Abstract
Over the past 150 years, our ability to produce and transform engineered materials has been responsible for our current high standards of living, especially in developed economies. However, we must carefully think of the effects our addiction to creating and using materials at this fast rate will have on the future generations. The way we currently make and use materials detrimentally affects the planet Earth, creating many severe environmental problems. It affects the next generations by putting in danger the future of the economy, energy, and climate. We are at the point where something must drastically change, and it must change now. We must create more sustainable materials alternatives using natural raw materials and inspiration from nature while making sure not to deplete important resources, i.e. in competition with the food chain supply. We must use less materials, eliminate the use of toxic materials and create a circular materials economy where reuse and recycle are priorities. We must develop sustainable methods for materials recycling and encourage design for disassembly. We must look across the whole materials life cycle from raw resources till end of life and apply thorough life cycle assessments (LCAs) based on reliable and relevant data to quantify sustainability. We need to seriously start thinking of where our future materials will come from and how could we track them, given that we are confronted with resource scarcity and geographical constrains. This is particularly important for the development of new and sustainable energy technologies, key to our transition to net zero. Currently 'critical materials' are central components of sustainable energy systems because they are the best performing. A few examples include the permanent magnets based on rare earth metals (Dy, Nd, Pr) used in wind turbines, Li and Co in Li-ion batteries, Pt and Ir in fuel cells and electrolysers, Si in solar cells just to mention a few. These materials are classified as 'critical' by the European Union and Department of Energy. Except in sustainable energy, materials are also key components in packaging, construction, and textile industry along with many other industrial sectors. This roadmap authored by prominent researchers working across disciplines in the very important field of sustainable materials is intended to highlight the outstanding issues that must be addressed and provide an insight into the pathways towards solving them adopted by the sustainable materials community. In compiling this roadmap, we hope to aid the development of the wider sustainable materials research community, providing a guide for academia, industry, government, and funding agencies in this critically important and rapidly developing research space which is key to future sustainability.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Forward
Magda Titirici
Department of Chemical Engineering, Imperial College London, United Kingdom
Taking the European Union (EU) as an example, the advanced materials sector generates more than 2.5 million direct jobs and a GDP of over 650 billion euros. The biggest challenge we are facing now is how to sustain such a materials heavy economy without continuing to harm the planet and destroy its ecosystems.
Sustainable materials are materials that can be produced at scale without depleting non-renewable resources and without disrupting the environment. Manufacturing and using truly sustainable materials are key goals for our future global economy and for meeting Paris Agreement targets to transition to net zero by 2050.
Materials sustainability is a very multidisciplinary and complex research area. Such materials vary from bio-based materials (polymers, carbons, ceramics, etc) to recycled materials that can be reprocessed or reused without requiring additional mining and minerals depletion. Sustainable materials must be closely connected with their applications, especially when urgent replacements for critical materials are required, for example in the energy sector but also in constructions, packaging, and textiles. Future developments in materials sustainability will need to eliminate resource scarcity and be part of a circular economy value chain to solve important global challenges. Not only the scarcity of resources must be eliminated, but such materials need to perform across their useful lifetime at a lower cost, be manufactured with minimum energy/water/toxic elements input and be reusable/recyclable at the end of their first useful life.
We have a crucial mission to change our future technologies mix from energy to buildings, transportation, agriculture, and industrial sector. We must ensure we decarbonise across all these sectors while improving performance and improving economic value and reducing the environmental footprint to a minimum. To achieve this, we need to implement affordable and performant sustainable materials across all these sectors. Only then we will have a prosperous and climate neutral economy by 2050.
The key goal of this 'sustainable materials roadmap' is to present different ongoing research, progress, and remaining challenges in sustainable materials. This roadmap will start with a section defining a material criticality. We will then focus on the potential of biomass, especially the biomass not in competition with the food supply (i.e. biowaste) and its potential as a sustainable, widely abundant source for creating the next generation of materials to feed our ever more demanding society requiring advanced materials across different sectors of the economy. Hence, we will have a section elaborating on how best to extract valuable biomass components from the raw parent biomass in a reproducible and sustainable fashion. Using pure biomass components is key to ensure materials reproducibility given biomass variety from species to species and even within the same species from crop to crop. We will then discuss biomass derived materials based on biomass components cellulose and lignin as well as the potential of wood for the future of nanoscience and materials engineering. Before diving into different specific classes of materials we will also discuss the importance of borrowing inspiration from nature to impart biomass-derived materials with crucial properties such as maximised transport and diffusion, self-healing, ability to expand and contract upon function without damage to only mention a few. We will then dive into specific classes of materials from carbons to metal–organic frameworks (MOFs), polymers, nanocomposites, quantum dots (QDs) and present recent progress and challenges on preparing these from sustainable bio-based precursors. After discussing various classes of sustainable materials, the roadmap will focus on important emerging technologies of key importance to reaching the Paris Agreement goals by applications across different sectors such as renewable energy generation (wind and solar), storage and conversion in sustainable batteries and fuel cells, H2 storage, as well as creating a circular carbon economy for CO2 capture and conversion into useful fuels and chemicals and electrochemical ammonia production. Other crucial sectors of our economy such as portable electronics industry, construction materials packaging and textiles and fashion will also be addressed with focus on materials sustainability. The roadmap ends with discussing important issues about materials circular economy and best practice in recycling. Finally, the very important aspect of quantifying sustainability across all the stages of a material life from the raw material is made from to manufacturing, transport, use phase and end of life is discussed.
We hope to have provided some concrete examples of how materials sustainability can be improved with focus on bio sourcing and bioinspiration by providing specific examples of sustainable materials and emerging challenges, their applications in key emerging technologies along with important aspects of materials circularity, recycling and life cycle assessment (LCA).
This collective review provides an overview of the current state of the art, research direction and future perspectives of sustainable materials.
Materials criticality
2. Assessing raw materials criticality
Sterling G Baird and Taylor D Sparks
Materials Science & Engineering Department, University of Utah, Salt Lake City, UT, 84112, United States of America
Status
Raw materials criticality can significantly impact global markets [1]. For example, the historical cobalt crisis of 1977–1979 consisted of a violent rebellion in Zaire (0.009% global GDP at the time) that led to a price spike by 380% [1]. In 2011, China implemented export quotas and taxes on rare earth materials, increasing the price of permanent magnet Dy and Nd materials up by approximately 800% and 2050%, respectively, which took several years to resolve via a World Trade Organization trade dispute [2]. Both cobalt and rare-earth materials, along with many other elements, are foundational for renewable or low-carbon technology, and the dependence of low-carbon technologies (e.g. solar, wind, batteries) on critical materials affects the energy security of nations [3]. There is a growing shift towards alternative, sustainable renewable energy [2], battery [2, 4], thermoelectric [5], piezoelectric, ferroelectric, and drilling/cutting/grinding (e.g. automotive, oil, and gas) [6] technologies. Clearly, energy materials are heavily impacted; however, all materials and related applications will be affected by materials criticality. Therefore, for all materials, it is important to consider raw materials criticality questions such as:
- Is a particular element abundant or scarce? (Elemental scarcity).
- Is it sourced primarily from a single region? (Market concentration).
- Do the region(s) suffer from political instability or frequent, disruptive, natural disasters? (Supply disruption risk).
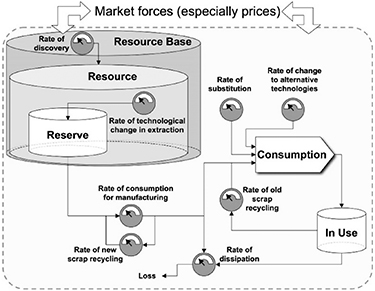
- How much can secondary sources relieve the above constraints? (Substitutes [7], recycling [2], see figure 1).
- How do the above factors depend on the short-, medium-, and long-term? (Temporal [7, 8]).
Figure 1. A flow analysis for materials criticality considerations demonstrates the dependence of materials criticality on and interdependence of many diverse factors, labelled as rates and displayed as gauges. Materials flow from resource base to resources and reserves, moving through manufacturing, recycling, consumption and in-use phases with circulatory connections in-between. Reprinted with permission from [1]. Copyright (2007) American Chemical Society.
Download figure:
Standard image High-resolution imageSpecifically, raw material criticality is a function of geographic, geological, environmental, political, temporal, and economic factors and is difficult to quantify (figure 1). Advancing understanding of the impact of raw materials criticality on industries for businesses, education, and governments [7] and increasing global data/analysis accessibility will enable:
- More efficient use of natural, monetary, and temporal resources;
- Avoidance of future supply-chain disruptions;
- Acceleration of materials discovery for game-changing technologies.
For a detailed report of materials criticality provided by the US Department of Energy (DoE), see [7].
Current and future challenges
Raw materials criticality needs to be assessed quantitatively in addition to qualitative measures so that data can be incorporated into algorithms to identify existing, high-risk materials as well as sustainable, alternative candidates. We present this in three distinct, data-driven components:
- (a)Identification and selection of meaningful features/metrics;
- (b)Data curation and sanitation;
- (c)Data analysis, interpretation, and visualisation.
Identification and selection of meaningful features/metrics
As a highlight of the ambiguity involved with defining 'raw materials criticality', Schellens and Gisladottir [9] gave a list of 23 distinct definitions across various review articles and gave a suggestion for a holistic definition. Alonso et al [1] identified 14 raw materials criticality features/metrics from 12 articles and sorted these into two categories: (a) institutional inefficiencies that cause resource unavailability and (b) physical constraints on amount and quality of resources. When a resource lacks certain institutional efficiencies, such as geographical supply and demand diversity or established recycling practices, the market is more susceptible to unpredictable events such as political change and natural disasters. Physical constraints were further sorted into static Malthusian (total amount and current rate of consumption), dynamic Malthusian (total amount and expected rates of consumption), and Ricardian (material quantity and quality) features, demonstrating the diversity of features that can exist. They also showed a material flow analysis (figure 1) which highlighted dependence on and interdependence of many, diverse factors. Some features may exhibit higher importance based on the application (as determined by feature selection algorithms), yet feature selection approaches may benefit from considering ease of obtaining and availability [10] of data in addition to importance.
Data curation and sanitation
Data may be subject to large uncertainty [1, 10], gaps [5], or systemic bias, any of which can depend on both material and temporal factors. For example, some materials (e.g. Li, Ni, Ga) are studied much more frequently than others (e.g. Cs, Tl, and Th) [8] and some material data has higher uncertainty (e.g. Herfindahl–Hirschman index (HHI) of Ga, Hf, Os) [5] or is missing data for certain years [5].
Data analysis, interpretation, and visualisation
Once data has been obtained and curated, screening can take the form of thresholds [1] and multi-objective optimisation for an application of interest [4–6]. Analysis and interpretation can be accomplished via machine learning algorithms or visual analysis of high-information density charts and tables. Algorithms or analyses which cater to input uncertainty or handle cases of sparse or missing data may be particularly useful.
Advances in science and technology to meet challenges
Hayes and McCullough [8] introduced a 'percent criticality' metric which they defined as the number of times an element was identified as critical vs the number of times an element was considered based on 32 comprehensive raw materials criticality studies. They analysed geographical, by-product recovery, and temporal trends using per cent criticality and found that rare earth elements, platinum-group metals, In, W, Ge, Co, Nb, Ta, Ga, Sb, Bi, Tl, and Mg are commonly considered critical.
The DoE's 2011 materials criticality document [7] identified Dy, Tb, Eu, Nd, and Y as near-term high criticality energy materials. They assigned qualitative measures of a material's importance to clean energy and supply risk (rankings between 1 and 4) and visualised these in gridded, criticality matrices for short- and medium-term forecasts.
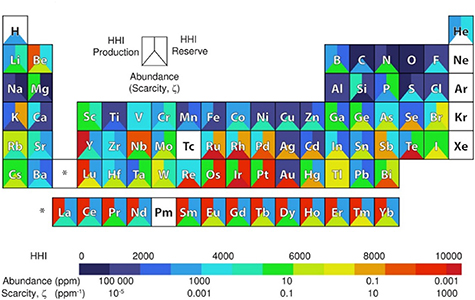
Successful examples of identifying new, promising materials have been demonstrated in the search of new, sustainable thermoelectric [5], battery [4], and superhard [6] materials using data compiled from the CRC Handbook of Chemistry and Physics and United States Geological Survey commodity statistics for elemental scarcity and the HHI, respectively (figure 2).
Figure 2. Elemental abundance and scarcity ξ (lower triangles), HHI production (left quadrilaterals) and HHI reserve (right quadrilaterals) data used for sustainable materials discovery is overlaid on the periodic table of elements. Unavailable data is portrayed as white. Reprinted with permission from [5]. Copyright (2013) American Chemical Society.
Download figure:
Standard image High-resolution imageGaultois et al [5] demonstrated that Te often correlates with high performance in thermoelectrics but has high scarcity. Despite being rare, the HHI value (a measure of market concentration) is low (good). In other words, the market is non-concentrated and less susceptible to local fluctuation.
Ghadbeigi et al [4] revealed that high energy battery materials (more typical of layered crystal structures) are scarcer than high power battery materials (more typical of olivine and spinel structures). They also showed that FeS2 can deliver good specific energy while having high abundance, and that Sb has more sustainable alternatives such as Si, Mn, Ni, Cu, and/or Zn.
Mansouri Tehrani et al [6] screened 1100 compounds for hardness values above 20 GPa and plotted the remainder in a high-information density plot (figure 5 of [6]). The abscissa (x-axis), ordinate (y-axis), colour, and plot marker size represented production HHI, scarcity, synthesis type (high-temperature/high-pressure vs high-temperature-only), and experimental hardness value (higher value = larger marker), respectively. By visually inspection, they identified best choices (diamond, c-BC5, c-BN, and BC2N), lower hardness alternatives (TiC, SiC, and TaB2), and poor choices (market-concentrated WB4 and transition metal candidates containing non-abundant Re, Ru, and Os).
Concluding remarks
Raw materials criticality is an important consideration for material selection. However, identifying and selecting criticality metrics, obtaining and curating data, and analysing and interpreting it can present significant challenges in the form of lack of availability, inconsistent reporting, and difficult to predict events (e.g. political uncertainty and natural disasters). Materials discovery approaches that incorporate criticality metrics have been successful in identifying new and sustainable thermoelectric, battery, and superhard materials. Improvements in raw materials criticality data availability and accessibility and increased use of criticality data in material informatics can enable more efficient use of resources, better supply-chain stability, and accelerated materials discovery for future technologies.
Acknowledgments
T D S and S G B gratefully acknowledge support from the National Science Foundation (CMMI-1562226 and DMR-1651668).
Biomass and bioinspiration as source for sustainable materials
3. Isolation of sustainable material precursors from biomass
Shirley Min Yang and Agnieszka Brandt-Talbot
Department of Chemistry, Imperial College London, United Kingdom
Status
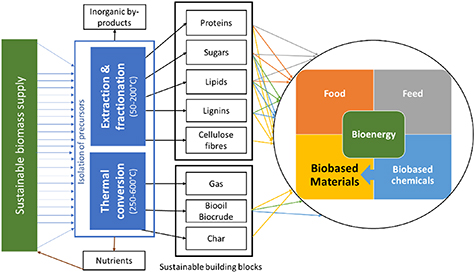
Precursor isolation is a central step of biorefining (figure 3). It determines the chemical characteristics of the precursors and affects economics and sustainability through energy and chemical inputs, process complexity and output yield and quality. The isolation consists of a variable number of separations and often a central thermochemical conditioning step. Precursor extraction and biomass fractionation aims to supply selected biomass components unaltered and hence these processes operate at lower temperatures (50 °C–200 °C) [11]. Thermochemical conversion aims to transform a whole biomass by applying high temperatures (250 °C–600 °C), generating mixed, chemically altered fractions, with any separation occurring afterwards [12]. Production of bio-oils (from wood pyrolysis) has reached commercial scale, but nearly all of it is used in fuel applications. There is one instance where high value flavour compounds are isolated from pyrolysis oil. The most developed biocrude and bio-syngas processes are currently at demonstration scale.
Figure 3. Isolation of key precursors within existing and future biorefineries (adapted from IEA). Reproduced with permission from [11].
Download figure:
Standard image High-resolution imagePrecursors obtained via extraction/fractionation are classified according to biomass supply and chemical structure. First-generation precursors are derived from plant parts used for food production. Sucrose is a first-generation sugar obtained by pressing sugarcane or sugar beet. The juice is used for fermentation, making sucrose the most accessible precursor for biobased polymers. Starch is a sugar polymer contained in grains, such as corn, and hydrolysed to fermentable glucose using enzymes. Starch is also used directly as a biobased thermoplastic [13]. First generation lipids are obtained from lipid-rich crops by pressing, spontaneously forming a layer separate from water. Solvent extraction can improve yield. After lipid or starch isolation, plant protein is often concentrated in the residue and can be isolated using solubilisation with aqueous alkali followed by acid precipitation [14]. Soybean is the most popular source of first-generation lipids and plant proteins. Cotton is a first-generation source of cellulose fibres and is the major precursor for biobased fabrics.
Second generation precursors are derived from lignocellulosic (wood) biomass, arising in forests (wood chips) or as by-product of agriculture (straws). Wood fractionation aims to separate its major constituents, cellulose fibres, lignin, and hemicellulose. Wood cellulose fibres are isolated by industrial pulping through removing most of the lignin and hemicellulose [15]. Kraft, sulphite and soda pulping processes use concentrated aqueous solutions of inorganic chemicals, which are recycled. Some sulphite mills market water-soluble lignins (lignosulfonates) obtained after precipitation or ultrafiltration. Kraft lignin is usually incinerated as part of the chemical recovery but increasing quantities are available from Kraft mills upgraded with an acid precipitation unit. Second generation sugars produced at costs acceptable for fermentation, are available via water-based processes, usually steam explosion and dilute acid pretreatment.
Current and future challenges
Current biobased materials are typically made from precursors that have simple isolation methods: biobased plastics from sucrose or starch and biobased fabrics from cotton. However, due to the use of agricultural land and intensive farming methods to grow first generation crops, there is a significant limit to the quantities that can be obtained and also their overall sustainability potential.
Lignocellulosic biomass can provide sustainable precursors at much larger scale, but the cost to isolate precursors is increased. Capital costs are higher due to more processing steps, increased complexity of process equipment and the need for equipment to be resistant to pressure and chemicals. Operating cost can be higher due to increased chemicals and energy use. As a result, many lignocellulose fractionation processes generate precursors at prices that do not cater for lower value uses, especially provision of fermentable sugar. Increased energy requirements also negate some of the greenhouse gas (GHG) savings in the life cycle of the biobased product. Steam explosion coupled with enzymatic hydrolysis can generate fermentable glucose at attractive cost without harmful chemicals, but is only effective on some agricultural straws. Adding dilute sulphuric acid as a catalyst broadens the variety of lignocellulosic feedstocks that can be processed. The deposition of sugar derived humins, in addition to reprecipitated, condensed lignin, reduces sugar yields, while fermentation inhibitors complicate downstream processes. The so-called biorefinery lignin is impure, making its use as a material precursor challenging. While certain natural and engineered microorganisms accept C5 sugars (derived from the hemicellulose) when provided alongside the cellulosic glucose, the fermentation is slow and uneconomical despite years of development. Concentrated acid processes combine softening the lignocellulose matrix and a chemical hydrolysis in one step while using low temperatures, but effective sugar-chemical separation is difficult. Fractionation processes using non-aqueous solvents struggle with solvent recovery, which must exceed 99 wt% even for inexpensive solvents such as ethanol. There is also concern for thermal and chemical stability of some solvents. For lipids and protein, processes that isolate them from second generation biomass, such as algae and protein rich wastes, are currently not commercial. Pyrolysis and hydrothermal liquefaction produce complex, partially unknown mixtures of organics with water, which are difficult to separate and may not be stable during storage, hampering their use as a chemical feedstock.
Advances in science and technology to meet challenges
Additional methods to isolate precursors from lignocellulose have the potential to increase the quantity of available sustainable precursors and provide unique chemistries. Focussing on hemicellulose and lignin quality and not just cellulosic fibres and sugars may provide breakthroughs in the fuller valorisation of lignocellulose.
Solvent based fractionation technologies often fractionate a wide range of woody feedstocks while producing purer cellulose fibres and novel lignins, but solvent stability and recovery (<99%) is key. Non-volatile, low-cost solvents such as waste glycerol and ionic liquids (ILs) may be a solution, as their solvent recovery is innately high, if their stability and recyclability is proven [16]. Concentrated acid processes using HCl as a distillable acid may allow for effective acid recovery. Feedstock independent fractionation processes that are technically and commercially viable would boost the biorefining industry.
Generating a separate C5 sugar stream from the C6 stream using pre-extraction can overcome the issue with slow C6/C5 co-fermentation. It may also be useful to produce furfural from the pentosan fraction, a versatile, underappreciated biobased building block with existing commercial applications [17]. Catalytic conversion of sugars rather than fermentation may also boost attractiveness of some isolation processes, for example concentrated acid processing. Cost-effective and sustainable lignin purification and fractionation will provide more useful lignins. Lignin first fractionation technologies may provide specialised lignins for certain performance materials or phenolic building blocks at high yields [18]. For monitoring consistency across the industry, better standardisation of analytical techniques is needed, particularly for less developed and chemically diverse precursors, such as lignins, bio-oils and biocrudes.
To provide low-cost, sustainable precursors, there is a case for utilising any low value food and lignocellulosic wastes, which includes peels, press cakes and shells, as well as construction and demolition wood waste. The use of marine biomass (seaweed, algae, crustaceans) has potential to provide sustainable precursors if efficient cultivation and isolation technologies are developed. Utilisation of second-generation lipids and protein from algae and food processing residues could increase sustainable supply of these non-lignocellulosic precursors.
Important innovations will be needed in the process engineering, especially in the separation steps which are vital in any precursor isolation. Increasing biomass loading while maintaining fractionation performance will reduce costs. This can be achieved through reactor design optimised for thermochemical conversion and extraction of high solid slurries, such as screw extruder reactors [19]. Lowering the water consumption during washing and effective precursor drying will help boost economics and minimise energy use. Fractional separations of pre-conditioned bio-oils and crudes may yield chemically pure precursors that can be used in materials production.
Concluding remarks
In the past, focus has been on providing food sugars and cellulosic glucose as biobased material precursors. This needs to be expanded to include all biobased precursors that have the potential to be isolated at a meaningful scale. We need to develop cost- and environmentally benign fractionation technologies that can offer high quality streams of as many constituents of a biomass as possible. Close collaboration between chemists, biologists and engineers is needed to overcome technology bottlenecks. Collaboration with upstream (farmers, forest owners) and downstream stakeholders (fermentation, chemical conversion, material assembly) is crucial to match biobased products with the right feedstock supply and the most suitable precursor isolation method.
Acknowledgments
The authors thank Dr Michael Wise and Dr Florence Gschwend from Lixea and Prof Jason Hallett for valuable input. Agnieszka Brandt-Talbot acknowledges funding by Imperial College London through an Imperial College Research Fellowship.
4. Lignin-a precursor for materials future
Omid Hosseinaei1 and David P Harper2
1 RISE Research Institutes of Sweden, Drottning Kristinas väg, 61114 28 Stockholm, Sweden
2 Centre for Renewable Carbon, University of Tennessee, 2506 Jacob Drive, Knoxville, TN 37996, United States of America
Status
Global attention to environmental challenges such as pollution, deforestation, and anthropogenic climate change has prompted international efforts to use sustainable sources of energy, chemicals, and materials. Biomass is the main and most available renewable resource for providing sustainable alternatives to fossil-derived liquid fuels and materials. Lignin is the second most abundant polymer in the plant cell wall and a by-product of pulp and paper manufacturing. Worldwide, lignin is expected to be produced at 225 million tons per year by 2030 with an estimated value of about USD 913 million just by 2025 [20]. Currently, lignin's main use is as a fuel for producing energy and electricity in pulp mills. However, lignin's high production volume and current low price make it attractive as feedstock for multiple applications (figure 4). In addition, lignin has other features making it attractive, such as high carbon content, aromatic structure, numerous depolymerisation, and repolymerisation possibilities. Most of these applications are still in the early stage of development and not commercialised, but the move toward commercialisation has been accelerating in recent years. The advances in the area also affected by the fast-growing needs for new technologies such as energy storage systems and smart materials [21, 22]. Also, the need for a sustainable value chain resulted in developing biological and biochemical approaches for conversion of lignin to value-added products [23, 24].
Figure 4. Some of the main demonstrated applications of lignin.
Download figure:
Standard image High-resolution imageThe pulp and paper industry, especially softwood kraft process, accounts for the vast majority of produced lignin, but the emerging bio-economy is rapidly adding lignin from hardwood and herbaceous plants to this market. To compete with fossil fuels, high biomass yield and lignin valorisation are necessary to enabling cost-competitive biofuels [25]. Lignin, as the main by-products of biorefineries, needs to obtain relatively higher market value for its products than carbohydrates since fuels generally have narrow profit margins. Still, carbohydrates markets are well established where higher-value chemicals and materials intermediates can help to support fuel production. The growing need to use all biomass components has spurred the development of new lignin isolation methods and chemical and biochemical conversion technologies.
Current and future challenges
Lignin's heterogeneous structure along with the challenge in isolating it from plant polysaccharides contributes to the challenges in valorisation. This heterogeneity arises from differences in botanical source that also dictates the isolation process. Lignin is based on varying amounts of different monomeric units, interunit linkages, and functional groups which harsh conditions are needed to deconstruct biomass and isolate the lignin. Contaminates, organic and inorganic, present from the biomass and pulping process often remain in lignin and add an additional layer of complexity. All of these factors increase variability and greatly impede depolymerising lignin into simple chemical building blocks for producing chemicals, fuels, and materials [24]. This makes finding new separation methods along with post refining and purification essential steps to valorise lignin [26]. For many products, the inherent variability in a natural polymer presents the biggest hurdle to overcome. Lignin's non-uniform structure along with readily oxidised linkages and a wide range of molecular weight creates challenges during a seemingly insurmountable challenge when process as a thermoplastic, such as in extrusion or melt-spinning, by producing volatiles, foaming during processing and crosslinking.
Lignin possesses strong intermolecular interactions, occur by hydrogen bonding and π–π stacking, that restrict thermal fusion and solubility of lignin in processing, conversion, and characterisation of lignin. On the other hand, presence of different functional groups in lignin lends to high reactivity as it can uncontrollably go under different condensation/repolymerisation reactions during both isolation and downstream processing.
