Small implantable magnetometers have been proposed in Zuo et al (2020), but they still need to be clinically tested. This technique is less sensitive to sensors' shift or sweat but may be strongly influenced by the environmental magnetic noise and the magnetic field of the Earth.
Peripheral neural interfaces measure the electrical activity of the motor peripheral with an invasive approach (table 1). There are three types of electrodes: extraneural, like CUFF or flat interface nerve electrodes (FINEs), which embrace the nerve; intraneural, which run longitudinally (longitudinal intrafascicular electrodes (LIFEs)) or transversally (transverse intrafascicular multichannel electrodes (TIMEs) or Utah slanted electrode array (USEA)) through the nerve; and regenerative, such as SIEVE or Microchannel, attached between the two extremities of a severed nerve (del Valle and Navarro 2013, Raspopovic et al 2021b). Nguyen et al (2020) enabled an amputee to control a 15 DoFs prosthesis, by using 4 implanted LIFE arrays, 2 in the medial nerve and 2 in the ulnar one. The negative aspects of this method reside in its profound invasiveness and in the acquisition of noisy signals.
3.1.1.3. Brain signals
The first neuroprosthetic application on humans was reported by the group of Donoghue, who demonstrated that tetraplegic individuals implanted with arrays of microelectrodes over the motor cortex were able to remotely control the movement of a cursor on a screen (Hochberg et al 2006). This clinical trial was soon followed by another from the same group reporting the control of reaching and grasping actions of a robotic arm (Hochberg et al 2012). The group of Schwartz also showed similar results of an individual with tetraplegia successfully controlling a seven DoF robotic arm (Collinger et al 2013). In all these examples, intracortical brain signals were used, i.e. action potentials of individual neurons were detected with an array of electrodes inserted into the brain, usually in the motor cortex (table 1).
Less invasive measurements of cortical currents using electrocorticography (ECoG) have been widely used for neuroprosthetic control in the lab. ECoG detects the electrical activity of the brain with strips of electrodes laid on the brain's surface, usually in the motor cortex area. ECoG signals have been used for hand gesture recognition (Bleichner et al 2016), for the control of a virtual prosthesis (Wang et al 2013) and of a robotic limb (Fifer et al 2013), and also with a detached prosthesis with active digits (Hotson et al 2016).
ECoG provides an ideal trade-off between the invasiveness of intracortical recordings and the poor spatial resolution of electroencephalography (EEG) (Thakor et al 2014). However, whether non-invasively collected signals convey enough motor information to control a neuroprosthetic hand is still debated (Fukuma et al 2016).
EEG measures the electrical activity of the brain with an external helmet made of electrodes (table 1). In a ULP application, a motor imagery task is typically used, and the subject only needs to think about the movement. EEG signals corresponding to the intention of the movement are therefore used to drive the end-effector. Recently, McDermott et al 2021 were able to extract from EEG recordings relevant brain states in real-time and indicated such states as prospective therapeutic targets for motor neurorehabilitation (McDermott et al 2021). Similarly, the group of Wolpaw showed that paralyzed patients could use EEG signals to control a cursor in three-dimensional space (Mcfarland et al 2010), suggesting that noninvasive EEG-based BCIs can be exploited for control of robotic devices or neuroprostheses. EEG-based neuroimaging is indeed emerging as a useful tool for robotic device control, as demonstrated by Edelman et al (2019).
Another promising technique is the functional near-infrared spectroscopy (fNIRS), which detects activity-related brain oxygenation. The instrumentation is the same used for NIRS, i.e. a near-infrared led, and a photodetector are used to measure the amount of scattered back light and, therefore, the amount of IR light absorbed by the hemoglobin in the brain, which increases during brain activity. In 2020, Syed et al (2020) used these hemodynamic brain responses to control a ULP for trans-humeral amputees with three DoFs, gaining eight out of ten classified movements in real-time.
These examples demonstrate that groundwork for brain control of motor prosthetics has been laid. However, it has been limited to the lab and mostly addresses paralyzed patients. Nevertheless, there is a growing interest in brain-derived measures for prosthetic applications and different recording techniques have been investigated for ULP control.
3.1.2. Other techniques under investigation
Besides the detection of physiological changes in residual muscles of the stump or in the brain during movements, described above, there are many other input sources and techniques capable or with the potentiality to control an upper limb prosthesis. Some of these are mainly used in the research field and since they lack usability, they do not find a real application in everyday life of amputees, or they are conceived for patients without the possibility to exploit other more convenient and intuitive sources (i.e. tetraplegic people). Some of them, instead, have been still only proposed as proof-of-concept.
The most studied approach is based on the use of IMUs. IMU sensors are cheap, small and can therefore by easily embedded in the prosthesis. They can increase the amount of data useful to successfully discriminate between different gestures of ULP during distinct phases of the reaching movement. These devices exploit accelerometers, gyroscopes and magnetometers to understand which is the actual altitude, position and orientation of the prosthesis. These sensors deliver information through quaternions and they are often used together with EMG to improve the classifier robustness (Georgi et al 2015). Zhang et al (2011) depicted the possibility to manipulate objects and perform complex tasks using both IMU and EMG sensors. As a matter of fact, the accelerometers can capture information that sEMG sensors cannot easily detect, such as hand withdrawal or rotation (Chen et al 2007). It has been shown that the use of IMU sensor coupled to EMG is more advantageous than increasing the number of EMG sensors (Fougner et al 2011). Similar results have been achieved by Krasoulis et al (2017), who have combined EMG and IMU to feed pattern recognition systems (see section 4.3.1). They demonstrated that this combination could significantly improve the real-time completion rates compared to the traditional methods, exclusively based on sEMG signals. Moreover, the data coming from IMU can be used alone to control a single module, usually the wrist or the elbow (Merad et al 2018) or to realize other types of control for rehabilitation purposes, such as shadow control, in which the control policy consists in replicating the movement captured by the IMU sensors (Rapetti et al 2020). These devices were also placed on feet to directly control an ULP by implementing precise foot movements (Resnik et al 2014). The adoption of IMU sensors is specifically promising in sensor fusion approaches, as discussed in section 3.1.3. Besides EMG signals, IMU data have been also combined with NIRS (Zhao et al 2019) and MMG (Wilson and Vaidyanathan 2017, Woodward et al 2017).
Table 2 summarizes the use of IMU and other input sources investigated for the control of ULP, many of which are described in (Grushko et al 2020).
Table 2. Alternative input sources investigated for the control multi-DoF prosthesis devices.
| Input source | Measured property | Sensors' placement | PROs | CONs | Sensor fusion | Examples |
|---|---|---|---|---|---|---|
| IMU | Specific force, angular rate, orientation of the body | Up to 8 IMU sensors located on feet | Non-invasive, simple, low cost | Problems during walking, not intuitive, unnatural | EMG, NIRS, MMG | (Resnik et al 2014) DEKA Arm control |
| Myokinetic control | Change of muscle morphology trough magnetic fields | Permanent magnet markers implanted over targeted muscles and external three-axis magnetic field sensors placed in the socket | Intuitive control, force and position feedback | Magnetic interferences, misalignments between socket and initial position, invasive | — | (Tarantino et al 2017, Clemente et al 2019) |
| Voice | Throat vibration | Piezoelectric sensor on the throat | Ease of use sequence of movements | External noise, input sound level, unintuitive control | EMG | (Mainardi and Davalli 2007) |
| Voice commands | Microphone near mouth | IMU | (Alkhafaf et al 2020) | |||
| Tongue | Pressures made by the tongue | Board of coils on the palate and activation unit on the tip of the tongue | Mobile, wireless, invisible | Unintuitive control, uncomfortable | EMG | (Johansen et al 2016, Johansen et al 2021) |
| Feet | Pressures made by the feet | Insole made of force sensing resistors | Simple low cost | Unintuitive control, problem during walking, need of accurate calibration | IMU EMG | (Carrozza et al 2007) |
| Optical myography (OMG) | Skin surface deformations caused by underlying muscle contraction | Single low-resolution camera and marker-based tracking methods | Simple low cost | No space for camera in the socket, low robustness | — | (Nissler et al 2016, Wu et al 2019) |
3.1.3. Integrative sources
An integrative input source is not used as the main responsible for the actual command of the prosthesis, but it is used to help and to facilitate its control, which usually depends on EMG signals. The integrative input sources work in parallel and together with the main ones, integrating their information and implementing the so-called data-fusion or sensor-fusion methods, see also section 4.3.3. Table 3 summarizes integrative sources found in the literature that have been used for ULP control.
Table 3. Integrative sources of information used to improve prosthesis control.
| Integrative input source | Instruments and measured information | Application | PROs | CONs | Fusion | Examples |
|---|---|---|---|---|---|---|
| Computer vision | Two cameras used to collect images and estimate depth | Estimation of size, distance and grasp type for a semi-autonomous control of the prosthesis | Ease of use fixing of errors without looking at the prosthesis automatic help in controlling the prosthesis | Expensive, cumbersome and uncomfortable | EMG IMU | (Markovic et al 2014) Stereovision (depth?) |
| Depth estimated by the color intensity of the pixel collected by the camera | (Mouchoux et al 2021) Depth and color camera RGB | |||||
| Eye movements | 4 Superficial electrodes for the measuring of the corneo-retinal standing potentials between the front and the back of the human eye | Estimation of the position/length/width/orientation of a final target and preparation of the preshape and direction of the hand | Ease of use automatic help in controlling the prosthesis | Distinction with random eye movements, cumbersome and uncomfortable | EMG | (Hao et al 2013) Electro-oculography |
| Camera mounted on a pair of glasses measuring the reflection of infra-red (IR) light from the eyeball | (Krausz et al 2020) Eye tracking glasses | |||||
| Optical sensor | Led-based optical sensor mounted on fingertips | Slip detection and eventual automatic suppression | Accurate, robust simple, low cost and power consumption | Poor detection with transparent surfaces | EMG | (Sani and Meek 2011) LED motion detection sensor |
| Miniature reflective optic sensor that combines an Infrared LED and a phototransistor in the same package. | (Nakagawa-Silva et al 2018) Reflective optic sensor | |||||
| IMU | Accelerometers, Gyroscopes, Magnetometers | Decreased #sensors, better controllability, artifact detection | Non-invasive, simple, low cost, motion artifact deletion | Prone to error cumulate over time | EMG, NIRS, MMG | (Krasoulis et al 2017, Krasoulis et al 2019b) up to 6 gestures |
3.2. Prosthetic sensing
Natural movements occur with a bidirectional flow of neural information, i.e. motor commands on one direction and sensory feedback on the other. In prosthetic applications, while many efforts have been spent to provide signals carrying motor intentions, a less explored path is the integration of sense of touch into the prosthesis (Clemente et al 2015). This lack is highly responsible for the missing perception of the prosthesis as part of one's own body and is also precluding a closed-loop control of the prosthesis.
