Abstract
Oral cancer causes over 350 000 deaths annually worldwide. Although most cases are in Asia, the incidence of oral cancer is rising across the world. Despite recent advances in screening methods, oral cancer remains a significant cause of mortality and morbidity. The 5-year survival rate (50%–60%) has not improved over the past several decades. Early detection and accurate diagnosis of the disease can improve the survival rate and patients' quality of life. This article provides a topical review of current and emerging techniques for screening and diagnosing oral cancer. Currently available technologies have only been moderately useful towards identifying oral cancer early, motivating the development of novel approaches to address this goal. In this article, we provide an overview of adjunctive screening aids, including biofluid (saliva and serum) diagnostics, vital staining, brush biopsy, chemiluminescence, and tissue autofluorescence. Furthermore, we discuss diagnostic imaging modalities, such as computed tomography, magnetic resonance imaging, positron emission tomography, ultrasound (including traditional B-mode imaging, color Doppler, and elastography), photoacoustics imaging, and optical coherence tomography, and artificial intelligence-based methods, which are either being used clinically or are under development for oral cancer staging. The physical and biological basis underpinning each technique are discussed, along with their advantages and limitations in the technological and clinical context. The review concludes with a discussion of the future perspectives in this rapidly evolving field.
Export citation and abstract BibTeX RIS
1. Introduction
The International Classification of Diseases defines oral cancer as 'cancer of the oral cavity and pharynx, including cancer of the lip, tongue, salivary glands, gum, floor and other areas of the mouth, oropharynx, nasopharynx, hypopharynx, pharynx and other buccal areas' [1]. The most common form is oral squamous cell carcinoma (OSCC), representing more than 90% of all head and neck cancer [1]. Oral cancer accounts for a significant fraction of the global burden of cancer, especially in Asia (e.g. India) and the Pacific Islands (e.g. Papua New Guinea) [2]. The age-standardized incidence rate is 20 per 100 000 people in India [3], and is 5.8 per 100 000 men and 2.3 per 100 000 women worldwide [2]. Despite significant advances in treatments, the long-term prognosis for patients with oral cancer remains poor.
The World Health Organization has identified early detection as a primary effort to control the oral cancer burden [4]. Early detection plays a significant role in assessing risks for malignant transformation [5]. However, the disease is typically diagnosed at advanced stages; over 60% of the patients are diagnosed with stage III and IV cancer [6]. Small asymptomatic lesions that are unintentionally ignored may transform into malignant tumors. The development of effective diagnostic tools could help clinicians in detecting potentially malignant lesions (PMLs) early [6]. Subsequent intervention could decrease morbidity and mortality, improve the patients' quality of life, and reduce financial burden.
This article provides a topical review of current and emerging tools for screening and diagnosing oral lesions. For more detailed discussions on either adjunctive aids or specific imaging techniques, the reader is referred to previous reviews [5, 7–10]. The present review encompasses the etiology of oral cancer and notable screening and diagnostic tools, including recent and emerging modalities (figure 1).
Figure 1. Classification of current and emerging oral cancer screening and diagnostic techniques. qMIDS = quantitative malignancy index diagnostic system.
Download figure:
Standard image High-resolution image1.1. Etiology and oral carcinogenesis
Tobacco use is a major risk factor in oral carcinogenesis [11], accounting for millions of deaths per year [12]. Immunosuppressed individuals are at a higher risk of developing oral cancer [12]. Tobacco smoke contains hazardous aromatic hydrocarbons and nitrosamines. The metabolites of these compounds covalently bond with deoxyribonucleic acid (DNA) of oral keratinocyte stem cells, resulting in the formation of DNA adducts [13]. Concurrent consumption of tobacco and alcohol has a synergistic effect on oral cancer pathogenesis. It has been implicated as a risk factor in 80% of males, 61% of females, and 74% of the overall population [14]. The consumption of alcoholic beverages leads to an increase in the permeability of the oral mucosa [11, 15] and a high risk for developing cancer in tissues, including the oral cavity, larynx, pharynx, and esophagus [16].
Human Papilloma Virus (HPV) and Epstein Barr Virus (EBV) infections are recognized risk factors for OSCC. The International Agency for Research on Cancer (IARC) has classified HPV 16 as a 'known cause' and HPV 18 as a 'possible cause' of oral cancer. HPV leads to carcinogenesis by two virus-encoded proteins, E6 and E7, which promote the degradation of tumor suppressor gene products p53 and pRb (retinoblastoma protein) [12]. EBV is a carcinogenic cofactor that has been shown to immortalize B cells, thereby acting as an agent of tumor progression rather than tumor initiation [17]. Excessive exposure to ultraviolet radiation has been implicated as a cause of lip cancer [12]. A positive association between processed red meat and oral cancer risk has been attributed to the carcinogenic nitrites and N-nitroso compounds present in animal fat and cholesterol [18]. Polycyclic aromatic hydrocarbons and heterocyclic amines produced during cooking also play a mutagenic role [19]. In addition, lichen planus and submucous fibrosis are other inflammatory disorders associated with an increased risk of OSCC [20, 21].
OSCC develops over several years, during which neoplastic changes occur in the oral cavity [22]. Sites with a high incidence of OSCC include the lip, lateral edges of the tongue, floor of the mouth, and lingual vestibule [23]. In Southeast Asia, where the use of chewable tobacco and betel nut is common, the buccal mucosa and gingivobuccal sulcus are most commonly involved [24]. Genetic alterations adversely affect various signal transduction pathways, leading to uncontrolled cell division, apoptosis, local invasion, and metastases [25]. OSCC originates from non-aberrant keratinocytes, resulting in epithelial hyperplasia, dysplasia, carcinoma in situ, and metastases. OSCC occurs because of a complex multistage process at the molecular level and is typically preceded by PMLs such as white (leukoplakias) and red patches (erythroplakia) [26, 27]. The malignant transformation rate for erythroplakia ranges from 20% to 68%, and that of leukoplakia from 3% to 33% [28, 29]. Transformation rates are higher in the case of erythroleukoplakias or speckled leukoplakias [23]. Histological atypia present in the basal and parabasal keratinocytes is known as mild dysplasia [30]. In the middle layer, it is called moderate dysplasia, and when it extends to the surface, it is categorized as advanced dysplasia and carcinoma in situ [30].
Oral cancer may have a multicentric origin. Neoplastic changes occurring at the molecular level do not necessarily produce clinically visible lesions [23]. Dysplastic and malignant modifications may be detected in normal mucosa at sites far away from the removed OSCC [23]. Additionally, several biopsies from visually normal mucosa showed histologically abnormal tissue [31].
1.2. Conventional methods for screening and diagnosis
Currently, screening and diagnosing cancerous lesions in the oral mucosa is primarily based on clinical oral examination (COE) (figure 1). Visual examination and palpation are the primary methods in COE, wherein the physician looks and feels for ulcers, lumps, lesions, and enlarged lymph nodes. However, some precancerous lesions may appear innocuous and can be overlooked [32]. The early stage of oral cancer is challenging to diagnose because of the lack of symptoms and clinical presentation prominently observed in the advanced stages, such as swelling, ulceration, pain, and induration (tissue hardening) [32]. Of those identified as PMLs, a small fraction is progressive, and COE cannot differentiate them from occult lesions. Additionally, these methods can produce variable results and cannot identify the deep extension of the disease [8].