The current availability of lignin sources is another challenge in developing lignin-based products. Lignin is burned as a source of producing energy and steam in pulp and paper mills. Pulp and paper mills usually produce more energy than they need. Lignin-based products can potentially reduce their energy production, though unlikely, and result in a need for another energy source. High-value lignin products would increase the returns on biofuel and pulp production [25].
Other factors that challenge the commercialisation of lignin-based products are colour, usually a dark to light brown, and odour, usually associated with the kraft process, which can limit the application of lignin in thermoplastics. The performance of the lignin-based materials is another restricting factor since these products need to compete with current commercial material. For example, the properties of lignin-based carbon fibres are still much lower than commercial petroleum-based (polyacrylonitrile) carbon fibres, but may possess other properties needed to be competitive in price and some important property compared to non-renewable sources [27].
Advances in science and technology to meet challenges
To overcome challenges related to the heterogeneous structure of lignin different approaches have been suggested and studied. Starting from the biomass, which is the source of lignin, engineering the feedstock is a solution for producing more homogeneous lignin [26]. The bioengineering in lignin biosynthesis pathway can further improve by developments in new CRISPR-based gene editing technologies [28]. Engineering biological solutions in depolymerisation of lignin is another means to overcome the challenges arising from heterogeneity of lignin in conversion of lignin to any product [23]. Biological funnelling has been proposed as a means to selectively convert lignin to chemicals and fuels within the biorefinery [23]. Refining and fractionation of lignin have been tried in many applications for obtaining a narrow molecular weight and higher homogeneity for different applications such as thermoplastics and fibres [27, 29]. Lignin can be fractionated into different molecular weight fractions using successive sequence of solvents after isolation of lignin or by other methods such as membrane filtration or pH fractionation during isolation. New green chemicals and solvents along with biological depolymerisation are the near future of pulping and will have added benefits of reduced environmental impact and improved lignin for downstream processing [25].
Increasing lignin purity is another important factor for better valorisation. The purity of lignin can be increased by improving lignin isolation technology or combined with post refining. Also, different pre-treatment technologies, feedstocks, and isolation methods greatly improve the purity of lignin. For example, organosolv process is known to produce lignin with a low level of contaminants. It mainly works well with herbaceous and hardwood feedstocks but has higher yields than kraft for all components. Nevertheless, developing commercial lignin purifications methods, such as LignoBoost, Borregaard, CIMV lignin, for recovery of commercial lignin. Developing new products based on new isolation technologies enables producing more lignin than needed for energy production and solves immediate availability issues.
Chemical modification of lignin is essential in some lignin valorisation, especially conversion of lignin to thermoplastics/thermosets or for alloying with other polymers. Chemically modifying lignin aims to overcome many processing barriers including heterogeneity, reactivity, compatibility, and to improve processability for many applications [29]. For example, reducing the intermolecular interactions in lignin improves fusibility in thermoplastic processes, while adding photo active groups allows for use as a photocurable resin system [30].
Concluding remarks
The field of lignin valorisation and producing lignin-based materials covers a wide range of materials, chemicals, and fuels. Promising results have been reported in this area and commercialisation of lignin-based products, especially in some area such as thermoplastics, thermosets, and some chemicals such as vanillin is rapidly growing. Factors such as environmental concerns and move toward sustainable materials with changes in legislations are greatly accelerating the transition toward renewable materials and energy. The developments in new technologies such as energy storage, smart materials, packaging, and biomedical have opened new possibilities for lignin products, which even has been extended to cosmetics and pharmaceuticals. Many developments are currently overcoming the challenges related to using lignin, which covers a broad range of current issues such as heterogeneity, functionality, odour reduction to facilitate lignin valorisation. The move towards using more sustainable materials, chemicals, and energy sources is pushing innovation that leads to new lignin materials to replace existing fossil-based materials and even new markets in the very near future.
Acknowledgments
D P H acknowledges support from the United State Department of Agriculture National Institute of Food and Agriculture Hatch Project 1012359.
5. Cellulose-based materials
Richard M Parker and Silvia Vignolini
Yusuf Hamied Department of Chemistry, University of Cambridge, Cambridge CB2 1EW, United Kingdom
Status
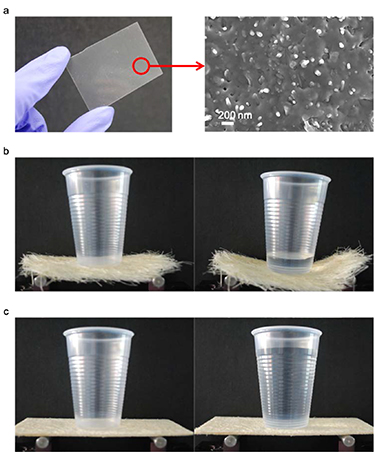
Cellulose, a naturally occurring high molecular weight homopolysaccharide, is the most abundant biopolymer available on Earth. It is widely produced by plants, where it is employed to give structure to the cell wall, but also can be found in some species of bacteria and aquatic animals. In plants, cellulose is produced in a hierarchical fibrillar form where the smallest element is a linear semi-crystalline polymer formed of β-1,4-anhydro-D-glucopyranose units [31]. The degree of polymerisation, which is measured as the number of monomer units per chain, depends on the cellulose origins and generally varies between 10 000 and 15 000 for wood and cotton-derived cellulose, respectively. The linearity of the chain is maintained by a combination of hydrogen bonds and hydrophobic interactions, which promotes parallel packing into elementary fibrils with a diameter of 3–20 nm. These nanofibrils are made of elongated crystalline domains separated by more amorphous regions, with the degree of crystallinity dependent on the source. The naturally occurring crystalline form is cellulose I, however this is a thermodynamically metastable structure and as such can be converted to the more stable cellulose II or cellulose III. The elementary fibrils are arranged into bundles forming a cellulose fibre. This fibre, with an overall diameter in the range 5–20 μm is produced by rosettes in the primary cell wall of the plant, where it is combined with pectin and hemicelluloses.
Cellulose has been widely exploited throughout history, although typically in an unprocessed form, such as cotton for textiles or wood for construction and paper manufacture. However, over the last few decades cellulose and its functional derivatives have been used directly to produce polymer films (e.g. cellophane) or as rheology modifiers in food and pharmaceuticals. More recently, nanocellulose has received strong interest due to offering a renewable, biodegradable, and biocompatible route to functional nanomaterials—key to transitioning to a more sustainable society [32]. Industrially-produced nanocellulose can be subdivided into two classes: (a) high-aspect ratio cellulose nanofibres (CNFs), which offer exceptional strength and stiffness for their weight (figure 5(a)), and (b) cellulose nanocrystals (CNCs), which are highly crystalline, elongated nanoparticles that can form a stable colloidal suspension in water (figure 5(b)). CNCs have potential in a wide-range of applications from rheology modifiers to emulsion stabilisers, and even offer potential as a sustainable alternative to synthetic pigments.
Figure 5. Naturally occurring cellulose fibrils comprise a hierarchical structure, allowing for nanocellulose to be extracted in the form of either (a) high aspect ratio CNFs or (b) colloidally stable CNCs. Images courtesy of Yating Zhang and Thomas Parton.
Download figure:
Standard image High-resolution imageCurrent and future challenges
From food and cosmetics to paint and textiles, colour is used to not only enhance aesthetics, but also act as a gauge for quality, attractiveness, freshness, or taste. While the colorant industry has long relied on the use of complex synthetic dyes or inorganic pigments to produce such visual effects, there is a growing demand for alternatives that can be presented to the consumer as natural, ethical and sustainable. To address this challenge, inspiration can be drawn from nature where 'structural colour' is responsible for many of the most vibrant colorations, from the metallic wings of butterflies and the vibrant feathers of birds to the iridescent epidermis of plants. In these natural examples, colour is produced not by absorption, but by the specific interference of light with precise nanoscale architectures. For example, within certain fruits and leaves, birefringent cellulose fibres are assembled into a periodic helicoidal nanostructure within the cell wall, enabling intense reflection of blue light [33]. Interestingly, aqueous suspensions of colloidal CNCs have been shown to spontaneously assemble on the nanoscale to mimic this natural helicoidal architecture. Upon drying, this structure can be retained, enabling the intense reflection of visible light (figure 6(a)). Using this approach, colours from across the entire visible spectrum can be produced and combined with visual effects such as iridescence and metallic shine [34].
Figure 6. (a) Structurally coloured CNC films can be tuned to reflect across the full visible spectrum by altering the initial formulation. (b) Arrays of CNC microfilms (diameter = 600 μm) can be printed to produce responsive dot-matrix images. Reproduced from [39]. CC BY 4.0. (c) Roll-to-roll (R2R) fabrication can be used to continuously encapsulate an iridescent mesophase of aqueous hydroxypropyl cellulose (roll width 14 cm). Reproduced with permission from Dr Hsin-Ling Liang.
Download figure:
Standard image High-resolution imageWhile the ability for CNCs to self-assemble into coloured films has been known for nearly two decades, critical technological locks have held back cellulose-based pigments from reaching industrial scale and entering the market [35]. The production of CNCs themselves has only recently reached industrial-scale production, with the largest supplier CelluForce able to output over 1 tonne d−1 since 2012. However, like any biologically sourced material, CNCs can be batch sensitive as their properties are related to the source and the methodology of extraction [36]. The key challenge now is to develop large-scale and cost-effective fabrication of CNC-based pigments in terms of production throughput and yield, while maintaining the desired optical uniformity and tailored visual appearance. This will require strategies to overcome the inherent limitations of self-assembly driven processes, control over the drying kinetics and mitigation of new physical phenomena that arise at larger scales, such as flow and shear, wetting and adhesion, or delamination and cracking.
Advances in science and technology to meet challenges
To move beyond small-scale batch production, several strategies can be envisaged, including: (a) continuous film deposition, (b) ink-jet printing of microfilm arrays and (c) emulsification to form dispersible pigments.
Cellulose-based packaging materials are industrially produced primarily via continuous roll-to-roll (R2R) manufacturing. While this methodology has been successfully demonstrated by encapsulating a mesophase of hydroxypropyl cellulose (figure 6(c)) [37], translating it to the large-scale organisation of CNCs to produce photonics films and coatings is challenging [38]. This arises from the slow timescales (hours to days) typically required by the complex evaporation-induced process of self-organisation into cholesteric liquid crystalline tactoids and subsequent long-range self-assembly into a well-aligned film. This can only be overcome through new insight into the underlying mechanisms that will allow the initial formulation of the CNC suspension to be optimised in terms of concentration, ionic strength, phase behaviour and the incorporation of functional additives. Finally, to achieve commercially viable fabrication over a large area (i.e. m2), it will be necessary to address principal issues such as the non-uniform visual appearance arising from shear upon deposition, lateral flow, and concentration gradients.
An alternative approach to scaling-up is to produce arrays of sub-millimetre CNC films (figure 6(b)) [39]. This could be achieved at scale by droplet-on-demand inkjet printing, whereby polychromatic CNC arrays would be used to create vibrant, structurally coloured dot matrix images, with applications ranging from highly reflective signage to anti-counterfeiting technology. To produce such designs, it will be necessary to develop 'CNC photonic inks' that are compatible with commercial inkjet printers, in terms of rheology (e.g. viscosity. <5 cP) and chemistry (e.g. 6–8 pH). Furthermore, strategies to reproducibly overcome the high drying rate (<1 min) of such small volumes will be needed, such that the final visual appearance of the array can be designed.
While CNC pigments can be prepared by dicing films into a dispersible glitter [38], a more disruptive strategy is to produce CNC pigments directly through the confinement of the self-assembly process within discrete micron-scale water droplets [40]. Upon drying, each microdroplet would produce a single, structurally coloured CNC microparticle [41]. The advantage of this approach is that it can readily build upon existing industrial emulsion technologies (e.g. homogenisation or membrane emulsification) to produce a non-iridescent powder that can be directly incorporated into existing formulations (e.g. cosmetics and paints). However, to optimise the visual appearance it will be necessary to first understand the role of surface buckling, overcome inherent scattering (i.e. whiteness) and maximise the colour intensity in terms of particle size and geometry.
Concluding remarks
By exploiting the most abundant biopolymer on the planet, cellulose, and replicating the natural assembly processes found within the plant cell, researchers are developing a new generation of sustainable 'photonic' pigments. These CNC-based pigments have great potential for mass-market applications such as cosmetics (US $429.8 billion by 2022), food coloration (US $3.75 billion by 2022), and printing ink (market-size: US $20.4 billion by 2022). Interestingly, membranes composed of CNFs have also recently gained significant attention due to their ability to scatter white light with high efficiency [42]. Given that the high refractive index nanoparticles commonly included as scattering enhancers within commercially available white products (e.g. TiO2) have recently raised serious health and environmental concerns [43], this is extremely relevant for industrial applications as such as paints and sun creams. Finally, by incorporating such biocompatible and biodegradable alternatives to synthetic colorants into products, it allows industry to directly respond to growing environmental concerns over microplastic pollution.
Acknowledgments
The authors acknowledge support from the European Research Council (ERC-2014-STG H2020 639088; ERC-2017-POC 790518) and the Engineering and Physical Sciences Research Council (EPSRC EP/R511675/1). The Biotechnology and Biological Science Research Council (BBSRC BB/V00364X/1).
6. Wood science and engineering for nanotechnology
Lars A Berglund and Yuanyuan Li
Wallenberg Wood Science Center, KTH Royal Institute of Technology, 100 44 Stockholm, Sweden
Status
Wood is by far the most successful biological material for structural applications. Worldwide, almost 500 million cubic meters of sawnwood was consumed 2018, together with 400 million cubic meters of wood panels (plywood, particleboard, fibreboard etc). Also on a weight basis, this even exceeds the plastics consumption. The main drivers have been low cost in combination with favourable mechanical properties per weight of material. Historically, the development of wood composites such as plywood, laminated veneer lumber and glulam was an important step forward, since the variability in mechanical properties caused by defects in wood structure is reduced significantly. Particleboard is a clever use of waste in the form of wood particles and fibreboard is a lightweight fibre composite material for buildings and furniture. Previous research on wood modification includes thermal treatment, metal salt impregnation, chemical acetylation and many different surface treatments, mostly to reduce moisture effects on degradation for prolonged service life outdoors.
The societal need for sustainable development creates opportunities for replacement of fossil-based plastics and composites by materials from renewable resources, such as wood. Expanding the use of wood to new applications could therefore reduce environmental stress. For example, the use of wood nanocellulose in large, load-bearing structures will be limited by the energy needs, carbon footprint and cost associated with disintegration of wood and wood fibres.
Nanotechnology is successfully commercialised in coatings, microelectronics and photonics, where sophisticated but elaborate bottom-up fabrication is common and materials are mechanically delicate. Wood offers unique potential for top-down approaches resulting in large-scale structures, where not only the nanostructure, but also the structural hierarchy of wood is exploited. The vision is to combine load-bearing function with new functionalities in applications where the wood component is a device. Functions possible in wood nanomaterials can be exemplified by liquid transport, electrical functions, thermal storage, fire retardancy, magnetic surfaces, energy harvesting and other functions introduced by chemical modification, functional polymers or nano-particles [44]. Filtration and molecular scale separation is also of interest. Although full-scale nanotechnologies may take some time to develop, there is a strong need to develop the underlying nanoscience.
Current and future challenges
A route for top-down preparation of wood nanomaterials is illustrated in figure 7, although it may not be necessary to carry out all steps. Wood is subjected to delignification, followed by functionalisation, which can include chemical modification, nanoparticle impregnation or precipitation from salt solution, monomer impregnation and polymerisation, either one method only or combinations of methods.
Figure 7. Scheme for top-down preparation of wood nanomaterials. Lignin is removed to form a nanoporous, delignified wood substrate. This can be functionalised by, for instance, polymers, nanoparticles or by carbonisation. Reproduced from [45]. CC BY 4.0.
Download figure:
Standard image High-resolution imageAn important step for wood nanoscience is the creation of a wood substrate by mild delignification of the wood cell wall, which leads to increased pore space and also space available for modification. One research challenge is to clarify effects from delignification and drying approaches on the nanostructure in terms of pore size distribution, cellulose nanofibril aggregation and chemical environment. Wood nanostructure can be controlled: (a) in the microscale pore space, where functional nanoparticles or other compounds can be added, or (b) in the wood cell wall. Chemical and/or physical engineering of the cell wall during processing stages is particularly important, since controlled distribution of nanoparticles, polymers, dyes etc is critical in order to achieve new functionalities or extending the property range. During these stages, processing for nano-structural control of the wood nanomaterial is important. Appropriate characterisation techniques for nanostructure include small angle x-ray scattering, neutron scattering, and Raman microscopy in addition to high resolution electron microscopy techniques [44].
Sustainable development is critical, and new materials can contribute in terms of reduced environmental stress. Examples include the use of green chemistry processes under mild conditions, eco-friendly nanoparticles, biobased polymers or no added polymers at all, and an overall focus on integrated processes with minimum energy requirements. For some applications, improved mechanical properties per weight of material is important since less material is needed or energy for transportation is reduced. Materials design concepts which provide long service life are advantageous, and this often means that biodegradability may not be feasible. Recycling or downcycling are important contributions to sustainable development, where interface tailoring has been shown to facilitate recycling of wood fibre materials with well-preserved structure and properties in subsequent service lives [46].
A different route, in the context of materials categories, is to convert wood into carbon or ceramic materials while controlling nanostructure [44]. This dramatically widens the attainable range of properties and functions, and is of interest in materials for energy. This category of materials, however, is competing with other related materials and devices, rather than polymeric materials.
Advances in science and technology to meet challenges
The wood substrate in figure 7 is the starting point for wood nanotechnology. Much of the scientific and technical progress in nanocellulose can be transferred into the creation of tailored wood-based nanostructures.
Take transparent wood as an example. Delignification or bleaching is important for wood cell wall tailoring to eliminate light adsorption and improve wood permeability/accessibility and create pore space for further modification. One key is to avoid wood tissue disintegration into fibres. Various delignification methods have been developed, including mild peracetic acid, sulphite and kraft pulping, 'organosolv' approach, and bleaching (NaClO2, NaClO, H2O2, PAA/H2O2) [47]. The H2O2-based bleaching has advantages, since toxic by-products are avoided and the yield is high. Recently, new routes have been developed towards increased specific surface area in the substrate, including cellulose dissolution and regeneration [45] and tempo-oxidation [48]. The cellulosic wood substrate can be compressed to form high-strength materials [44], and the collapse can even be spontaneous during drying [48].
After delignification, the wood cell wall can be further modified to reduce moisture affinity and facilitate monomer impregnation. Examples include acetylation [49] and biobased anhydrides [50]. In-situ polymerisation inside the cell wall of wood substrates has been thoroughly reviewed [51]. The wood substrate has also been modified by diffusion of nanoparticles into the cell wall, precipitation of metal salts [45] or addition of other active compounds, such as optical dyes [52]. Many other functional components have been used, as was recently discussed [44]. Figure 8 shows the examples of functional materials and devices based on transparent wood.
Figure 8. Examples of functional materials and devices based on transparent wood [52]. John Wiley & Sons. [Copyright © 2018 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim].
Download figure:
Standard image High-resolution imageScalable and sustainable processing concepts for wood nanomaterials are important, since in the longer term perspective, nanoscience eventually needs connections to industrial applications. Introduction of a polymer matrix can serve as an example. For example, this can take place by thermoset precursor impregnation or monomer impregnation, which are possibly solvent-assisted. After impregnation, polymerisation is initiated thermally, by mixing or by photo-curing. Such methods are used industrially for fibre composite prepregs, and lamination schemes have been developed for nanostructured plywood. Better understanding of how processing influences nanoscale distribution of polymer inside the wood cell wall is needed, and the nature of chemically and thermodynamically favourable conditions. These aspects are important for optical properties, since even small scale defects have an effect.
For outdoor structural applications in moist environment, perhaps the nanostructured polymer matrix composites concept has the highest application potential. Jungstedt et al recently reported a Young's modulus of 20 GPa and a tensile strength of 260 MPa [53].
Applications and new material concepts
Many new functional material concepts have been developed for wood, including solar steam generation towards water purification, ionic heat-to-electricity conversion for energy harvesting, energy efficient buildings through light transmission, radiative cooling, or thermal energy storage, smart windows with electrochromic or sensor functionalities, and wood as monolith reactors for gas conversion and water splitting. A thorough and up-to-date review with numerous other examples is available [44].
Concluding remarks
Wood nanotechnology research is under rapid development, and many new material concepts have been introduced just recently. Top-down wood nanotechnology is an interesting prospect, where the hierarchical wood structure is exploited for the development of sustainable nanomaterials. Energy consumption and carbon footprint for the materials need to be minimised by development of processing methods, material component combinations and applications. Molecular and nanostructural control of functional component distribution in wood are important engineering science goals, as well as scalable materials and processing concepts. The lesson from general nanocomposites research is that candidate materials, exemplified by transparent wood, also need stimulation in the form of feasible applications so that problems which hamper industrial development can be addressed.
Acknowledgments
This project (WoodNanoTech) has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (Grant Agreement No. 742733). We acknowledge funding from KTH and Knut and Alice Wallenberg foundation through the Wallenberg Wood Science Center at KTH Royal Institute of Technology.
7. Biomimetic materials
Huai-Ling Gao, Li-Bo Mao and Shu-Hong Yu
Hefei National Research Center for Physical Sciences at Microscale, University of Science and Technology of China, Jinzhai Road 96, Hefei 230026, People's Republic of China
Attraction and inspirations
Learning from nature is an eternal theme in the development of human society. Biological materials found in nature have long been recognised as a rich source of inspiration for the development of new materials with enhanced properties [54, 55]. Scientists are always fascinated by their distinctive and elegant property combination. Over millions of years of evolution, biological materials have developed a wide variety of efficient strategies for optimising their properties and functions in terms of density, mechanics, hydrophobicity, colour, etc [56]. As representative examples, bone and nacre (part of abalone shell) are surprisingly damage-tolerant natural structural materials, which are formed by organisms at mild conditions from very limited options of raw materials, while exhibiting unprecedented combinations of low density, high strength and excellent toughness [54]. They inspire enormous biomimetic endeavours aiming to achieve lightweight and mechanical robust synthetic materials for structural applications, especially sustainable materials that exhibit potential to replace petroleum derived plastics [57]. Another case in point is gecko feet, which presents a robust attachment and easy detachment ability to arbitrary dry surfaces [58]. In addition, the surface of lotus leaves has excellent water repellency and self−cleaning ability, which has motivated great efforts to develop superhydrophilic materials for self−cleaning and anti−fogging applications [59]. Intriguingly, the wings of butterflies are able to produce attractive structural colour based on well-defined and sophisticated hierarchical structure [60], which is another typical example to show the charm of biological materials. Moreover, many biological materials are also able to self-diagnostic, self-repairing and self-adaptive according to the changes of physiological conditions. These biological functions are highly desirable for synthetic materials, but are far beyond what would be achieved synthetically at present.
Understanding and bio-mimicking
Discerning the underlying mechanisms of biological materials is a prerequisite for the design of advanced biomimetic materials. Until recently, deciphering the relationships between biological structures and their properties is still a hot research topic [61–63]. It has been certified that almost all biological materials have some common structural characteristics [60]. They are commonly natural nanocomposites, comprising strong and stiff crystalline nanoscale building blocks bonded by very small volume of soft and compliant biopolymer interfaces. These constituents were arranged in sophisticated hierarchical architectures with structural dimensions ranging from the nanoscale to the macroscale. The unique hierarchical architectures together with their intricate interfaces are the prime factors biological materials utilised to optimise their mechanical behaviours or other functions. It is because that naturally available constituents for building biological materials are confined to very limited, mundane and sustainable species, such as calcium salts, silica, polysaccharides, proteins, etc.
Nacre from abalone shell is the mostly extensively studied and imitated natural structural material since its microstructures seem easier to mimic (figure 9(a)). It is essentially a layered biological ceramic with unique 'brick-and-mortar' microstructure, which consists of 95 vol.% of mineral aragonite (CaCO3) platelets, bonded by a thin (∼10–50 nm) layer of ductile organic phase. Surprisingly, it shows unusual and attractive combinations of stiffness, hardness and toughness, far beyond that of aragonite [60]. The highly mineralised CaCO3 platelets provide strength, stiffness and hardness, and their precisely controlled interfaces (organic mortar, nano-asperities, mineral bridges, tablet interlocking, etc) allows for efficient toughening mechanisms [54]. Based on these principles, numerous strategies have been developed to copy the multiscale features of natural nacre, aiming at obtaining artificial nacre with desired mechanical performance.
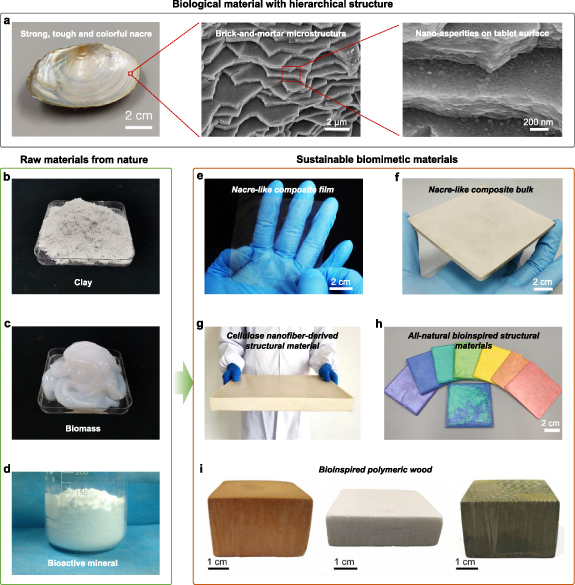
Figure 9. Sustainable biomimetic materials made from natural raw materials. (a) The morphology and microstructure of a natural nacre (Anodonta woodiana). Reproduced from [76]. CC BY 4.0. (b) The powder of mica microplatelets. (c) A piece of bacterial cellulose (BC). (d) The powder of brushite nanoplatelets. Reproduced from [67]. CC BY 4.0. (e) A multifunctional nacre-like composite film made from alginate and mica nanosheets. Reproduced from [65]. CC BY 4.0.(f) A nacre-like composite bulk made from biopolymers (alginate and chitosan) and brushite nanoplatelets. Reproduced from [67]. CC BY 4.0. (g) A lightweight, tough, thermostable, and sustainable bulk structural material made from pure CNFs. Reproduced from [57]. CC BY NC. (h) Bioinspired structural materials with tunable colours made from CNFs and TiO2-mica, which possess great potential for plastic replacement due to their high comprehensive performance. Reproduced from [76]. CC BY 4.0. (i) Multifunctional bioinspired polymeric woods with tunable constituents made from traditional resins and nanoparticles. Reproduced from [82]. CC BY NC.