More recently, the scientific community has started exploring different methods to equip prosthetic devices with perception of tactile and pressure information (Schmitz et al 2008, Tee et al 2012, Hammock et al 2013, Lucarotti et al 2013, Taunyazov et al 2021), although often resulting in very complex, unreliable, or unpractically cumbersome solutions. The few solutions tested on real prosthetic setups impacted on their anthropomorphism and dexterity.
To integrate touch sensors into robotic and prosthetic devices (figure 2, end-effector feedback) (Lucarotti et al 2013, Iskarous and Thakor 2019, Dimante et al 2020), different technologies have been investigated and employed (Ciancio et al 2016), namely capacitive (Jamali et al 2015, Maiolino et al 2013), resistive (Beccai et al 2005, Tee et al 2012, Zainuddin et al 2015), piezoelectric (screen printed piezoelectric polymer, PVDF) (Alameh et al 2018), and magnetic sensors (Ahmadi et al 2011). Other examples include technologies based on electrical impedance (Zainuddin et al 2015, Wu et al 2018), pressure and electrical impedance (Lin et al 2009), optical fibers (Bragg fiber (Massari et al 2019)), micro-electro-mechanical systems (texture sensing (Mazzoni et al 2020)) combined with spiking based on Izhikevich neuron model (Gunasekaran et al 2019) and optoelectronic (Alfadhel and Kosel 2015).
Examples of the application of these sensors into prosthetic devices include the E-dermis (piezoelectric sensors integrated on the Bebionic's fingertips) (Osborn et al 2018), E-skin (integrating different types of sensors) (Iskarous and Thakor 2019), and BioTac (impedance sensor integrated on the Shadow Hand (Robot 2022)) (Fishel and Loeb 2012).
Among commercial devices, the SensorHand Speed (Ottobock 2021) made by Ottobock is the only one including tactile sensors based on resistive technology (Ottobock 2021).
Therefore, tactile sensation is the first step toward novel and more efficient control strategies that do make use of feedback information (Raspopovic et al 2014). To this end, artificial intelligence can be exploited to detect the grasp of different objects from sensor data (Alameh et al 2020).
3.3. Sensory feedback
Sensory feedback patterns are designed to enrich the perceived responsiveness of the device and the subjective experience of its use as a limb (Antfolk et al 2013b, Svensson et al 2017, Raspopovic et al 2021a). Such a result derives from the elicitation of physiological and psychological reactions that promote embodiment processes (described in section 5.1). Furthermore, such stimulations (haptic feedback in many cutting-edge devices) are designed as a fundamental component of bidirectional human-machine interfaces empowering prosthetic control (Navaraj et al 2019). Establishing such a closed-loop can trigger learning processes even for artificial sensations (Cuberovic et al 2019), pointing at somatosensory plasticity processes. These phenomena provide the user with an engaging guidance within a natural interaction, facilitating the execution of prosthetic maneuvers during calibration, training, and daily use. Importantly, such an enhanced practice will ease the production of consistent biosignals that will progressively become easier to interpret as user commands.
However, current commercial prostheses generally do not incorporate an explicit haptic feedback but the incidental feedback, like visual and the sound cues, could be exploited by the user to estimate the prosthesis state (Wilke et al 2019). For example, the acoustic feedback provides a guidance on how to reach target during the rehabilitation session, in this way the rehabilitation step can be more interactive and engaging if appropriately designed (never obnoxious, possibly plausible). Overall, the next sub-sections will discuss the design of sensory feedback in prosthetics, distinguishing invasive and non-invasive stimulation modalities.
3.3.1. Non-invasive methods
Non-invasive feedback restoration for upper limb amputees is a hot topic in the research community, and yet it has not achieved broad clinical application (Sensinger and Dosen 2020). Many solutions have been proposed, but the main problem lays in their poor robustness. (Ribeiro et al 2019) highlighted the most widespread types of non-invasive feedback, described in table 4.
Table 4. Non-invasive methods for sensory feedback in ULP.
| Feedback sense | Instruments and feedback information | Application | PROs | CONs | Examples | |
|---|---|---|---|---|---|---|
| Touch (cutaneous stimulation) | Vibrational | Eccentric rotating motors, proprioception, force | Array over the forearm or over the arm | Non-invasive, robustness control, brief training period, intuitive, cheap, small | (Bark et al 2014, Markovic et al 2019) up to 3 DoFs or different force levels | Non-physiological, need calibration, coupled intensity and rotation frequency, position displacement |
| Mechanotactile | Linear actuator, pressure sensation, spatial touch sensation | Detected areas to reproduce real touch sensation, array over the arm | Non-invasive, intuitive, brief training period, decoupled intensity and frequency | (Antfolk et al 2013a, Svensson et al 2017, Tchimino et al 2021) different pression level, touch sensation | Need spatial and intensity calibration, bulky, position displacement | |
| Electrical | Transcutaneous stimulation using bipolar electrodes, pressure, slip, proprioception | Array over the forearm or arm | No electrode displacement, low power consuming, high sensor skin contact, intensity or frequency modulation | (Jorgovanovic et al 2014, Xu et al 2015, Garenfeld et al 2020) touch location, pression, proprioception | Noise during acquisition, long calibration, not localized sensation | |
| Sound (acoustic) | Acoustic speaker, proprioceptive movements | Laptop speaker to guide the training acquisition and improve the pattern recognition strategy | Low cost, no calibration, intuitive | — | (Gigli et al 2020) multiple arm positions | |
| Vision (visual) | Camera on board, external camera | Head-mounted displays, laptop displays, virtual reality, augmented reality | Increase perceptual experience, engagement, intuitive, promote training | Bulky, not portable, uncomfortable | (Clemente et al 2016, Markovic et al 2017, Sharma et al 2018, Hazubski et al 2020, Sun et al 2021b) trajectory, force | |
The most investigated feedback relies on the sense of touch and therefore consists of cutaneous stimulation. This can be performed with different modalities namely, vibrational, mechanotactile or electrical stimulation.
The vibrational feedback is generally implemented with the addition of eccentric rotating motors placed in contact with the skin surface of the stump (Ribeiro et al 2019). This method is generally employed to augment the robustness of the control system by providing the user with additional information regarding the position of the prosthetic device but it lacks intuitiveness, as the association between perceived sensation and the corresponding information has to be learned by the user. For example, in Bark et al (2014), the motors were placed in four distinct areas of the stump to guide the user through the desired trajectory while grasping object and the results showed a significant decrease in the root mean square angle error of their limb during the learning process. More recently, Markovic et al (2019) proposed a joint-oriented feedback criterion consisting of three vibromotors placed on the arm to provide the information on which joint is currently activated by the user, thus restoring proprioceptive sensation. The experiment was performed by 12 able-body subjects and 2 amputees controlling 3 DoF prosthesis, and it was found that the myoelectric multi-amplitude control outperformed the pattern recognition method when the feedback was applied.
Differently from the vibrational, the mechanotactile feedback is based on the application of linear actuators on the skin and provides pressure sensation. Antfolk et al (2013a) exploited this technique and proposed a multisite mechanotactile system to investigate the localization and discrimination threshold of pressure stimuli on the residual limbs of trans-radial amputees. They demonstrated that subjects were able to discriminate between different location of sensation and to differentiate between three different levels of pressure. This study demonstrated that it is possible to transfer tactile input from an artificial hand to the forearm skin after a brief training period. Recently, Svensson et al (2017) used it to translate the interaction between a virtual reality (VR) environment and a virtual hand into user sensation. The authors showed that by placing the tactile actuators in correspondence with the areas of the skin involved in object manipulation, subjects were able to feel a real touch sensation that increased their sense of body ownership. For example, pressure applied to the prosthetic fingers was perceived as a tactile sensation on the skin (Svensson et al 2017).
The electrical feedback is based on transcutaneous stimulation. The elicited sensations range from perception of pressure (Jorgovanovic et al 2014) to slip sensations (Xu et al 2015), depending on the electrical parameters (i.e. current amplitude, pulse frequency, pulse width). One advantage of this approach with respect to the vibrotactile and mechanotactile ones is the lack of moving components avoiding problems of electrode displacement and, thus, improving the sensors-skin contact. Nevertheless, it is important to take into account that the noise introduced by the electric stimulation can corrupt the acquisition of muscular activity, causing errors if the ULP is myoelectrically controlled. Moreover, the perceptions are not strictly confined to the zone under the stimulating device but they can spread in a wider region if the area above a nerve is considered.
Another sensory modality exploited for feedback delivery is the acoustic one. Gigli et al (2020) recently tested a novel acquisition protocol with additional acoustic feedback in 18 able-body participants to improve myoelectric control. The protocol consisted in dynamically acquiring EMG data in multiple arm positions while returning an acoustic signal to urge the participants to hover with the arm in specific regions of their peri-personal space. The results showed that the interaction between user and prosthesis during the data acquisition step was able to significantly improve myoelectric control. Auditory feedback has also been employed to convey artificial proprioceptive and exteroceptive information. Lundborg et al (1999) and Gonzalez et al (2012) employed auditory feedback by encoding the movement of different fingers into different sounds. The method demonstrated that the inclusion of auditory feedback reduces the mental effort and increase the human-machine interaction; furthermore, better temporal performance and better grasping performance were obtained.
In the last years, there have been some examples exploiting vision to deliver sensory feedback. Indeed, visual stimulation can be provided as explicit feedback through screens during game-like exercises, helping the prosthetic user to learn how to control the device (e.g. adjusting trajectory or grasping force) (Markovic et al 2018). However, adding sensory information to the prosthetic user's perceptual experience in real contexts requires solutions like augmented reality (AR, occurring when computer-generated items overlay a real setting) or mixed reality (MR, a term that represented different combinations of real and virtual items) (Milgram and Kishino 1994, Speicher et al 2019). AR and MR environments, implemented through wearable solutions like head-mounted displays, can support the actual control of a prosthetic device through visual feedback that does not occlude the real context (Clemente et al 2016, Markovic et al 2017, Hazubski et al 2020). However, they can also be used for prosthetic use training (Anderson and Bischof 2014, Sharma et al 2018)—in such a case, VR (a fully computer-generated setting) can offer visual feedback too (Lamounier et al 2010, Sun et al 2021b), especially within game-based frameworks (Nissler et al 2019) for engaging the users and motivating their activity.
3.3.2. Invasive methods
There are different technologies that can be employed to provide a sensation directly to the nerve (Cutrone and Micera 2019, Raspopovic et al 2021a). The most used employ intrafascicular electrodes, such as TIME and wire and thin-film LIFE, which can both record muscle activity (e.g. iEMG) and stimulate nerves. Other solutions are characterized by the fact that the electrodes are placed around the nerves, such as cuff electrodes and FINEs.