A definitive oral cancer diagnosis is achieved by histological examination of biopsied specimens from suspicious tissues. Typically, biopsy is indicated for any lesion that persists in the oral mucosa for more than 3 weeks [23]. However, biopsy is invasive and not suitable for regular screening of high-risk populations [33]. Other limitations include sampling bias, processing time, and interobserver and intraobserver variability [34]. These limitations emphasize the need for developing adjunctive diagnostic aids.
1.3. Genomics-based approaches
Genomics-based approaches can be used both for screening asymptomatic patients as well as diagnosing symptomatic oral cancer patients. Promoter hypermethylation of two genes, namely HOXA9 and NID2, are present in oral PMLs, which may serve as valuable biomarkers in OSCC [35]. Genetic alterations occur primarily due to DNA-based changes classified as point mutations, gene amplification, fusion, deletion, insertion, and single nucleotide polymorphisms (SNPs) [36]. Tumor-specific features can be observed in DNA, such as p53 and tumor suppressor genes in somatic mutations, abnormal promoter methylation, microsatellite alteration, presence of the tumor-related viral DNA, and mitochondrial DNA mutations [37].
Complementary DNA microarrays provide information on whole transcription activity in a particular sample. Most OSCC studies are based on expression arrays. This method allows the investigation of the genes of a larger part of the genome and detects abnormalities and new functions [38]. An increase in the extracellular matrix (ECM) glycoprotein tenascin (TN) expression has been observed during tumor progression. Also, an increase in the ECM protein osteopontin confirmed detection of the protein in premalignant and malignant lesions arising from oral epithelium. It was demonstrated that tumor-derived osteopontin was able to inhibit macrophage function and enhance survival of SCC metastases. A loss of keratins 4, 13, and 15 was also observed during tumor progression. Four other potential tumor suppressor genes were noted, which are as follows: BRUSH-1, MXI1, oxidative stress-response gene, and occluding [39].
Whole-exome sequencing studies revealed that TP53, CDKN2A, PIK3CA, HRAS, and NOTCH1 are the most frequently mutated genes [40, 41]. In another study, 63% of OSCC were reported to have had alterations in genes that control mitogenic signaling, including activating mutations in HRAS, PIK3CA, and BRAF. Shaleen et al performed a study on SNPs in 500 oral cancers (confirmed by histopathology) and 500 healthy controls who were tobacco users for at least 10 years. They reported that the SNPs rs2124437 (RASGRP3), rs1335022 (GRIK2), rs4512367 (PREX2), rs4748011 (CCDC3), and rs1435218 (LNX1) were significantly associated with tobacco habits [42]. Oncogenes and tumor suppressor genes, such as CCND1, EGFR, RAS, VEGF, p53, CDKN2A, STAT3, and Rb, have been implicated in oral cancer [25, 43]. Sasahira et al found that prospero homeobox 1 (Prox1) and forkhead box (FOX) C2 regulate angiogenesis and/or lymphangiogenesis in OSCC [44]. Their findings suggested that Prox1 and FOXC2 play key roles in OSCC progression. However, the detailed role and function of Prox1 and FOXC2 in cancer remains elusive [44].
Tumor budding is histopathologically evident that shows the pattern of invasion, and it also highlights morphological features that represent an aggressive invasive phenotype [45]. In several studies, the scoring for tumor buds has been performed in biopsied sections stained by hematoxylin and eosin (H&E). However, challenges in visualizing structures with adequate contrast make evaluating H&E sections difficult. To overcome this limitation, immunohistochemistry with cytokeratin markers is used [46, 47]. Priyanka et al reported the amplification of 8q24.3, deletion of 8p23.2, and dysregulation of DERL3, EIF5A2, ECT2, HOXC9, HOXC13, MAL, MFAP5, and NELL2 genes in later stages of OSCC using integrated genome-wide analysis. Their findings can be used in treatment monitoring for patients with potentially malignant leukoplakia and OSCC patients with a high risk of lymph node metastasis [48]. Kasradze et al provide a systematic review on this topic [36].
1.4. Quantitative malignancy index diagnostic system (qMIDS)
The qMIDS was developed for objective assessment of the malignancy status of biopsy samples [49]. qMIDS shows potential to be used as a diagnostic tool for symptomatic patients. qMIDS provides a digital index based on the expression of the forkhead box M1 (FOXM1) oncogene to assess the risk of squamous cell carcinoma (SCC) [49]. The FOXM1 transcription factor is one of the top upregulated oncogenes across 39 types of cancer and is a significant predictor of poor cancer prognosis [50]. qMIDS has been useful in distinguishing between benign (low risk) lesions, such as oral lichen planus or fibro-epithelial polyps, and premalignant (high risk) oral dysplastic samples [49, 51]. At a score cut-off of 4.0, qMIDS exhibited a high detection rate (>90%) and a low false-positive rate (<3.5%) [49, 51]. qMIDS scores of tissue specimens from two patient populations (i.e. Chinese and European patient groups) were found to be nearly similar, indicating that the results were not influenced by ethnicity [49, 52]. Furthermore, differences in qMIDS scores were not affected by age or gender [49, 52]. Because qMIDS scores are derived from biopsy samples, sampling bias and patient discomfort can be the drawbacks of this technique.
1.5. Biofluid diagnostics
Despite general awareness about the benefits of regular health checkups, deeply seated diseases are not typically diagnosed until morbid symptoms appear in the advanced stages of the disease. Molecular changes in the oral tissue typically precede morphologic criteria for diagnosis. Therefore, analysis of tumor biomarkers can be useful for screening asymptomatic patients at an incipient stage [53]. Biofluid diagnostics has the potential to be an effective screening tool because of reported alterations in the composition of saliva and serum in patients suffering from malignancy [7, 54].
Research is underway to facilitate detection of OSCC at the early stages by analyzing the molecular biomarkers in biofluids, such as saliva, blood plasma, and serum. Oral cancer diagnosis using saliva offers the advantages of easy accessibility, safe handling, easy storage, and non-clotting ability. Saliva diagnostics is the least invasive, enabling the monitoring of biomarkers for disease surveillance more frequently than other types of biofluids. Saliva is a representative biofluid of serum, and the majority of its components are derived from serum through transcellular or paracellular routes. Saliva has direct physical contact with oral lesions and can reflect molecular changes before malignant transformation [55]. Saliva is classified into cellular components, including exfoliated epithelial cells, lymphocytes, and erythrocytes, and acellular components, such as water (99%), proteins (∼0.3%), inorganic ions, enzyme cofactors (∼0.2%), metabolites, and nucleic acids [56]. OSCC can be detected by identifying changes in the salivary proteome, transcriptome, metabolome, and microbiome [57].