Download figure:
Standard image High-resolution imageUnlike nature, we have more options to utilise environmentally friendly, readily available and mechanically superior components, including graphene oxide [64], clay nanosheets [65], alumina nanoplatelets [66], brushite nanoplatelets [67], chitosan [68], alginate [69], nanocellulose [57], etc, as building blocks (figures 9(b)–(d)) to fabricate high-performance and sustainable artificial nacre-like materials (figures 9(e)–(h)). In addition, a variety of interfacial modification methods also have been developed to optimise their mechanical performance [70–72]. Some useful and frequently applied bottom-up assembly strategies, such as layer-by-layer assembly [68], evaporation induced self-assembly [73], vacuum filtration [70], spray coating [65], etc, are proved to be simple, efficient and versatile for fabricating high-performance nacre-mimetic films with large-size and multifunctions (figure 9(e)), but difficult to produce nacre-mimetic bulk materials. In response to this challenge, the slurry-based freeze-casting [66] or magnetic-field-assisted [74] slip-casting combined with further sintering technique have been exploited as promising techniques for the fabrication of bulk artificial nacre. These methods could manufacture centimetre-thick nacre-mimetic bulk materials with high level of microstructural control across multiple length scales, achieving impressive mechanical properties. However, they are still relatively complicated and further scale-up has been obstructed by intrinsic barriers. In view of this, we presented an efficient and versatile bottom-up approach to solve this problem via combining well-developed evaporation-induced self-assembly with further lamination technique [67]. By laminating the pre-fabricated nacre-mimetic films that can be produced in large-scale and with good microstructural control, large-sized bulk nacre-mimetic composites with comprehensive mimicry of both the hierarchical structures and toughening mechanisms of natural nacre could be fabricated (figure 9(f)) [75]. Recently, we demonstrated that high-performance and sustainable structural materials with scalable size can be directly made by directionally pressing nanoscale building blocks derived from biomass and natural clays (figures 9(g) and (h)) [57, 76]. These biomimetic materials show great potential to substitute petroleum-based plastics for future engineering applications due to their high comprehensive performance.
While most of the above techniques involve the anisotropic assembly of building blocks, in nature, nacre is built via a biomineralisation process in living organisms, which is completed at mild conditions. In this respect, biomimetic mineralisation fabrication strategy has some incomparable advantages, especially for some heat-labile materials. However, creating a macroscopic artificial nacre through this technique is still challenging. Recently, a mesoscale 'assembly-and-mineralization' approach was proposed to address this challenge, and bulk synthetic nacre that highly resembles both the chemical composition and the hierarchical structure of natural nacre was successfully fabricated [77].
Apart from nacre, many other biological materials, such as wood [78], bone [79], enamel [80], exoskeleton of crustacean [81], etc, exhibit a more remarkable degree of sophistication compared with the layered 'brick-and-mortar' structure of natural nacre, making imitation of them with great challenge. Instead of using 2D nanoscale building blocks, one-dimensional (1D) fibrous nanoscale building blocks are wildly adopted in these natural structural materials to generate more fascinating mechanical functions. For instance, compact bone is composed of two major hierarchically arranged nanophases, including 1D collagen fibrils and 2D plate-shaped hydroxyapatite (HA) nanocrystals. They are assembled together periodically into a fibrous structure at the nanoscale with fibres of varying orientations arranged in a lamellar fashion. The sophisticated structures impart bone with both intrinsic mechanisms to promote ductility at molecular to nanometre scales, and extrinsic mechanisms to arrest the growing cracks at larger length scales [54]. Thus, unprecedented fracture resistance is achieved. However, artificial bone have not really been produced so far owing to the structural sophistication of natural bone. A recently reported work from our group showed that biomimetic woods, made of traditional resins and functional nanoparticles, could achieve excellent performance far beyond that of natural wood, although their structural hierarchy is still at a low level compared to that of natural wood (figure 9(i)) [82]. Another example is the stomatopod dactyl club which is regarded as a formidable damage-tolerant biological hammer [81]. It can defence against catastrophic failure during repetitive high-velocity offensive strikes. It was found to have three mechanically distinct domains with an ultrahard external layer for maximum impact force, a modulus mismatch region for crack deflection, and a periodic helicoidal region for further crack shielding. The helicoidal region consists of unidirectional chitin fibre sheets arranged helicoidally in a twisted plywood (Bouligand-type) structure with each sheet rotated by a small angle from the sheet below. This unique helicoidal architecture provides effective toughening mechanisms to hinder catastrophic crack propagation by constantly rotating the crack front. This unique natural structural design motif offers important hints to design highly impact-resistant composite materials for structural applications. There have been several recent attempts to fabricate composite materials following this inspiration. Three-dimensional (3D) printing technique has shown good potential to produce biomimetic Bouligand-type structures, achieving distinctly enhanced toughness [83–85]. However, limited control over structural accuracy and limited selection of constituents restrict its application. A recently proposed brushing-induced assembly combined with further rotated laminating method revealed its capability to precisely control the arrangement of 1D bioactive mineral micro/nanofibres in biopolymer matrices, resulting in bulk biomimetic materials with similar Bouligand-type structure and toughening mechanisms resembling that in nature [86].
Demands and challenges
At present, there is an urgent need for new structural, functional and sustainable materials to serve the rapid development of many high-tech fields, such as aerospace, transportation, biomedicine, and energy storage and conversion [54]. Previous achievements clearly demonstrate that biomimetic strategies can provide promising routes to design and manufacture advanced materials to fulfil diverse modern engineering demands. Despite impressive advances in the laboratory, biomimetic materials are still far from achieving the high degree of architectural control as those of biological materials, failed to yield expected material properties. In the future, deeply understanding and precisely mimicking the hierarchical structures, multiscale interfaces, and the underlying design principles of these elegant biological materials will still be the research hotspots in the field of materials science. In order to achieve engineering application, efficient techniques for large-scale manufacturing of biomimetic materials with practical bulk form must be exploited. In addition, scalable preparation and utilisation of high-performance raw materials from cheap, abundant and sustainable natural resources, e.g. clay minerals, crop straw, marine food waste, etc are also highly desirable.
Sustainable classes of materials
8. Sustainable carbon materials
Noel Díez1, Guillermo A Ferrero2 and Marta Sevilla1
1 Instituto de Ciencia y Tecnología del Carbono, Spain
2 Humboldt-University Berlin, Germany
Status
Carbon materials are ubiquitous in our society since they are among the most versatile kinds of materials due to their variety of structures, forms and properties, which are a consequence of the highly flexible coordination chemistry of carbon atoms. They are used in a wide range of applications including energy, environment, bioscience, medicine, aerospace and defence, vehicle manufacture, electronics, and sports. Given the energy challenge we are facing, we would like to highlight the importance of carbon materials in the energy field, they are the main material of choice in most energy storage and production systems [87, 88]. Accordingly, the demand for them has steadily increased and will rise even more in the future with their use in emerging technologies (energy storage devices, electrocatalysis, photocatalysis, biofuels, smart textiles, etc) and most probably in new technologies to come. With the exception of some activated carbons, carbon materials (e.g. carbon nanotubes, carbon fibres, carbon onions, synthetic graphite, carbon black, graphene, etc) are industrially manufactured from non-renewable precursors and the manufacturing processes are not sustainable, often involving harsh or energy-intensive conditions (e.g. chemical vapour deposition, electric-arc discharge techniques, strong oxidants/reductants, etc). There is therefore a need to develop more sustainable approaches for the production of high-performance carbon materials, which consider the whole life cycle (from precursor to manufacturing and end-of-life disposal), in order to meet the growing demand without compromising the environment or human health. The use of earth abundant, renewable resources will guarantee the necessary supply. Advances in the efficiency of manufacturing methods with lower waste generation, energy consumption and smaller GHG footprints will not only benefit the environment, but will also result in low cost materials, opening up new applications otherwise prohibitive for certain allotropes (e.g. graphene as electrode in energy storage devices or carbon nanotubes (CNTs) in microelectronics).
Current and future challenges
The production of carbon materials frequently requires complex methodologies and/or the use of different fossil fuels as precursors. The development of new synthesis routes with biomass as the carbon precursor has emerged as an environmentally-friendly and economical alternative. In this regard, many different types of biomass have been reported as carbon precursors with advantageous properties and in a variety of morphologies (figure 10). In addition, the natural abundance of heteroatoms in biomass represents an advantage in producing doped carbon materials with beneficial properties. However, the high moisture content of biomass is an obstacle for conventional pyrolysis, which translates into higher energy consumption. Moreover, this process is normally characterised by low yields, as a result of the high oxygen content and low aromaticity of biomass.
Figure 10. General overview of different biomass precursors and the sustainable carbon materials derived from them (carbon capsules, carbon spheres, graphene, hierarchical porous carbon materials, nanofibres, nanosheets and multi-walled CNTs (MWCNTs)). Reproduced from [92] with permission of The Royal Society of Chemistry [93]. John Wiley & Sons. [Copyright © 2015 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim]. Reprinted from [94], Copyright (2015), with permission from Elsevier. Reprinted from [95], Copyright (2017), with permission from Elsevier. Or [96] John Wiley & Sons. [Copyright © 2009 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim]. Reprinted from [97], Copyright (2012), with permission from Elsevier. Reproduced from [98] with permission of The Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageOne alternative for handling these feedstocks is the hydrothermal carbonisation process [89]. However, it is relatively slow and generates a product that is unsuitable for certain surface-dependent applications (e.g. energy storage), thus requiring the use of additional pore generation treatment.
In addition to bio-char, bio-oil is another valuable product obtained from the pyrolysis of biomass [90]. Although bio-oil has been initially used for the production of fuel, it has been recently proposed as an interesting alternative for directly producing functional carbon materials and bio-pitch. In spite of this, the process yield (∼7%) is far from ideal at the moment and further investigation is needed.
Sustainable graphitic materials can also be produced from renewable precursors (e.g. vegetable oil) and, furthermore, in a variety of different forms (nanofibres, nanotubes or graphene) [91]. However, the high temperature needed for the graphitisation step hinders widespread application. The development of synthesis processes with lower energy penalties coupled with the search for sustainable, greener catalysts (i.e. catalytic graphitisation) and reducing agents (for graphene production) are challenges that need to be overcome.
Even though the harnessing of biomass represents a step forward in sustainability, scalability and the need for high-yield processes are still challenges for large-scale production. The majority of the studies published in the literature are based on homogeneous feedstocks or individual precursors (e.g. glucose, saccharose). The heterogeneous properties of biomass and waste materials is however a drawback, even when the same source is selected and when the reaction conditions are similar. Thus, the initial composition of a selected biomass feedstock can be affected by several factors such as the location and climate where it is harvested, the part of the original product used (e.g. stem or flowers) or the type of feed (e.g. animal wastes). Besides, the aforementioned variations in combination with the presence of inorganic compounds (from alkali to heavy metal) can result into differences on the final chemical composition of the carbon (N, S, P, Al, Si, Na, K, Ca), distinct yields or variations on the textural properties (e.g. after etching of CaCO3) which might result in irreproducible synthesis and therefore requiring an additional pre-treatment step.
Advances in science and technology to meet challenges
Integrating the production of carbons in material-driven bio-refineries would significantly alleviate the high energy consumption and waste generation associated with the pyrolysis of biomass. Such a production scheme would help to reduce the price of the carbons obtained from biochars, thereby boosting their position in the market. Along these lines, the thermochemical treatments aimed at the production of char from biomass should be optimised in terms of operational parameters (e.g. higher heating rates or pressurised conditions to increase carbon fixation) or by coming up with improved carbonisation routes. In terms of the latter, using polymerisation agents (even obtained from the acid/alkaline hydrolysis of biomass) can increase the yield and strength of biochars, and it is their strength that currently limits their use in large sectors like the iron and steel industries [90]. Thermal condensation of bio-oils leads to bio-pitches with lower S and PAH content than coal tar pitches, which makes them a greener alternative for the preparation of pitch-derived carbon materials (C/C composites, needle cokes, synthetic graphite, etc). However, the preparation of carbons from bio-oils is in the very early stages, and further investigation of the reaction pathways of complex bio-oil solidification needs to be done first [99].
Emerging carbonisation technologies should be implemented according to the characteristics of the feedstock and the desired final properties in the carbon. Hydrothermal carbonisation avoids energy intensive drying pre-treatments and produces carbons with a rich surface chemistry. For biomass with good microwave absorption capacity, microwave-assisted pyrolysis could reduce the cost and time of production significantly. However, current issues with temperature control and the formation of hot spots in large scale production should be resolved first [100].
The search for more sustainable and environmentally friendly synthesis processes involves not only the carbon precursor, but also demands a complete review of the use of other toxic/harmful substances involved in the preparation of the carbon materials. This is the case for highly porous carbons. In the preparation of these carbons, corrosive chemical activating agents can be replaced with milder but effective reactants, as has been shown in recent scientific studies [101]. Implementing greener templating strategies (e.g. soft or salt templates), as well as using low-cost catalysts from natural rocks in the synthesis of nanocarbons, will allow more scalable and more environmentally-friendly progress in the development of carbons with controlled morphological and textural properties.
Concluding remarks
While the era of coal is coming to an end, the era of carbon materials is starting to flourish. Carbon materials hold the key to progress in many technologies that can improve our standards of living while protecting the environment. However, in order for that to happen, carbon materials must be produced more sustainably, based on using biomass/biomass wastes, and efficient, green manufacturing processes. Integrating carbon material production into biorefinery schemes will help take full advantage of biomass for carbon production, reducing energy consumption and waste generation. Despite great progress already having been made at laboratory scale, further involvement, and especially more investment, is necessary from industry to advance towards commercialisation.
9. Waste upcycling into metal–organic framework (MOF) materials
Petra Ágota Szilágyi
School of Engineering and Materials Science, Queen Mary University of London, Mile End Road, London E1 4NS, United Kingdom
Status
MOFs are a class of hybrid materials, highlighted as desirable in applications ranging from catalysis, gas storage and separation, sensing, energy storage, drug delivery, etc [102]. Their most exciting and unique properties such as chemical and structural modularity are enhanced and exploitable through their high porosity and crystallinity; they are built up of inorganic nodes, i.e. metal cations or metal-oxide clusters, interconnected by organic linkers, i.e. polycarboxylic acids or heterocycles. However, these materials are costly for large-scale applications as their synthesis often requires expensive reactants and harsh conditions. Incorporating waste-derived reactants in their synthesis would make their production both greener and cheaper, thereby potentially enabling their applications.
One of the most common linker is terephthalic acid (i.e. 1,4-benzenedicarbocylate, BDC) or its functionalised analogues, which in turn modify their physical and chemical characteristics, diversifying potential applications. As a number of polyesters feature BDC monomer units (PET: polyethylene terephthalate, PPT: polypropylene terephthalate, PBT: polybutylene terephthalate, PTT: polytrimethylene terephthalate), their use as a feedstock for MOF linkers is therefore desirable. To date, syntheses have made use of PET as BDC feedstock (figure 11).
Figure 11. Schematic diagram of the upcycling of terephthalic acid based polyesters to MOFs.
Download figure:
Standard image High-resolution imageTo demonstrate the potential of using polymer waste as feedstock for MOF synthesis, first an indirect approach has been developed. It consists of the initial depolymerisation of PET yielding BDC and subsequent purification and MOF synthesis (following conventional solvothermal routes). This has successfully led to MOFs built up of inorganic nodes of both metal cations (e.g. Cu(BDC) [103]) and metal-oxide clusters (e.g. UiO-66(Zr) [104]).
As the above approach still relies on the costly conventional MOF syntheses, de Vos et al [105] developed a one-pot route for direct PET-to-MOF conversion. This led to the synthesis of MOFs with cations for inorganic nodes (e.g. MIL-53(Al/Cr/Ga), MIL-47(V) [105, 106]) both in hydrothermal and solvothermal conditions, while MOFs with metal-oxide-cluster nodes such as the defectful hcp UiO-66(Zr) [107] and MIL-101(Cr) [106] obtained solvothermally and hydrothermally, respectively. Nevertheless, to date, no reports have been published of direct PET-to-MOF conversion in aqueous environment under mild conditions.
Finally, it is worth mentioning that de Vos et al [105] also found that employing nitric acid in the one-pot conversion leads to the nitro-functionalisation of BDC.
Current and future challenges
The production costs (a combination of the price of reagents, and environmental and energy costs) of MOF is still excessive for wide-scale industrial use. While the application of polyester waste as linker feedstock addresses the challenge of reactant costs, the environmental and energy cost of the synthetic process, such as toxic solvents and/or harsh reaction conditions, remains a concern. This is exacerbated by the requirements for the depolymerisation reaction (either in an independent step or simultaneously with the MOF assembly). Therefore, one of the main challenges of waste conversion to MOFs can be identified as enabling greener reaction conditions. This may mean employing lower pressure and temperature, and/or avoiding toxic solvents, such as DMF. Ideally, however, conversion conditions should be set to take place near ambient pressure and temperature, and in aqueous media. As stated above, various MOFs with metal-cation inorganic nodes have been achieved in one-pot PET-to-MOF conversion in aqueous media, however the sole successful conversion of PET to a MOF with metal-oxide-cluster inorganic nodes remains that to MIL-101(Cr), which is unique as MIL-101(Cr) is typically synthesised hydrothermally and thus its synthetic conditions may coincide with PET depolymerisation. A conclusion can therefore be drawn, namely that the assembly of MOFs based on metal-oxide-cluster inorganic nodes, such as the UiOs, is challenging in aqueous media, particularly when using PET as linker feedstock.
For applications of MOFs, particular properties; binding sites, colour, etc, are required, which may be tuned by changing the framework chemistry, or the addition of organic functional groups. The direct synthesis of functionalised MOFs from PET feedstock is therefore a challenge, which may add further value to this upcycling approach. The indication that by judiciously selecting the reagents of the conversion mixture, it is possible to append –NO2 functional groups on the BDC linker [105]. Further study is therefore necessary to screen the possible reagents leading to functionalisation.
However, to date, only PET has been successfully employed as BDC feedstock, yet other polyesters, i.e. PPT, PBT and PTT, may also be desirable precursors. Finally, a great challenge of PET-to-MOF remains the relatively low yield, which needs further breakthrough.
Advances in science and technology to meet challenges
One of the major challenges in the direct upcycling of polyesters to MOFs is the development of synthetic conversion processes that take place under milder and greener conditions, while maintaining the required crystallinity and surface areas. This challenge is particularly acute for MOFs built up of metal-oxide-cluster inorganic nodes, such as UiO-66(Zr). Recently, significant advances have been made in understanding the fundamentals of the formation of inorganic nodes and how it may enable the assembly of the MOF framework. In particular, for the UiO structures, it has been shown that the pre-formation of a hexanuclear Zr-complex enables aqueous and green synthetic routes, and it may be achieved through the careful design of reaction conditions, such as the application of modulator acids, e.g. acetic acid, the pH of the reaction mixture, etc [108]. Such strategies could be translated into developing environmentally friendly waste-to-MOF upconversion approaches.
The applications of MOFs are most realistic when their chemical and structural diversity, and the resultant high specificity in their interactions with substances, are accounted for. Therefore, it is important that MOFs with various chemical functionalities are synthesised through the direct conversion of plastic waste. While such functionalisation typically concerns the decoration of the aromatic linkers with organic functional groups, examples have arisen in which the inorganic nodes may be grafted with functional molecules, such as N,N-dimethylethylenediamine, an organic base [109]. Taking into account the possibility for base-catalysed depolymerisation of PET, it should be considered that such functional grafting agents are included in the plastic upcycling process.
A final consideration for the application of MOFs is their physical shape. While most syntheses yield powder samples, such forms are difficult to integrate into devices. In order to synthesise MOFs in a useful shape, such as monoliths, thin films, fibres, etc, a new area of interest has emerged. In particular, shaped metal oxides have been successfully used for MOF synthesis with a dual function, i.e. as reactants (for the inorganic nodes) and templating agents [110]. Such approach however has not been reported for linker feedstock, e.g. wherein shaped PET is templating the MOF morphology while supplying the linker for framework growth.
Concluding remarks
The direct conversion of plastic waste into MOFs serves two simultaneous purposes; it will tackle the issue of plastic pollution by adding value to the feedstock in the process, while it could enable the wide-spread use of MOFs, currently hindered by production costs, by lowering their price. Particularly, if functionalised MOFs can be obtained in a controlled and direct route, their syntheses through waste upcycling may become a major production approach. In addition, as many waste polyesters are produced and used in the form of fibres, often woven ones (e.g. textiles, carpets), if the shape of this feedstock may be used as morphological template for MOF synthesis, the potential and commercial value of plastic-to-MOF conversion would increase significantly.
Finally, in order to make this process truly environmentally friendly, care should be taken to develop processes for the reuse of other monomer units, e.g. ethylene glycol in the case of PET.
Acknowledgments
The authors thank the EPSRC for funding: EP/S018204/2 'Sustainable Processing of Energy Materials from Waste'.
10. Sustainable biopolymers
Connor J Stubbs and Joshua C Worch
School of Chemistry, The University of Birmingham, Birmingham, United Kingdom
Status
Polymers, colloquially referred to as plastics, are ubiquitous in modern society with uses ranging from routine (such as packaging) to high performance (in medicine or transportation) applications. The first synthetic plastics appeared in the early 20th century and the contemporary industry was largely established by mid-century. However, the overwhelming majority of society's accumulated plastics are derived from non-renewable feedstocks, specifically by-products produced from the refinement of fossil fuels (>99% of the estimated ∼8300 million metric tons produced up until 2017 [111]). Not only are fossil fuels a limited resource, they are environmentally damaging to harvest and consume. Moreover, the plastic products that they yield pose lasting ecological issues considering they are usually not degradable and their usage is mostly linear. In response, the future materials economy is envisioned to transition from linearity to circularity whilst simultaneously drawing from renewable, rather than finite, biomass resources (figure 12).
Figure 12. Overview of bioplastics (BPs) production including synthetic polymers with novel composition from biomass feedstocks.
Download figure:
Standard image High-resolution imageNaturally occurring polymers, also referred to as biopolymers (BP), such as polysacharrides (carbohydrates) or polypeptides form the chemical structural basis of many valuable bio-derived materials including starch, cellulose, algin, chitin and collagen. Before the development of synthetic plastics, these biopolymers were routinely processed into useful products via simple chemical modification. Even today, many biopolymers retain their commercial worth in numerous industries (especially in textiles and building materials) but ultimately lack the mechanical diversity of non-degradable plastics. In the future, natural biopolymers should become increasingly important feedstocks en route to the production of emergent synthetic BPs. Specifically, sustainable building blocks for BP synthesis can be furnished via the depolymerisation (saccharification) of carbohydrates and the derivatisation of the isolated monosaccharides [112]. One of the most well-known and highly regarded examples is the manufacture of poly(lactic acid) (PLA) from lactic acid, which is conveniently derived from plant starches and is already produced on a commercial scale [113]. Although the current market for synthetic BPs captures <1% of the annual plastics production by volume, this is predicted to increase substantially [114].
Biopolymer feedstocks are also being formulated into identical chemical precursors to yield conventional fossil fuels (figure 12) [115]. Under such circumstances, conventional plastics produced from sustainable monomers are also termed BPs and there are currently sustainable synthetic pathways to most commodity plastics, for example bio-polystyrene and bio-polyethylene. Many essential plant oils (in particular terpenes and terpenoids) can be isolated and/or chemically functionalised to yield sustainable building blocks for the synthesis of advanced materials [116]. Since they possess structural likeness to fossil fuel building blocks, their corresponding reactivity is often parallel to traditional petrol-derived vinyl monomers (such as styrene) and this makes terpenoids potentially amenable to existing plastic manufacturing processes. Vegetable oils or triglycerides harvested from agricultural plants (such as soybean, corn, or palm) are also important raw materials for polymers, although this application is presently overshadowed by their use as biofuels and/or conversion to other platform chemicals [117].
Current and future challenges
Great progress has been made in increasing the sustainability of the polymers to afford BPs, either with new compositions or as bio-derived variants of conventional plastics. However, their properties and/or production costs are not yet competitive enough to be viable drop-in products. Moreover, many of the signature advancements in this area have only been achieved as 'bench-scale' or 'proof-of-concept' demonstrations. While this should give the field hope since the chemistry solutions have been largely offered, scaling these processes for commercial use poses a grand challenge—from both a technological and economical perspective.
The manufacturing processes that are in place for refining biomass for BP production are inherently expensive, with PLA and polymers from isosorbide (another starch derivative, see figure 12) being rare exceptions that are economically viable [118]. There are also reservations that products may compete with food production (such as maize, palm or sugar cane to list a few). However, many of the same agricultural products are already used for bioethanol production without serious consequence to food production even though a similar argument was previously raised in this regard. Nevertheless, the economics are even more dire when compared next to fossil fuel monomers (such as ethylene, propylene, styrene, etc) and their associated plastics, which are almost free for manufacturers to obtain and produce [115]. Thus, one of biggest barriers in transitioning to sustainable BPs is the overwhelming presence of cheap petrochemicals. In fact, plastic manufacturing goliaths are planning to invest nearly $400 billion in petrochemical plants in coming years, effectively aiming to double the market capacity [119]. With this in mind, it is difficult to imagine rapid growth of the BPs industry. One potential mitigating force is that bioderived plastics do provide excellent environmental returns in the form of significantly lower overall GHG emissions compared to fossil-fuel plastics [112].
Beyond economic considerations, most sustainable BPs (of novel composition) often possess inferior thermal and mechanical performance compared to conventional fossil-fuel counterparts. The highly sought-after combination of heat resistance, ductility and strength, that is typified by non-degradable polyolefins, is difficult to imitate. For example, although PLA is a strong BP, it softens well below the boiling point of water, is exceptionally brittle, and possesses poor barrier properties necessary for translation into packaging applications. The incorporation of plasticising additives, non-degradable fillers or multilayered materials provide general solutions to improve the properties of PLA and other BPs [120], however these strategies can be environmentally damaging within their own rights [121]. The key challenge will be to create more durable sustainable materials whilst ensuring the entire product is degradable, without causing environmental damage at any stage of the life cycle (such as the production of microplastics).
Advances in science and technology to meet challenges
Technological advances to improve BPs are mostly focused on two interrelated fronts: (a) identifying and scaling more efficient chemical transformations and (b) creating higher performing materials that are also more recyclable and/or degradable via careful monomer design. There is also a substantial translation divide between bench-top chemistry solutions and materials applications on scale. Simply put, chemists and engineers need to communicate more proficiently with strategic goals in mind to afford economically viable platforms. The same general sentiment should also be applied in bridging the 'translational gap' between academic and industrial research.