The first example of ULP with sensory stimulation dates back to 1979 and it was based on the remapping between pressure signals acquired by prosthesis sensors to an amplitude-frequency modulation. This consisted of a series of pulses delivered with a pulse rate proportional to the increment of the pinch force and provided through dry electrodes placed over the skin in correspondence of the median nerve, as described in Shannon (1979). Later, the group of Micera employed thin-film intrafascicular electrodes longitudinally implanted in peripheral nerves (tf-LIFE4) to deliver electrical stimulation. With this method, they were able to elicit sensation of missing hand in the fascicular projection territories of the corresponding nerves and to modulate the sensation by varying the pulse width and pulse frequency (Benvenuto et al 2010). Importantly, this method avoids muscle crosstalk, fundamental for guaranteeing myoelectric control. More recently, new bioinspired paradigms have been suggested to better induce natural sensations (Raspopovic et al 2021a). In particular, the study of Oddo et al (2016) showed that it is possible to restore textural features recorded by an artificial fingertip. This device embedded a neuromorphic real-time mechano-neuro-transductor, which emulated the firing dynamics of SA1 cutaneous afferents. The emulated firing rate was converted into temporal pattern of electrical spikes that were delivered to the human median nerve via percutaneous microstimulation in one trans-radial amputee.
Valle et al (2018) suggested a 'hybrid' encoding strategy based on simultaneous biomimetic frequency and amplitude modulation. This kind of stimulation was perceived more natural with respect to classical stimulation protocol, enabling better performance in tasks requiring fine identification of the applied force. This paradigm was tested and validated during a virtual egg test (Valle et al 2018), where the subject needed to modulate the force applied to move sensorized blocks. This encoding strategy not only improves gross manual dexterity in functional task but also improved the prosthesis embodiment, reducing abnormal phantom limb perceptions.
Similarly, Osborn et al (2018) implemented a neuromorphic feedback paradigm based on Izikevich neuron model to generate the current spike train to inject directly in the median and ulnar nerves, using beryllium copper (BeCu) probes. Their prosthesis proposes a neuromorphic multilayered artificial skin to perceive touch and pain. Their transcutaneous electrical nerve stimulation allows to elicit innocuous and noxious tactile perceptions in the phantom hand. The multilayered electronic dermis (e-dermis) produces receptor-like spiking neural activity that allows to discriminate object curvature, including sharpness in a more natural sensation spanning a range of tactile stimuli for prosthetic hands. The authors were able not only to restore finger touch discrimination and objects recognition, but also to provide a pain sensation when the prosthesis touched sharp objects. In particular, they found that pain sensation is generated by a stimulation of 15–20 Hz.
Tan et al (2014) suggested that simple electronic cuff placed around nerves in the upper arm can directly activate the neural pathways responsible for hand sensations. This neural interface enabled the restoration of different sensations at many locations on the neuroprosthetic hand. Different stimulation patterns could transform the typical 'tingling sensation' of electrical stimulation into multiple different natural sensations, enabling the amputees to perform fine motor tasks and improving the embodiment.
In George et al (2019) a biomimetic method was described to restore both force and haptic sensation. The sensory feedback was implemented to restore the force sensation and promote objects recognition: USEA electrodes were used to deliver stimulation proportional to the variation of contact force exchanged between the prosthesis and the object during manipulation. Instead, the haptic sensation was based on the distribution of stimulation delivered during contact with the object with a fixed frequency and amplitude. The characteristic of this encoding scheme is based on electrical biphasic, charge—balanced of 200 or 320 µs phase durations. The biomimetic model describes the instantaneous firing rate of the afferent population using the contact stimulus position, velocity, and acceleration simulating all tactile fibers to any spatiotemporal deformation of the skin and hand. This strategy allows the amputee to augment the active exploration experience and to discriminate object size and stiffness.
Liu et al (2021b) have shown that primary afferents encode different stimulus features in distinct yet overlapping ways: scanning speed and contact force are encoded primarily in firing rates, whereas texture is encoded in the spatial distribution of the activated fibers, and in precisely timed spiking sequences. When multiple aspects of tactile stimuli vary at the same time, these different neural codes allow for information to be multiplexed in the responses of single neuron and populations of neurons. Exploiting this sensory architecture with invasive methods may lead to the development of prosthetic devices able to truly evoke natural sensations.
Another promising approach is targeted sensory reinnervation (TSR), i.e. the sensory version of TMR, which consists in coupling a pressure sensor placed on the prosthetic device to surgically redirected cutaneous sensory nerves (Marasco et al 2011). This technique strongly helps discrimination of objects size and stiffness during active exploration, especially if the tactile feedback is biomimetic (George et al 2019). Recently, Marasco et al (2021) have developed a prosthetic system based on both targeted sensory and motor reinnervation. TSR was used to deliver both touch and kinesthetic feedback. The authors showed that the system was able to significantly improve device control and promote embodiment.
These results indicate that, in order to close the loop on user and provide useful sensation (regardless the specific feedback modality), an optimal feedback control policy is necessary (Sensinger and Dosen 2020), as discussed in section 4.4.
4. Prosthetic control strategies and algorithms
Although the focus of this section is on the active prosthesis, it is worth mentioning that an important portion of the amputees still uses body-powered prosthesis (Carey et al 2015). These are cable-operated devices usually equipped with split hook or hand as terminal part (Millstein et al 1986).
Ranging from standard control approaches (e.g. dual-site control (Scott and Parker 1988)) to simultaneous control of multiple degrees of freedom (e.g. pattern recognition (Hahne et al 2018)), the literature offers disparate solutions for ULP control depending on the type of input signal and the sensors density.
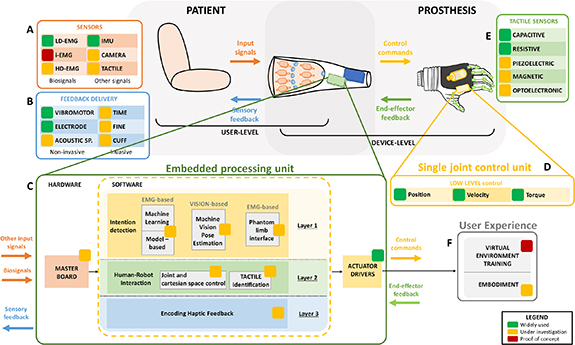
In general, prosthetic control is performed at different levels. The low level refers to motor actuation (figure 5(D)) and, more in general, to the control of the active degrees of freedom of the device; the medium level consists of the translation of movement intentions into joint references and gestures (figure 5(C)); the high-level control translates input signals collected from the user (figure 5(A)) into movement intentions (figure 5(C), yellow panel—layer 1). In the next sections, we describe these different levels of control and provide examples of the different strategies that can be used.
Figure 5. Architecture of ULP control: actuation and feedback. Input signals collected from the user (A) are processed into the embedded processing unit (C) to generate control commands for the single joint control unit (D). Feedback information coming from the prosthesis or its interaction with the environment (E) are also processed in the embedded processing unit (C) to deliver sensory feedback (B). The embedded processing unit (C) can be set up by different layers: layer 1 (intention detection, yellow panel) is the software turning the input signals (A) sampled by master board into detected movement intentions, by means of specific control algorithms (e.g. machine learning or deep learning (DL) algorithms); layer 2 (human-robot interaction, green panel) is the software responsible of processing prosthesis position (joint and Cartesian space control) and external information (tactile identification, (E)); layer 3 (encoding haptic feedback, blue panel) is the software responsible for encoding the information processed in layer 2 into sensory feedback. The output of the embedded processing unit are control commands (mediated by actuator drivers) both to move the device and to provide sensory feedback. This has a direct impact on the user experience (F) in terms of learning how to use the device (training) and of user-prosthesis integration (embodiment).
Download figure:
Standard image High-resolution image4.1. Low-level control: from control commands to motor actuation
The low-level control combines the well-known strategies implemented in the automation industry to operate autonomous machines, e.g. industrial robots. We will not detail the structure and mathematical formality of these control architectures. However, if the readers are curious, a more complete and detailed analysis of robot lower-level control is provided by the comprehensive work of Siciliano et al (2010).
In brief, at the base of these controls, there is always an active and controllable actuator, that for upper limb prosthetic solutions coincides—most of the times—with an electrical motor (either brushed or brushless) often coupled to a dedicated transmission system (e.g. a planetary gear) to reach the desired torque-speed characteristic. It is possible to present the low-level control of ULPs as the combination of three possible nested controllers: the current, the speed and the position control loops (figure 5(D)).
The current control loop takes care of reliably tracking desired current trajectories. To be implemented, it requires the presence of reliable and precise current measurement sensors. The current control also provides a relatively good force/torque control of the system, being the current absorbed by the actuator directly proportional to the generated output torque. On top of the current controller, it is usually found a speed control loop to regulate the rotational speed of the motor and, thus, the speed of the actuated system. The combination of an external speed controller with an internal current control guarantees the possibility of safely operate the actuating unit in terms of desired speeds and torques. Sometimes, on top or in substitution to the speed controller, systems also implement a position control loop. The position controller guarantees the tracking of desired angular trajectories. It is therefore preferable to use the speed controller if the goal is to precisely track given trajectories in specific time intervals. The implementation and application of speed and position controllers can be performed either before (fast shaft) or after (slow shaft) of the transmission system. The decision depends on the availability of sensing devices (e.g. angular sensors such as encoders or resolvers) to measure the required physical quantities.
All these controllers are implemented in a negative feedback architecture and typically controlled by means of PID controllers, whose proportional (P), integrative (I) and derivative (D) parameters are tuned to reach the desired system response in terms of control reactivity (rise time and settling time), precision (steady-state error and overshoot) and stability. It is worth mentioning that a negative feedback architecture is typically only bounded to the low-level control of the prosthesis, while higher level controllers and especially high-level control (see section 4.3) are often treated in an open-loop fashion, where the user directly generates the reference control signal without any feedback verification. The generated reference commands will then be directly sent to the low-level controller.
4.2. Mid-level control: from movement intention to control commands
The mid-level techniques (figure 5(C), yellow panel—layer 1) aim to synthetize the control commands to suitably activate the electric motors of the multiple DoFs ULP (actuation drivers in figure 5(C)). These signals are the input of the aforementioned low-level control.
A major classification of the mid-level control strategies for multi-DoFs robots divides them in two categories: joint-space and task-space (Cartesian) controllers (Siciliano et al 2008, Corke and Khatib 2011).