1.5.1. Salivary proteomics
Several recent proteomic studies have studied OSCC and other oral lesions for early salivary biomarkers. Proteomics studies the cellular levels of all protein isoforms and post-translational modifications encoded under a given set of conditions by the cell's genome. Although a genome is more or less unchanged, protein levels in a cell can change drastically [58]. Genes also get turned on and off during the cell's response to its environment, contributing to the diversity of proteins. Proteomics studies reported thus far have identified 17 upregulated protein biomarkers. The most promising protein biomarkers were interleukins 6, 8, and 1b, cyclin D1 thioredoxin, and profilin 1 [59].
Yu et al identified 49 saliva proteins as potential OSCC biomarkers [60]. Salivary proteins (e.g. cell surface proteins, cytoskeleton fragments, intracellular proteins, and proteases) are differentially expressed in OSCC patients [37, 61]. Specific salivary proteins (M2BP, MRP14, profilin, CD59, and catalase [62]) and metabolites (valine, lactic acid, and phenylalanine [63]) exhibit high accuracy in detecting oral cancer. Upregulated salivary levels of cell cycle regulatory proteins have been identified in oral cancer patients, which include Cyclin D1 and ki67, glycolytic enzyme lactate dehydrogenase, matrix metalloproteinase (MMP)-9 and reduced DNA repair enzymes, eight oxoquanine DNA glycosylase (OGG1), and tumor suppressor protein maspin [37].
Salivary protein concentrations of both MMP1 and MMP3 were shown to be highly elevated in OSCC patients relative to cancer-free controls [64]. Significantly higher salivary levels of IL-1, IL-6, IL-8, and TNF-alpha in oral cancer were found in contrast to dysplastic oral lesions and controls [37]. Aziz et al demonstrated that salivary cytokines (IL-10 and IL-13) in oral cancer patients showed remarkable elevation [65]. Deepthi et al investigated the role of salivary tissue necrosis factor-alpha (TNF-α) in oral cancer and leukoplakia [66]. Significantly higher levels of salivary TNF-α were noted in dysplasia relative to precancer and healthy controls. Receiver operating characteristic (ROC) analysis revealed that TNF-α could serve as a valuable biomarker for differentiating leukoplakia from healthy controls (area under the curve (AUC) 0.968, 95% confidence interval (CI): 0.930–1.000), as well as for differentiating OSCC from healthy controls (AUC 0.997, 95% CI: 0.989–1.000). Furthermore, salivary TNF-α levels increased with increasing histological grade of differentiation in OSCC as well as leukoplakia. High sensitivity (100%) and specificity (96.7%) of this approach provide evidence of the promising role of salivary TNF-α as a useful biomarker in OSCC [66].
Several other proteins have previously been reported to be elevated in OSCC patients (e.g. squamous cell carcinoma antigen 2 [SCC-Ag2, calcyclin, Rho GDP dissociation inhibitor, heat shock 70 kDa protein 1, annexin I, cathepsin G, peroxiredoxin II, thioredoxin, short palate, and lung and nasal epithelium carcinoma-associated protein). These target proteins may assist in elucidating the molecular mechanism of the disease, which could be significant for clinical applications [67].
1.5.2. Salivary transcriptomics
Salivary transcriptomics is an emerging robust, cost-effective, and noninvasive biomarker sighting tool, which could potentially be used in the prognosis and diagnosis of OSCC [68]. The differentially expressed transcripts from the saliva samples of OSCC patients can serve as a potential biomarker. Specific RNA biomarkers (IL6 [69], IL8, IL1B, DUSP1, HA3, OAZ1, S100P, and SAT [70, 71]) are upregulated in the saliva samples of OSCC patients. Researchers have investigated the clinical relevance of salivary microRNAs (miRNAs) as diagnostic markers in oral cancer. miRNAs are 19–25 nucleotides long, endogenous, single-stranded, non-coding RNAs that regulate the gene expression post-translation [72, 73]. Several studies have shown that miRNAs in salivary exosomes could serve as novel biomarkers for identifying OSCC. Exosomal miRNA into saliva and blood appears because of apoptotic and necrotic cell death [73]. During tumor progression, dysregulated miRNAs are involved in tumor initiation and modulation of oral cancer malignancy. Liu et al found that salivary miR-31 levels significantly increased in OSCC patients and decreased after tumor excision, indicating that miR-31 originated from the tumor [74]. Recently, He et al reported overexpression of miR-24-3p in OSCC neoplasms and that an increase in salivary exosomal miR-24-3p in vitro resulted in enhanced proliferation of OSCC cells. ROC analysis showed that miR-24-3p could be a potential biomarker in OSCC (AUC of 0.74) [75]. Duz et al identified miR-139-5p to differentiate tongue SCC patients from healthy controls (preoperative AUC: 0.805 and post-operative AUC: 0.713) [76]. However, some miRNAs (e.g. miR-125a and miR-200a) are significantly reduced in salivary levels of OSCC patients [77]. A detailed systematic review by Patil et al discusses the role of salivary transcriptome as potential biomarkers in prognosis and diagnosis of OSCC [68].
1.5.3. Serum diagnostics
Several studies have identified blood plasma and serum biomarkers that may have diagnostic value. Serum diagnostics can be used both as a screening as well as a diagnostic tool. Chang et al proposed two core-fucosylated glycoproteins in blood plasma—apolipoprotein A-IV and LRG1, as potential oral cancer biomarkers [78]. A significant difference was observed in the quantities of these two glycoproteins in oral cancer patients and healthy subjects (sensitivity of 83.3%, specificity of 89.7%, AUC of 0.89) [78]. Serum samples from groups with oral cancer demonstrated higher growth factors (e.g. growth-differentiation factor 15 (GDF 15) [79] and vascular endothelial growth factor (VEGF) [80]), p53 antibody [81], cyclin D1 [82], sialic acids [83], and proteins (e.g. C-reactive protein [84], decoy receptor 3 [85], and SCC-Ag [82]). However, other proteins (e.g. adiponectin [86]) and annexin A1 mRNA expression [87] were reduced. Fernandez-Olavarria et al provide an exhaustive review of major serum biomarkers in oral cancer [9].
Additional research and clinical validation are necessary to determine if these biomarkers are suitable for larger-scale clinical implementation. Moreover, the exact spatial location of lesions cannot be identified with these techniques.
1.6. Vital staining
Vital staining involves using a dye, such as toluidine blue (TB) or tolonium chloride, to stain and highlight abnormal tissue regions. TB is a blue metachromatic dye that binds to nucleic acids. The nucleic acid content of dysplastic and anaplastic cells is higher as compared to normal cells. TB stains abnormal more than normal cells (figures 2(A) and (B)), which helps identify any mucosal changes. Additionally, intracellular canals are comparatively wider in the malignant epithelium, allowing greater penetration and more retention of the dye in abnormal cells [88]. The extent of staining depends on the degree of epithelial surface involvement, i.e. benign lesions show faint coloration, whereas dysplastic lesions and carcinomas show intense coloration. Vital staining can be used for screening asymptomatic patients.