The principal pathway to mitigate inherent economic drawbacks will be to develop more cost-effective processes for converting biomass to monomer precursors. This can be achieved by using a waste biomass source as the monomer feedstock, such as in PLA which can be generated from food waste [122], or through advances in catalysis to achieve higher reaction yields and better product selectivity while requiring a lower energy input (figure 13) [123]. Sustainability should also be designed into this framework by adopting green chemistry concepts and principles where possible [118]. This should further bolster the environmental advantage, or argument, for sustainable BPs compared to petrochemical-based polymers. Finally, simply performing reactions on ever-increasing scale should concomitantly improve the overall efficiency and lower the cost of feedstock refining efforts.
Figure 13. Emerging solutions for BPs design and synthesis to improve sustainability.
Download figure:
Standard image High-resolution imageThe subsequent transformation of biomass feedstocks to polymerisable monomers for renewable BP production could also benefit from chemistry advancements (figure 13). A rethink and/or redesign of targeted monomers should be considered in light of the aforementioned limiting properties of most BPs. More to the point, the available monomer stock can heavily dictate the accessibility of BP products. The judicious design of monomers that can be more easily polymerised and depolymerised (i.e. polymers near equilibrium) should offer better prospects for recycling, and thus sustainability (figure 13). On the other hand, further advances in polymer chemistry should continue to afford effective paths to sophisticated polymer architectural control that can enable property improvement compared to current BP formulations. For example, the performance of many BPs can be tailored and enhanced by controlling the 3D arrangement of atoms (stereochemistry) using controlled polymerisation techniques [124]. Furthermore, the synthesis of sustainable multi-block biopolymers also provides a path to make the mechanical performance more competitive [125].
Concluding remarks
The field of sustainable BPs has made giant leaps in the past few decades with numerous BPs beginning to trickle into the consumer market. However, a genuine sustainable plastics economy is only attainable through: (a) more competitive properties and facile recyclability from improved polymer design; (b) plastic degradation that is orthogonal with the environment; and (c) using sustainable sources obtained from biomass (figure 13). This monumental challenge can be surmounted through intense interdisciplinary communication, collaboration and action to bridge the divide between what is both sustainable and feasible. Unfortunately, the systemic overhaul necessary to achieve this goal will likely come in gradual developments. Although there are serious economic barriers and some lingering performance issues centred around sustainable BPs, progress is tangibly accelerating and this encourages optimism. Remember, the modern polymer industry leaped from discovery to industrial maturity within just a few decades. The journey to a more sustainable biopolymer economy, one that is carefully designed for a more circular framework, may appear daunting but it is attainable.
Acknowledgments
C J S and J C W acknowledge funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme under Grant Agreement No. 681559.
11. Sustainable conjugated polymers (CPs)
Yunping Huang1 and Christine K Luscombe1,2
1 Materials Science and Engineering Department, University of Washington, Seattle, Seattle, WA 98195, United States of America
2 Okinawa Institute of Science and Technology Graduate University, 1919-1 Tancha, Onna, Kunigami District, Okinawa 904-0495, Japan
Status
CPs are semiconducting materials featuring both flexibility and solution processability, breaking the dated conception that semiconductors are inorganic and rigid [126]. Moreover, this unique property combination has given birth to the previously unprecedented idea of flexible electronics. Simultaneously, this field pursues efficient device manufacturing via solution processing in contrast to high vacuum and/or high temperature processing used in inorganic semiconductors. CPs are indispensable in organic light emitting diodes [127], enabling flexible and light-weight lighting and display technologies now available in the commercial market. Other novel CP-powered technologies, including organic solar cells [128] and organic field transistors [129] are currently undergoing the commercialisation process, taking lab-scale research into a viable product. To date, the champion efficiencies of single-junction organic solar cells are around 16%; highest mobilities in organic field transistors are up to 20 cm2 V−1 s−1 for p-type and approaching 10 cm2 V−1 s−1 for n-type CPs.
In contrast to traditional semiconductors made from silicon and rare-earth elements, CPs are made primarily from organic elements, especially C, H, N, O, and S. Therefore, CPs can alleviate or eliminate concerns about deforestation, loss of biodiversity and pollution related to silicon and rare-earth production in the current semiconductor industry. The semiconducting nature of CPs is enabled by p-orbital overlap between adjacent C, N, O or S atoms (figure 14), allowing electrons to delocalise along the polymer chain. Electron delocalisation is further enhanced in the condensed state, due to the ability for interchain π–overlap between polymer backbones.
Figure 14. Top: Illustration of how extended π orbitals in CPs function. Bottom: select monomers used to construct CPs with different properties determined by the structure of the conjugated backbone.
Download figure:
Standard image High-resolution imageTo meet the requirements of different applications, monomers with different π structures (see examples in figure 14) were incorporated into the conjugated backbone to tune the optoelectronic properties of resulting CPs, such as absorption, emission, charge mobility, energy levels [127, 128]. This requires efficient polymerisation methods applicable to a wide variety of conjugated monomers. Moreover, high-selectivity methods are crucial for the synthesis of defect-free CPs, essential for champion performances. Furthermore, the economic and environmental impacts of CPs directly influence the commercial viability of these technologies, therefore in addition to researching recyclability and biodegradability [129], developing low-cost and eco-friendly synthetic methodology for CP syntheses is also important in advancing this field [130, 131].
Current and future challenges
Figure 15 illustrates the polymerisation methods commonly applied in the synthesis of CPs along with their usage frequency over the past 15 years. Suzuki and Stille polymerisations have been the most common techniques for CP synthesis, and so far, the majority of the record-breaking CPs are synthesised with these methods. These high performances are enabled by few defects in the CPs: (a) after the substrates undergo ex situ conversions into halogenated, organotin or organoboron precursors, side products can be removed through purification before polymerisation. (b) The reactivity of C–X (X = halogen) and C–M (M = SnR3, B(OR)2) bonds is significantly enhanced compared to the other C–H bonds on the substrates, which is crucial for controlling the site of bond formation on each monomer. These two factors reduce branching defects in CPs. Moreover, utilising two different functional groups is important for reducing homo-coupling defects, preparing structures with desired alternating repeat units.
Figure 15. Comparison between polymerisation method of CPs and the major defects involved. Homo-coupling and branching defects may exist in Suzuki or Stille polymerisation as well, but the amounts are not as significant as in DArP and CDCP. It is worth mentioning that CDCP is different from oxidation polymerisation, the latter of which is uncontrollable and leads to a significantly larger number of defects [130]. Their usage frequency in the recent 15 years are also included (search results from Google scholar as of October 2020).
Download figure:
Standard image High-resolution imageHowever, these pre-functionalisation procedures compromise the economic and environmental sustainability of CPs: (a) they lengthen synthetic routes and require extra reagents and working hours, increasing production cost; (b) explosive organolithium and toxic organotin used in the pre-functionalisation steps are detrimental to workplace safety and environmental wellness [130, 131].
To overcome these two concerns, direct arylation polymerisation (DArP) [131] and cross dehydrogenative coupling polymerisation (CDCP) [130] were developed (figure 15). Compared to tradition polymerisation methods, DArP and CDCP activate C–H bonds in situ in polymerisation. This bypasses the syntheses of organotin or organoboron precursors, alleviating the environmental concerns regarding organolithium and organotin. However, undesired C–H bonds in the molecule can also be activated during DArP and CDCP, creating branching defects in the resulting CPs. Eliminating branching defects is one of the critical challenges for C–H functionalisation in polymer science. Since CDCP utilises monomers with the same functional group (two C–H bonds), in great contrast with traditional polymerisation methods (C–X bond and C–M bond) and DArP (C–X bond and C–H bond), it is challenging for the catalyst to differentiate between the monomers and therefore homo-coupling defects are more pronounced in CDCP.
Advances in science and technology to meet challenges
Catalyst development is essential to address the challenges regarding selectivity and defects, and several papers have successfully synthesize CPs with minimal defects from DArP [132] and CDCP [133]. Still, DArP and CDCP are both at early stages in the development roadmap comparing to Suzuki or Stille polymerisation, and their current major research focuses are:
- (a)Expanding substrate scopes and increase site selectivity. DArP and CDCP have limited substrate scopes, requiring the presence of highly reactive C–H bonds or directing groups. Meanwhile, suppressing undesired bond formation on during polymerisation is essential to reduce defect and enhance the performance of CPs. Therefore, challenges regarding substrate dependency and site selectivity need to be overcome through development novel catalysts prior to the wide range application of these methods.
- (b)Exploring earth abundant catalyst systems. Considering catalysts made from expensive and rare palladium are widely applied in Suzuki coupling, Stille coupling, DArP and CDCP, efforts are focusing in developing earth abundant catalysts (e.g. copper [134]) for DArP to further optimise the economic and environmental sustainability of CP syntheses. However, to our knowledge CDCP utilising an earth abundant metal is still an untapped research area.
Sustainable synthetic methodology alone is inadequate for the vision of sustainable CPs. Sustainable thinking in CP molecular design is just as important to their economic and environmental sustainability. At present, a majority of high-performance CPs are synthesised with lengthy synthetic routes, which increased production expense and the total amount of by-products. Researchers have been experimenting creatively on shortening the synthetic route and lowering the environmental impacts in the syntheses of organic conjugated molecules, by utilising abundant natural products with conjugated structures (e.g. indigo [129], theobromine [135], and biobased furans [136]) with impressive performances achieved. Moreover, incorporating natural products introduces biodegradation pathways for resulting molecules [129]. This thinking pattern can be also transferred into the design of CPs considering they share similar design principles. It is always important to continue developing novel molecular designs that increase the sustainability of CPs and leave their performance uncompromised. In addition, green and biomass-derived solvents (e.g. 2-methyltetrahydrofuran) are encouraged and effective as alternative solvents for developing more environmentally sustainable polymerisation methods [134].
Concluding remarks
CPs are a class of materials that comprise features of semiconductors and plastics, and this unique combination is indispensable for the development of flexible electronic devices. At present, the syntheses of high-performance CPs require longer syntheses and chemical reagents that are toxic and explosive, adversely affecting their economic and environmental impacts. Green polymerisation methods such as DArP and CDCP are now faced with inadequate selectivity and limited substrate scope, where significant efforts have been invested in recent years. In addition, the sustainability profile of these polymerisation method can be further optimised by replacing the use of noble metal catalysts with earth abundant metal elements like copper. Last but not least, sustainable thinking in CP molecular design is equally important—designing simplified synthetic routes is straight-forward and efficient in lowering the economic and environmental footprint of CP syntheses.
Acknowledgments
The authors would also like to acknowledge the financial support from the Clean Energy Institute, NSF Center for Selective C-H Functionalization (CHE-1700982), NSF Partner for Innovation Award (2043422) and a Materials Research Science and Engineering Center (DMR-1719797).
12. Sustainable cellulose nanocomposites
Koon-Yang Lee
Department of Aeronautics and the Institute for Molecular Science and Engineering, Imperial College London, SW7 2AZ, London, United Kingdom
Status
Nanocellulose, i.e. cellulose fibres in the nanometre scale, is a family of abundant, lightweight and high-performance bio-based fibres that possess the broad chemical modification capacity of a cellulose molecule and the high specific surface area of a nanomaterial. It can be obtained either top-down or bottom-up. In the top-down approach, wood pulp is passed through either high-pressure homogeniser, microfluidiser or stone grinder to liberate the cellulose fibrils [137]. Wood pulp-derived nanocellulose (figure 16(a)) is more commonly known as either nano- or micro-fibrillated cellulose (NFC/MFC), depending on the mechanical action employed. The bottom-up approach utilises cellulose-producing bacteria from the Komagataeibacter genus to convert low molecular weight sugars into cellulose fibrils [138]. These microbially-synthesised nanocellulose (figure 16(b)), otherwise known as BC, is an ultrapure form of cellulose without impurities such as hemicellulose or traces of lignin that are often present in MFC and NFC.
Figure 16. (a) An aqueous gel of nanocellulose consisting of ca. 98 wt.-% water, along with a high magnification scanning electron microscopy (SEM) image showing the morphology of the nanocellulose; (b) BC pellicle with a water content of 99 wt.-% and an SEM image showing the morphology of BC. Reprinted from [139], Copyright (2020), with permission from Elsevier. Reprinted with permission from [140]. Copyright (2019) American Chemical Society.
Download figure:
Standard image High-resolution imageA major driver in the field of nanocellulose science and technology is the possibility of exploiting the high tensile properties of cellulose crystals for various advanced composite applications. Theoretical calculations and numerical simulations estimated the modulus and strength of cellulose crystals to be up to 300 and 22 GPa, respectively [137]. Experimentally, the tensile modulus of single nanocellulose was measured to be ca. 100–160 GPa. Recent work based on ultra-sound induced fragmentation of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) oxidised nanocellulose estimated the tensile strength of single nanocellulose to be 1.6–6 GPa, depending on the source of nanocellulose [141]. Therefore, nanocellulose is often regarded as the prime candidate for the production of high-performance sustainable nanocomposites that could replace fossil-derived engineering materials. At a nanocellulose loading of 60 vol.-%, the tensile modulus and strength of (sustainable) cellulose nanocomposites have been reported to be as high as 12 GPa and 200 MPa, respectively [137]. As a comparison, poly(L-lactic acid) (PLLA), a fully bio-based polymer with the highest mechanical performance that already has widespread use, possesses a tensile modulus of 4 GPa and a tensile strength of 63 MPa only.
Current and future challenges
Despite the promising mechanical performance, nanocellulose has not yet been commercialised as reinforcement for sustainable cellulose nanocomposite production. In fact, the commercial applications of nanocellulose are currently limited to low value applications, such as thickeners for gel inks in ballpoint pens (Mitsubishi Pencil Co. Ltd), diaphragm in audio speakers (Onkyo Corp.), additives in toilet wipes (Daio Paper Corp.), adult diapers (Nippon Paper Industries Co. Ltd) and in-sole for shoes (ASICS). The bottleneck in the application of nanocellulose for sustainable nanocomposite applications are: (a) the cost and (b) the transportation of nanocellulose. Currently, nanocellulose is priced at US$100 kg−1 (dry basis) [142]. For NFC and MFC, this stems from the high energy refinement process to convert wood pulp to nanocellulose. For BC, the cost stems from capex as BC is grown most efficiently in static culture at rather low production rate. Nanocellulose is therefore not cost competitive in the high-volume low profit margin sustainable materials market as high loading fraction of nanocellulose is required [137]. Cheaper reinforcing fillers, such as talc and natural fibres, are available for the sustainable composites industry. In terms of transportation, nanocellulose will form a strong nanofibre network that can no longer be reprocessed upon drying. This is known as hornification, the formation of irreversible hydrogen bonds between adjacent cellulose fibres. Nanocellulose must therefore be kept wet after production in its never-dried form, typically contains up to ∼98 wt-% water, prior to subsequent use. This reduces the cost-efficiency of nanocellulose as 98% of the total transportation cost of never-dried nanocellulose is transporting water, which does not add value to the final sustainable cellulose nanocomposites.
Advances in science and technology to meet challenges
It can be anticipated that the high cost of nanocellulose can be offset by designing high performance materials for high volume application but containing only low loading fraction of nanocellulose whilst offering dramatically improved mechanical performance that conventional materials cannot achieve. One can estimate the theoretical tensile modulus ( ) of cellulose nanocomposites using the Cox–Krenchel model:
) of cellulose nanocomposites using the Cox–Krenchel model:

In the model,  = fibre orientation factor,
= fibre orientation factor,  = tensile modulus of the fibre,
= tensile modulus of the fibre,  = tensile modulus of the matrix and
= tensile modulus of the matrix and  = fibre volume fraction. The term
= fibre volume fraction. The term  is related to the limited stress transfer efficiency due to the fibres having a finite length. It can be written as:
is related to the limited stress transfer efficiency due to the fibres having a finite length. It can be written as:



whereby  = fibre length,
= fibre length,  = fibre diameter,
= fibre diameter,  = shear modulus of the matrix,
= shear modulus of the matrix,  = Poisson's ratio of the matrix and
= Poisson's ratio of the matrix and  = packing of fibres.
= packing of fibres.
Using an imaginary  = 4 GPa, which represents the tensile modulus of PLLA,
= 4 GPa, which represents the tensile modulus of PLLA,  = 114 GPa [143],
= 114 GPa [143],  =
=  (assuming hexagonal packing of fibres),
(assuming hexagonal packing of fibres),  = 0.34,
= 0.34,  = 50 nm (see figure 16) and an estimated
= 50 nm (see figure 16) and an estimated  value of 5 µm, the tensile modulus of a cellulose nanocomposite comprising of 5 vol.-% nanocellulose in a PLLA matrix (figure 17(a)) is estimated to be 5.7 GPa. Such low nanocellulose loading would not increase the cost of manufacturing significantly over neat PLLA. Couple this with the comparable tensile properties to a 30 wt.-% randomly oriented glass fibre-reinforced polypropylene composite
37
, such sustainable cellulose nanocomposite with radically improved performance should easily penetrate the mass market as it could serve as a more sustainable alternative to fossil-derived engineering materials. Yet, this high tensile properties at such low nanocellulose loading are rarely achieved experimentally due to the less-than-ideal dispersion and our limited understanding of the mechanics of nanocellulose in a polymer matrix. Further work in the consistent exploitation of single nanocellulose tensile properties in a composite setting is required to breakthrough this bottleneck in the commercialisation of sustainable cellulose nanocomposites.
value of 5 µm, the tensile modulus of a cellulose nanocomposite comprising of 5 vol.-% nanocellulose in a PLLA matrix (figure 17(a)) is estimated to be 5.7 GPa. Such low nanocellulose loading would not increase the cost of manufacturing significantly over neat PLLA. Couple this with the comparable tensile properties to a 30 wt.-% randomly oriented glass fibre-reinforced polypropylene composite
37
, such sustainable cellulose nanocomposite with radically improved performance should easily penetrate the mass market as it could serve as a more sustainable alternative to fossil-derived engineering materials. Yet, this high tensile properties at such low nanocellulose loading are rarely achieved experimentally due to the less-than-ideal dispersion and our limited understanding of the mechanics of nanocellulose in a polymer matrix. Further work in the consistent exploitation of single nanocellulose tensile properties in a composite setting is required to breakthrough this bottleneck in the commercialisation of sustainable cellulose nanocomposites.
Figure 17. (a) An exemplary 5 vol.-% nanocellulose-reinforced PLLA composites, where the nanocellulose can be seen dispersed in the polymer matrix. (b) Nonwoven natural (sisal) fibreboard without nanocellulose binder. Deformation can be seen when small amount of water is added into the plastic cup. (c) Polymer-free nanocellulose-enhanced nonwoven fibreboard. No deformation was observed even when the water level was almost full in the plastic cup. The hornification between nanocellulose bind the loose fibres together, producing a rigid and robust fibreboard. This is adapted from Lee et al [144].
Download figure:
Standard image High-resolution imageAnother potential solution to the cost challenge of nanocellulose is to fully utilise the effect of nanocellulose hornification. Whilst the hornification of nanocellulose is detrimental in the processing of cellulose nanocomposite, the strong hydrogen bonds between adjacent nanocellulose could be used to bind loose or waste fibres. This concept has previously been explored to bind the otherwise loose natural fibres [144], as well as waste chicken feathers [145] to produce rigid and robust polymer-free fibreboards (figures 17(b)–(c)). Only a small amount of nanocellulose is required and this effect, to our knowledge, is only exclusive to nanocellulose due to its strong hornification effect. The next logical step in the evolution of such nanocellulose technology could be in the maximisation of nanocellulose hornification to produce polymer-free, sustainable and durable composite fibreboards. This may further revolutionise how we think about utilising waste fibres as a resource, which can also act as carbon sink.
The second challenge is to transport never-dried nanocellulose. Strategy based on capping the hydroxyl groups of nanocellulose [146] has been explored to prevent hornification but at the expense of altering the surface properties of nanocellulose. Never-dried nanocellulose has also been embedded in a water-soluble polymer [147] (essentially creating a composite), which can be dissolved away to recover nanocellulose. Whilst this approach is interesting, the nanocellulose-to-water mass ratio is typically ∼0.002, implying that to transport 2 kg of nanocellulose (dry), 1 tonne of water needs to be evaporated to embed the nanocellulose in the polymer (not cost-competitive!). However, if a high boiling point liquid could form a thin film around individual nanocellulose, essentially 'sizing' the nanofibres, hydrogen bonds between adjacent cellulose fibres could be circumvented, thereby preventing hornification. This was explored through the use of water-miscible low molecular weight liquid polyethylene glycol (PEG), resulting in nanocellulose-PEG filter cake achieved a solid content of up 70 wt.-%, without nanocellulose hornification [139]. Whilst this is an improvement over the state-of-the-art, this solution is not perfect as the solid content is still relatively low and a washing step was still required to completely remove the PEG. Nevertheless, this work has hinted the possibility of 'sizing' nanocellulose as a mean to transport nanocellulose cost-effectively. If a solution to transport never-dried nanocellulose filter cake at a solid content of >95 wt.-% is available, this will further contribute to the successful commercialisation of sustainable cellulose nanocomposites.
Concluding remarks
The strong reinforcing effect of nanocellulose for polymers was first reported over 30 years ago. The first wave of nanocellulose research focussed on the understanding of the physical and chemical properties of nanocellulose, as well as nanocellulose-polymer interactions to a certain degree. As our knowledge broadened, the emphasis on the second wave of nanocellulose research switched to the creative utilisation of nanocellulose to maximise materials performance. The nanocellulose community has now reached a critical mass. There are currently various industrial partners with pilot plants producing nanocellulose, waiting for a 'killer application' to commercialise nanocellulose, increasing its market uptake. Looking forward, the third wave of nanocellulose research will focus on the cost-competitiveness of nanocellulose, especially in the high volume sustainable cellulose nanocomposite application. The keys in the successful commercialisation of sustainable cellulose nanocomposite are:
- Sustainable design—reduce complexity, efficient use of materials and minimise the use hazardous solvents.
- Manufacturability—ensure that sustainable cellulose nanocomposite production can be scaled up easily in an economically feasible manner.
- Radical effects—provide distinct mechanical performance other materials cannot achieve, especially in the context of sustainability.
Acknowledgments
The authors would like to the UK Engineering and Physical Science Research Council (EP/S025456/1) for funding.
13. Sustainable quantum dots (QDs)
Hui Luo
Department of Chemical Engineering, Imperial College London, SW7 2BX, United Kingdom
Status
QDs are a class of fluorescent semiconductor materials with the general size of 2–10 nm, exhibiting optical and electronic properties that differ from bulk particles due to quantum mechanics [148]. The most well-studied QDs including CdSe, CdS, PbS, Si, and GaAs, possess strong and tunable and robust fluorescence properties, which have potential applications in solar cells, light-emitting diodes (LEDs), sensing and bio-imaging. However, most of the semiconductor QDs contain metals from group II–VI and III–V in the periodic table, which dictate their high toxicity and large life-cycle environmental impact [149]. Therefore, it is imperative to design and fabricate QDs from more sustainable resources.
The discovery of fluorescent carbon-based dots (CDs) in 2004 brought people's attention to this novel zero-dimensional carbon material [150], which soon becomes a rising star. CDs are called carbon QDs in the early years of discovery. However, new studies revealed their fluorescence is not entirely governed by quantum confinement, and there's large diversity in CDs' structure. Thus, CDs are now often used as the genetic term.
Similar to semiconductor QDs, CDs usually have an average particle size below 10 nm, synthesised by either 'top-down' methods involving cutting large carbon materials such as graphite into small fragments, or 'bottom-up' approaches, with growing small precursor molecules such as glucose into large-conjugated domains. Building up CDs from small molecular precursors is considered to be more sustainable and environmentally friendly.
Because of this synthetic diversity, CDs possess various chemical structures, ranging from mainly crystalline, a hybrid of sp2/sp3 carbon, to mostly amorphous, and typically have different functional groups on the surface. The schematic diagram in figure 18 summarises the structural differences between semiconductor QDs and CDs, along with their distinct fluorescence mechanisms. For CDs, their structural complexity makes it more difficult to tune the fluorescence property, but their carbon-based structure also endows them with advantages such as low toxicity, high (aqueous) solubility, facile modification and low cost. Thus, it is essential to develop CDs with well-controlled optical properties to complement or replace semiconductor QDs in the above-mentioned applications to achieve a more sustainable life-cycle across all stages.
Figure 18. Illustration of the structure and fluorescence mechanism of semiconductor QDs and CDs.
Download figure:
Standard image High-resolution imageCurrent and future challenges
Although clearly more sustainable than their inorganic counterparts, critical challenges remain to be addressed for CD further development and implementation in the desired applications mentioned above.
Firstly, the inhomogeneity of most of the synthesised CDs makes it difficult to acquire precise information about their chemical structure. Since their electronic and optical properties are closely correlated with their structure, this challenge has hindered the fine-tuning of their performance towards the desired applications [151]. Suitable purification methods and thorough fundamental studies are thus required to unveil the true structural features of different CDs, in order to investigate their evolution during formation for improving their reproducibility. Secondly, a clear structure-property correlation for each type of CDs needs to be established, similar to the quantum confinement mechanism for semiconductor QDs, to allow accurate control of their emission behaviour by tuning their structural features, such as crystallinity, particle size, heteroatom doping and surface functionality. For each type of CDs, to avoid pitfalls and misleading results, universal protocols, from synthesis methods to characterisations, need to be shared across the entire research community to allow benchmarking and correct data interpretation [152].
Following upon this, the third challenge is to fine tune the CDs optical properties based on their structural features, to overcome the issues in wide emission range and low quantum yield, as well as establish a clear excitation-emission relationship.
The fourth major challenge relates to up-scaling of the CDs production, which currently impedes their commercialisation. The CDs yields in the current synthesis approaches are too low to achieve a sustainable production stream. Strategies to improve the synthetic methods for higher CDs yield with minimal waste are thus necessary.
Fifth, from the application aspect, improving the performance of CDs is a key priority to meet the standards for different applications. This includes higher quantum yields across the entire light spectrum for LED applications, accurate response upon stimulation for bio-sensing and bio-imaging, as well as long-term stability for solar cells and photo(electro)catalysis. For example, CDs are excellent photosensitisers and co-catalysts when coupled with semiconductor materials in solar energy conversion, but its stability over hundreds of hours, especially at high catalytic rates, has been rarely discussed in the existing literature, leaving a knowledge gap for further industrialisation [153].