Joint-space control strategies directly feed the commands to each of the actuated joints, namely DoFs, of the upper-limb robotic device. It is a direct approach that does not require any particular mathematical manipulation. In such a scenario, the mid-level control receives information from the high-level (see section 4.3), then it assigns specific commands to each low-level controller (see section 4.1). The logic used to assign the control commands is strongly based on the kind of information coming from the high-level side. Nonetheless, it will most likely reduce to a set of independent commands for each of the actuated joints.
On the other side of the spectrum, we have task-space based control strategies. In this case, the control commands for each of the joints are the results of a mathematical manipulation that involves the transformation from the Cartesian space to the joint space. If the aim is to regulate the Cartesian trajectory, the controller will need to translate the Cartesian trajectories into joint angles, by means of a process known as inverse kinematics. If instead the aim is to regulate the Cartesian force, the controller will transform the Cartesian forces into joint forces (or torques) utilizing the process of inverse dynamics.
Both these approaches are well known to robotic applications and will not be treated in detail in this review. Nonetheless, the authors suggest the comprehensive works of Corke and Khatib (2011) and Siciliano et al (2008) to get the fundamentals of the aforementioned approaches.
In general, Cartesian based controls are more intuitive for the external user, namely any subject interacting with the robot as an external tool. In fact, the robot behavior can be more naturally interpreted being the forces or the trajectories referred to the three-dimensional space we are used to deal with. However, from a computational and complexity point of view, task-space controllers require a bigger effort and introduce limitations to their application, e.g. singularities, redundancies. On the other hand, joint space control behavior is less intuitive to predict but it is easier and less complex to implement.
Which approach is better for upper-limb prosthetic devices is still unclear. However, it is important to notice that, even if Cartesian controls are more intuitive from an external perspective, they might appear more complex from an internal perspective, such as the one of a prosthesis user, where the motion of the arm is more likely imagined in terms of joint motions and not Cartesian ones.
4.3. High-level control: from input signals to movement intentions
This section summarizes the most assessed techniques for ULP control. Considering the prostheses available on the market but also the research activities, the main input source exploited to control such devices is the EMG. On the basis of the EMG type multiple control strategies can be employed, and the last decades of studies on active prostheses mainly focused on the control strategy design and development.
The most common control strategy is based on dual site control which consist in two electrodes placed in two antagonist muscles (Scott and Parker 1988). This solution allows the control of the motor in two directions according to the muscle amplitude of the selected electrodes. The synthetized reference usually is proportional to the amplitude of the muscle signal in term of speed or force. With the introduction of multiple DoFs, a co-contraction strategy has been implemented to switch between controlled joints (Resnik et al 2018). This allows the control of a single DoF at a time using two electrodes as in dual-site control. When both muscles are simultaneously contracted the control signal switches the joint to be controlled. This is a simple solution yet unnatural and lacking intuitiveness.
Another diffused strategy to control prosthesis with multiple active DoF is the finite state machine (Moon et al 2005). Commercially available ULPs implement this strategy to switch the position of the thumb to reproduce different types of grasp (Ottobock 2020a, 2020b, Ossur 2020b). For example, the Michelangelo hand allows to switch the thumb position when a signal of opening is triggered with the hand in a fully opened configuration (Ottobock 2020b).
With the aim of increasing the number of controlled DoFs, many different methods were proposed, such as muscle synergies, feature extraction (FE), multi-amplitude threshold control and machine learning methods. Muscle synergies capture muscle activation invariance during motor production and can be exploited as control variables for ULP, with aim of obtaining a biomimetic human-like behavior (d'Avella and Bizzi 2005). The main idea is to extract motion primitives from muscle synergies and combine them to generate complex arm movements (Jiang et al 2013, Liu et al 2021a). Furui et al (2019) propose a biomimetic control based on muscle synergies to extract motion primitives and combine them to generate complex movements. FE methods foresee the computation of some EMG-based metrics that reflect movement intentions (Guo et al 2015). Multi-amplitude threshold methods work as dual-site control, but they associate different amplitudes of the input signal to different DoFs (Markovic et al 2019). Although robust, these techniques are poorly used because they lack intuitiveness (Markovic et al 2019). Machine learning methods will be described in the following paragraph.
4.3.1. Machine learning algorithms
Figure 6 illustrates the main machine learning methods employed for ULP control. These methods generally solve a pattern recognition problem in which, given the input signal, an output movement have to be identified.
Figure 6. Division of machine learning approaches for ULP control.
Download figure:
Standard image High-resolution imageThe first Pattern Recognition (PR)-based control schemes arose around the second half of 1960s (Scheme and Englehart 2011). In this configuration, the acquired EMG signals are elaborated by the controller to determine the action to be performed by the prosthesis. The five pillars of this computation process are: pre-processing, data segmentation, FE, classification, and post-processing. Each step is briefly described in table 5.
Table 5. Pattern recognition steps.
| Pre-processing | During this phase, the incoming signals are firstly filtered to delete the interferences, such as acquisition noise and artifacts. |
| Data segmentation | This process divides the signals into time-windows, overlapping or adjacent (Parajuli et al 2019). |
| Features extraction | It reduces the signal information into a set of representative features in time domain (e.g. variance, zero crossing, etc.), frequency domain (e.g. mean frequency, spectral properties, etc) or time-frequency domain (e.g. the wavelength transform, an alternative to the traditional Fourier Transform useful for noise-removal and data compression (Hartwell et al 2018)), as described in Boostani and Moradi (2003). Importantly, this part can greatly affect the computational costs. |
| Classification | This is the crucial step for the classifier, where the controllers recognize and classify the signals input information and generate an output for the actuators. |
| Post-processing | It has the main goal to reduce as much as possible the misclassification. An example is the majority vote strategy, in which the current output is calculated on the previously most recognized class. The majority vote scheme is used for eliminating spurious misclassifications caused by too short windows on which the most recurrent class is selected; it employs the previous classification results and evaluates the current output on the basis of the previously most recognized class (Englehart et al 2003). |
EMG-based pattern recognition controllers are now investigated by many groups and are even available in commercial prostheses (Coapt 2017, Ottobock 2019, i-biomed 2021).
The PR-based controllers apply linear and non-linear methods to classify the EMG signal into a possible large number of movements. The two main families of classification methods used in this context are regression (Hahne et al 2014) and classification techniques (Hudgins et al 1993). While the former is usually simple to implement and train, the latter are generally more difficult to employ. The embedding of neural networks (NN) in an ULP strictly depends on the structure of the algorithm (number of layers and neurons), since complex architecture requires high computational effort (Hagan et al 1997).
Statistical regression models usually produce good results in terms of high accuracy percentages. However, the out-of-laboratory results are particularly poor, because these techniques are extremely sensitive to changes of the input signals (Parajuli et al 2019). Motivated by this issue, in the last decade, many groups focused on classification-based techniques to implement more reliable decoders. Importantly, training classifiers requires longer than training linear models, however, the formers can achieve better results during real-time execution. Different classifiers have been exploited in ULP control such as support vector machine, regularized least squares, whereas the gold-standard is the linear discriminant analysis (Scheme and Englehart 2011, Cloutier and Yang 2013b, Di Domenico et al 2021). Among NN, the most common architecture is the multi-layer perceptron (MLP) (Amrani et al 2017, Shahzaib and Shakil 2018). The MLP is a supervised machine learning (ML) technique, which exploits labeled data to train the algorithm. It is characterized by three types of layers: input, hidden and output layer. The first one contains the same number of neurons as the input signals (for example, features extracted from EMG signals), the second stage can have one or more layers where there are all the trainable neurons, while the last layer comprises all the output nodes representing the results (for example, classification likelihood of each class of movement). Neurons of a certain layer are fully connected to the neurons in the next layer via nonlinear activation functions. However, as for the regression algorithms, the performance results obtained in the lab are not easily replicated in the real-life scenario. Moreover, the complexity of the controlled prosthesis (e.g. the number of DoFs) corresponds to a higher number of neurons in the NN, with important consequence not only on the computational burden, but also on the memory consumption.
When considering an increase in the number of controllable DoFs, current pattern recognition approaches demonstrated poor performance (Piazza et al 2020). As a matter of fact, to enhance the classification rate (i.e. number of correctly recognized movement) a greater content of information should be handled. The higher the amount of input data, the more complex would the ML algorithm be.
Therefore, HD-sEMG can be exploited to increase the amount of muscular information but this comes at the cost of higher computational burden. It has been proven that the use of this type of data can be helpful in increasing the robustness against electrode shift (Pan et al 2015), allowing an improvement of the classification by exploiting spatial images of the muscular contractions (Geng et al 2016), and for retrieving measures of motor unit potentials, which can be difficult to assess without invasive techniques (Merletti et al 2008).
Different techniques can be exploited to extract motor units' activity from the HD-sEMGs. The main used decomposition algorithm is the blind source separation (with the Convolution Kernel Compensation described by Holobar and Zazula (2007)) which seems to be the most suitable since it does not make any prior assumptions on the action potential shapes. The main problems related to this technique is the lack of a useful output for the prosthesis control, since the decomposition provides an extraction of principal features of the EMG signals. On the one hand, the algorithm returns reliable information about neural activity, but, on the other hand, it increases the computational burden required to the system. Indeed, the Holobar algorithm has been used together with ML algorithms to control robotic arm in real-time (Barsakcioglu and farina 2018).
Another approach includes the exploitation of ML algorithms where the input EMG signals are considered as numeric values and the definition of the output is based on a Black Box technique. Therefore, the mathematical tools contained in the Black Box do not take into account the biomechanics of the amputated limb and they are not specific for prosthetic applications.
It is relevant to feed the ML algorithm via a set of EMG signals (muscular patterns) specific for different prosthesis movements in such a way that the classifier does not misclassify. However, it is not always feasible to acquire the same signals for each movement due to different sources of errors (i.e. muscle fatigue, sweating, electrode misalignment). Indeed, more complex classifiers belonging to the DL field are exploited to make the control more robust. A possible application can be the use of convolutional neural network (CNN), which exploits dimensionality reduction to extract complex features from the activation maps of the HD-sEMG without dramatically increasing the computation time (Olsson et al 2019). This type of algorithm is also ideal for increasing the number of DoFs (and therefore the number of classes to be recognized) while keeping a quite high accuracy rate (Hartwell et al 2018). Moreover, Zhai et al (2017) has proved that the exploitation of CNN can help in removing issues of daily life noise, updating its feature map to include this new information, avoiding the need of periodical readjustment.
Adaptive technique based on reinforcement learning (Vasan and Pilarski 2017, Wu et al 2022) has been recently investigated, with the aim of facilitating the learning process of prosthetic use. This approach is promising as it points towards the development of a 'human–prosthesis symbiosis in which human motor control and intelligent prosthesis control function as one system', as defined by the group of Huang et al (2021).