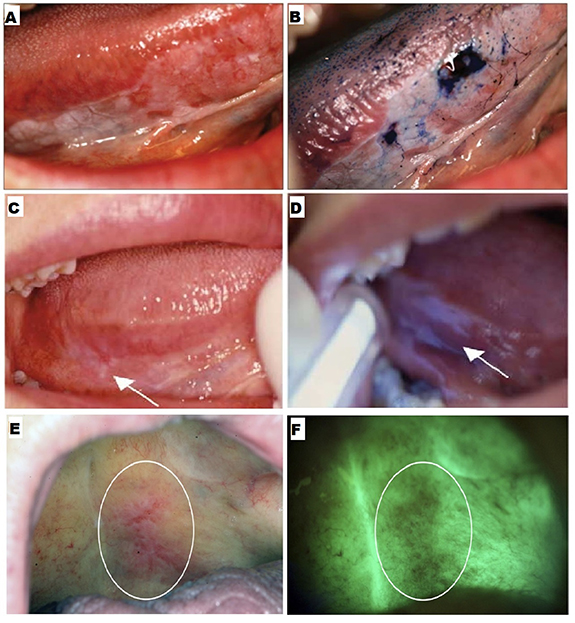
Figure 2. Adjunctive aids. (A) An erythroplakia lesion on the right lateral border of the tongue, and (B) the same lesion viewed after toluidine blue staining. Reproduced from [10]. CC-BY-NC-ND 3.0. Copyright © 2013, The Author(s). (C) Clinically non-evident lesion in right lateral posterior tongue, and (D) the same lesion identified by Vizilite with irregular bluish-white reflection. Reprinted from [99], © 2004 International Association of Oral and Maxillofacial Surgeons. Published by Elsevier Inc. All rights reserved. (E) An erythroleukoplakia viewed under visible light and (F) the same lesion with autofluorescence. Reprinted from [102], © 2011 Mosby, Inc. Published by Elsevier Inc. All rights reserved.
Download figure:
Standard image High-resolution imageThe diagnostic efficacy of TB staining has been investigated in several studies. Lingen et al noted that the sensitivity and specificity of TB staining reported previously ranged 78%–100% and 31%–100%, respectively [6]. Rosenberg et al reported sensitivity values ranging from 93.5% to 97.8% and specificity ranging from 73.3% to 92.9% [89]. However, high false positive results were observed. Upadhyay et al reported a false positive value of 32.6%, due to to hyperkeratosis, hyperplasia, lichen planus, and traumatic ulcers [90]. A false negative value of 26.1% was primarily because of mild dysplasia [90]. Despite variability in the reported diagnostic efficacy of TB staining, it is considered an important adjunct to COE. It is recommended that any lesion with a positive TB stain should be considered for biopsy [91].
1.7. Brush biopsy
Brush biopsy is used routinely to assess lesions noninvasively. The OralCDx brush biopsy uses the exfoliative cytology method for collecting trans-epithelial cell samples from suspicious regions. A brush with nylon bristles is rubbed onto the lesion's surface, and the epithelial cells trapped within the bristles are released into a bottle with a liquid fixative. Thereafter, the cells in the sample are separated from the fixative liquid and nonspecific debris. The disaggregated cells are stained and examined under a microscope by a pathologist. OralCDx is beneficial for investigating mucosal abnormalities that have low-risk features [92]. It is essential for patients with multiple lesions with no history of oral cancer and who would not willingly accept undergoing several scalpel biopsies. The advantages of brush biopsy include its simplicity, noninvasiveness, and relatively low cost. Brush biopsy can be used as a tool for screening asymptomatic patients as well as diagnosing symptomatic cases.
The diagnostic efficacy of OralCDx has been inconsistent amongst different studies. In a pivotal clinical trial conducted at 35 centers in the United States, OralCDx demonstrated sensitivity greater than 96% and specificity over 90% [93]. Thereafter, OralCDx received approval from the American Dental Association. Subsequent work reported similar results, demonstrating the high accuracy of this technique [94]. However, subsequent studies reported lower diagnostic efficacy [95, 96]. Specifically, Hohlweg-Majert et al reported 52% sensitivity and 29% specificity [95], and Poate et al stated 71.4% sensitivity and 32% specificity [96]. Despite inconsistencies in diagnostic efficacy, OralCDx can identify mucosal lesions in high-risk patients [97]. However, the rate of false positives increases in low-risk populations [97]. Although this technique helps in the definitive diagnosis of visible lesions, it cannot diagnose deep-seated and microscopic lesions [88]. In cases where atypical and positive results are reported, a scalpel biopsy is typically recommended [6].
1.8. Chemiluminescence
Chemiluminescence is the emission of visible light following a chemical reaction. Chemiluminescent techniques, such as those used in the product Vizilite®, have been devised to detect oral cancer at the early stages [98]. The patient's mouth is rinsed with 1% acetic acid solution to enhance light penetration, removing debris and disrupting the glycoprotein barrier. Vizilite® contains hydrogen peroxide and acetyl salicylic acid, which react and emit diffuse bluish-white light with a wavelength between 430 and 580 nm for approximately 10 min [99]. Dysplastic and neoplastic cells with abnormal nuclei reflect this light because of a high nucleus-cytoplasm ratio. Abnormal squamous epithelium tissue appears aceto-white, whereas normal epithelium appears darker (figures 2(C) and (D)) [98]. Vizilite® can serve as a tool for screening asymptomatic patients.
Several limitations are associated with the use of this technique. Although Vizilite® has been reported to have high sensitivity ranging from 77% to 100%, its specificity ranges from 0% to 27% [3, 99, 100]. In a study by Farah et al, ViziLite improved the visualization of lesions in high-risk populations [100]. However, it could not differentiate between benign, inflammatory, potentially malignant, and cancerous mucosal conditions. Mehrotra et al reported that Vizilite was ineffective in detecting dysplasia or malignant cells [101]. Although the lesions previously identified by standard light were enhanced by up to 60%, no additional lesions were detected.
1.9. Tissue autofluorescence
Native fluorophores in the oral epithelium and submucosa can be excited upon exposure to light in the UV-visible range, causing the tissue to fluoresce [102]. Carcinogenesis induces modifications in their concentration and fluorescence properties. Autofluorescence imaging and spectroscopy have been used to analyze tissue autofluorescence. VELscope® is a commercial device that uses tissue autofluorescence for screening oral precancerous lesions based on the structural and metabolic changes of the epithelium and connective tissue upon interaction with light. When stimulated with intense blue light (400–460 nm), normal oral mucosa appears pale green (figures 2(E) and (F)) [88, 92]. Conversely, dysplastic and malignant lesions appear relatively darker because of reduced autofluorescence, which can serve to screen asymptomatic patients.
The reported sensitivity of VELscope® for identifying malignant and dysplastic lesions ranges from 50% to 100% [101–103]. A disadvantage of this technique is its relatively low specificity (15%–80%) [101–103]. Ford and Farah concluded that this technique is unreliable in diagnosing dysplasia without any clinical interpretation [104]. In another study, VELscope® effectively determined oral leukoplakias and erythroplakia, but it could not distinguish between dysplastic and benign lesions [103].