Advances in science and technology to meet challenges
Intensive research efforts have been made in the last decade to address the challenges mentioned above, and some progress has been achieved across different areas. Qu and Sun have summarised the recent progress in the formation mechanism and chemical structure of CDs from 'bottom-up' routes with various precursors, where the fluorophores in different types of CDs have also been proposed and discussed [154]. These fundamental studies provide insights on the precise chemical structures of CDs, paving the way towards accurate property control by tuning the reaction parameters. In a recent report, Wu et al established a machine-learning model based on hydrothermal-synthesised CDs, which is capable of screening the relationships between various synthesis parameters, experimental outcomes as well as process-related properties such as the fluorescent quantum yield [155]. Under the guidance of machine learning, the authors have obtained CDs with strong green emission with quantum yield up to 39.3%, demonstrating the great potential of machine learning in accelerating the development of high-quality CDs.
As machine learning provides a possibility for fast screening different reaction parameters for CDs fabrication, the mass production challenge for a high CDs yield with minimal waste stream also needs to be addressed. Efforts have been made in the scientific community to develop industry-relevant synthesis routes. Vomiero, Gong et al have reported a gram-scale synthesis of CDs with yield of approximately 18% through a space-confined vacuum-heating approach [156]. Those CDs with a high quantum yield of ∼65% and large Stokes shift of 0.53 eV are used as luminescent solar concentrators for solar cells, which can achieve the photo-conversion efficiency (PCE) of 1.13%.
Advances in developing high-performance CDs have also thrived over the past few years, expending their potential applications from conventional areas such as LEDs, photocatalysis, bio-imaging, towards emerging fields such as anticounterfeiting, tumour therapy and self-healing materials, as shown in figure 19. These remarkable achievements unlocked the vast potential of CDs in the future society.
Figure 19. A summary of potential future applications of CDs. Reprinted from [157], Copyright (2020), with permission from Elsevier.
Download figure:
Standard image High-resolution imageConcluding remarks
In summary, sustainable CDs from eco-friendly precursors have been intensively studied, which have shown promising applications in LEDs, photocatalysis, bio-imaging, anticounterfeiting, tumour therapy, self-healing materials and many more. Although many intriguing electronic and optical properties have been investigated, some critical issues and significant challenges remain to be addressed before achieving a lab-to-industry transition. This includes (a) understanding the formation process and revealing their precise chemical structure, (b) identifying the fluorescence mechanism, (c) accurate controlling of the optical properties, (d) increasing the production yield, as well as (e) improving the performance in terms of activity, sensibility and stability. Recent advances have already established major milestones in these areas, but more systematic researches related to these issues are still needed to release the full potential of this charming material.
Applications: the need for sustainable materials for emerging technologies; renewable energy generation, storage and conversion
14. Sustainable wind turbines
M J Platts
Institute for Manufacturing, Alan Reece Building, 17 Charles Babbage Road, Cambridge CB3 0FS, United Kingdom
Status
In the public mind, infrastructure has always been there, is totally reliable, will always be there... and is utterly invisible. It is a 'given'... so it does not need to be understood. This is a fatal blindness when something new, such as wind energy, needs to be developed at infrastructural scale, as attention is misdirected to things that do not matter, away from things that do.
The wind turbine industry is now a very large global industry, with over 650 GW of wind energy capacity installed and over 50 GW p.a. being added—over 25 000 wind turbines a year—by an industry employing over 1.3 m people and turning over more than $100 bn p.a., and with a very large R&D budget within the industry itself, so most of the important technical data are not in the public domain. The industry is set to grow several-fold in the coming decades. But little is known about its carbon footprint as an industry. How good is it, and is it getting better?
With wind turbines, the blades that rotate in the sky catch the attention as well as the wind. They are made of advanced composites and they are the exciting bit. But if you are interested in sustainability, it is the hundreds of tonnes of steel in the tower and hundreds of tonnes of reinforced concrete in the foundations—the boring bits—that dominate the carbon footprint... and if you construct an access road to your wind farm across a peat bog you make the carbon footprint even worse... but nobody pays any attention to the carbon footprint, because wind energy is defined as 'clean'... so 'thought' is unnecessary.
So, the key problem is the public blindness. No figures are made available for the carbon footprint 'cost' per MWh of a proposed wind farm, because nobody asks for them. The public may have many emotive opinions about it but they can have no informed discussion about it. Whilst it is true that information is necessary for this, what is essential in the first place is wanting to know. The driving public insistence needs to be 'Show us the numbers'.
Current and future challenges
One aspect is particularly important, and that is lifetimes. In civil engineering shorthand, 'infrastructure' is what you build for your grandchildren. There is an intention of permanence. Things that last 20–25 years—which is the design lifetime of current and planned wind turbines—are not 'infrastructure', they are consumables. We speak of being zero carbon by 2050. But with design lifetimes of 20–25 years, all the wind turbines currently installed and in planning will be totally derelict by 2050. You need to understand that it is not only the rotors, the gearboxes, the generators, the electronics and so on that fatigue to death, the towers and foundations do too. Everything is derelict. In Denmark there is a well-established wind turbine demolition industry removing the 1990s turbines that are now derelict, and this includes blowing up the concrete foundations.
With designing against fatigue, it in fact only involves the addition of a small percentage more material to double the fatigue life, which would take the life out from 20 to 40 years. If you are only looking at the economics this small increment to the first cost is undesirable. But if you are looking at the carbon footprint this doubling of life halves the carbon footprint 'cost' per MWh of electricity delivered to the user. And those turbines would then be still turning in 2050, not yet in need of replacement. Down this road, the word sustainable begins to have meaning. But nobody asks the question.
Advances in science and technology to meet challenges
Concerning the technology itself there are some exciting parts to the story. The wind turbine blade industry has created a completely new sector of the global composites industry, currently producing 3/4 m tonnes per annum of highly develop technical composites entirely different to both the aerospace and automotive industries, and it is set to grow several-fold in coming decades. This is around 75 000 wind turbine blades a year - 300 a day - averaging some 60 m long and 10 tonnes in weight. As energy capture capacity factors have increased from typically 20% to over 50%, developments in better carbon fibre, better glass fibre and particularly in better resin chemistry, plus more advanced aerodynamic design, have enabled blades to become highly optimised, efficient structures, steadily reducing their carbon footprint per MWh of electricity produced, and there is more to come.
Unfortunately, it is not matched by the carbon footprint per MWh of the wind turbine tower-and-foundation support structures, which is increasing as they get bigger. Globally, they already use over 5 m tonnes of steel and 15–20 m tonnes of reinforced concrete annually. As commodity materials they are cheap, but they have a high carbon footprint. These are not optimised minimum mass structures. They remain simple, unoptimised structures in which their mass is driven by a cube law as wind turbines get larger, whereas the energy capture, which is driven by the swept area of the rotor, only rises as the square. And as the turbines have got bigger, towers have got proportionally taller, increasing in height from 0.8 times rotor diameter to 1.2 times. Lifting the rotor higher in the sky has increased the energy capture, but now the tower and foundations dominate the carbon footprint [158].
The story offshore is similar. Figure 20 shows the massive sub-sea foundation cylinder produced for a 6 MW offshore wind turbine. The 7.8 m in diameter and 82.2 m long, it goes down through more than 30 m of water and more than 30 m into the soft sea bed. At 1302.5 tonnes of steel, it weighs far more than the wind turbine and tower it is to support and is dominant in the carbon footprint analysis.
Figure 20. A 1302.5 tonne monopole. Reproduced with permission from [159]. [Source: EEW SPC].
Download figure:
Standard image High-resolution imageThese towers and foundations are not minimum mass structures and they need to be. Their design is shape constrained, not material constrained. Improvement is only possible with changing the geometry. Using guyed tower designs for instance, as is used in some smaller wind turbines, the quantity of material in the tower could be halved and the foundations reduced to less than a quarter. To achieve better sustainability figures, this is where future attention is needed.
Concluding remarks
To propose achieving a zero-carbon economy by 2050 and then completely ignore the carbon footprint of the technology being developed to get you there, is absurd. Every wind farm proposal has, on the first page, a figure showing the cost per MWh of the electricity it will produce, and that figure is made public. It is just as easy—and just as important—to put a second figure beside it showing the carbon footprint 'cost' per MWh of electricity it will produce, so that this too can be discussed. It should be public policy to ask for this figure, calculated in a standardised way that is audited to ensure comparability with other proposals, so that, together, they create a public 'carbon cost' awareness that will allow a scientifically and technically sound zero-carbon strategy to be developed.
Acknowledgments
The insights concerning the differences between what drives the economic cost and the carbon footprint 'cost' have emerged over time from a small number of student projects using example data made available privately for teaching purposes. More widely, practitioners do not write papers. Their strategic thinking is revealed by their design decisions and manufacturing choices, both fruitful and problematic, evolving over decades. Luckily, wind energy is blessed by having a 'journal-of-note'—windpower monthly—that has grown through the decades with this industry, covering all conceivable aspects of both technology and policy worldwide, and trusted by all. The whole industry has been the constant teacher of this practitioner. Windpower monthly is the reference that honours them all.
15. Sustainable inorganic materials for system integrated photovoltaics (PV): bismuth based solar absorbers
Devendra Tiwari1,2, Dmitry Kovalevskiy2 and David J Fermin2
1 Department of Mathematics, Physics and Electrical Engineering, Northumbria University, Ellison Place, Newcastle upon Tyne NE1 8ST, United Kingdom
2 School of Chemistry, University of Bristol, Cantocks Close, Bristol BS8 1TS, United Kingdom
Status
PV remains one of the fastest-growing energy markets worldwide, which is overwhelmingly dominated by monocrystalline Si absorbers reaching PCEs of 24.4% across modules as large as 13 177 cm2 [160]. While Si technologies will remain dominant in the utility sector, alternatively technologies are required for integration in densely populated areas as well as for powering every sector of the economy. One of these sectors include building-integrated PV, which requires several functionalities beyond PCE, such as light-weight, flexibility, low-toxicity, high stability [161].
Lead hybrid perovskites have recently become one of the leading emerging technology, reaching PCE values of 16.1% in 802 cm2 modules [160]. Lead hybrid perovskites are characterised by large absorption coefficients, high carrier mobilities, high dielectric constants and long carrier-lifetimes, which are linked to the electronic properties of Pb2+ ion [162]. Given the similar chemical properties of Bi3+ and the significantly lower toxicity in comparison to Pb2+ ions, there is a strong drive towards identifying Bi-based inorganic solar absorber which can match the optoelectronic properties of lead hybrid perovskites. Furthermore, bismuth-chalcogenides and halides have been implemented in several technological applications such as thermoelectrics, ionising-radiation detectors, as well as topological and spintronic devices. Some of these applications display electronic and bonding structure relevant to solar PV.
Figure 21 shows the state-of-the-art PCE of prototype PV devices featuring inorganic Bi-based compounds. AgBiS2 have shown the highest PCE value reported (6.3%), while BiFeO3 all-oxide PV devices have reached 4% [165, 169]. Figure 21 also contrasts the experimental values and the corresponding Shockley–Queisser limit based on the material bandgap. The trends clearly illustrate the scale of the challenge not only on the preparation of high-quality materials but crucially on rationalising power conversion loss mechanisms as well as to establishing effective device design principles.
Figure 21. Difference between best PCE values reported for Bi-based PV devices and the corresponding Schockley–Queisser limit. PCE were obtained from [162–164] (AgBix Iy , Cs3Bi2I9, Cs2AgBiBr6) [165], (Bi2S3; Cux BiSy ; AgBiS2) [166], (BiI3) [167], (BiSI) [168], (BiOI) and [169] (BiFeO3).
Download figure:
Standard image High-resolution imageCurrent and future challenges
Three key challenges can be identified towards the development of efficient Bi-based PV devices, namely carrier lifetime, anisotropic carrier mobility and interface engineering. The minority carrier lifetime associated with most reported Bi-absorbers, suitable for single-junction PV devices (Eg < 2 eV) such as BiI3, BiSI, BiOI, BiPS4, are in the range of several nanoseconds [166–168, 170]. Only a few wide bandgap materials such as BiFeO3 and Cs2AgBiBr6 have shown lifetimes from 10 ns up to 1 μs [162, 164]. Lee et al suggested that the shorter carrier lifetime in Bi-based compounds relative to Pb-based perovskites is connected with the relatively smaller contribution of the Bi 6s2 lone-pair to the valence band, which leads to deeper point defects acting as recombination centres [162]. On the other hand, it is yet to be assessed the effect of surface defects on the carrier lifetime of these complex materials. For instance, significant improvement in AgBiS2 device performance has been achieved through surface passivation [165]. Our recent study on BiPS4 also points towards a significant contribution from surface recombination [170]. Thus, it is imperative to understand the process leading to a decrease in the carrier lifetime of Bi-compounds to develop appropriate preparation methods and surface passivation strategies.
Bismuth materials can be prepared as 0D (i.e. QDs) such as the case of Cs3Bi2I9, 1D (BiSI), 2D (BiI3) or 3D (Cs2AgBiBr6) structures. In the case of 1D and 2D materials, the carrier effective masses along the directions of van der Waals interaction are significantly larger i.e. lower charge-mobilities than in the directions of the covalent bonding. Most of the growth methods reported so far lead to textured thin films with random crystal orientation. To improve charge transport across the devices, the new deposition strategies should be developed with control crystal orientation [165, 166, 169].
Challenges associated with device engineering can be seen by the band edge energy offset of the Bi-compounds to conventional charge-transporting layers illustrated in figure 22. It can be seen that Bi-compounds are characterised by deep-lying valence band edge, which is not optimally aligned with hole-transporting layers such as 2,2',7,7'-tetrakis[N,N-di(4-methoxyphenyl)amino]-9,9'-spirobifluorene, abbreviated as spiro-omeTAD. We have obtained promising performances in the case of BiSI and BiI3 employing poly(9,9-di-n-octylfluorenyl-2,7-diyl), commonly referred to as F8, which shows a better band alignment [166, 167].
Figure 22. Band edge energies offset of several Bi-compounds along with conventional electron- (ETM) and hole-transporting materials (HTM) [165, 170]. CBI, ABS, CABB, ABI, BPS and F8 stand for Cs3BiI9, AgBiS2, Cs2AgBiBr6, Ag3BiI6, BiPS4 and poly(9,9-di-n-octylfluorenyl-2,7-diyl), respectively. Reprinted with permission from [170]. Copyright (2020) American Chemical Society.
Download figure:
Standard image High-resolution imageConcluding remarks
Bi-based materials offer a range of exciting structural and optoelectronic properties which can be exploited in the context of thin-film PV. Despite similar electronic properties between Bi3+ and Pb2+ cations (i.e. ns2 lone-pairs), we strongly believe that implementing the same strategies as for Pb-perovskites cells will not lead to efficient PV devices. In this sense, understanding the processes limiting carrier lifetime (bulk v surface defects), as well as designing strategies for oriented crystal growth are two important areas of research. Furthermore, we also propose a more rational approach to engineering the boundaries to these materials. For instance, compositionally graded heterojunctions such as those developed for high-efficiency CdTe and copper indium gallium diselenide (Cu(In,Ga)Se2) (CIGS) solar cells, rather than abrupt heterojunctions as in Pb-perovskite cells, may hold the key for taking these systems to technology relevant performances.
Acknowledgments
DT and DJF are grateful to the UK Engineering and Physical Science Research Council (EPSRC) for support through the grants EP/L017792/1, EP/ V008692/1, and EP/V008676/1. DT is also thankful to the Royal Society of Chemistry for support through grant E20-9404.
16. Sustainable battery materials
Heather Au, Hande Alptekin and Maria Crespo-Ribadeneyra
Department of Chemical Engineering, Imperial College London, SW7 2AZ, United Kingdom
Status
To achieve the target of carbon neutrality by 2050, a shift in energy production to renewable sources is imperative. Sustainable batteries will play a prominent role in our changing energy economy; indeed, batteries could enable 30% of the required reductions in carbon emission in the transport and power sectors [172]. In pursuit of sustainable technologies, it is essential to consider availability of resources, environmental efficiency of manufacturing processes, scalability, toxicity, safety, and end-of-life pathways.
Li-ion batteries (LIBs), which currently dominate the market, depend on graphite and transition metal oxides for the anode and cathode, respectively. However, natural graphite has been classified as a critical resource by the EU, not least because there are no natural resources within the EU, and while synthetic graphite is an alternative, it is more expensive to produce, and derived from petroleum; thus, recovery of graphite may become more important in the future. Of the existing cathode materials, lithium cobalt oxide, lithium nickel cobalt manganese oxide and lithium nickel cobalt aluminium oxide contain cobalt, which is considered an endangered resource and depends heavily upon mining in the Democratic Republic of Congo [173]. Cathodes based on lithium manganese oxide and lithium iron phosphate rely on elements which are earth abundant, and therefore present a more attractive alternative to traditional Co-based electrodes.
The environmental impact of the other components within a battery must also be evaluated. Typically, polyvinylidene fluoride (PVDF) is used as the binder, but processing requires the use of toxic and teratogenic N-methyl-2-pyrrolidone, and the fluorinated degradation compounds complicate disposal. Similarly, lithium electrolytes contain fluorine-based anions such as hexafluorophosphate (PF6 −), bis(trifluoromethane)sulfonimide (TFSI−) and bis(fluorosulfonyl)imide (FSI−), which can degrade to toxic and reactive products. In addition, commercially available LIBs use polyolefin separators, derived from petrochemical sources, while copper, of limited availability, is used for the current collector.
Driven by these shortcomings, alternative battery chemistries have therefore been the subject of much research, although sustainability considerations have always been placed below material performance. However, the urgency of the climate crisis is pushing sustainability criteria to the forefront to achieve a healthier battery economy.
Current and future challenges
Given the forecasted demand for energy storage, the availability of lithium itself is uncertain, while uneven global distribution raises geopolitical concerns. The enormous and growing body of research into new battery chemistries thus focuses on other alkali metals, Na and K, as well as multivalent-ion batteries (MVIBs) based on earth abundant Mg, Ca, Al or Zn [174]. The development of these technologies suffers from several problems: Na- and K-ion batteries (NIBs and KIBs) have a lower energy density, so new active materials must be developed; MVIB have higher specific capacities, but these chemistries are still in their infancy with stable, high-energy cathodes and non-corrosive high-voltage electrolytes remaining to be discovered. Alongside the development of suitable high-performance materials, efforts must also be concentrated on improving the overall sustainability credentials related to three main aspects: materials resource management, production energy cost, and end-of-life pathways (figure 23).
Figure 23. Considerations for designing future sustainable batteries in terms of materials resource management, processing and end-of-life pathways.
Download figure:
Standard image High-resolution imageThe materials currently used in existing batteries are derived from steadily depleting sources. Often, materials obtained from mining can only be found in geographically limited regions and may rely on unethical labour. These materials must be replaced with alternative and renewable precursors, ideally evenly distributed globally.
Streamlined, low-energy manufacturing must be targeted for fully sustainable materials. In the production of carbons, as active materials or conductive additives, this requirement is especially difficult, since energy-intensive high temperature annealing is necessary. Decreasing the volumes of solvents or switching to aqueous media will reduce the environmental impact; however, many cathode materials are extremely sensitive to water, and parallel materials discovery must be pursued to develop cathodes able to withstand aqueous processing. Additional consideration must also be given to by-products, which must be easy to dispose of safely, or harnessed for other applications.
Lastly, disposal or recycling routes must be redesigned. At present, existing LIBs are difficult to dismantle, contain harmful components, and do not biodegrade, posing a large-scale problem of waste disposal [175]. Greater emphasis must be placed on designing batteries either for facile disassembly, or to be fully biodegradable. End-of-life design is strongly linked to materials resource management, because if materials can be efficiently recovered, then reliance on depleting sources may be alleviated.
Advances in science and technology to meet challenges
New technologies have emphasised material source as a key consideration; NIBs replace Li with abundant Na, while sulphur is a readily available cathode for Li–S batteries. Carbon materials, found across all battery chemistries, are increasingly shifting towards biomass precursors [176]. However, to achieve the requisite conductivity, alternatives to high temperature treatment must be explored, such as hydro-, solvo-, ionothermal synthesis, and catalytic- or microwave-assisted processes [177].
Water-processable, biomass-derived or synthetic biodegradable polymers can exhibit excellent chemical and mechanical properties, already outperforming PVDF when used as binders in carbon, Sn or Si anodes [178]. Further, lignin- or cellulose-derived mats may be a renewable alternative to polyolefin separators or foil current collectors [179]. Future advances will be achieved by introducing functionalities onto these polymer scaffolds tailored to specific battery chemistries.
Polymers with enhanced ionic conductivity could also replace organic electrolytes, improving both sustainability and safety. Despite their cost, ILs are also promising; easy to recover and recycle, their environmental impact is lowered. Replacing organic electrolytes for aqueous alternatives is also attractive, although the limited operating voltage window remains challenging; water-in-salt electrolytes are one potential solution to overcoming the problem [180].
While the energy demands of battery production cannot be completely eliminated, offsetting them against longer battery life will improve sustainability. Efforts to prolong lifetime should concentrate on protecting component structure and stabilising interfaces, for example by introducing self-healing ability, as well as in-situ monitoring to aid diagnostics [181].
Once battery life is exhausted, full biodegradability of cell components must be targeted: one example study produced an all-organic LIB based on 3,4,9,10-perylenetetracarboxylic dianhydride, graphite and cellulose [179]. Alternatively, the battery must be easily disassembled to allow extraction, purification and recycling of individual parts. However, designing a cell to be mechanically and chemically robust during operation, yet easily dismantled after use, is highly challenging. Approaches based on electrode patterning or transient bonds could potentially allow strong interactions when cycling, but easy separation of components for recycling. It is clear that end-of-life pathways cannot be an afterthought to the rest of the battery manufacturing process: disposal or recycling routes must be central to the initial material or cell design.
Concluding remarks
In the development of batteries, performance will always be the primary consideration. However, emphasis on sustainability is growing and both factors must now drive the discovery of new materials. For materials to be deemed fully sustainable, they must be derived from readily available abundant or renewable sources, by ethical means; processing must be energy efficient with no harmful by-products; and they should be easily biodegradable or recoverable, to allow repeated use.
While not falling directly within the sustainability remit of the material itself, developing approaches to prolong the battery lifetime will offset the energetic cost of production against useful life. Understanding the degenerative effects of extreme thermal, mechanical, and electrochemical stress during operation, or simply ageing, will be key to designing these strategies. Therefore, to achieve truly sustainable battery materials, efforts must be made not only in advancing sustainable materials discovery and processing but critically examining the entire life cycle of the battery to design for a circular economy.
Acknowledgments
H Au would like to thank the Faraday Institution's LiSTAR Project (EP/S003053/1, Grant FIRG014) for funding. H Al acknowledges the funding provided by the Republic of Turkey Ministry of National Education. M C R is grateful to the EPSRC for funding (EP/S018204/2).
17. Sustainable materials for H2 storage
Valeska P Ting
Department of Mechanical Engineering, University of Bristol, Bristol BS8 1TR, United Kingdom
Status
As a sustainable fuel vector, hydrogen has the potential to be a key technology in the race to limit climate change. It can be a means of balancing supply and demand of energy from renewable sources such as wind and solar power and is an alternative low-carbon fuel for applications that are challenging for electrification (such as long-haul flights or long-distance road transport) [182]. However, the safe and efficient storage of hydrogen (a light, flammable gas) remains a significant engineering challenge—one that could benefit from new materials-based solutions. While state-of-the-art for hydrogen storage on-board vehicles involves storage as a cryogenic liquid or as high-pressure gas, the specialised, lightweight carbon fibre-wound composite tanks needed for these applications are expensive to produce. In addition, to increase the storage density of the H2, high pressures (of typically 350 or 700 bar) or exceedingly low temperatures (∼20 K, in the case of liquid H2) are required. These extreme storage conditions present safety concerns and increase costs [183]. As an alternative approach, storage of hydrogen in solid-state materials offers the ability to contain practical amounts of hydrogen at lower pressures than compressed gas tanks, thus allowing for safer storage and lower cost, more conformable fuel tanks [184].
In terms of sustainability, solid-state hydrogen storage systems involving physical adsorption of gases can result in less material waste and greater cyclability then systems based on chemical storage (e.g. metal hydrides or complex hydrides), where storage efficiency may degrade over time or generate waste intermediates. In addition, since physical adsorption is based on weak interactions between H2 and the surface of the material, uptake and release of the hydrogen is rapid, can be simply controlled using changes in pressure or temperature and can be cycled reversibly without loss of performance. However, there are still outstanding challenges to be met, and with legislated deadlines for decarbonisation fast approaching, the search for materials-based solutions will become even more intensive.
Current and future challenges
Of the current challenges in adsorptive hydrogen storage, three key areas of relevance to sustainability involve: (a) identification of materials with sufficient storage capacity under close-to-ambient conditions; (b) optimisation of materials performance; and (c) large-scale manufacture of the materials for practical application (see figure 24).
Figure 24. Inter-related challenges and advanced tools in the development of sustainable materials-based solutions for hydrogen storage.
Download figure:
Standard image High-resolution imageIn terms of identification of appropriate materials, the challenge is presented by the sheer variety of porous materials available, with carbon-based materials and metal organic frameworks (MOFs) perhaps being the most heavily researched over the past few decades. In an effort to provide benchmarks for the development of hydrogen storage systems, the United States Department of Energy (US DOE) has compiled guidance on some of the key technical targets for use in light-duty road transport [185], with system capacities expressed in terms of gravimetric (kg H2 kg−1 system) and volumetric (kg H2 l−1 system) targets. To date, development of porous materials for hydrogen storage has focused on the maximisation of surface areas, pore volumes (as a proxy for storage capacity) and increasing isosteric enthalpies of adsorption to improve the amount of hydrogen stored at ambient temperatures (e.g. by incorporating open metal centres/metal-decorated surfaces). Discovery of materials that have high hydrogen capacities has traditionally involved costly and challenging experimentation, to uncover empirical relationships such as the dependence of hydrogen capacity with Brunauer, Emmett and Teller (BET) surface area and the dependence on extremely small pores. (i.e. micropores of diameter <2 nm) [186].
With regard to optimisation of performance, the storage capacities of materials such as nanoporous carbons and MOFs are maximised at low temperatures and are correlated with the material's structure. Experimental evaluation of the storage capacities of the materials under different operating pressure and temperature ranges is time-consuming and may not lead to an optimal materials solution. A closely-related aspect is the reproducibility or trustworthiness of experimental measurements of porous materials; data that is misleading or irreproducible can lead to much wasted effort and resources [187].