Other DL algorithms take into account time series with feedback loops with prior hidden layers (Sun et al 2021a). This architecture allows storing the history of the input signals by considering the information of previous time instants, also resulting in performance improvements with respect to simpler DL architectures (Amado Laezza 2018).
Recently, novel DL strategies have also been proposed for ULP: recurrent NNs process temporal or sequential information; temporal convolutional networks take advantage of a one-dimensional convolution layer running along the time dimension to learn the time dependence of a given input signal (Li et al 2021); transformers are attention-based architectures applied to HD-sEMG data (Burrello et al 2022, Montazerin et al 2022).
Overall, the main problem related to ML applied to the bionic field is the evident gap between the results observed in a closed safe environment, such as a laboratory, and in real daily life (Resnik 2011).
4.3.2. Model-based approaches
To overcome the limitations of ML algorithms for ULP control, some groups investigated the model-based approach, which consists of an accurate description of the muscles and bones involved in the movements starting from the Hill model of muscle fiber (Winters 1990). For example, the neuromusculoskeletal model extracts from the residual EMGs the activation dynamics of the limb (Pan et al 2018, Zhao et al 2022). The activation dynamics combined with the kinematics of the limb produces the contraction dynamics. This consists of the modification of fiber length involved in the motion along the specific DoFs. In particular, Sartori et al (2018) implemented a control strategy based on the physiology and kinematics of a real hand and tested it with an amputated subject performing some complex grasping tasks. This approach needs a calibration step to scale the model to the subject specific activation EMGs. Results showed great stability over the noise introduced by sensors or movements artifacts. Moreover, the amputee was able to reproduce simultaneous multi-DoF gestures. The limitation of this approach is its susceptibility to electrode shift and fatigue condition that affects the EMG acquisition. The real-life scenario is yet to be tested, but preliminary results appear very promising (Sartori et al 2018).
4.3.3. Sensor/data-fusion and other techniques
For ULP control using different input sources together with or without EMG signals, other methods can be adopted. In case of force myography, the same algorithms used for EMG input can be applied. For example, machine learning techniques can be used to analyze and synthetize output starting from FMG input (Cho et al 2016). The adoption of other input signals different from EMG clearly requires the implementation of ad-hoc methods for their processing. For example, voice control introduces audio analysis method to detect and translate command into prosthetic movements (Mainardi and Davalli 2007, Alkhafaf et al 2020). Further, tongue control allows the motion of the prosthesis using a wireless controller resembling a dental retainer and providing the functionality of a wireless joystick or keyboard (Johansen et al 2016).
The high complexity of ULP control has led to the development of sensor fusion approaches, in which input signals of different nature are simultaneously collected and then processed to estimate the intended movement more reliably and accurately.
On low-density sEMG, we can find robust and semi-autonomous control solutions based on custom multi-amplitude algorithms, as those implemented on the Michelangelo hand with closed-loop multi amplitude control (CMAC) (Markovic et al 2019, Mouchoux et al 2021). The adoption of IMU sensors may lead to further improvements, such as the automatic adaptation to unexpected external factors, including sweat, muscle fatigue, mental stress, electrode re-positioning and weather conditions. The state-of-the-art algorithms have to cope with these challenging issues. Therefore, the combination of EMG and IMU as input to a classifier could provide useful localization information of the hand position, which could delete possible false positives, actively improving the obtained accuracy (Krasoulis et al 2017, 2019b). Moreover, it has been observed that integrating EMG, IMU and artificial vision sensors could benefit both the classifier accuracy and the increment of available DoFs (Mouchoux et al 2021). Other promising research advancements demonstrated that mixing EMG with FMG could lead to an improved multi-DoFs control as proposed by (Nowak et al 2020). Similarly, Jiang et al (2020) proposed a sensor fusion approach among EMG and FMG. Moreover, by fusing FMG and IMU, other interesting results were presented by (Ferigo et al 2017). In addition, other research activities treated NIRS fused with EMG (Guo et al 2017b) and IMU respectively (Zhao et al 2019).
In conclusion, a data fusion aims at compensating some of the main limiting factors of single input approaches (such as EMG-based or others) as these latter suffer from artifacts, electrodes shift, etc.
4.4. Control strategies for the sensory feedback and closed-loop approaches
Recent developments in the prosthetic field have focused attention on sensory feedback restoration. In particular, many groups began studying how to provide the user with information about the interaction between the prosthetic system and the physical world. This information needs to be collected (figure 5(E)), processed (figure 5(C), green panel—layer 2) and encoded into control signals (figure 5(C), blue panel—layer 3) for the feedback system (figure 5(B), e.g. vibromotors, electrostimulation, etc).
The control strategy implemented to encode this information depends on the type of sensation to restore as, for instance, tactile feedback (pressure, temperature, pain) or proprioception feedback (gestures, joint movements). To this aim, different solutions have been developed.
Mamidanna et al (2021) focused their research activity on the force feedback that the prosthesis applies to the grasped objects by using vibromotors attached to the forearm skin. To do that, an encoding scheme of the current absorbed by the prosthetic motor was translated into vibromotors amplitude. Other sensorized solutions have been developed to directly translate the prosthesis interaction to user sensation like artificial skin able to translate the distribution of pressure and intensity to tactile and pain sensations on users with invasive interfaces (Jiang et al 2019). Similarly, Markovic et al (2019) implemented a proprioceptive feedback translating prosthesis movements into vibration orientation and shape to be intuitively interpreted by users.
In addition to prosthetic feedback, some groups are working on user feedback in terms of providing information about how the prosthesis is controlled by means of closed-loop approaches. For example, Schweisfurth et al (2016) have tested on amputees a ULP system in which EMG input used to drive the prosthesis was translated into intensity of vibromotors activation. In this configuration, the amount of EMG activity detected is directly proportional to prosthesis grasping strength and to intensity of vibration amplitude. In another work, the control commands generated by the user and translated into joint angles were encoded as proprioceptive information delivered through electrical stimulation (Garenfeld et al 2020). This allowed user to understand if the intended control command was correctly detected by the algorithms.
Similarly, Tecnalia developed a ULP system with sensory feedback by merging into a unique device EMG acquisition and electrical stimulation (Štrbac et al 2016). Although this solution significantly reduced the problem of encumbrance, it still faces some issues mainly related to the artifacts that the stimulation produces on the EMG signal and that cannot be removed using standard signal processing algorithms (Li et al 2019).
As for decoding of movement intention from input signals, the interpretation of feedback information needs a calibration procedure aimed at familiarizing the user with the ULP device. In this context, it is fundamental to guide the user to: (a) produce the correct input signal to perform the desired movement, and (b) to intuitively convert the feedback signal into useful information for motor planning. A user-centered approach can maximize and speed-up the learning process, as detailed in next section.
5. User-centered solutions in upper limb prosthetics
As observed in previous sections, multiple efforts in research and development offer heterogeneous technological solutions to enable a proficient control of an ULP device. However, selecting a sub-set of these solutions is compulsory for implementing and validating them. Accordingly, this section will discuss user-centered solutions based on the technologies described in the previous paragraphs, highlighting the opportunity of overcoming a separation (often, an opposition) between (for instance) user-centered features and technical ones, or between ADL-related performance and biomimetic one. Nevertheless, we decided to proceed in the selection of each solution by pragmatically moving from one perspective toward the other.
Fundamentally important criteria for performing such a selection should come from the analysis of the prosthetic user experience (figure 5(F)). Indeed, special attention should be paid to the user needs in order to promote the daily use of prosthetic devices, a prerequisite for checking the validity of any technological solution presented in previous sections.
In particular, the prosthetic technology acceptance constitutes a dramatic issue in this domain. Overall, ULP technology acceptance (Longfellow 2014) is tied to several interdependent functional factors related to ease of use (sensory feedback, control), dexterity (motion complexity, force output, actuation speed, manipulation), body integration (anthropomorphism, autonomy, weight), technology transfer (cost, reliability). Further factors embrace several domains, namely clinical (age, level of amputation, fitting timespan), cultural (education, social conditions, living environment, country development), and personal (psychological attitudes, subjective expectations, occupation, activity, environment).
Low acceptance can contribute to the abandonment of a prosthetic hand, erasing any chance of improvement in the control skills of the users (Castellini 2020). Thus, it is important to promote an intrinsically motivated and continuous ULP practice, which must be experienced by the users as immediate and rewarding in order to achieve high degrees of technology acceptance (rodgers et al 2019) and integration (Shaw et al 2018). The users must also feel engaged enough to surpass the impact of feeling social stigma or the doubts on the functional impact of the system on daily life. Intuitive patterns of system control play a critical role in this context to facilitate a spontaneous use of the system and to improve the user experience (Krasoulis et al 2019a).
Obviously, the absence of appropriate acceptance, usability, and user engagement creates a barrier for the introduction (and the further development) of any technological improvement in prosthetics.
5.1. Toward user-centered upper limb prosthetics
In order to improve the ULP acceptance, different approaches can be adopted, especially in terms of user research (Figliolia et al 2019). A review of Cordella et al (2016) provided a rich set of guidelines for enhancing the prosthetic hand technology acceptance through the analysis of the user requirements, considering literature and case studies like Luchetti et al (2015). Among these requirements: the capability to accomplish basic grasping actions during activities of daily living with minimal visuo-attentional focus, high dexterity, appropriate strength control; biomimetic features of sensory feedback and anthropomorphism; duration and reliability of the device and its component; technical features with impact on comfort like heat dissipation and motor noise reduction.
All features must be designed according to individual preferences. These preferences can depend on demographic factors, type and level of amputation, pain symptoms, and type of prosthesis (e.g. body-powered or myoelectric) (Biddiss and Chau 2007, Biddiss et al 2007, Davis and Onge 2017, Uellendahl 2017, Smail et al 2020, Kerver et al 2020). The amputees' preferences must also be investigated to design virtual and augmented environments for prosthetic use training (Garske et al 2021b). If appropriately devised, game-like engaging exercises can motivate the user to train, feeding the prosthetic with consistent biosignals that efficiently represent different types of grasps, an advantageous condition for ML based control (Tabor et al 2017). This can possibly happen with a successful generalization if the training is adequately designed with solutions like task switching (Heerschop et al 2021). Overall, the training designers should focus not only on playfully engaging the user to train the muscles, but also on accurately representing prosthetic use tasks to enable the related skills transfer (Garske et al 2021a). Furthermore, the parameters of meaningful and, possibly, ecological interactive settings can be experimentally controlled by the clinician or the researcher (Resnik et al 2011, Bouwsema et al 2014, Markovic et al 2017, Paljic 2017, Nissler et al 2019, Boschmann et al 2021, Phelan et al 2021). In addition, interactive settings can be adjusted to the individual needs and reactions. To understand the individual needs in prosthetic use (training and daily activities), the improvement of user research methodologies themselves becomes a priority to promote effective co-creation frameworks. Recent works (Jones et al 2021) described surveys and workshops to investigate the point of view of amputees and all stakeholders (clinicians, academics, experts and managers in industry and charity), observing a gap between laboratories and the real life of prosthetic users (whose issues are typically misrepresented by media too). Interestingly, initiatives like the Cybathlon competitions for assistive and prosthetic technology users are also devised for overcoming such a gap (Riener 2016).