1.10. Computed tomography (CT)
In CT, x-ray image slices are acquired at several different angles to reconstruct a tomographic image of the body. Each image slice is created by measuring x-ray attenuation through the tissue. CT is used for oral cancer diagnosis in evaluating the extent of infiltration in the buccal space, bone invasion, and extension to the retromolar trigone (figure 3) [8, 105–107]. Several studies have confirmed that CT is reliable in staging malignancy and detecting bone erosion [105, 106, 108]. Mukherji et al demonstrated that CT detected mandibular bone invasion with 96% sensitivity and 87% specificity [106]. Brockenborough et al reported diagnostic values in close range (95% sensitivity and 79% specificity) [109]. In addition, Trojanowska et al showed promising results using CT perfusion imaging to discriminate between benign and malignant lesions [110]. CT can thus be used as a diagnostic tool for evaluating symptomatic patients.
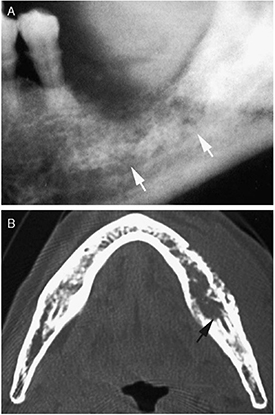
Figure 3. X-ray radiography (A) and CT image (B) of bone invasion (indicated by the arrows) caused by carcinoma of the mandibular gingiva. Reprinted from [107], Copyright (2009), with permission from Elsevier.
Download figure:
Standard image High-resolution imageAlthough CT effectively assessed tumor bone invasion at the advanced stages, it did not provide additional information to clinical examination for tumors less than 2 cm without bone involvement, rendering CT limited for early detection [105]. Further, CT is inadequate in characterizing soft tissues in the oral cavity, and image artifacts due to metallic dental fillings hamper adequate diagnosis [8, 111].
1.11. Magnetic resonance imaging (MRI)
MRI provides detailed images of the body and performs superior to CT in soft tissue differentiation and tumor contrast [8]. MRI is non-ionizing and works on the principle of nuclear magnetic resonance. Hydrogen nuclei in tissues align with an applied magnetic field. Thereafter, radiofrequency (RF) exposure is used to move the nuclei out of equilibrium. Subsequently, energy is emitted when the nuclei relax and is detected by receiver coils surrounding the tissue. MR images are reconstructed from spatial variations in the phase and frequency of RF energy. Tissue contrast is generated by exploiting the difference in relaxation times in different tissues. A contrast agent (e.g. gadolinium) can be used to enhance tumor features and assess microvascularity [8]. MRI can be used for the diagnosis of oral cancer in symptomatic patients. It is effective in staging for malignancy, identifying tumor locations, and measuring tumor thickness (figure 4(A)) [8]. It is comparable to CT in delineating regional disease and detecting lymph node metastases [112]. MRI has been reported to achieve 82% sensitivity and 63% specificity for evaluating muscle infiltration [113]. For detecting mandibular invasion, Vidiri et al reported 94% sensitivity and 60% specificity [114]. A subsequent study by Nae et al demonstrated that combining MRI with CT improved diagnostic efficacy (100% sensitivity and 72% specificity) [115]. However, false negatives were high and attributed to minor cortical inflammatory changes unrelated to the tumor and improper sampling of cortical bone. Some studies suggest using MRI and CT as a standard method for evaluating tongue neoplasms [116, 117]. Moreover, combined CT and MRI is better than conventional oral examination (64% sensitivity, 87% specificity) in lymph node staging [116]. However, these techniques fail to detect primary tumors and micro-metastases, which do not alter tissue at specific imaging planes or invade adjacent regions [118].
Figure 4. A 72 year-old man with gingival cancer. (A) Axial T1-weighted MRI of a gingival tumor exhibiting muscle infiltration and mandibular bone invasion (black arrow). (B) Axial fused PET/MR demonstrating an intense FDG uptake of the same tumor (arrow). Reprinted from [128], Copyright (2013), with permission from Elsevier.
Download figure:
Standard image High-resolution imageLwin et al evaluated the accuracy of MRI in determining tumor thickness in OSCC by comparing it with histological data [119]. Most tumors appeared thicker in MRI relative to histopathology, and the shrinkage factor varied for different oral sub-sites. Lwin et al concluded that the need for dissection could not be determined from the MRI staging or tumor thickness. Although MRI could provide valuable information in large tumors, it may still miss additional small tumors less than 10 mm [119].
The efficacy of dynamic contrast-enhanced (DCE) MRI in distinguishing between three types of head and neck cancer, i.e. SCC, undifferentiated carcinoma, and lymphoma, was assessed [120]. Lee et al found that the AUC (calculated at 90 s) showed significant differences in DCE MRI parameters between undifferentiated carcinoma and SCC (accuracy of 78%), as well as between lymphoma and undifferentiated carcinoma (accuracy of 97%) [120].
Although MRI is practical for pre-surgical planning, it is unclear whether it is helpful for early detection [119]. The use of MRI can be limited by expense, availability, length of procedure, and incompatibility with ferromagnetic dental fillings and other metallic implants [116, 121]. Hence, the role of MRI in early tumor detection needs to be assessed further.
1.12. Positron emission tomography (PET)
PET is a functional imaging modality based on the measurement of tissue metabolic activity. A radioactive compound (radiotracer) (e.g. 18fluorodeoxyglucose (FDG)) is injected intravenously along with a carrier molecule. The unstable nucleus emits a positron that encounters a free electron in the tissue region of interest. These two particles annihilate each other, resulting in two high-energy photons that move in opposite directions, which are detected by the PET scanner. Tomographic image reconstruction methods are then used to estimate the distribution of the radiotracer [122]. PET can be used for diagnosis of symptomatic patients.
The diagnostic efficacy of PET in identifying primary tumors has been compared with CT and MRI. PET using FDG (FDG-PET) has been reported to achieve 80% sensitivity and 86% specificity, in contrast to 75% sensitivity and 79% specificity obtained with CT and MRI [123]. In the same study, PET outperformed CT/MRI in overall pretreatment evaluation of patients, but not in detecting metastases. In another study, PET was more effective than CT and MRI in detecting primary tumors and metastatic nodes [118]. Dammann et al conducted a study to evaluate the efficiency of FDG-PET, CT, and MRI in detecting head and neck SCC [124]. CT and MRI results were found to be equivocal, while FDG-PET provided additional information to conventional examination in 31% of patients [124]. Furthermore, PET showed higher potential in correcting CT results than MRI because of its high specificity [124]. Ng et al found that FDG-PET is superior to CT/MRI in detecting palpably occult neck metastases [125]. However, PET is only recommended as a supplementary diagnostic procedure in case of ambiguous findings by CT or MRI.