A further consideration in terms of sustainability of such materials solutions is the sheer scale of the application. If even a fraction of the vehicles in production today were converted to hydrogen fuel cell vehicles employing porous materials for on-board storage of hydrogen, the material demand for the storage component would be of the order of hundreds of thousands of tons of adsorptive materials. The challenge is thus to find a materials solution that addresses all of these needs, while acknowledging the speed at which we need to act to meet our net zero targets.
Advances in science and technology to meet challenges
Understanding the key structural characteristics that will lead to a practically useful hydrogen storage material can usefully direct the search for new materials. For large-scale screening of systems for H2 storage, the application of machine learning [188], structure-based computational approaches to prediction of storage capacity [189] and use of data mining [190] for high-throughput analysis can be far more time- and materials - efficient than experimentation alone. Such computational approaches have been used to inform targeted synthesis, for example in predicting the structure and properties of a NU-100 MOF that was later shown to have a record high surface area and gravimetric capacity (13.9 wt% at 100 bar and 77 K [191]) exceeding the 2025 US DOE targets of 5.5 wt% (though it should be recognised that the DOE targets refer to the whole system, rather than the material alone). In terms of validation, variability in experimentally-determined surface areas and hydrogen storage capacities also lead to difficulties in successfully comparing different types of porous materials; thus, recent efforts toward standardising methods for calculating and reporting surface areas and hydrogen uptakes are recognised to be valuable and of universal benefit [187].
In terms of optimising performance under real-world conditions, better understanding of systems integration has also helped steer research. An example is the recent focus on usable deliverable hydrogen capacities (which consider the need for hydrogen to be delivered to a fuel cell at pressures greater than atmospheric) rather than total hydrogen capacities (often measured with respect to a vacuum) [186]. An example of the application of computational prediction alongside practical targets has led to the development of NU-1501- Al MOF material displaying gravimetric and volumetric hydrogen capacities (14 wt%, and 46 g l−1) that exceed the US DOE targets using adsorption and desorption at 100 and 5 bar pressure, respectively, and temperatures of 77 K and 169 K [192].
Finally, with regard to the scale of production, carbon materials are advantageous as they can be fabricated from waste biomass feedstocks, are non-toxic and can be produced on a large scale. However, new methods including mechanochemical synthesis, spray-drying or continuous approaches have also been reducing the environmental impact of large-scale production of more complex porous materials such as MOFs [193]. Due to the scale of the application, assessment of the whole life cycle of the material will play an important part in determining which future solutions are implemented.
Concluding remarks
The urgency for hydrogen storage solutions that can operate at the volumes needed to allow widespread application must drive smarter and more holistic approaches to materials development. Advances in computational modelling, data mining and prediction of storage capacity are expected to make the search far more targeted, rapid and efficient, while standardisation of experimental testing methods will also reduce wasted effort.
However, materials development cannot be focussed on developing materials purely for higher capacity. Considering all aspects of the sustainable production, use and cyclability of any materials-based solution is imperative to ensure that in solving one problem, we are not creating additional sustainability issues.
Acknowledgments
The author gratefully acknowledges financial support from the UK Engineering and Physical Sciences Research Council (EP/R01650X/1) in the form of an EPSRC Research Fellowship in Hydrogen Storage.
18. Sustainable catalysts for fuel cells
Tim-Patrick Fellinger
Bundesanstalt für Materialforschung und -prüfung (BAM), Unter den Eichen 87, 12205 Berlin, Germany
Status
Sustainable catalysts are omnipresent as enzymes, efficiently facilitating most complex reactions at ambient conditions. Also, the life-spending (di)oxygen reduction reaction (ORR), is facilitated by enzymes like cytochrome-c-oxidase, which shows turnover frequencies ∼500 s−1, 20x higher compared to platinum nanoparticles and still twice as active as bulk platinum at 0.8 V vs reversible hydrogen electrode (RHE) [194, 195]. Therefore, it is no surprise that the development of sustainable catalysts for hydrogen-oxygen fuel cells was originally inspired by an enzymatic motif. Jasinski showed in 1964 that the macrocyclic cobalt-phthalocyanine molecule, an analogue of the oxygen-binding heme molecule, is able to catalyse the ORR [196]. Modern analytical techniques today leave little doubt, that the square-planar coordination of four nitrogen atoms around a central metal-ion (M–N4 motif) found in heme is the functional unit of so-called atomically dispersed M–N–C catalysts [197]. Enzymes decay relatively quickly (because of their organic backbone), however the active-sites in M–N–Cs are embedded into inorganic carbon, which is also part in durable commercial Pt/C catalysts. While substantial reduction of precious metal amounts is feasible to allow wide application of fuel cell cars, M–N–Cs are the most promising class of precious-metal-free fuel cell catalysts today.
Practically relevant polymer-electrolyte-membrane fuel cells (PEMFCs), still rely on high loadings of platinum, the pure metal with the highest catalytic activity towards the ORR. The reduction of platinum is progressing, however for reasons of cost competition rather than for sustainability. In context of growing efforts to utilise hydrogen in alternative energy grids, this will however become a topic of increasing importance. Despite the substantial progress in the last 15 years [197–200], sustainable precious-metal-free catalysts currently still lack competitive performance (activity, selectivity and stability) as compared to platinum-based catalysts [201]. However, recent developments suggest that M–N–C catalysts with practically relevant catalytic activity and stability may be reached in the future, even for demanding PEMFC applications such as in the automotive sector. Realisation of equally performing M–N–Cs compared to commercial Pt catalysts would facilitate the transition to sustainable energy schemes by the balancing of the intermittent supply of sustainable electricity. The scientific and engineering steps to develop such functional M–N–C based technology may cause breakthroughs also in other chemistry-related energy conversion technologies having potential for substantial socio-economical changes in general.
Current and future challenges
M–N–C catalysts can be regarded as the most studied subgroup of the recently emerging atomically dispersed catalysts or single-atom catalysts. The activity has been limited by stagnating catalyst loadings, hence limited active-site density (SD). This is because side-phases increasingly form throughout the synthesis if higher catalyst loadings are targeted, which is typically stated as dilemma of M–N–Cs or Fe–N–Cs, the most common type of M–N–C as cathode catalyst in PEMFCs. New preparation protocols recently lead to a steady increase in reported SDs, especially for cases in which Zn ions were present in the precursor mixture [201–203]. It has been shown that Zn2+ can imprint the N4 binding site for active metal ions, while being less reactive towards harmful side-phase formation [203].
Active-site imprinting therefore in principle allows to increase the density of active sites until the intrinsic conductivity of the [FeNx ]Ny Cz becomes limiting. The quantification of utilisable active-site densities is a current topic of research, a fundamental criterion to determine site-specific catalytic activities as well as stabilities. Several methods have recently been developed and can be applied to M–N–Cs without contamination with harmful inorganic side-phases [199, 204]. However, there is still a large range of active-sites with different chemical structures to be considered, so that specific activities and durability still need to be considered average values [205]. Besides M–N4 sites, in which the coordinating N-atoms can be of either tetrapyridinic or tetrapyrrolic nature, the presence of penta-coordinated M–N5 sites, trigonal M–N3 sites and sites with other or mixed coordinating atoms such as C, O, S, etc are discussed. A future challenge is to quantify site-specific catalytic activity, selectivity and stability to be able to target the most active, selective and stable catalytic sites. This might have a disruptive impact on the applicability of sustainable fuel cell catalysts, depending on the performance of the best catalytic site. The ultimate challenge is the preparation of catalysts by design, which would allow for the adoption of more complicated enzyme principles employed by nature to unleash the potential that lies behind the still very sluggish reaction kinetics for the ORR even for platinum-based catalysts.
Advances in science and technology to meet challenges
Assuming that the SD of future Fe–N–Cs is only limited by their intrinsic conductivity, a projection for future catalyst performances can be made. A conductivity limit may be speculated at 50 carbon atoms per FeN4 site, e.g. [FeN4]N4C50, corresponding to about seven mass per cent of iron at maximum. Further assuming a reasonable catalysts layer density of 0.4 g cm−3 [202] gives a theoretical SDvol of 0.52 mmol sites cm−3. With an empirically found (average) turnover frequency of 1.6 e− s−1 (at 0.8 V vs. RHE) [204] and full site utilisation, a catalyst layer thickness of τ =75 µm would then be sufficient to match the kinetics of commercial PEMFC cathodes (τ =10 µm, 50 wt.% Pt/C), without running into severe mass transport issues [200]. The moderate stability of current Fe–N–Cs is currently far from practical requirements of 5000 h [201].
However, increased SDs in combination with improved selectivity have the potential to shift the situation into an adequate range. The stability may increase proportional to the SD, while selectivity enhancements may have a larger effect. The ultimate know-how to meet future challenges would be the synthetic control to define chemical composition and structure on the sub-nanometre level. To develop accurate synthetic protocols for the chemistry of M–N–C materials standardised methods and more powerful analytical tools for the characterisation of the amorphous, lightweight materials may arise to support such development. The merger of macromolecular carbon chemistry and pyrolytic carbon chemistry may result in progress if 3D systems are increasingly addressed. In a first step, the selective synthesis of single-site M–N–C catalysts is a requirement to rank the variety of different active-sites (by means of activity, selectivity and stability) and account for the potential with regards to commercial application. In the second step a scalable and inexpensive (relatively 'costless') preparation of the respective highly structured and heavily loaded single-site catalyst could allow the use of thinner layers in practical fuel cell application.
Advanced synthetic techniques may further allow for the design of more complicated, enzyme-inspired electrocatalysts e.g. mimicking the active-sites of cytochrome-c-oxidase or transfer other enzyme-principles to electrocatalytic chemical conversions.
Concluding remarks
M–N–C catalysts are the most promising class of sustainable fuel cell catalysts applied in PEMFCs. The technological immaturity of alkaline fuel cells currently still omits the practical use of metal-free carbon materials as well as other sustainable catalysts. M–N–C catalysts allow to combine achievements of nature by means of enzyme reactions with the accomplishments of human technology by means of electricity and conductive synthetic matter. The coming decade is expected to reveal, if the heme-inspired catalytic M–N4 site in combination with a synthetically optimised conductive carbon matrix will be sufficiently performing to justify the high costs of other required fuel cell components, resulting in a commercially viable product. Regarding the progress of the field in the last ∼15 years, the current projections, and the library of other naturally employed catalytic sites, a disruptive impact of M–N–C catalysts on the chemical energy conversion technologies seems to be a reasonable scenario.
Acknowledgments
Tim Fellinger would like to thank the German Federal Ministry of Economic Affairs and Energy (BMWi for funding within the Verbundproject innoKA (Project No.: 03ET6096A).
19. Bioinspired sustainable materials for ammonia production
Jesús Barrio1,2, Olivia Westhead1, Claudie Roy3 and Ifan E L Stephens1
1 Department of Materials, Imperial College London, England
2 Department of Chemical Engineering Imperial College London, England
3 Energy, Mining and Environment Research Center, National Research Council Canada
Status
Ammonia is a widely utilised chemical that allowed the growth of world population in the last century due to its application as fertiliser and energy carrier. Currently, ammonia is produced in centralised industrial plants through the Haber–Bosch process, which combines atmospheric nitrogen with hydrogen derived from the combustion of hydrocarbons at high temperatures and pressures (400 °C and 150 bar) in the presence of an Fe catalyst. The highly energetic triple bond of N2 (941 kJ mol−1) requires high temperatures and high pressures to enable its dissociation and subsequent reduction, implying large infrastructure and centralised production. Moreover, since H2 is derived by steam reforming methane, it results in 1% of global energy consumption and the production of 1.4% of global CO2 emissions [206]. The production of such a chemical utilising renewable feedstocks and electricity is therefore highly appealing, as it would allow production on site, rather than in centralised structures, and under ambient conditions. Consequently, low temperature electrochemical nitrogen fixation has emerged as a sustainable alternative to the Haber–Bosch process, where electrons reduce atmospheric N2 in the presence of protons on the surface of an electrode, acting as a cathode and the protons would ideally be derived from oxidising water. This process would have tremendous economic and social implications in sub-Saharan Africa due to the lack of transportation network and its potential for producing ammonia from renewable energy to be used as fertiliser and seasonal energy buffer.
Singh et al estimated, if 100% Faradaic selectivity towards nitrogen reduction was achieved, operating at 1 V total cell overpotential, the power needed to feed a standard field with ammonia for a year would be 145 W ha−1, which could be provided by a 5 m2 solar cell. Obviously the size of the solar cell would be larger if the electrolyser had a lower Faradaic selectivity and higher overpotential [207]. In addition to selectivity and overpotential, sustaining high current densities is important to minimise capital costs: the US DoE set the target of 300 mA cm−2 at 90% FE of N2 to NH3, similar to state-of-the-art water electrolysers [208]. Thus far, the only solid electrode in the literature which can unquestionably reduce N2 to NH3 is an in-situ deposited lithium electrode in an organic electrolyte with ethanol as a proton source [206, 209]. Conversely, no aqueous system employing a solid electrode has yielded quantities of NH3 that are sufficiently high to be distinguishable from adventitious contamination [210]; almost all electrodes simply yield the competing side reaction, H2 evolution. There is therefore a need to develop novel, sustainable materials which enable efficient nitrogen electroreduction. In this sense, nature may provide suitable inspiration: nitrogen reduction is carried out under ambient conditions by the nitrogenase enzyme through its Mo–Fe–S–C catalytic cofactor with up to 65% Faradaic selectivity [211]. The emulation of nitrogenase on a solid electrode could lead to the efficient activation and hydrogenation of N2 (figures 25(a) and (b)).
Figure 25. Structure of the catalytically active cofactor within the nitrogenase enzyme (a) [211]. Schematic representation of the synthesis of a carbon-based bioinspired electrocatalyst, yet to be realised experimentally (b).
Download figure:
Standard image High-resolution imageCurrent and future challenges
One of the major challenges in the field of nitrogen reduction is that all metallic surfaces display scaling relations between the adsorption energy of the sole intermediate of H2 evolution, *H and all the nitrogen containing intermediates of nitrogen reduction, e.g. *N2, *N2H, *N2H2, *N, etc [212]. As such, in an aqueous electrolyte, *H poisons N2 reduction, favouring H2 evolution.
The only electrochemical process that has been rigorously shown to produce ammonia is a Li-mediated approach in an organic electrolyte with ethanol as a proton source [206]. This is putatively due to the formation of a solid electrolyte interphase which can inhibit the transport of protons towards the active site, emulating the hydrophobic peptide matrix of nitrogenase that controls the release of H+ species through a proton wire [211]. Additionally, this interphase can potentially be tailored by manipulating the chemical composition of the electrolyte, borrowing insight from lithium ion battery science [213]. However, the use of lithium restricts operation to lithium plating potentials, building in an intrinsic overpotential and low energy efficiency, which prevent its industrial scale-up. Moreover, water oxidation would be a far more convenient source of protons than ethanol oxidation.
Advances in science and technology to meet challenges
The discovery of a solid catalyst that emulates nitrogenase would be a game changer. We propose that the key enabling features would be (a) highly reactive metal site consisting of two contiguous Fe atoms, coordinated to inert sulphur (b) restricting access to protons, oxygen and electrons [211]. Density functional theory (DFT) calculations have revealed that isolated dimers surrounded by an inert substrate exhibit exceptionally low barriers for N2 dissociation (figure 25(b)) [214]. The difficulty of preparing these catalysts lies in the tendency of isolated metallic moieties to aggregate owing to their large surface energy. To avoid such aggregation into nanoparticles, loadings are often kept low (1–2 wt%), which presents further challenges in the precise elucidation of their chemical and electronic state through advanced characterisation techniques. The construction of an appropriate support which sustains a high number of binding sites for stabilising isolated dimers is critical. In this regard, nitrogen-doped carbons hold the potential to become an ideal platform for carrying this task, owing to their high conductivity and hierarchical porosity and facile tuning of their chemical composition and pore size. Additionally, while the stability of enzymes attached to electrodes is low, we envision that a solid inorganic electrode might be more durable. Moreover, to maximise circularity, the materials used should be amenable to recycling. However, the production of these materials often entails complex methodologies; consequently sustainable C–N based precursors and new synthetic routes that can provide crystalline products with well-defined pores and homogeneous distribution of heteroatoms with lone electron pairs (N, S) in a large scale are sought after [88].
Due to the number of different sources and quantity of contamination, it is critical to carry out electrochemical experiments with quantitative isotopically labelled 15N2 as the feed gas to be certain of nitrogen reduction; it is essential that multiple checks are performed and that the ammonia concentration increases with reaction time or charge passed, both in the labelled experiments. Techniques such as NMR, FTIR or mass spectrometry can distinguish between 15NH3 and 14NH3 for the accurate and rigorous quantification of NH3 [206].
In order to understand the working mechanism of sustainable carbon-based bioinspired catalysts for nitrogen reduction, we can borrow methodologies from adjacent fields of electrochemistry and catalysis. For instance, in-situ surface enhanced infrared absorption spectroscopy can probe the potential dependent formation of adsorbates such as *NH or *N2H2 [215]. Furthermore, in-situ electron paramagnetic resonance spectroscopy, a technique well used in homogeneous catalysis [216], could elucidate the environment around the active metal centre.
Concluding remarks
While low-temperature electrochemical approaches have recently emerged for ammonia production, the realisation of an efficient bioinsipired sustainable catalyst remains a formidable challenge. The success of the Li-mediated approach may be because it affords the controlled diffusion of protons to the electrode, emulating the hydrophobic peptide matrix in the secondary structure of enzymes. Going further, the formation of isolated metallic dimers in an inert C–N or C–S based substrate could emulate the active site of the catalytic cofactor within the MoFe-nitrogenase, which is unique in activating and promoting the reductive hydrogenation of the highly stable N2 molecule. By combining the power of the metal dimer catalyst and lithium mediated approaches, it may be possible to mimic the nitrogenase enzyme over a robust, recyclable, solid electrode. Nitrogenase currently outperforms all anthropic attempts at green ammonia synthesis under ambient conditions. If a solid electrocatalyst could achieve the same per-site activity and efficiency as nitrogenase, it would be revolutionary (figure 26).
Figure 26. NH3-producing electrolyser with a bioisnspired material acting as a working electrode where the N2 reduction takes place: N2 + 6 H+ + 6e− → 2 NH3. O2 evolution occurs at the anode: 2 H2O → O2 + 4 H+ + 4e.
Download figure:
Standard image High-resolution imageAcknowledgments
The authors would like to acknowledge financial support from the Engineering and Physical Sciences Research Council (EPSRC) (EP/M0138/1 and EP/S023259/1), the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (Grant Agreement No. 866402) and the National Research Council Canada through the Materials for Clean Fuels Challenge Program.
20. Sustainable materials for CO2 capture
Sabina Alexandra Nicolae1,2,3
1 Queen Mary University of London, School of Engineering and Materials Science, Mile End Road, London E1 4NS, United Kingdom
2 National Institute of Materials Physics, 077125 Magurele, Romania
3 Department of Chemical Engineering, Imperial College London, SW7 2BX, United Kingdom
Status
Global emissions of CO2 are increasing year by year adding up to the greenhouse effect and leading to serious environmental problems. Global average temperatures have increased with about 1.2 °C since the pre-industrial revolution, underlying the negative impact of the anthropogenic activities on the environmental quality [217, 218]. There are five scenarios about the evolution of CO2 atmospheric concentration by 2100, according to Climate Action Tracker (figure 27): if no climate policies are considered, a warming with about 4.1 °C–4.8 °C is expected by 2100; current climate policies project a warming with about 2.8 °C–3.2 °C by 2100; in the third scenario, if all countries keep on their current targets, established in the Paris Climate Agreement, the average warming projected by 2100 is below 2.8 °C; 2 °C consistent will limit the average warming by 2100, but requires a significant increase in ambition of the current plans within the Paris Agreement and the 1.5 °C consistent scenario represents the case where the average warming will be limited to 1.5 °C by 2100, if an important and rapid reduction in global GHG emissions will happen [219].
Figure 27. Global GHG emissions and warming scenarios; reproduced from climateactiontracker.org. Climate Action Tracker (2021). 2100 Warming Projections: emissions and expected warming based on pledges and current policies. November 2021. Available at: https://climateactiontracker.org/global/temperatures/. Copyright © 2021 by Climate Analytics and NewClimate Institute. All rights reserved.
Download figure:
Standard image High-resolution imageOver the years, numerous international efforts were dealing with the reduction of CO2 atmospheric emissions, including Agenda 21 (1992), Kyoto Protocol (1997), Bali Road Map (2007), Copenhagen Accord (2009), Paris Agreement (2015), European Green Deal and UK, Japan, Korea and US 2050 Net Zero (2019) and China 2060 Net Zero (2020). These represent action plans targeting the diminution of the greenhouse effect, air and water depollution, clean and sustainable energy production. Carbon capture and separation can be achieved via post-combustion, pre-combustion and/or oxyfuel combustion, for capturing the CO2 from flue gases, and direct air capture (DAC), dealing with CO2 adsorption from atmospheric air. The CO2 separation technologies include absorption with alkaline compounds (carbon scrubbing), membrane gas separation, carbonate looping technology, cryogenic distillation and adsorption. Carbon scrubbing is a mature technology used in industry, based on the absorption of CO2 with alkanolamine. Absorption process received recognition due to the capacity of absorbing large gas emissions from industrial processes and power plants operations. In addition, the cost and the possibility to operate in power plants with low infrastructure make this separation method highly used. Membrane gas separation gained a lot of interest. Membrane Technology and Research Inc. [220] reported on a rubbery polymer membrane, 'Polaris' with impressive CO2 permeance (3.3 × 10−7 mol m−2 s Pa), and about 50 selectivity CO2/N2. The good performance comes from the membrane microstructure which consists of an inorganic material in the form of micro- and nanoparticles (discrete phase) integrated into a polymeric matrix (continuous phase). Various solid porous materials have been proposed for the removal of CO2 via adsorption, including MOFs, covalent-organic frameworks (COFs), zeolites and porous carbon materials. The high chemical and thermal stability of zeolites coupled with low-cost production, porous structure and structural diversity make them attractive for CO2 capture applications. MOFs and COFs have merits like large CO2 capacity and selectivity, uniform pore size, rich porosity, easy recyclability, good stability and low energy input for regeneration. On the downside, zeolites, MOFs, and COFs show a decrease in the CO2 adsorption capacity in moisture conditions. Porous carbon materials have emerged as low-cost CO2 adsorbents. They present common advantages with the above-mentioned solids, plus they can retain important amounts of gas in humid conditions. In addition, carbon-based adsorbents present good stability, high CO2 capture capacities at high pressures and low-cost regeneration.
Current and future challenges
Present CO2 capture and separation technologies suffer from lack of sustainability corroborated with limitations of the process performance and selectivity, in some cases. For example, carbon scrubbing involves high cost and energetically intensive processes. Moreover, the solvents (alkanolamines) are toxic and corrosive. Separation membranes have proved a high stability, long operational life and improved selectivity, but still require optimisation. Further research is needed to overcome challenges such as inability to separate low concentrations of CO2 in the gas feed (below 20% the membrane technology becomes unsuitable) which makes it incompatible with the post-combustion capture process. Solid CO2 adsorbents, like zeolites and porous carbon adsorbents, present high chemical and thermal stability and low production cost, plus good performance towards carbon capture. Zeolites are standardly synthesised via hydrothermal processes, which can be energy and water intense. Moreover, their powdered form represents a limitation when it comes to implementation in large-scale applications where processable monolith or membrane like formats are preferred. Porous carbon materials show high CO2 adsorption capacities at high pressures, even under moisture conditions, but their lack of selectivity and small low-pressure working capacity remain challenging to solve.
In addition, the production of some carbon materials is based on the usage of different fossil fuels precursors. Alternatives, like starting from biomass as carbon precursor or synthesis via hydrothermal carbonisation to diminish the high energy consumption and the emission of different volatiles compounds during pyrolysis, have been adopted. Despite these efforts, the high moisture content in biomass, and the long duration process of hydrothermal transformation to ensure maximum yield under high temperature adds up to the current challenges of designing truly sustainable and low-cost adsorbents. The applications of MOFs in gas storage are based on their pore structure and the functionalisation of the inner and outer surface with different groups, improving in this way the gas adsorption performance. The CO2 capture is highly influenced by the humidity conditions and by the open metal sites in MOFs, that bond preferentially and much more strongly with H2O than CO2. COFs are equally used in CO2 capture, based on their textural properties (large surface area and pore volume). Among all solid adsorbents, COFs are characterised by sustainable character [221]. Their stability is sometimes a challenge. Some COFs like boroxine or boronate ester are not stable under humid conditions, implying that more research needs to be done in order to implement these materials at industrial level [221]. Moreover, the structure of the porous adsorbents is crucial for their application in carbon capture. It has been reported that the best results are usually obtained with solid adsorbent which have in their structure a high number of micropores, predominantly narrow micropores. MOFs, COFs, zeolites and porous carbons can have this type of structure, but improvements in their carbon capture properties can be achieved by tuning their porosity. This could add up to the synthetic process and can also involve alkaline templating compounds with a hazardous character. The separation methods can be employed in DAC, as well. For example, amine-based CO2 capture incorporated in solid sorbents is often used in DAC. The solid sorbent can be silica, nanocellulose, MOFs and resins. The main challenge is that DAC is thermodynamically unfavourable. In addition, the feasibility of the material is very much dependent on the active materials that captures CO2.
Advances in science and technology to meet challenges
There is a clear need of more efforts for improving the present technologies for CO2 adsorption and addressing the current challenges in the field. In this way, with respect to the up to date performance of the solid adsorbents, the research community reports as much as 7.9 mmol g−1, for MOF-based adsorbents (Mg-MOF-74); around 5.9 mmol for zeolite 13X, 4.4 mmol g−1 for COFs and 5.2 mmol g−1 for waste derived porous carbons, all at 25 °C and 1 bar [222]. Starting from here, more attention is put into developing efficient and sustainable synthetic routes for solid CO2 adsorbents. Valencia et al [223] reported the synthesis of a new sustainable bio-based hybrid foam, consisting of cellulose and gelatine, infused with high amount of zeolites which has an excellent CO2 adsorption capacity and selectivity. The zeolite synthesis via hydrothermal treatment through a microwave assisted heating system or using low-cost templates has been reported, and scaled up by BASF (Ludwigshafen, Germany) [224]. In addition, porous carbons with high number of micropores can be manufactured using less harmful additives (K2CO3, Na2CO3, Na and K bicarbonates, potassium oxalate, potassium bicarbonate, potassium acetate), or strategies like self-activation of biomass or salt template-assisted chemical activation [225].