The users' involvement in iterative activities of design and evaluation of products and of product services is highly important (O'Sullivan et al 2017). Such activities must be planned for checking and improving the usability of prostheses as medical devices according to the international standards (Pelayo et al 2021) and for estimating the impact of user experience on the technology acceptance (Longo 2018, Lah et al 2020). Obviously, user-centered evaluation methodologies and metrics must be adjusted to the specific case of ULPs (Resnik 2011, Zahabi et al 2019), especially considering how their user interface is not based just on buttons, plugs, and leds and their behavior and feedback are eminently biomimetic in hand-like manipulation tasks.
The functional resemblance of the ULP design to a real hand is a wise strategy for promoting a positive interaction between user and prosthesis. Such an approach (implicitly and explicitly) aims at building artificial limbs that are spontaneously used by the amputees as their own. Such a 'prosthetic ownership' experience is deeply investigated within the domain of the embodiment research, crossing disciplines like cognitive psychology and robotics according to the roadmap in Beckerle et al (2018).
The embodiment phenomenon can be constituted across its components, i.e. self-location, ownership, and agency, by the sensation that an artifact is integrated in one's body scheme (Kilteni et al 2012, Maimon Mor and Makin 2020, Toet et al 2020). Overall, the technology embodiment promotes intuitive control with improved user experience and acceptance (Makin et al 2017, Nelson et al 2020, Toet et al 2020). About ULPs, the embodiment improves: (a) movement control (Grechuta et al 2017), (b) object discrimination and manipulation (Tan et al 2014), (c) manual accuracy and sensitivity. Furthermore these processes contribute to: (d) the reduction of the phantom-limb pain (Page et al 2018) and (e) the mitigation of the risk of prosthesis abandonment (Mcdonnell et al 1989, Beckerle et al 2019).
Obviously, we must ponder how to measure and to stimulate the prosthetic embodiment. Overall, the embodiment evaluation is typically entrusted to methods (questionnaires, biosignal analysis, proprioceptive drift) based on the rubber hand illusion (RHI) studies (Botvinick and Cohen 1998, Tsakiris and Haggard 2005, Ehrsson et al 2008, Romano et al 2021). RHI can be also implemented on its different versions—e.g. virtual hand illusion (Pyasik et al 2020, Beckerle 2021) and robotic hand illusion (Romano et al 2015, Huynh et al 2019). However, these methodologies are still debated in cognitive studies (Gallagher et al 2021) which show the complexity of the processes underlying the embodiment itself.
Understanding such processes is required for designing appropriate strategies to enhance the embodiment of an artificial limb. First of all, it must be said that daily prosthetic practice, individual characteristics (like the cause of limb absence), and multisensory feedback congruency play fundamental roles in this process, which does not necessarily require cosmetic improvements or specific control patterns (body-powered or myoelectric) (Dornfeld et al 2016, Engdahl et al 2020b, Moore et al 2021, Zbinden et al 2021). Embodiment training strategies can also be explored in virtual and augmented settings (Barresi et al 2021), even if the generalization of their effects to actual prostheses must be investigated. Importantly, establishing optimal techniques to promote the embodiment of an artificial limb is a way to fully engage the user in exploring the prosthetic device and its potential, further improving its embodiment too within a virtuous circle.
However, it is necessary to consider what technological challenges must be faced for achieving a truly 'biomimetic' experience as the prosthetic embodiment to improve the use and the acceptance of an artificial limb.
5.2. Promoting prosthetic use and acceptance through improved mechatronics and control
To improve ULPs, two main classes of approaches can be taken: one focusing on mechatronic development and the other on control implementation. From the mechatronic side we suggest optimized actuation, anthropomorphism, human-like grasping behavior, and biomimetic performance as key factors to take into account for promoting ULP use and acceptance, while from the control perspective we identify robust control strategies and use of smart prostheses. Moreover, we believe the inclusion of multimodal sensory feedback a fundamental prerequisite of next generation prostheses. Each approach contributes to approximate the prosthetic user experience and the user-prosthesis system performance to, respectively, the sensations provided by a natural limb and its spontaneous and effective usage. However, all approaches face issues that must be solved in order to obtain a robustly controlled prosthetic system easily accepted by the user. These approaches thus constitute research challenges, summarized in table 6 with their actual potential solutions.
Table 6. Current issues affecting ULP mechatronics and control.
| Challenge description | Current and possible solutions | |
|---|---|---|
| MECHATRONICS | Optimized actuation | |
The actuation architecture (i.e. the number of actuators employed) influences the overall performance of the device in terms of:
| There are two possible strategies to optimize the actuation of ULPs. From a qualitative point of view, a fully actuated system might lead to independent control of each single joint thus allowing to replicate the full amount of gestures of a real hand. However, this solution is typically characterized by a heavier and bulkier or poorly performing system. Moreover, this configuration prevents the use of power motors to generate human-like grasping strength. On the other hand, an underactuated device might guarantee compactness, light weightiness and the possibility to achieve more efficient actuation and therefore higher performance, at the cost of passive uncontrolled degrees of freedom. Both actuation solutions are still affected by acoustic noise during prosthetic movements and this constitutes room for improvement for future development. | |
| Anthropomorphism | ||
| Anthropomorphism also represents a key design feature for an ULP. In fact, users are more prone to adopt and utilize anthropomorphic devices that anatomically and functionally resemble their missing limb as much as possible (Varol et al 2014). | In two useful reviews on mechanical and anthropomorphic aspects of prosthetic hands, Belter and colleagues proposed a list of guidelines to achieve, by mechanical and mechatronic means, the desired hand anthropomorphism in terms of size, weight, shape and kinematic capabilities (Belter and Dollar 2011, Belter et al 2013). The group of Metta proposed a systematic approach to benchmark different robotic and prosthetic hands in terms of shape, feature and performance, observing a continuous need for weight, payload and generic grasps improvement while maintaining an anthropomorphic appearance (Vazhapilli Sureshbabu et al 2019). However, it should be mentioned that a minority of ULP users do not recognize anthropomorphism as priority, focusing their needs on functionality. In some cases, the ULP is deliberately unconventional and worn as a fashion gadget or stylized wearable art pieces (De Oliveira Barata 2021). | |
| Human-like grasping behavior | ||
| Human-like grasping behavior represents the aesthetic capability of the ULP to synergistically operate and adapt its configuration and to robustly perform different sets of grasping tasks. | Underactuated solutions greatly simplify the accomplishment of this due to their intrinsic capability of conforming to the object to be manipulated during grasp (Catalano et al 2014, Weiner et al 2018, Laffranchi et al 2020). On the other hand, in systems with such architecture, human-like behavior is limited exclusively to the grasping function. | |
| Biomimetic performance | ||
| The biomimetic performance is intended as the capacity of reaching the desired biomechanical force and speed requirements in the different activities of daily living. | Achieving the force and speed of the biological hand in an anthropomorphic prosthesis is an extremely challenging task. High integration density can be facilitated by adopting mechatronic architectures with high efficiency. This can be achieved by using once again underactuation, which centralizes the power source (motor) and minimizes the number of transmission components which dissipate mechanical power (Laffranchi et al 2020). | |
| CONTROL | Robust control strategies | |
| The control strategy is a fundamental element to provide the ability of simultaneous actuation of multiple joints and to improve the activities of daily living (ADLs) of the amputees. It is strongly linked to the onboard mechatronics, and in particular the number of active joints plays an important role. In the case of fully actuated systems, the relationship between inputs and outputs is complex and unintuitive, due to the limited amount of input information, for example carried from the superficial EMG signal (i.e. extrinsic muscles, residual muscular activity) (Farina et al 2004). Typically, control strategies on commercial devices are based on buttons (Ottobock 2020a) or smartphone apps (Ossur 2020a) to respectively configure the hand grasps and gestures. Considering an underactuated system, the relation between inputs and outputs for trans-radial case is typically based on the real residual muscle of the forearm. In this case, the research is focused on finding a connection between EMG and movements (Marinelli et al 2020, Nguyen et al 2021). Considering a trans-humeral or interscapular/shoulder disarticulation, the loss of muscles related to the actuation of hand and wrists increases the complexity of relation between inputs and outputs, resulting in a less intuitive control. | Innovative solutions based on ML algorithms are routinely used for trans-radial amputation and have been proposed not only for underactuated but also for fully actuated ULPs to translate the user intentions into single finger movements (Nguyen et al 2021). These methods are intuitive and functional, but they are still not widespread in the market due to their limited robustness over time, requiring frequent recalibration of the entire system (Marinelli et al 2020). Possible solutions in this regard are constituted by maximizing the user experience during training (Del Vecchio et al 2021), or by incremental learning strategies for device control that allows continual adaptation to the changes in the input signals (Gijsberts et al 2014). For trans-humeral amputation, the target muscle reinnervation (TMR) is the most promising solution for simultaneous control of a multi-DoF prosthetic system (Mereu et al 2021). Also in this case, ML algorithms represent an interesting approach to relate the muscular activity of the reinnervated limb into more intuitive and physiological movements. | |
| Smart prostheses | ||
| Current prostheses lack of the possibility to automatically process an incoming stream of information, differently from the human hand that is equipped whit automatic behavior in response to certain stimuli. For example, our hand immediately reacts when touching a burning heat source, even before we consciously perceive the thermal sensation. This kind of features can be a precious improvement of the current solutions in shared-control (Cipriani et al 2008, Yang and Liu 2021). | It would be desirable to have a prosthesis able to take decisions in those situations that require an immediate response. This would free the user from the need of constantly monitoring prosthesis status, limiting the damaging events and allowing the user to operate the device only for voluntary motor production, consequently reducing the mental effort. Equipping the prosthesis with specific sensors and related processing can enable shared control solutions aimed at completing the action-perception coupling with the missing contribute of sensory information. We define this solution as feedback-to-prosthesis. This can be obtained by sensors embedded on the prosthesis, which can measure the interactions between the device and the external world. This can be done exploiting different measurements ranging from the motor current (Ajoudani et al 2013, Laffranchi et al 2020, Deng et al 2020), to tactile/pressure sensors (Tomo et al 2018), Inertial measurement units (IMU) to understand the actual pose of the prosthesis (Krasoulis et al 2019b), or artificial vision (Mouchoux et al 2021) to understand which is the shape and orientation of the nearby objects. | |
| Feedback | Multimodal sensory feedback | |
| The sensory feedback, namely the possibility to restore the feel of interaction with the external world, represents the last key element of ULPs. Current ULP systems rely solely on vision as feedback information. However, we cannot consider just visual (unimodal) feedback to catch the complexity of the human-machine-environment interactions. Multimodal sensory feedback is necessary to empower the prosthetic control training and to trigger the embodiment processes. | Many groups are now exploring novel solution, both invasive and non-invasive, to provide sensory information about prosthetic movement. Regardless the specific methodology used, it is fundamental to achieve an intuitive or easily learnable strategy to associate the perceived feeling with a specific posture of the controlled device. The feedback-to-user can also be adjusted to the user through intelligent solutions, for instance through EMG biofeedback strategies based on the individual monitoring of the physiological input (Dosen et al 2015). | |
These technological approaches constitute the premise for many kinds of breakthrough in prosthetics. Obviously, we need to consider that tradeoff calculations must be made for selecting the most rational set of features that can be combined for providing a satisfying (without creating excessive expectations in users and any stakeholder) and (also economically) sustainable design of the devices. However, the features we described, and their user-centered synergies, can make us foresee the perspectives on bionic hands innovation that will be discussed in next section.