Several studies have investigated combining the functional information of PET with the anatomical images of CT and MRI. Pentenero et al found that PET-CT combination could precisely determine the extent of primary tumor and the depth of invasion [126]. However, PET-CT combination fails to determine microinvasive lesions (1–3 mm thickness) [127]. Fused PET-MRI is a highly effective technique for the assessment and staging of head and neck cancer (figure 4(B)), as compared to PET-CT and MRI alone [128]. Despite improved diagnostic capabilities of combined PET-CT and PET-MRI, these techniques are secondary to evaluation with CT and MRI [122]. Although these combined techniques help identify metastases and guide treatment, they have been found ineffective for diagnosing the early stages of the disease [122]. Perez et al provide a comparative study about the utility of the three imaging techniques (CT, MRI, and PET) in detecting primary tumors, bone invasion, and neck metastasis [129].
1.13. Ultrasound
Ultrasound imaging involves the propagation of high-frequency sound waves (typically in the MHz range) by a piezoelectric transducer, which are transmitted through the body. The ultrasound waves interact with tissue structures, and the backscattered waves are received by the same transducer. Ultrasound can be used to extract structural and dynamic functional information and can be used both as a screening and a diagnostic tool. Ultrasound is widely used clinically in assessing head and neck cancer due to its real-time capabilities, versatility, adaptability, repeatability, and safety [121]. Ultrasound systems are also relatively inexpensive compared to the other imaging modalities discussed above. It can be performed on patients with metallic implants or contrast agent hypersensitivity [121].
Several studies reported using ultrasound imaging to measure the thickness and delineating boundaries of oral tumors, which can help predict the risk of metastasis and guide surgical resection margins [130–134]. Shintani et al demonstrated that intraoral ultrasound (7.5 MHz) could measure tumor sizes as thin as 1 mm in the buccal mucosa and 2 mm in the tongue and mouth floor of 39 patients [130]. The thickness measurements by intraoral ultrasound were highly correlated (R = 0.98) with histopathological findings [130]. On the other hand, CT and MRI had difficulty detecting tumors less than 5 mm thick and differentiating them from surrounding normal tissue [130]. Later studies reported similar findings. Lodder et al showed a high correlation (R = 0.87) between intraoral ultrasound (7–15 MHz) and histopathological thickness measurements of tumors of 65 patients with early-stage oral cancer, as compared to MRI (R = 0.54) [131]. Furthermore, Yesuratnam et al performed preoperative intraoral ultrasound (7–15 MHz) and MRI on 79 and 81 patients, respectively [132]. They reported that the tumor thickness measured by ultrasound was highly correlated (R = 0.8) with that of histology. Whereas, T1 post-contrast and T2-weighted MRI measurements were moderately correlated (R = 0.69 and R = 0.64, respectively) with histological results. In addition, Joshi et al found a good correlation (R = 0.73) in the length of the tumor between intraoral ultrasound (5–9 MHz) and histological measurements [133]. They identified tumors as deep as 19 mm in the tongue and buccal mucosa of seven patients. However, they found a weak correlation (R = 0.12) in tumor width, which could be tested further with a larger patient cohort [133].
The accuracy of intraoral ultrasound in measuring tumor thickness relative to histopathology was higher than that of MRI [132, 135]. Yesuratnam et al found a difference of 1.28 mm in mean tumor thickness between ultrasound and histological measurements for 79 patients [132]. Whereas, a difference of approximately 3 mm in mean tumor thickness was reported between MRI (i.e. T1 post-contrast and T2–weighted MRI) and histological measurements for 81 patients [132]. In another study, Noorlag et al assessed the depth of tumor invasion (DOI) by histology and found that it correlated well with the tumor thickness as measured by preoperative intraoral ultrasound (15 MHz) (R = 0.78) and MRI (R = 0.72) [135]. For thin tumors with a histological DOI of less than 10 mm, the mean difference between ultrasound and histology measurements was 1.6 mm, which was lower than the mean difference of 3.1 mm between MRI and histological measurements.
Color Doppler ultrasonography has been proven sensitive and specific in identifying malignant oral tumors based on blood flow indices [136–138]. An increase in tumor vascularity correlates with metastatic potential at the advanced stages of cancer [138]. However, the utility of Doppler ultrasonography in early detection remains unascertained.
Lam et al evaluated the use of high-frequency ultrasound (frequencies exceeding 20 MHz) for assessing early neoplastic changes associated with SCC using a hamster buccal pouch model [139]. A 41 MHz transducer was used to detect tumor progression as a result of carcinogen application. This high-frequency transducer achieved enhanced imaging resolution than standard clinical ultrasound frequencies (i.e. spatial resolution of 37 µm axially and 65 µm laterally). Structural changes in tissue layers were observed between pathological states using high-frequency ultrasound. A benign precancerous growth was differentiated from an invasive SCC that was only 300 µm away [139]. Additional preclinical and clinical studies are necessary to confirm the utility of high-frequency ultrasound to evaluate early oral lesions.
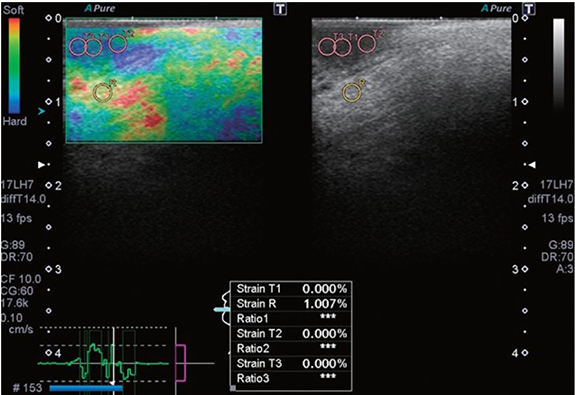
Tissue stiffness increases as cancer progresses, including at the early stages. Recent studies have evaluated the use of ultrasound elastography for estimating the stiffness of oral lesions. Strain elastography is based on imaging tissue strain in response to an external compressive force [140]. Softer tissues respond with higher strains than stiffer tissues. Strains are measured by analyzing displacements of ultrasound RF echoes from tissues in real-time. A ratio of the strains of the tumor and surrounding healthy tissue is used to gauge relative differences in stiffness. Aktar et al were the first to investigate strain elastography for SCC of the tongue of patient volunteers [140]. They found that normal tongues had higher strain ratios than those with SCC. Later studies explored the diagnostic feasibility of intraoral strain elastography for early-stage tongue carcinoma (figure 5) and reported clear discrimination between non-malignant lesions and carcinoma [141, 142]. Strain elastography is simple to implement and provides relative stiffness images in a manner analogous to palpation. However, strain images are confounded by artifacts caused by geometry effects, tissue nonlinearity due to excessive strain, and non-uniform stress distribution in tissues [143].
Figure 5. A strain elastogram (left) and B-mode image (right) of tongue squamous cell carcinoma in a 59 year-old man. The average strain of the normal tissue (R) is higher than that of the carcinoma (T1-3). Reprinted from [142], Copyright (2018), with permission from the Korean Academy of Oral and Maxillofacial Radiology.