This results in minimising the expenses associated with the manufacture, easy cleaning of the generated waste and a reduction in the reaction time. Moreover, based on the acidic character of CO2, incorporation of basic adsorption sites has been intensively proposed, such as nitrogen doping into the carbon structure or impregnation with alkaline compounds, targeting superior performance [222]. In addition, advances addressing the gaps between the scientific and engineering CO2 capture research have been reported. In this way, a number of 25 adsorbents have been considered, including MOFs, zeolites and activated carbons, and have been screened in four different scenarios (a natural gas combined cycle flue gas, a high rank pulverised coal ultra-supercritical flue gas, a cement plant and an integrated steel mill), considering the CO2 purity and recovery plus the cost. The outcome revealed that the cost of using solid adsorbents remains quite high compared to amine-based process [226]. In parallel, advances in DAC have been reported. This technology started out at \600 per ton of carbon, and now costs around \200 a ton. The price is still high, but companies like, Swiss Climeworks, Canadian Carbon Engineering and American Global Thermostat are working on getting the costs down by selling the captured CO2. Nowadays, the first commercial plant near Zurich captures 1000 metric tons of CO2 per year, used in a greenhouse to boost the yields by 20%. The same company (Climeworks) has now 14 DAC facilities, and the Italian plant uses the CO2 for the manufacture of fuel for trucks.
Concluding remarks
Although, over the years many technologies and researchers have been working on lowering the CO2 atmospheric concentration, this is still a pressing problem, threatening the ecosystem and the quality of life on earth. Nowadays, the amine-based carbon scrubbing process is still the most used technology for CO2 removal, at industrial scale. Despite their good affinity, zeolites, MOFs, COFs, and porous carbons cannot compete with amine-based systems, because of their high synthetic cost, and their porous structure which behaves different depending on the process conditions (gas concentration, temperature, pressure, impurities). Overall, research needs to be invested in the development of materials with good performance towards CO2 removal, which show improved stability, low cost production, easy reusability and sustainable character for real life applications, such as pre- and post-combustion in power plants applications or DAC.
Acknowledgments
This project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Grant Agreement No. 721991.
21. Understanding the challenges for multi-carbon products (⩾C2) formation during electrochemical carbon dioxide reduction
Saurav Ch Sarma1, Rose P Oates2 and Ifan E L Stephens2
1 Department of Chemical Engineering, Imperial College London, London SW7 2AZ, United Kingdom
2 Department of Materials, Molecular Sciences Research Hub, Imperial College London, London W12 0BZ, United Kingdom
Status
The Paris Agreement, signed in 2015, aims to limit the global temperature rise to 1.5 °C. This is ideally possible when 12 billion tonnes of climate-warming CO2 are continuously removed from the atmosphere every year through decarbonisation, sequestration and conversion to value-added chemicals. One example of an established conversion initiative is by BluePlanet which has developed carbon-negative building material to construct the Terminal 1 of San Francisco International Airport. Similarly, the Opus 12 start-up uses a low-temperature electrochemical reduction process to produce 1 tonne of ethylene (precursor of plastics) from 3 tonnes of CO2. This provides an attractive alternative to the conventional high-temperature/high-pressure technology to produce energy-dense ethanol, acetic acid and acetone with high market value.
Interestingly, Cu is the only catalyst that can electrochemically produce such multi-carbon products with a reasonable turn-over frequency (TOF). Many recent studies have reported Cu-based alloys/bimetallics with higher Faradaic selectivity/efficiency (FE) towards multi-carbon products. However, Faradaic selectivity is not a measure of the intrinsic catalytic activity. Hence, TOF and partial current density are also important figures-of-merit for the comparison of activity; to date, no catalysts have surpassed the activity of polycrystalline Cu [227].
Alkaline electrolyte enhances the formation of multi-carbon products due to the promotional effect of the cation (Na+, K+ or Cs+). However, in such an alkaline condition, a significant amount of CO2 is lost due to the formation of CO3 2−/HCO3 −, which is an energy-intensive process. Further, the anions move across the anion-exchange membrane, evolving CO2 at the anode. This has a huge implication on the output gas-flow (used for quantification of FE) and single-pass conversion efficiency of an alkaline electrolyser, increasing the cost of the CO2-converted products. The issue can be resolved through a tandem device, where (a) in the first electrolyser, CO2 is converted to CO, either using a solid oxide electrolyser at 700 °C or a low temperature device below 80 °C and (b) in the second electrolyser, the CO produced from is reduced further to produce multi-carbon products. However, this integrated technology is still at a low technology readiness level (TRL-2). Consequently, optimisation of the reaction parameters is crucial to achieving the target set by the Energy-X expert panel of the EU for the next 5 years: low operational voltage (<2.5 V), a high current density of 500 mA cm−2 for a continuous duration of >1000 h with a single-pass efficiency of 40% [228].
Current and future challenges
The field has multi-faceted challenges to overcome: ranging from catalyst design to reactor engineering. A few of the major issues are briefed below:
- (a)Selectivity: Enhancing selectivity towards multi-carbon products is essential to reduce the cost of separation. Polycrystalline Cu has many distinct active sites including defects, undercoordinated sites and grain boundaries [229]. Ager et al [229] observed that the presence of these sites are responsible for steering reaction pathways to specific products via the stabilisation of different intermediates. Thus, it becomes necessary to enhance the selectivity by reducing the number of distinct active sites and enhancing homogeneity within the catalyst [227].
- (b)Stability: The stability of a catalyst over an extended operational period is challenging under reductive reaction conditions [230] and maintaining the stability of a molecular catalyst is far more challenging. Degradation is mainly observed due to restructuring of the surface, detachment of the catalyst due to hydrogen evolution or surface poisoning by adsorbed CO2 reduction intermediates. Restructuring and aggregation of nanoparticles are enhanced when the constituent elements have different surface energies or binding to reaction intermediates. Stronger anchoring to the support should enhance stability. Operando studies, such as in-situ TEM coupled with x-ray absorption spectroscopy and in-situ IR are crucial to understanding the deactivation mechanism of the catalyst during the reaction [230].
- (c)Current density: There is a limit to which surface areas can be maximised through nanostructuring without incurring transport localised CO2 or proton depletion. In recent years, researchers have been working on gas-diffusion electrodes and membrane electrode assemblies to circumvent mass-transfer limitation and achieve high current density (⩾500 mA cm−2).
Advances in science and technology to meet challenges
Rate-determining C–C coupling step for multi-carbon product formation is highly dependent on the adsorption energy of CO and its concentration near the catalyst surface. A mere error of 0.15 eV in theoretical adsorption energy calculation may lead to a 300 times error in the TOF calculation [227]. Since a single catalyst can have many types of planes exposed, each with different CO adsorption energy, this makes predictions quite complicated. Thus, relative comparison can be made, but designing materials based on DFT predictions still remains in its nascent stage.
Following strategies can be used to enhance the formation of multi-carbon products (figure 28):
- (a)Introduction of secondary elements: Yeo et al [231] introduced Zn as a dopant in Cu lattice to generate an in-situ source of CO near the catalyst surface. CO2 gets converted to CO on the Zn active sites due to the relatively weak binding energy of CO. With this strategy they could enhance the selectivity towards ethanol. Thus, optimisation of the local geometric arrangement of atoms is crucial to manipulate the adsorption energy of the intermediates.
- (b)Tandem catalysis: In tandem catalysis, two different catalysts are coupled together for subsequent CO2 reduction. Ager et al [232] proved this concept by coupling Cu and Ag together. Ag was fixed at a higher negative potential to maintain a high flux of CO near Cu surface which is then further reduced to form multi-carbon products.
- (c)Nanocavity formation: Sargent et al [233] experimentally observed that nanocavities can confine soluble C1 and C2 intermediates, such as aldehydes or CO, thus increasing their concentration to form multi-carbon products, such as propanol.
- (d)Metal-doped carbons: Metal-doped carbons are known to produce highly selective CO at industrially relevant current densities (700 mA cm−2) [234]. However, the coordination environment can be modified to mimic the nature of V-nitrogenase enzyme, where the metal–metal bond facilitates the C–C coupling and produce ethylene (major product), ethanol and propane from CO reduction [235]. We envisage the same functionality of dual-atom catalysts can be incorporated in functionalised carbon. However, stabilising such a species during the harsh reducing environment is crucial to achieving high efficiency. Tuning the micro-environment around the active sites can be done through defect engineering, manipulating metal-support interaction and varying the anchoring atoms.
- (e)Membrane optimisation: High CO2/CO partial pressure is crucial for facilitating C–C coupling. Unlike alkaline membranes, bipolar membranes can reduce CO2 losses by resolving the issue of CO3 2− formation and crossover in alkaline electrolyte. However, further optimisation with thickness, chemical composition etc are required to reduce the extra over-potential it adds for driving the water dissociation at the bipolar membrane [236].
Figure 28. Schematic representing strategies to enhance multi-carbon products.
Download figure:
Standard image High-resolution imageConcluding remarks
Electrochemical CO2 reduction is a promising carbon-neutral electrochemical technique capable of producing desirable, energy-dense, multi-carbon products with the potential to be integrated into the present-day industry. To improve upon the activity and selectivity towards the multi-carbon products, an alternative to the Cu catalyst is desired. The intrinsic activity (turnover frequency) of such a catalyst should be compared along-with the Faradaic selectivity and partial current density. It is also crucial to test the electrochemical activity and stability of the best catalysts in commercially relevant conditions and at a higher current density. We foresee that by stabilising metal-doped carbon catalysts, which emulate nitrogenase, researchers may enable achieving higher selectivity for multi-carbon products.
Acknowledgments
The authors would like to acknowledge financial support from the Engineering and Physical Sciences Research Council (EPSRC) (EP/M0138/1 and EP/R513052/1), the European Union's Horizon 2020 research and innovation programme through the European Research Council (ERC) (Grant Agreement No. 866402) and the Marie Skłodowska Curie—Individual Fellowship scheme (Grant Agreement No. 896637) and the Government of Canada through its National Research Council (NRC, CH-INT-106-1).
Demand for sustainable materials across other sections
22. Biodegradable sustainable electronics
Chen-Gang Wang, Zibiao Li and Xian Jun Loh
Institute of Materials Research and Engineering, A*STAR (Agency for Science, Technology and Research), 2 Fusionopolis Way, Innovis, #08-03, Singapore 138634
Status
Electronics are indispensable tools in modern society. While the electronics become more powerful and affordable, the lifetime of electronics is becoming shorter, even within a few hours for single-use transient devices. Most electronic devices contain a variety of toxic elements and organics in complex structures, which is the key hurdle for electronic waste (e-waste) treatment. In 2019, approximate 50 million tonnes of e-waste was produced and less than 20% of e-waste was recycled, remanufactured and reused appropriately [237]. Landfill and incineration are currently the main methods for e-waste treatment, which brings potential soil and groundwater contamination as well as bioaccumulation of hazardous molecules. It is noted that e-waste represents around 2% of solid waste streams but it produces 70% the hazardous waste during landfill processes [238]. To address these environmental threats, sustainable electronics, which made with non-toxic and biodegradable materials, are emerged as eco-friendly alternatives to conventional electronics [239–241]. In comparison with conventional electronics consisting of inorganic materials such as heavy metals and ceramics, sustainable electronics use organic materials coupling with less toxic metals. Polymeric materials with intrinsically light-weight, flexible, adapted for R2R processes, and even biodegradable have been well-developed for constructing functional sustainable electronics in the past decade [242].
Current and future challenges
Electronic devices generally consist of several components with conducting, semiconducting, and dielectric functions on a substrate. Substrate generally constitutes the majority of weight ratio in the entire device; therefore, the use of biodegradable substrate materials is vital for sustainable electronics. A variety of naturally derived polymers, e.g. cellulose, alginate, silk and chitosan, have been utilised as sustainable substrates due to their enzymatic degradability. Although natural polymeric materials are low-cost, flexible and biocompatible, the insufficient strength and thermal stability limit their utility on chips and electronic displays. On the other hand, synthetic polymers, such as polycaprolactone (PCL), poly(hydroxybutyrate), poly(4-hydroxybutyrate), poly(L-lactide) (PLA), PEG, poly(vinyl alcohol), polydimethylsiloxane, and poly(lactic-co-glycolic acid), have been engineered as the substrates in sustainable electronics. Synthetic biodegradable polymers are advantageous in the physical and thermal properties, designable structures and stable qualities.
One of the challenges on sustainable electronics is the evaluation of the toxicity of the degradation products, especially for biomedical devices [243]. The degradation by-products of environment friendly polymeric materials are naturally abundant compounds (i.e. sugars, proteins and peptides) and chemicals (i.e. monomers and oligomers) with low or no toxicity. In comparison with a broad material scope on organic substrates, biodegradable conducting and semiconducting materials are relatively limited. Metal matrix composites and inorganic materials such as Si, SiO2, Mg and MgO were employed as bioresorbable and biocompatible substrates and components in electronic devices [244]. Rogers et al developed implantable silicon electronics containing transistors, diodes, inductors, capacitors, and resistors for biomedical applications [245]. The device degrades rapidly in deionised (DI) water and simulated body fluid as shown in figure 29(a). Nguyen et al reported a biodegradable and biocompatible piezoelectric pressure sensor constructing by a sandwich structure with three molybdenum electrodes and two piezoelectric PLA films [246]. This sensor is applicable for pressure measurement in a wide range of 0–18 kPa and completely degrades after use. Moreover, the sensor was implanted inside the abdominal cavity of a mouse to monitor the pressure of diaphragmatic contraction and to produce useful electrical stimulation for tissue regeneration, while the sensor degraded gradually in vivo without significant presence of inflammation. Two-dimensional (2D) materials such as molybdenum disulphide (MoS2) and black phosphorus QDs also exhibited superior mechanical strength, flexibility, and biodegradability [247–249]. These emerging 2D materials were integrated into biocompatible and biodegradable transient bioelectronics. Although the conditions to achieve fully biodegradable are varied due to different materials and testing environments, most of the bioelectronics can degrade and convert to non-toxic molecules under physiological (phosphate-buffered saline (PBS) or saline at 37 °C) or enzymatic environments from days to months (figure 29(b)).
Figure 29. (a) Images of a degradable electronic oscillator device on a thin silk substrate and its dissolution process in DI water. From [245]. Reprinted with permission from AAAS. (b) Schematic and optical images of a MoS2-based biodegradable sensor. Degradation was performed in a PBS solution (pH = 7.4) at 70 °C with different dissolution times (0–72 h). Reproduced from [247]. CC BY 4.0. (c) Chemical structure, hole mobility across 0%–90% strain and degradation of the semiconducting polymer. Degradation conditions: in PBS (red line) and 0.5 M NaOH (black line) at 37 °C for 12 weeks. BCP: block copolymer; T: 2,5-bis(trimethylstannyl)thiophene; TT: thienothiophene. Reprinted with permission from [250]. Copyright (2018) American Chemical Society. (d) Image of an optical device and time-sequential collapse images of BA-embedded electronics in DI water at room temperature. Inset: device image under a dark condition. Reprinted with permission from [251]. Copyright (2021) American Chemical Society.
Download figure:
Standard image High-resolution imageAdvances in science and technology to meet challenges
Although many organic–inorganic hybrid devices demonstrate excellent biodegradability, the excessive dosage of degraded inorganic by-products may exhibit physiological toxicity and health concerns. Biodegradable organic conductors, semiconductors, dielectrics and their integrated devices have therefore recently gained significant attention [129]. Notably, conductive and semiconductive organic molecules and polymers containing rigid conjugated moieties are often brittle and stiff. The increasing needs on implantable flexible electronics boosts the development of next generation (semi)conductive polymers with high processibility and biodegradablility. Lipomi et al reported a stretchable and biodegradable semiconductive block copolymer containing rigid and semiconducting diketopyrrolopyrrole (DPP) segments and soft and degradable PCL segments. This polymer exhibit over 100% elongation and a hole mobility of near 0.1 cm2 V−1 s−1, showing in figure 29(c) [250]. The PCL segments is degradable in physiological conditions and the remained DPP segments are intact. Recently, Hwang et al developed a water-triggered, rapid destruction system with biologically safe, dissolvable citric acid (CA) and sodium bicarbonate (SB) to construct transient electronics. For example, they successfully integrated CA and SB with supporting polymer matrices and degradable Mg coil to create an optical device, as shown in figure 29(d) [251]. When the device immersed in water, CA and SB could rapidly dissolve in water and generate carbon dioxide. Meanwhile, Mg also reacted with dissolved CA to produce hydrogen gas, resulting in on-demand degradation after use. These biodegradable and flexible materials represents promising advances toward developing next-generation sustainable electronics for foldable devices, electronic skins and implantable biosensors.
Concluding remarks
Innovation and collective efforts from researches, industry and end-users are highly demanded to overcome the obstacles of e-waste. Nowadays, electronics is becoming more hybrid and smaller to match the requirement of personal wearable devices and micro-electronics, leading to difficulties on recycling. Therefore, besides active recycling and raising social awareness, sourcing for alternative materials and technologies to traditional electronics is a feasible route toward sustainable electronics. Electronics constructing with (bio)degradable materials is a particularly attractive solution because the waste could be treated with enzymatic or chemical methods and covert to safe by-products. In the future, sustainable electronics should be designed and fabricated with suitable materials to match not only performance and strength but also degradation conditions in different physiological environments. Apart from biodegradability, innovations on eco-friendly processes for sustainable electronic manufacturing are also important in reducing the environmental impact while increasing the value of sustainable electronics.
Acknowledgment
The research work of the authors in this paper are funded by A*STAR.
23. Sustainable construction materials
Rupert J Myers1 and Niko Heeren2
1 Department of Civil and Environmental Engineering, Imperial College London, United Kingdom
2 Industrial Ecology Programme, Department of Energy and Process Engineering, NTNU, Trondheim, Norway
Status
More materials are used in construction than in any other human activity. Construction materials have been used throughout human history [252]. Hunter-gatherer societies used natural materials such as wood, soil, and stone to construct temporary shelters for many thousands of years, consistent with their mobile lifestyles. From ∼10 000 BC, agrarian societies built permanent structures with a greater diversity of construction materials, including timber, bricks, glass, and Roman concrete. More types and higher quality materials have been used since the industrial era, notably Portland cement concrete (including steel reinforced), structural steel, and plastics. Within the last few decades, materials such as self-compacting concrete and cross-laminated timber have been developed and become commonplace in construction.
Accordingly, the main construction materials used today—concrete and mortar, timber, steel, plastics, bricks, glass, stone, soil, and aluminium—are produced from raw materials that are typically regionally or globally available in massive quantities. They are usually classified as non-hazardous [253], simple to use, and relatively inexpensive, such that they can be accessed by most of the population. Construction materials must also have good 'durability'. This is especially important since construction materials must ensure structural integrity for typically many years in e.g. walls, and infrastructure, tunnels, where maintaining a low risk of failure is imperative.
In 2015, the production of construction materials led to ∼5 Gt CO2-eq. GHG emissions [254]. This corresponds to roughly half of the GHG emissions from materials production and ∼10% of the global total. GHG emissions from materials production approximately doubled between 1995 and 2015, i.e. at a faster rate than the global total, which rose by ∼40% over the same time period [255]. Prevailing urbanisation and development trends [256], e.g. expansion of urban land area; higher density living; increasing demand for floor area, material goods, and services, particularly in less developed economies and countries with growing populations; alongside increasing generation of lower GHG emitting electricity and heat (e.g. wind); indicates that GHG emissions from the production of construction materials will both continue to increase in absolute amount and relative share of the global total in the future. These issues—climate change, urbanisation, development, and population growth—demonstrate the importance of systemic construction materials research.
Current and future challenges
Transformative changes to how we produce and use construction materials are needed to meet future demand with lower environmental impact. This requires actions from all stakeholders across the life cycles of buildings and infrastructure systems, from raw material extraction, material production and consumption, and through to end-of-life/waste management. It is thus necessary to produce more durable, inexpensive, and less GHG emitting ('low carbon') construction materials, while at the same time reducing their consumption. Here, we distinguish the former supply-driven measures, which mainly lie in the cradle-to-gate or material production stages (but also in waste management) and are the traditional focus of engineering and materials science research, from demand-driven measures, which involve changes to demand for materials and exist throughout product life cycles.
Recent industrial ecology research has focussed on improving quantitative understanding of the effects that supply- and demand-side measures may have on the life cycle GHG emissions from use of construction materials [254, 257]. Practically all emissions occur upstream and therefore are mostly able to be influenced by industry. Nevertheless research shows that strategies to improve material-service efficiency rather than decarbonisation of material production, i.e. material efficiency or 'circular economy' strategies, such as more intense and prolonged use, reuse, or recycling of construction materials, can substantially reduce demand for primary material production. For example, it has been reported that net-zero carbon scenarios for year 2050 can be achieved with 60% of the necessary GHG emissions reduction contributed from material efficiency strategies [258] leaving the remainder needing to be achieved by supply-side measures such as novel technologies and carbon capture and storage. Despite the growing body of literature in this area, important questions remain about the systemic effects and implementation of material efficiency strategies [259]. Table 1 illustrates some open research questions and measures in this area.
Table 1. Summary of challenges related to sustainable construction materials, differentiating those related to systematic science, the supply-side, and the demand-side. GHG is greenhouse gas emissions.
| Category | Strategy | Description | Challenges | Solutions | Potential | References |
|---|---|---|---|---|---|---|
| Systemic science and academia | Information discovery | Identification of systemic interdependencies (e.g. between construction materials selection and operational energy demand). | Knowledge of systemic issues are currently insufficient (e.g. biomass demand competition across different sectors (energy, chemicals, construction); carbon cycles and storage in buildings). | Systemic research to identify effective measures, further research needs, and create public awareness. | High. Identification of synergies and anticipation of future issues (rebound and lock-in effects, etc). | [259–261] |
| Implementation and action | Implementation of research results. | Market mechanisms hinder uptake of new technologies and products. | Increased interdisciplinary research (e.g. collaboration between engineers, economists, psychologists, etc to explore feasibility and actions for politicians and consumers). | High. Overcoming information gaps and increasing effectiveness of academic research. | [262] | |
| Dissemination | Research is often poorly communicated to the public and decision makers, resulting in poorly informed consumer decisions. | Simplifying findings from research studies into accurate and actionable insights. | Interdisciplinary research, stakeholder involvement, strengthening the science-policy-practice system, new ways to disseminate science. | Medium. More effective research, impact-driven research, informed politics. | [262] | |
| Demand | Reuse | Buildings can be repurposed, and components (e.g. steel beams, windows) can be reused, substituting production of new materials and avoiding their associated emissions. | Legislation, creating markets, quality assurance, potential rebound effects due to increased transport or energy demand related to building operation. | Research on quality assurance of reuse components, establishing web platforms for trading components. Flexible architecture and creative design allowing repurposing of buildings. | Medium. Emissions can be avoided entirely. | [263], [258], [254] |
| Recycling | Availabilities of end-of-life materials and energy intensities of recycling processes determine recycling benefits. Recycling is both a demand- and supply-side measure: resource recovery is a responsibility of the consumer and the recycling process is operated by industry. | Avoid downcycling, since it degrades material quality over time, sustaining demand for primary material. Some recycling processes have a negative net benefit (e.g. concrete containing recycled aggregates typically have similar impact relative to virgin concrete mixes [263, 264]. | Improve collection rates, use less environmentally impactful recycling technologies, optimisation of transport, life-cycle assessment. | Varies. Metals recycling rates are already relatively high in most countries, while plastic recycling still has a large potential. Most recycling processes cause non-negligible GHG emissions, limiting overall potential. For some construction materials and regions recovery rates can be increased. For some materials, e.g. timber, implementation of cascade use must be explored. | [254] | |
| Sufficiency | Less floor area per person and densification of urban areas. | Sufficiency has social, economic, and ethical implications that are often controversial. | Smart architecture enables denser buildings. Shift from single-family (especially detached) to smaller single-family and multi-family homes, less rural structures, higher occupation in buildings. | High. Per capita floor area differs widely between countries, with some countries developing at a rapid rate. | [254] | |
| Prolonged lifetime | Using buildings or components for longer reduces overall demand. | For energy efficient buildings the material emissions may outweigh the operational ones. Therefore, operating an inefficient building for longer may backfire. Often buildings reach end-of-life for socio-economic reasons rather than technical ones. | Case-by-case analysis, avoid lock-in effects. | Uncertain. Context dependent. | [254, 259] | |
| Material substitution | Use of materials with less embodied carbon (e.g. wood rather than concrete). | Established industries and standards hinders the uptake of new construction materials. Higher skilled personnel (designers, etc) are generally needed. | Ensure national legislation allows for alternative materials (e.g. standards for fire and earthquake safety of high-rise timber buildings). Create awareness with architects and stakeholders. | Medium to High. Depends on the materials substituted (e.g. steel for wood beams). Biogenic carbon sequestration effect is generally not considered, but is important. | [254, 265, 266] | |
| Supply-side | Concrete and mortar | Concrete is the most used construction material by mass and volume. Most concrete contains approx. ∼10–15 mass% cement, which is responsible for up to 60%–90% of its CO2 emissions. | Most of the CO2 emissions arising from the life cycle of concrete arise from Portland cement clinker production. Reducing these cement-related GHG emissions is key. | Substitution of Portland cement clinker, which may be facilitated by transitioning from ready-mix to precast concrete use; improve production efficiency alternative fuels use in Portland cement production including electricity and hydrogen produced from renewable energy; carbon capture and storage. | High. Current clinker-to-cement ratios of ∼0.75 can be readily reduced to ∼0.5 using LC3 technology. | [258], [267, 268] |
| Timber | A number of studies show that wood buildings have lower life-cycle emissions, due to lower specific mass (weight) and because the building envelope can fulfil thermal and structural function simultaneously (e.g. insulation between beams). | Production of wood products is energy intense and requires large amounts of processing and transport. Timber yields substantial advantages particularly when considering the end-of-life phase and biogenic carbon storage. Timber supply needs to be massively expanded to substitute concrete at scale. | Reducing upstream impacts from timber production (e.g. no fossil fuels for drying). Use locally sourced timber. Implement end-of-life management strategies (e.g. pyrolysis for permanent carbon fixation). Develop legislation for high-rise timber buildings. | High. While industry has been improving their processes (e.g. use of waste wood for drying), further optimisation can yield important savings. Wood can be an important carbon sink. | [261, 266] | |
| Metals | Metal production involves considerable GHG emissions. | Metal production is energy intensive. Steel production requires fossil fuels. Not all metals are recovered at end-of-life for recycling. | Decarbonising energy supply, use alternative processes (e.g. hydrogen-based steel production | Medium. Metal production impacts can be greatly reduced by increasing recycling, substitution, and technology innovation. | [254, 259] | |
| Bricks | Bricks are well-known, common, simple, and durable construction materials | Most GHG emissions associated with brick production originate from fossil fuel use in the high-temperature processes in the kiln. | Alternative fuel and energy use derived from renewable and low carbon sources; carbon capture and storage; use of alternative bricks and blocks, for example derived from lower-carbon cements and biomass. | High. Alternative bricks and blocks from lower-carbon materials. Medium. microwave assisted gas firing and greater electricity use. | [267] | |
| Plastics | Plastics demand in the construction sector has been increasing considerably. | Plastic recycling is currently largely under-developed due to a lack of technologies and low collection and recycling rates for some plastics. Furthermore, downgrading in recycling is problematic. | Recycling rates must be increased. New chemical processes and understanding of polymer chemistry are required. | High. Emissions from the use of plastics are expected to increase considerably in the future. | [269] | |
| Glass | Glass is used in most buildings, produced using the float process. | Most GHG emissions from glass production come from use of fossil fuels, so reducing these is key. | Use of alternative fuels and energy; carbon capture and storage. | Medium. Emissions from fuel use can be reduced. Process emissions from use of Na2CO3 as a raw material are difficult to mitigate. | [267] |
38Austria: https://asia.nikkei.com/Spotlight/Environment/Climate-Change/Mitsubishi-Heavy-to-build-biggest-zero-carbon-steel-plant; Sweden: www.hybritdevelopment.com/.