6. Perspectives on tomorrow's upper limb prosthetics
By analyzing the state-of-the-art techniques for ULP input (section 3.1) and feedback (section 3.2) signals, it emerges that there exist two parallel directions for future development, namely the non-invasive and invasive approaches. This is due to different reasons: first of all, because non-invasive solutions may provide the amputees with a plug and play device ready to be used for ADLs, while invasive solutions still need to overcome technological barriers before they can be routinely adopted by the majority of amputee population. Moreover, the specific choice of non-invasive vs invasive strategy is highly dependent on the level of amputation. For example, for trans-humeral amputees, TMR represents the most promising opportunity for restoration of lost functionality, which could not be achieved with non-invasive approaches. In the following, we describe possible direction for future prosthetics. In particular, for non-invasive solution we suggest the use of multiple input sources and of sensory feedback. For invasive solution, we recommend strategies promoting a direct translation of user intentions into prosthetic movements. These directions are also outlined in table 7.
Table 7. Perspectives on tomorrow's upper limb prosthetics.
| Future perspectives | Short-term non-invasive solutions | Long-term invasive solutions |
|---|---|---|
| Increase of input sources | Multiple input data to estimate the movement intentions; data fusion. | Increase the number of myoelectric input sites; neuroprosthetics. |
| Restoration of sensory feedback | Build artificial feedback, i.e. proprioception and tactile sensations by use of vibrotactile feedback or skin stretching. | Restore natural sensation, i.e. proprioception (by kinesthetic illusion), spatial sensation and phantom limb cortical representation (by refer touch strategies). |
| Closed-loop | Implementation of a bidirectional communication between user and prosthesis to restore a link between motor and sensory counterparts. | |
| User-prosthesis co-adaptation | Promote learning with engaging/immersive training and rehabilitation protocols (from user to prosthesis). | |
| Adaptive control, advanced PR algorithms (from prosthesis to user). | ||
| Co-adaptive feedback (feedback-to-user and feedback-to-prosthesis). | ||
| Miniaturization | Miniature technology to limit the encumbrance within the socket. | |
| Modular architecture | Modular prosthetic system enabling the progressive replacement of the non-invasive input and feedback sources with implanted ones. | |
| Standardization of amputation and surgery procedures | Standardization of level of amputation to help designing sockets that are simultaneously comfortable, anthropomorphic, and spacious (to integrate the circuitry and the power system). | |
| — | Chronic and reliable implants. | |
6.1. Short-term non-invasive solutions
The most widespread technique for non-invasive control is based on sEMG. This technique has lots of advantages, such as low cost, direct correlation between muscle activation and movements, and intuitive control, as described in sections 3.1.1.1 sEMG and section 4.3. Nevertheless, EMG-based systems lack of robustness, due to EMG susceptibility to artifacts of biological nature (sweating, hair, muscle fatigue), instrumental source (electromagnetic disturbances), intrinsic to the device (movement artifacts, electrode shift, variations in contraction depending on the orientation of the arm), or intrinsic to the control algorithm (optimization of the classifier).
For this reason, there is ample room for improvement in non-invasive approaches for ULP control. One research direction points toward the use of sensors fusion techniques, in which multiple input data is taken into account to estimate the movement intentions, as described in section 4.3.3. However, the use of great number of sensors may be impractical for creating an embedded system for everyday applications. Future ULP should be equipped with multi-modal control based on minimal number of sensors (Jiang et al 2012, Di Domenico et al 2021). Moreover, recent studies are investigating the miniature technology to limit the encumbrance within the socket (Marinelli et al 2021).
With the aim of improving ULP control, we pose that a fundamental element is the feedback. This will allow a closed-loop interaction between user and prosthesis, a essential prerequisite for promoting the integration of the prosthesis into the body scheme and for facilitating the controllability of the entire system. In this direction, many studies have pointed out the usefulness of vibrotactile stimulation for providing sensory feedback information (Sensinger and Dosen 2020). This technique is cheap, easily integrated into a socket, and its modulation in frequency and intensity allows to provide various information.
In the last years, wearable technology has largely expanded in many fields, influencing also prosthetics. For example, the CTRL Labs have realized a wearable wristband, which reads EMG signals and translates them into finger movements (Melcer et al 2018), later improved by the Facebook Reality Labs by means of advanced ML algorithms (Basu 2021). This technology could be exploited for prosthetic applications with a strong impact for ADLs usage.
Another promising approach consists in providing sensory information by stretching the skin of the stump. This can be done with a wearable haptic device producing rotational skin stretch according to the movement of the controlled device (Kayhan et al 2018, Battaglia et al 2019).
These examples show that innovative ULP solutions can be adopted for restoring lost functionality in the short-term using non-invasive approaches.
6.2. Long-term invasive solutions
The great advantage of invasive approaches is a direct bidirectional contact with the nervous system. This comes at the cost of several issue related to the surgical procedure. Nevertheless, there are some promising approaches whose invasiveness drawbacks are counterbalanced by considerable improvements in device functionality, usability, and embodiment.
Moreover, although still far from clinical usage due to technological barriers, brain-based approaches seem a promising solution for prostheses of the future.
6.2.1. Peripheral bionics implants
Among the invasive approaches, surgical procedures aimed at augmenting the signal containing the motor commands have gained popularity in the last decades. Indeed, TMR is now routinely adopted in case of trans-humeral amputation, and it allows to increase the number of myoelectric input sites, which can be exploited for multi-DoFs control. Differently from invasive procedures for electrodes implantation, TMR is permanent, turning the reinnervated muscles into natural bioamplifiers of motor commands. It is also adopted for phantom limb pain reduction (Mereu et al 2021).
Similarly, the more recent RNPI represents another promising technique for bioamplification of motor signals and its successful demonstration in experimental trials encourages their potential adoption in clinical practice (Vu et al 2020b).
As for delivery of feedback information, invasive approaches represent the more intuitive and natural solution towards the retrieval of sensations, as demonstrated in (Osborn et al 2018, Nguyen et al 2021). However, the main limitation toward the diffusion of these techniques is represented by their invasiveness, i.e. poor compatibility of electrodes, risk of infection due to external cables, scar tissue formation on the nerve, etc.
6.2.2. Neuroprostheses
Ideally, a prosthetic limb should be a perfect replication of the natural limb, both in terms of control and perception, such as Luke Skywalker's arm in the Star Wars saga. In this scenario, control signals should directly derive from the brain and communicate the intended movement to the robotic device, while sensory information should be encoded into stimulation patterns delivered to the brain. The field of neuroprosthetics, among other things, aims at addressing these fascinating goals and in the last 50 years several progresses have been made, indicating that these visionary scenarios might one day become true.
Fetz (1969) demonstrated that monkeys could voluntarily modulate the firing rates of neurons in the primary motor cortex, in the absence of movement. At the same time, Humphrey et al (1970) were able to predict arm displacement from the activity recorded from small populations of neurons in the motor cortex. These exciting and pioneering works thus proved the possibility of controlling artificial devices with the mind and eventually led to a rapid flourishing of investigations aimed at interfacing the brain with machines. These studies culminated with the first demonstration by the group of Nicolelis, of a robotic arm controlled with signals produced by an ensemble of neurons recorded from the motor cortex of a rat (Chapin et al 1999). At the beginning of this century, BMIs were thus born and scientists were therefore hoping that in few decades, fully functional bionic limbs would have been routinely adopted by amputees and paralyzed individuals (Nicolelis and Chapin 2002). Sadly, this is not at all how the story ended. Indeed, more than 20 years after the first demonstrations of brain-controlled devices, we still do not have the technology nor the computational capabilities to effectively control artificial devices with cortical brain signals.
However, in the last few years, some groups have presented promising examples of paralyzed individuals with neural implants in the motor and premotor cortices controlling artificial limbs for several months/year, while other groups worked on non-invasive applications on neurological populations, as detailed in section 3.1.1.3. Although the target population of these studies is mostly composed by stroke or paralyzed patients, exploitation of results for prosthetics applications clearly emerges, i.e. the possibility to perform device control by reliably and timely accessing to the subject's motor intentions. These examples indeed demonstrate that groundwork for brain control of motor prosthetics has been laid. However, it has been limited to the lab and mostly addressing paralyzed patients, for whom there are currently not viable solutions to enable dexterous device control as for amputees, whose residual motor functions can be successfully leveraged for prosthetic control signals.
In sum, brain control approaches are still far from clinical and personal applications, not only because of the poor controllability that they exert over the prosthetic device, but mainly because of the cumbersome apparatus they need for their collection and processing. However, the dream of brain-controlled devices has spread outside the academic labs and has contaminated also visionary entrepreneurs from venture capitals and tech giants, with the consequent birth of some important companies interested in brain-interfacing technology, such as Neuralink (Musk 2021), Facebook Reality Labs (Zuckerberg 2021), and Google DeepMind (Deepmind 2021). In conclusion, cutting edge research that we are currently witnessing both in academic and non-academic contexts may thus soon push the envelope of neuroprosthetics up to its diffusion in our everyday life, with important consequences also for amputees.
6.3. User-prosthesis co-adaptation
Designing user-centered prosthetic devices and user-centered prosthetic trainings is necessary to guide an appropriate learning of the system, as explained in section 5.1. Indeed, motivating the user to exercise and to get practice in using the bionic hand constitute the main way to improve the prosthetic control. Such approach can facilitate and accelerate the co-adaptation between humans and machines, as described in the following.