Download figure:
Standard image High-resolution imageUltrasound shear wave elastography has recently been investigated for oral cancer diagnosis. This technique involves applying an external mechanical source to generate propagating shear waves in tissue. Tissue is detected by ultrasound imaging, and the measured speed of the shear wave is used to estimate tissue elasticity [143]. Thus far, only one study by Ogura et al has reported using shear wave elastography for tumor evaluation in the oral cavity [144]. They found that the stiffness of the tumors was more than ten times those of benign lesions. However, delineating tumor boundaries in the elasticity images was challenging, and thus, their results may have been confounded by boundary artifacts. Therefore, more studies are necessary to elucidate the role of shear wave elastography on oral cancer staging.
1.14. Photoacoustic imaging (PAI)
PAI is a relatively recent imaging approach that combines light and ultrasound. Tissue is exposed to a focused light beam (typically a laser), which is absorbed in tissue, thereby causing thermoelastic expansion and relaxation of tissue [145]. This phenomenon generates a wideband ultrasonic signature that is detected for imaging. PAI offers excellent image resolution and higher penetration depths than conventional optical imaging modalities. This approach can generate endogenous contrast from biomolecules, such as hemoglobin and melanin, which can be used for functional imaging of angiogenesis and blood oxygenation. PAI has been applied in preclinical research and in the clinical diagnosis of various cancers [145].
A few studies have investigated the feasibility of PAI for assessing oral lesions [146, 147]. Fatakdawala et al combined PAI with high-frequency ultrasound (41 MHz frequency) and fluorescence lifetime imaging to extract functional, structural, and biochemical information from oral lesions in a hamster cheek pouch model [146]. An increase in PAI signal intensity was associated with increasing tumor vascularity in precancerous lesions and invasive carcinoma, according to histological images. In addition, a reduction in high-frequency ultrasound backscatter intensity and an increase in average fluorescence lifetime corresponding to nicotinamide adenine dinucleotide (NADH) emission were observed in precancerous lesions relative to normal tissues. In a later study, Guo et al designed a miniaturized probe for dual-modal PAI and ultrasound imaging and demonstrated its clinical feasibility on imaging tongues of healthy volunteers [147]. These multimodal approaches could provide complementary structural and functional information for detecting and discriminating the different stages of carcinogenesis [146]. However, further studies are necessary to assess the clinical utility of this emerging technology for screening and diagnosis.
1.15. Optical coherence tomography (OCT)
OCT is an interferometric imaging modality that provides high-resolution (10–20 µm) structural images of targeted tissues up to a depth of 3 mm [148–151]. The contrast in OCT images arises from the difference in light absorbing and scattering properties of tissues [148]. Time domain (TD-OCT) and Fourier domain (FD-OCT) have been investigated for assessing oral pathologies. In TD-OCT, a broadband source emits light towards a beamsplitter, in which one beam is directed towards a reference mirror at a known distance and the other is directed towards a tissue sample. The interference patterns of the reflected light from the reference mirror and multiple depths in the tissue sample are analyzed to produce an image [151]. Transverse scanning is performed to create 2D or 3D tomographic images of tissue samples. OCT could potentially be used for screening asymptomatic patients and diagnosing symptomatic patients.
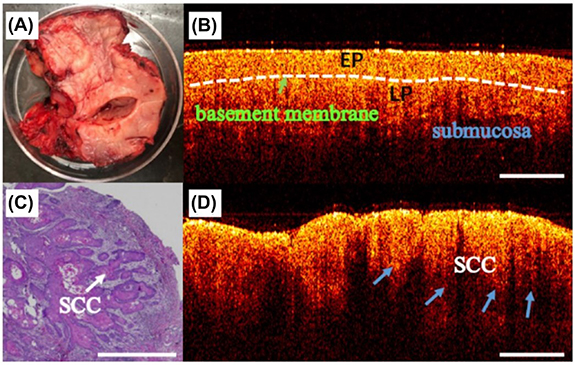
Preclinical studies using the hamster cheek pouch model demonstrated that OCT images can display progressive structural changes in oral tissue during carcinogenesis, which correlates well with histological findings [150, 151]. In particular, the disruption of the layered structure in the oral mucosa and thickening of the epithelium were observed. In a clinical study with 50 patients, Wilder-Smith et al demonstrated that TD-OCT was effective in differentiating between carcinoma in situ and non-cancerous lesions with a 93% sensitivity and 93% specificity [149]. Furthermore, TD-OCT images closely agreed with the histopathology of biopsied samples.
Compared to TD-OCT, FD-OCT has a higher imaging speed and signal-to-noise ratio and is less likely to be confounded by motion artifacts [150]. Spectral domain (SD-OCT) utilizes the frequency components of backscattered light to create an image of the tissue [148]. Swept source (SS-OCT) combines the capabilities of TD-OCT and SD-OCT, which has been shown to differentiate pathological oral lesions from healthy tissue (figure 6) [152–154]. However, Hamdoon et al reported that SS-OCT was inadequate in grading PMLs, and definitive identification of the basement membrane was challenging because of the limited depth of imaging [152]. Further, hyperkeratosis associated with most oral premalignancies has a degrading effect on image quality [155]. Despite these limitations, OCT has been investigated for clinical use in endoscopic procedures [156] and intraoperative imaging of oral lesions [157]. Furthermore, portable OCT systems have been developed to improve access to low resource clinical environments, which have been shown to exhibit superior diagnostic performance than COE [158].
Figure 6. (A) A photograph of an excised oral tissue sample. Optical coherence tomography images of the same sample at two different locations with (B) normal mucosa and (D) squamous cell carcinoma (SCC). (C) Histopathological image of the SCC. EP: epithelial layer and LP: lamina propria. Reproduced from [157]. CC BY 4.0. © The Author(s).
Download figure:
Standard image High-resolution imageImage processing algorithms have been developed to classify oral precancerous lesions by extracting quantitative parameters from OCT images. Lee et al analyzed SS-OCT image data to differentiate between normal mucosa, mild dysplasia, and moderate dysplasia in 54 patients [159]. Patients with mild oral dysplasia can reverse their condition by refraining from harmful practices that lead to cancer, such as smoking and alcohol consumption [159]. Lee et al mapped the standard deviation (SD) of the OCT signal intensity to estimate the distribution of dysplastic cells. They found that based on an SD threshold level of 70%, their method differentiated mild dysplasia from moderate dysplasia with a sensitivity of 82% and a specificity of 90% [159].
Jo et al combined FD-OCT with fluorescence lifetime imaging microscopy (FLIM) to differentiate between healthy and cancerous lesions based on structural and biochemical activity in the oral epithelium in a hamster cheek pouch model [160]. They observed that structurally normal epithelia based on OCT exhibited higher collagen emission on FLIM as compared to SCC. Further, FLIM measurements indicated that malignant lesions exhibited an increase in NADH and flavin adenine dinucleotide. Subsequently, Pande et al developed automated feature analysis algorithms for FD-OCT [161] and FLIM data to classify benign, precancerous, and cancerous oral lesions in the hamster cheek pouch [162]. Combining OCT with FLIM enabled more accurate discrimination (87.4%) than OCT (81.0%) alone [162].
OCT angiography (OCTA) enables the visualization of vascular information to complement the structural features of the tissue. In a preclinical study by Chen et al, changes in the intraepithelial papillary capillary loops (IPCLs) at the different stages of oral carcinogenesis were quantified from OCTA images [163]. Epithelial thickness, vessel area density, average vessel radius, and tortuosity of the IPCLs in mice oral mucosa significantly increased between normal and premalignant lesions (p < 0.05) and between premalignant and cancerous lesions (p < 0.05) [163]. Further preclinical and clinical studies are needed to optimize and establish the use of OCTA for early detection.
OCT has been combined with PAI to overcome the limitations of OCTA, which includes the inability to extract absorption parameters corresponding to hemoglobin concentration and oxygen saturation [164]. Qin et al developed a dual-modal OCT and PAI system for oral disease diagnosis [164]. Although the authors only reported the feasibility of imaging the lip of a healthy volunteer and monitoring the healing process of a lip ulcer, their integrated system has the potential for clinical diagnosis of oral tumors [164]. Olivo et al provide a detailed discussion on the role of various optical imaging techniques in early oral cancer detection [165].
1.16. Artificial intelligence (AI)
Higher workload, complex tasks, and fatigue may adversely affect the outcomes of tests conducted by human observers [166]. Machine learning (ML) is a branch of AI wherein computers are trained to recognize patterns from training datasets (past data) which can be applied to the testing dataset (present sample) to accurately identify patterns from large, noisy, and complex datasets [167]. Current developments in ML have shown the feasibility of detecting cancers in the liver, breast, lung, brain, and skin [168]. ML has also been shown to improve the prediction of cancer susceptibility, recurrence, and mortality by 15%–25% versus conventional or alternative approaches [167]. In the near future, it may serve as a good screening and diagnostic tool to aid oral cancer detection. Recently, Martino et al performed segmentation on OSCC images by a deep learning method [169]. Gupta et al applied convolutional neural networks (CNN) on images of 672 oral epithelial tissue obtained from 52 patients with oral dysplasia. The images were classified into four categories: normal tissue, mild dysplastic tissue, moderate dysplastic tissue, and severe dysplastic tissue. Their model exhibited adequate performance with accuracies of 91.65% (for the training dataset) and 89.3% (for the testing dataset) [170]. CNNs enable 2D visual pattern recognition, making it suitable for image classification and segmentation [171]. Wieslander performed a study to evaluate two network architectures based on CNN, namely VGG and ResNet, and found that the latter performed better with higher accuracy (78%–82%) [172]. However, the sample size in the study was limited to six patients. In another study, Muthu Rama Krishnan et al used a hybrid feature extraction method to identify oral precancerous lesions (oral sub-mucous fibrosis (OSF)) from histopathological images. Tissue sections were classified into normal, OSFWD (without dysplasia), and OSFD (with dysplasia). They observed that combining texture and higher order spectra features and Fuzzy classifier resulted in detection accuracy of 95.7%, a sensitivity of 94.5%, and specificity of 98.8% [173]. Jeyaraj et al investigated hyperspectral patient images using CNN and showed promising results for early detection of oral cancer (accuracy of 91.4%, sensitivity of 94%, specificity of 91%) [174]. Panigrahi et al provide a detailed review of the ML and deep learning algorithms for analyzing histopathological images of OSCC patients [171].
2. Discussion and conclusion
Conventional examination has limited efficacy in discriminating oral mucosal lesions and assessing the deep extension of the disease. Biopsy remains the gold standard confirmatory test. Genomics-based approaches could identify tumor-specific biomarkers for helping in early diagnosis. The advent of qMIDS has enabled objective diagnosis based on biopsy samples. Despite these technological advancements, biopsy is limited by its invasiveness, sampling bias, time, and resource requirements. Adjunctive approaches are needed to assist in screening asymptomatic patients, enabling effective diagnosis, and reducing unnecessary biopsies. However, none of the currently available adjunctive aids provide sufficient accuracy for the early detection of PMLs. Although some diagnostic imaging techniques appear promising, approaches are needed to enable early detection, improve diagnostic efficacy, and reduce unnecessary biopsies.
Adjunctive diagnostic aids have the potential to identify malignant lesions noninvasively. However, they alone do not definitively diagnose the disease, especially at the early stages. A scalpel biopsy and further histopathological analysis follow any reported suspicions. Furthermore, these techniques are limited in examining the deep extension of the lesion. According to the American Dental Association, there is a lack of currently available diagnostic adjuncts that demonstrate sufficient accuracy for evaluating oral cavity lesions [175]. Salivary and serum biomarkers have the potential to be used in diagnostic testing for oral cancer. However, currently, most techniques have been validated only in a laboratory setting or in a pilot cohort of patients. Further large-scale studies are needed to investigate the clinical diagnostic utility of these techniques. Cytologic testing using the adjuncts is conditionally recommended only under exceptional circumstances.
Diagnostic imaging modalities show promise in examining the deep extension of oral lesions. CT has been proven effective in detecting bony involvement in malignancy, but has limited utility in soft tissue characterization, especially in the early stages. MRI helps assess soft tissue invasion and identify tumor locations. PET offers a functional imaging approach, but should be considered only if results from CT and MRI are inconclusive. CT, MRI, and PET are useful for diagnosing the advanced stages of the disease. Ultrasound can be used to delineate boundaries and measure sizes of tumors as small as 1 mm thick and up to 20 mm in depth with high accuracy. A handful of studies have demonstrated the potential of ultrasound color Doppler, strain imaging, and shear wave elastography for oral cancer staging. PAI can provide functional information of tumor vascularity, complementing the structural information obtained from ultrasound and OCT. OCT is effective in diagnosing oral cancer progression up to only a depth of 3 mm. However, further studies are needed to assess the efficacy of these imaging techniques.
New techniques are on the horizon, including various forms of elastography and molecular imaging. These approaches depend on different contrast generation mechanisms and are enabled by advances in instrumentation and computation. Integration of AI-based approaches with diagnostic markers or imaging is another promising prospect, which is only beginning to be explored [176, 177]. These technologies are in the preclinical and clinical testing stages for various cancers. However, very few studies have tested these approaches in the context of oral cancer. Research and development efforts in this domain in the next several years could likely enable improved diagnostic screening and staging approaches, leading to reduced healthcare costs and improved clinical outcomes for oral cancer patients.
Acknowledgments
The authors would like to thank the Gujarat State Biotechnology Mission (GSBTM), Govt. of Gujarat (GSBTM/JD(R&D)/610/20-21/344) and the Indian Institute of Technology (IIT) Gandhinagar for financial support. The authors would like to acknowledge Aditya Guduru for assistance in the preliminary stages of this literature review and Himanshu Shekhar, Ph.D., for helpful discussions and providing valuable comments on the manuscript. The authors also acknowledge Library Services at the Indian Institute of Technology Gandhinagar.
Data availability statement
No new data were created or analyzed in this study.