Humans implicitly learn how to control devices, even if initially they must adopt explicit strategies. Indeed, it must be underlined that ULP control training is an important step of rehabilitation. In particular, the ability of generating distinct muscle contractions increase with time and exercise. The use of functional tasks, like target achievement test (Simon et al 2011) or activities of daily living, allows users to learn how to produce repeatable patterns of contraction to better control the prosthesis. However, the learning process can be long and sometimes stressful, as described in (Zecca et al 2002). The development of more engaging training tasks and of a more immersive rehabilitation protocol could promote the learning process by increasing the engagement of the users (Roche et al 2019). In this context, user-centered design can truly make a difference in the effectiveness of a training procedure, as better discuss in section 5.
While humans have to learn ULP control, machines need to be trained with growing datasets for classifying the signals in terms of user's commands. For effective ULP control, PR-based algorithms currently represent the most effective solution, as they are able to recognize the human intentions on the basis of training data. An important aspect that can affect the accuracy of the classifiers is thus the way in which these data are collected. Indeed, the prosthesis control might not ensure good performances under different arm positions and several studies have been conducted on the evaluation of the impact of upper limb position during the data acquisition on classifier performance (Geng et al 2017). For example, as far as EMG is concerned, muscle activation is not completely the same when performing a given movement under different elbow and shoulder configurations. The signals on which the algorithm is trained are thus different from the ones obtained in a daily living scenario. Indeed, the classification performances strictly depend on the labeled data assigned to the specific movement. Moreover, because the method used for the acquisition strongly affects the classification accuracy, it is important to collect data under the same conditions of ADLs, i.e. by wearing the prosthesis, in order to have the training signals as similar as possible to the online ones. Cipriani et al (2011) highlighted that indeed EMG signals do not carry just information about the desired arm movement, but they also contain the muscular contribution to sustain the prosthesis weight. This aspect has to be taken into account in order to avoid misclassification and unwanted prosthetic movements, because as soon as the signals change, the classifier is no longer able to behave properly.
To cope with these problems, a possible solution consists in the adaptation of the control algorithm while using the prosthesis. This is of paramount importance, since the biggest issue of ULP control lies in the variability of the sEMG input signal due to electrodes shift, muscle fatigue, sweat, etc. ML algorithms are highly performing after training, but deterioration of the input signal or subsequent doffing/donning of the prosthesis can lead to misclassifications. For these reasons, amputees must regularly perform the training from scratch of the algorithm, which typically takes long time (Phinyomark and Scheme 2018). To address this issue, the incremental learning focuses on the adjustment of internal weights of the model without the need of re-training (Gijsberts et al 2014). In this way the training data is continuously updated, and the control system is capable to cope with possible sources of errors (i.e. unwanted changes in input signals).
A promising approach to innovate the ULP field is the adoption of co-adaptive features through bidirectional human-machine interfaces (De Santis 2021). In this case, the human and the machine reciprocally adjust their activity in order to improve their task-specific joint performance as a human-machine system. Co-adaptive features constitute a convergence of user activity and machine activity (including feedback toward the user and the machine), i.e. an 'agreement' between human and machine on the biosignals produced by the first and on their interpretation performed by the second. Co-adaptation can modulate both (human and machine) training processes within the same framework (Digiovanna et al 2008, Yeung et al 2019, Zbyszynski et al 2019, Igual Bañó 2021). This is performed through the feedback of human and machine on each other, further supporting the opportunity of designing feedback-to-user and feedback-to-prosthesis. Such a research effort requires innovative approaches to training processes as interactions between human and machine. In the next we present two building blocks for a user-prosthesis co-adaptation: closed-loop sensorimotor systems and interactive training.
6.3.1. Toward closed-loop sensorimotor prosthesis
While great progress has been made in recognizing human motor intention and translating it into prosthesis joint movements, sensory feedback restoration is one of the many challenges that many research groups are still addressing (see section 3.2) (Farina et al 2021). Obtaining a reliable and efficient way to artificially convey sensory information to prosthetic users would allow to develop smart devices able to truly mimic the behavior of human limbs, establishing the premise for a true co-adaptation between user and machine.
However, such a sensorimotor prosthesis would still need to face technological barriers for its development and use in ADLs. For example, all the circuitry and components needed to operate the device (i.e. acquire signals for motor intention decoding and translate sensory information into stimulation patterns) should fit into the socket space. One possible solution to obtain an embedded device is to rely on a hybrid approach. For example, a non-invasive sensorimotor prosthesis could use EMG signals to extract motor intentions and vibrotactile feedback to deliver sensory information. This configuration requires the embedding of an analog to digital converter (ADC) amplifier to acquire EMG data, and motor drivers to control vibromotors. All these components could be placed on the same board, overcoming the problem of electrical coupling.
However, although this design is very articulated, we believe that this is not the real end point for a fully-integrated prosthetic system capable of maximizing patient acceptance. It simply turns out to be a developmental test bench for what will be the next generation of prostheses on a longer temporal horizon, namely neuroprostheses. By creating a modular architecture, in fact, it will be possible to maintain the physical prosthetic system and to progressively replace the non-invasive input and feedback sources with implanted ones, namely: (a) the standard sensors for surface EMG replaced by an intraneural implant; (b) the hardware for feedback delivery replaced by a chronic implant; and (c) the hardware on which run the control strategies replaced by a smaller and more performing dedicated hardware able to process neuromorphic algorithms (e.g. Application specific integrated circuit (ASIC)).
The realization of bidirectional ULP systems able to restore both sensory and motor functions will open new research scenario for embodiment process and neuroplastic phenomena. Indeed, about neuroplasticity in amputees using bionic hands as prostheses, Di Pino et al (2009) highlighted how the reorganization of the central nervous system after the usage of the device can be the source of indices of prosthetic effectiveness in functional recovery. Furthermore, the effects of the device on the central nervous system can make the prosthesis work as a neurorehabilitative solution mitigating aberrant plasticity phenomena and facilitating positive neural changes. Finally, novel human-machine interfaces should consider neuroplasticity principles for restoring the efferences and afferences of the central nervous system with the lost limb in order to exploit them for connecting a prosthesis.
The processes described above are an excellent expression of the technologies that can lead to a user-prosthesis co-adaptation. They care for providing the human with sensations matching the motor activity and the events occurring on or for the artificial limb, which needs to learn how to offer appropriate feedback to the user. Next paragraphs will describe how this can happen.
6.3.2. Innovation in interactive training
Establishing interactions between user and prosthesis requires a human-centered design of the technology 'behavior'. Since the term co-adaptation implies that two or more entities are adjusting to each other, possibly learning through the interaction itself for reaching a goal that can be the improvement of the human-machine performance. The attention to the interactive aspects of learning and training was recommended in Castellini et al (2016). In this context, intriguing opportunities come from theoretical frameworks in psychology like the constructivism to define and improve the paradigm of interactive ML in prosthetic training and control (Nowak et al 2018, Bettoni and Castellini 2021). Accordingly, a myocontrol system should learn and forget on demand, under request of each one of the components of the human-machine system. For instance, the users can label the violation of their expectations on the prosthesis interpretation of their commands, starting a novel data collection cycle. Additionally, the machine itself can highlight the need of collecting further data (especially from correct execution of a training exercise) through acoustic feedback to the user. Through this, the myocontrol models are updated.
Moreover, certain biosignal features could guide an automated labeling of the violation of the observer's expectations without any explicit command, as in neuroprosthetic interfaces using Error-related Potentials (Chavarriaga et al 2014). Such an advance (still explored in laboratories) could enable self-calibrating intention detection processes, leading to a true (and fruitful) human-machine symbiosis. Such symbiosis is based on the online processing of users' neurocognitive states. The machine interprets such states for implicitly adjusting (without direct and declarative commands of the user) its activity to the individual current capabilities and preferences. Exploiting this process as a further example of the closed-loop previously envisioned, the human-machine system will become a fully functional unit able to enact manual and bimanual biomimetic behaviors (Chavarriaga et al 2014).
However, when the practical applications of this kind of implicit learning will move outside the laboratories, its advantages should be evaluated in real-world cases of prosthetic learning. Currently, a human-machine explicit communication component in initial co-training sessions can highlight an active role of the users with positive impact on their self-efficacy and engagement. In this context, biofeedback strategies (self-regulation of perceptually represented physiological changes) in co-adaptive systems could be especially useful for easing the integration of both implicit and explicit aspects within the user-prosthesis co-training (even during daily re-calibrations) (Kalampratsidou and Torres 2017).
6.4. Amputation matters: the key role of the surgeon
A further reflection that emerges in order to maximize patient acceptance is related to the clinical situation immediately preceding amputation. In fact, although it is clear that in case of injury/accident the doctor/surgeon has to manage a situation of immediate danger in which the priority is to secure the patient trying to save 'as much as possible of the injured limb', on the other hand, on the engineer side, the ideal would be to be able to standardize the level of amputation. In this case, in fact, it would be possible to avoid 'extreme' situations in terms of amputation levels: a very distal amputation does not allow the orthopedic technician, and therefore also the engineer, to have enough space to integrate the circuitry and the power system inside the cavity between the internal and external lamination of the reservoir itself, on the other hand, a very proximal amputation does not allow the orthopedic technician to create a socket capable of allowing the patient's stump to support the weight (thus not even having enough residual muscles from which to extract the EMG signal) of the dedicated prosthetic system. Therefore, having the possibility, even in case of extreme danger, to be able to define a 'standard' level of amputation in which the surgeon is able to have a slightly longer-term vision, would allow a greater number of patients to take advantage of these fantastic biomedical technologies. In fact, at present, many patients find themselves having to request an additional surgical operation, thus further compromising the patient's willingness to use these solutions.
7. Conclusions
In this manuscript, we have detailed and discussed several strategies to substitute and restore the functionality of human upper limb when missing. We specifically focused on input and feedback signals for bidirectional device control and on aspects regarding the user needs that should be addressed with a user-centered prosthetic design approach. We also stressed that in order to have a fully embodied prosthetic system it is essential to implement a synergistic collaboration of three components: mechatronics, control algorithm and the user perception. These have to be combined by a real rehabilitation process that is necessarily user-centered.
Acknowledgments
The authors gracefully acknowledge Marco Freddolini and Simone Tanzarella for useful discussion on ULP input signals and their processing.
The Open University Affiliated Research Centre at Istituto Italiano di Tecnologia (ARC@IIT) is part of the Open University, Milton Keynes MK7 6AA, United Kingdom.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions
A M, N B, F T, D D D, G B, and M S conceived the study. N B, A M and M S designed the figures. N B and A M prepared the figures. All the authors contributed to the writing, read and approved the final version of the manuscript.