Many supply-side challenges are material-specific. For mortar and concrete, the latter being by far the most used man-made material, these include: (1) greatly increasing substitution of Portland cement clinker for non-Portland cementitious materials like coal fly ash and calcined clay (the current global average substitution rate is ∼25 mass% [270] as well as developing and using non-Portland cements such as those based on Mg–Si rocks [271], since most CO2 emissions from the cementitious materials cycle originate from Portland cement clinker production [268]; (2) low-cost and mass scalable carbon capture and storage/utilisation technology that does not deleteriously affect material properties and thus product functionality; and (3) (kiln) electrification of Portland cement clinker production, since this can roughly halve process emissions.
Similarly, for steel, (1) use of alternative energy sources such as hydrogen, with or without carbon capture and storage, and (2) electrification of iron production, can make substantial progress towards decarbonising steel production since most of its CO2 emissions originate in the blast furnace [272]. (3) Improving reuse and functional recycling of steels is another key lever, although steel recycling rates are already high (50%–90% [273]) and demand for steel is far greater than end-of-life steel generation (1500 Mt yr−1 demand vs 298 Mt yr−1 end-of-life generation of steel in 2014 [274]), so the former upstream challenges have higher decarbonisation potential.
Demand for plastics, such as polyvinyl chloride—the most used plastic in construction (43% of the total [111])—also far outstrips supply of end-of-life material (299 Mt yr−1 demand vs 28 Mt yr−1 end-of-life generation of plastics in 2014 [274]), so upstream challenges are similarly important here. For plastics, a key challenge is (1) shifting production from fossil resources to alternative and renewable feedstocks such as biomass and end-of-life organic materials, since fossil resources are used to synthesise virtually all plastics today.
Holistic governance of land is needed if biomass resources are to be used, as well as for timber, since a key challenge for this material is (1) massively upscaling its supply, on the order of doubling or greater (in 2017 timber accounted for ∼2.4 mass% or equivalently ∼14 vol.% of all construction materials, figure 30 [275]). Understanding how land use should be managed to approach maximal delivery of socioeconomic services and minimal environmental impact is a key modelling challenge. Forestry will take time to upscale. It will also take time to (2) establish the standardisation needed for massively increased timber use in construction, hence for the foreseeable future construction should be envisaged as use of a palette of materials involving timber in combination with other construction materials. Finally, since most new construction will occur in developing economies (see the status section), (3) governance for forestry in these growing regions is undoubtedly an urgent and key challenge.
Figure 30. Global materials extraction in 2017. (a) Masses of materials extracted: (a—i) all materials, data from UNEP [275]; (a—ii) construction-related materials; and (a—iii) construction-related materials used in construction products. Masses (b) and volumes (c) of materials extracted that are both construction-related and used in construction products. The construction-related materials, their proportions used in construction products, and densities are: non-metallic minerals—construction dominant, approximated here as granite (100%; 2700 kg m−3) [275]; wood, approximated here as Sitka Spruce (50%; 415 kg m−3) [276]; ferrous ores, approximated here as hematite (50%; 5150 kg m−3) [277, 278]; non-ferrous ores, approximated here as chalcopyrite (37.5%; 2700 kg m−3) [279, 280]; and petroleum, approximated here as crude oil (20%; 870 kg m−3) [111]. The values shown here should be treated as rough estimates only since mass losses occur during their downstream production, fabrication, and manufacturing.
Download figure:
Standard image High-resolution imageAdvances in science and technology to meet challenges
While many strategies for reducing construction material-related emissions have been investigated in detail, systemic understanding is still scarce. It can be anticipated that strong competition for available resources (e.g. land) and technologies will result once all industries more fully transition to carbon neutral production. For instance, it is likely that demand for hydrogen will increase tremendously as many high temperature processes transition to use of this energy carrier (e.g. metal industry), while also aviation and automobile industries may become more dependent on it as a fuel. Another example of potential market competition is timber and lignite, since wood is a key renewable material. Chemical, automobile, construction industries are all investigating large scale use of wood to replace current fossil-based processes. At the same time biomass has become an important source for thermal energy supply in recent years. Industrial ecology is a branch of environmental science that aims to analyse life-cycle and systemic effects and is well suited to identify strategies that approach maximum climate benefit from strategic management of resource competition [281].
Major trends in construction such as digitalisation, off-site construction, and additive manufacturing are simultaneously addressing multiple supply- and demand-side challenges (see the current and future challenges section). For example, digitalisation, through the use of building information modelling to create digital twins of buildings, and eventually whole cities and larger regions in the future, is enabling precise tracking of materials and thus increasing data availability for improved design and sustainability assessments. Off-site construction, meaning manufacturing products (i.e. component production and assembly) such as buildings at least partially in a facility 'off-site' rather than at the use site, will increase material choice and improve build quality since it can shift the burden of performance specification from the material to the component level. This is especially important for cementitious materials, since their properties change over time and are greatly affected by the prevailing environmental conditions during setting ('curing conditions'); since such conditions can be much better controlled in an off-site facility, this will lead to a greater variety of cementitious materials (including non-Portland) being specified. Finally, additive manufacturing can improve material-shape combinations and thus material efficiency. These trends need to be accelerated and the technologies underpinning them, e.g. wire arc additive manufacturing, need to be further developed.
Supply-side challenges can also be met by advancing material-specific technologies. We provide some examples here. For concrete and mortar, new cements with lower Portland cement clinker content that leverage intelligent materials selection based on local availability of primary (mined, e.g. clay) and secondary (generated, e.g. coal fly ash) resources should be developed [282]. Novel combinations of non-Portland cement binder and non-steel reinforcement should be explored as a longer-term strategy. Electrolytic production of cementitious materials from renewable energy sources requires further research [283], as does increased utilisation of CO2 in cementitious products including both in the binder phase and in aggregates. For steel, development of more impurity tolerant alloys and improvements to metal collection and sorting processes can improve recycling rates, which may thus reduce steel production from primary resources. To accelerate upscaling and standardisation of engineered wood products such as cross-laminated timber, as well as to increase their quality, a more accurate and reliable capability to predict properties of structural members derived from multiple species, with different grades, and including defects is needed [284]. This applies to engineered wood products sourced from both primary and secondary materials, where the latter may have additional defects and contamination (e.g. metallic fasteners) from their previous use [285]. Technological advancements are also needed to reduce the environmental impact of timber production, including decarbonised wood drying and transport, and novel materials (e.g. non-fossil derived glues) [286].
24. Nature, an architecture in the making
Alice Grégoire and Clément Périssé
Cookies, Villa Médicis, Académie de France à Rome, Viale della Trinità dei Monti 1, 00187 Rome, Italy
Status
A primitive 'man' inventing architecture to overcome his nakedness and shield himself from the cruel elements is the seminal tale. From there, using a unique brain ability, a continuous lineage of designers have piled and arranged bones, stones, sticks, then mortars, bricks and concrete to protect themselves and live comfortably away from nature. The traditional architectural canon has always been about celebrating human's superiority over Nature. This historical opposition must be challenged, even reversed so as to be able to consider architecture as an emanation from nature.
Recent scientific and philosophical advances on the notion of 'living' allowed a considerable amplification of the meaning and extent of the term. The question nowadays is not much anymore to know what is 'living', but rather to be able to find what is not the by product or a remnant of various life forms. From sedimentary stones, made from dejections and shells of early molluscs, to oxides formed following the great oxidation event triggered by planetary bacterial populations, minerals are often the result of life activity 39 .
Architecture in that regard finds itself in a new situation now that we can consider it as a recomposition of materials stemming from the 'living'. The discipline that was once considered as a celebration of human's victory over nature, in the form of a sophisticated articulation of inert matter, has now become the recomposition of remains, traces of anterior lives with the role of welcoming and hosting human and non-human life forms.
Architectural material follows a trajectory, from a natural habitat towards a final manufactured and spatialised state. Reinscribing the act of building in continuity with the natural world, should interrogate the architect's position and its practices considering the current environmental challenges.
Current and future challenges
Architecture's shameless extractivist behaviour emanates mostly from the misconception that architectural material is inert. Stone, sand, gravel are considered as inanimate. They are only rated as per the force, the energy thus the CO2 required to extract, transport and transform them into architecture. Wood belongs also to the dead matter reign, as it is considered as a handy CO2 trap, once cut and sawed.
Totally detached from their local context, and for most of them heavily transformed, these supplies' relation with the environment is limited to the carbon footprint exhibited on their label.
Yet archaeology or building restoration consider architecture's ingredients from an entirely different perspective. Intimate chemistry between stones and mortars, complex oxidation processes, micro infiltrations and cracks reveal how materials are constantly changing. Spectrographic analysis can help historians to retrace material's origins and age. Both of those sciences are maintaining the link between the built environment and its natural ores, quarries (figure 31) and mines.
Figure 31. An abandoned quarry near Sermoneta (Lazio), Italy. ©Cookies 2020–21.
Download figure:
Standard image High-resolution imageThis link gives architecture a powerful connection with deep time and planetary history. From rather young travertines (figure 32) to the seminal basalt, architecture is reorganising the planet's timeline into new configurations. This directly links our everyday spaces, our built commons and our monuments with the cosmos where they all originated from.
Figure 32. A Travertine quarry in Tivoli, where travertine has been extracted since Antiquity. ©Cookies 2020–21.
Download figure:
Standard image High-resolution imageAdvances in science and technology to meet challenges
It seems to us that the tools to bridge architecture with its natural context are missing. A direct relation between the two exists in specific cases, such as for the ruins. These unique moments where remains of architecture, in most cases stone parts, are absorbed back in the soil have been thoroughly described and depicted by the art of the Romantics.
Calamities such as earthquakes, volcanic eruptions and at a smaller scale sinkhole are also moments of symbiosis between architecture material and nature, where the built artefacts are swallowed by the superficial crust layers and head towards deeper geotopes.
But this is a one-way relation. Why does it take such incommensurable forces to reunite architecture with its breeding ground?
We want to believe that with the help of geologists, meteorologists, biologists, archaeologists and artists, we can develop tools for architects, but also for non-architects which would allow them to reconnect the discipline with its origins. The most important of these tools should address representation. How to show, illustrate, communicate and demonstrate the intimate bonding between materials and design, for all audiences 40 .
How to read a landscape, a region's geomorphic qualities and understand what architectural potential they contain? What vocabulary, which lexical field is appropriate to describe an architecture in the making, seen from the natural environment?
Ahead of the carbon optimisation at the level of building products, there must be a new intellectual corpus that gives architects, engineers, manufacturers, builders and ultimately end users a way to read and understand material nature more intuitively, and therefore think of architecture in another, more sustainable way.
At a planetary scale, architecture is a superficial, rapidly eroded mineral layer. A thin layer of soon to be debris. Yet it is impact on the living is immense and threatening. Understanding and discussing architecture as an act of biopsy could pave the way to a more responsible design.
Concluding remarks
As we advance in our research, we are obsessed with two overarching interrogations. Is architecture Earth's latest geological layer? Has rock itself evolved in a complex and surprisingly rapid manner into a refined assemblage that has the form of buildings?
A large part of the rock formation is either the product of organic matter, a massive accumulation of dead matter, compacted over thousands of years by colossal forces or it has been greatly influenced by living organisms at a planetary scale. We want to believe that human's building actions are the most recent factor in steering rock evolution 41 . This allegory would put us in a more appropriate position regarding our extractivist behaviour, and ultimately in the manner we produce architecture.
Since their origins, humans showed a profound devotion to the beauty of minerals 42 . The immense amount of inhabited geological chimeras resulting from this obsession has since then taken the name of architecture...
Acknowledgments
We would like to thank the Académie de France à Rome, Villa Médicis for giving us the opportunity to develop our project, in the frame of our one year fellowship. This project stems from and is part of the work of Cookies, our architecture, design, scenography and research studio. Federico Martelli's ongoing research on preservation politics at Cookies has been seminal to this project.
Alice Grégoire and Clément Périssé are architects and fellows at the Académie de France à Rome, Villa Médicis for 2020–21. Together with Federico Martelli and Antonio Barone, they founded Cookies, an architecture, design, scenography and research studio based in Rotterdam, The Netherlands and Paris, France.
25. Sustainable packaging
Xiaoying Zhao1,2 and Yael Vodovotz1,2
1 The Ohio State University, Department of Food Science and Technology, 2015 Fyffe Road, Columbus, OH 43210, United States of America
2 College of Chemistry and Materials Engineering, Beijing Technology and Business University, Beijing 100048, China
Status
Currently, the major challenge of improving food system sustainability is to reduce food loss and waste. Around 30% of the food produced globally is lost/wasted along the supply chain [291]. Appropriate packaging can reduce losses at almost every stage of the food supply chain by extending shelf life and facilitating the safe food transit. However, the overall contribution of packaging to the food system sustainability is controversial, as packaging is linked with high levels of non-degradable plastic waste and low rates of recycling. More than 90% of food packages are made from petroleum-based non-compostable plastics, around 40% are disposed of after a single use, and more than 80% are landfilled [292], causing great environmental concerns. Therefore, food and packaging manufacturers are under great pressure to seek sustainable alternatives.
Sustainable packaging is a complex concept requiring critical thinking and systematic approaches [293]. Evaluation of packaging sustainability needs to consider (a) lifecycle impact of the package from raw materials to end-of-life management; (b) interactions between the package and the food; (c) impacts of the packaging on the business, people, and the natural environment including material sourcing, production, and waste management (triple bottom line) [293].
BPs are polymers that are either biobased (fully or partially), or biodegradable, or both (figure 33). Currently, BP packaging accounts for less than 1% of the packaging market. Among them, biobased and nonbiodegradable BPs, i.e. drop-ins, such as bio-PET and bio-high density polyethylene (HDPE), have the largest packaging market, as their identical chemical structure and properties to their petroleum-based counterparts can lower the risk for end users [294]. Braskem have produced commercial bio-PET and bio-HDPE which can replace their conventional counterparts [292]. Examples of drop-in BP packaging include snack bags and stretch films from Braskem, Avery Dennison, and FKuR [292].
Figure 33. Types and applications of BPs. Reprinted with permission from [292]. Copyright (2020) American Chemical Society.
Download figure:
Standard image High-resolution imageBiodegradable (in defined conditions) BPs are expected to play a greater role in the packaging industry. According to European Bioplastics, biodegradation is a chemical process in which materials are metabolised into CO2, water, and biomass with the help of microorganisms. For example, blends of PLA and polyhydroxyalkanoates (PHAs) have been used for coffee capsule and pouches [292]. Different biodegradability can result in different packaging applications. For example, shelf life of products can drive the packaging needs: BPs with high biodegradability, such as starch and cellulose-based BPs, can be used for food with short shelf life while those with lower biodegradability such as PHAs can be used for food with longer shelf life; BPs that require extended periods of time to biodegrade and can be recycled, such as PLA, can be used for food with a much longer shelf life [292]. It is worth mentioning that although biodegradability is a useful characteristic for packages in applications where plastic recycling or reusing is difficult, such as flexible packages and plastic wastes in marine environments, BPs are not a solution to the problem of plastic littering, as littering should not be promoted or accepted for any plastic waste in any environment. Instead, proper end-of-life management for all kinds of plastic waste is needed.
Additionally, novel food packaging techniques such as active and intelligent packaging are being used to combat food waste and improve food system sustainability. Active packaging interacts with the food and/or its direct environment to reduce food spoilage. Intelligent packaging can monitor the condition of food and inform food spoilage using colour or other indicators. It also allows real-time monitoring of food quality throughout the supply chain to reduce food-borne disease and food waste. Green, active, and intelligent packaging technologies can work together to yield a multipurpose food‐packaging system, to reduce food loss and create more sustainable food systems. This aim is seen the ultimate future goal for food packaging technologies.
Current and future challenges
Currently, the challenges for bio-alternative packaging mainly include:
- (a)Property and cost gap between bio- and conventional plastics for food packaging. Although drop-ins do not pose a gap in functional properties compared to conventional plastics as they have the same chemical, mechanical, and barrier properties, they are more expensive. For the compostable BPs, they mostly have lower toughness, lower flexibility, lower barrier, and higher price than the conventional plastics [292].
- (b)End of life management of BPs and their effect on current plastic management system. As drop-in BPs can be recycled in the same way as their conventional counterparts, their use does not disturb the current plastic waste management system. In contrast, biodegradable BPs have a much more complex end of life scenarios. Biodegradable BPs can potentially contaminate the current plastic waste recycling stream due to the lack of nationwide BP collection infrastructure and accurate BP waste separation technique [114, 292].
- (c)Lack of universal biodegradation definition, policies, and testing method. As BP market is not as mature as the petroleum plastic market and only accounts for a small section (<1%) of the market, currently, there is no universal definition for biodegradation or standards regulating the framework conditions and pass/fail criteria of biodegradability [292, 295]. To make accurate claims about biodegradability/compostability of a BP material, the biodegradation/composting location (home, industrial, etc.), condition, and time frame need to be specified.
- (d)Lack of comprehensive BP sustainability assessment approach. The sustainability assessment of BPs should consider their environmental, economic, and social impacts. Currently, most assessment approaches are not comprehensive enough to include all these three dimensions. For example, life-cycle-assessment (LCA) approaches, which has limitations for a direct comparison of bio- and conventional plastics [292, 296]. It is generally believed that BPs can potentially save 241–316 million tons of CO2-eq. each year and have lower non-renewable energy use than conventional plastics [292]. For example, the carbon footprint of packaging films made from LDPE and PLA were assessed with three different end of life management, i.e. incineration, landfill, and recycling [292]. LCA study showed when biogas collection from the landfill included landfilling, PLA film reduced carbon emissions the most [292].
- (e)Poor public understanding of BP definition, identification, and end-of-life handling, limits waste separation and restrict consumers' desire to pay for BPs [292].
- (f)Concerns over competition of BPs with food production if widely adopted. Currently, some of the marketed BPs, such as bio-polyethylene and PLA, use food resources such as corn or cane sugar for the production, causing concerns over food security and pressure on agricultural land if such products are scaled up significantly for replacement of petroleum-based plastics [297].
Advances in science and technology to meet challenges
To meet the challenges faced by the sustainable packaging, required advance in science, technology, and policy include:
- (a)Property reinforcement. The toughness and flexibility of the BPs can be enhanced through plasticisation, blending/compounding with other flexible and tough biopolymers, chemical copolymerisation, grafting, and cross-linking, incorporation of reinforcing agents, altering crystallisation behaviour, and other methods. The barrier properties can be improved by lamination with barrier plastics, metallisation, decreasing moisture/gas diffusion by incorporating nanofillers, introduction of hydrophobic materials, crosslinking, and other methods. The processability can be improved by chemical modifications, such as long chain branching, grafting, cross-linking, blending with elastomers, plasticising, nucleation to change crystallisation properties, and other methods [292, 298].
- (b)Cost reduction. Production of BPs by value-added use of agro-food waste residues can potentially reduce BP cost [299]. Additionally, BP prices will likely decline if economies of scale are achieved and logistics are fully developed [292]. Finally, price reduction can be fostered by product design, such as rigid BPs may require less materials than conventional plastics to produce products with the same rigidity [292].
- (c)End-of-life management. Ideally, BPs should be separated from other plastic waste at the household level to reduce their risk of contaminating the plastic recycling stream [299]. Biological treatments, like composting and anaerobic digestion, are considered the most suitable waste management option for biodegradable plastics [299]. Another method is chemical recycling which breaks down BPs into smaller hydrocarbon molecules that can be used to produce new materials. Additionally, an emerging and promising treatment is biological recycling, which involves in designing enzymes and microbes to selectively degrade BPs into new molecular feedstocks [292].
- (d)Meaningful education of consumers, manufacturers, stakeholders, and government about BPs is needed. Clear communication with consumers on the proper use of BPs, the importance of BP separation, and BP waste handling instructions is needed [292].
- (e)To comprehensively assess the sustainability of BP packaging, the assessment approach needs to (1) address the impact differences of bio- and conventional plastics through normalisation/weighting, (2) include BP end of life scenarios, (3) include more environmental impact indicators, (4) consider that the carbon resources of the BP materials can potentially be recycled and reused after degradation [292, 300].
Concluding remarks
We are at a crossroad for BPs. Packaging represents the largest portion (∼60%) of BP applications. Collaborations between academic and industry is required to advance the applications of BPs for packaging industry. Reliable and sustainable raw material supplies for BPs as well as appropriate BP waste collection and separation options are needed. The sustainable economic growth of the BP market also requires policy support. In the future, compostable, semidurable (BPs that can be both recycled and composted), and durable (BPs that are recyclable but nonbiodegradable or with very low compostability) BPs will attract increasing interest for packaging applications [292]. To address the sustainability challenges of agricultural production and end of life management, research efforts on improving energy efficiency in manufacturing, accelerating biodegradability, clear plastic labelling, and efficient recycling systems are desired [292].
26. Sustainable textiles for fashion
Rebecca Earley
University of the Arts, 272 High Holborn, London WC1V 7EY, United Kingdom
Status
The fashion/textiles industry presents enormous challenges in reducing energy consumption. Impacts from every stage of the garment's lifecycle need to be considered—extraction, production, use and disposal—and these vary according to fibre type, textile/garment finishing and construction, retail/use contexts, and end-of-life options.
The sector has grown exponentially in the last few years and is predicted to continue to grow:
In 2019, global fibre production was around 111 million tonnes. Fibre production has more than doubled in the last 20 years and is expected to increase by another 30% to 146 million tonnes in 2030 if business as usual continues [301, p 6].
The volumes of fashion/textiles create multiple environmental impacts: waste—through overproduction, over consumption, and inefficient processes—as well as resource depletion, water usage, chemical pollution, exploitative labour practices, deforestation, endangered species, animal suffering and habitat destruction. The current linear system within the textiles industry is highly reliant on non-renewable materials and energy [301, p 4].
Clothing consumption around the world is equivalent to 62 million tons, being responsible for the annual consumption of 79 billion cubic metres of water and the emission of 1715 million of CO2 tons emitted [302, p 8].
Emissions are greatest in the fibre to yarn manufacturing stage [303, p 58]. Assessing fibre impacts is difficult as key factors—boundaries, assumptions and in particular geographical locations—can vary hugely.
There is no one study that deals with the quantification of energy needs of different textile fibres. If such a study were available, then it would be possible to compare various fibres in terms of their energy needs [304, p 86].
Conventional cotton and virgin polyester are the two most commonly used materials within textiles industry today and account for around 75% of the global fibre production [301, p 6, 303, p 13]. Conventional cotton uses 60 MJ kg−1 of fibre to produce; organic cotton, 54 MJ kg−1. Polyester uses 125 MJ kg−1 [304, p 86]. If you include the other synthetics—nylon 66 (138 MJ kg−1), acrylic (175 MJ kg−1), etc—then together with cotton and polyester, this totals 89% of the market. These synthetic fibres are made from oil, using an estimated 342 million barrels every year [305, p 38].
Current and future challenges
Replacing fibres made from oil, focussing on organic and improved cotton and getting a more balanced palette of textile options lies at the heart of the current and future challenges. However, changes need to be made around the whole lifecycle and value chain, as clothing moves across continents and in and out of people's homes.
Impacts come from the heat and power needed for spinning, weaving, dyeing, finishing and constructions processes [306, p 8, 304, pp 86, 87]. Shipping and distribution impacts from transportation are increasingly relevant as the internet drives consumers to shop more globally [304, pp 65, 70]. Both the physical and online retail functions create impacts; washing machines, tumble dryers, irons and dry-cleaning uses energy too [304, pp 71, 72].
The sheer volume of clothing however, as much as the material and systems around them, are amongst the biggest challenges. The industry produces large volumes of waste through overproduction, as well as underutilisation (people not wearing and tiring quickly of their purchases) [305, p 36, 306, p 21].
At end-of life the textile collection systems on offer in the UK are the bin (in which case the clothes will go to landfill or incineration), resale, donations to a charity/organisation's bank/shop, or in council recycling schemes [307, p 16, 304, p 25, 305, p 104]. Yet of all the total global fibres produced less than 1% is recycled textile-to-textile [301, p 92, 305, p 20].
Advances in design strategy, science and technology to meet the challenges
The problem of fashion textile design and energy is so complex it needs to be viewed through a holistic lens and supported by design-driven approaches. Design decisions directly lead to between 50%–90% of impacts; aesthetic/functional choices made in terms of fibre type and mix, fabric colour/finishing, garment construction approach and market level, etc, all play a part in the overall environmental performance [308, p 11].
Designers need to work with scientists/technology experts to become fully aware of impactful decisions. Several advances have been made in recent years to bridge knowledge gaps by bringing designers together with scientists, business and policy makers. Projects like the Mistra Future Fashion (MFF) programme 43 and Trash-2-Cash 44 have created insights, roadmaps and methods. The TEN 45 were co-created to break down and detail the range of sustainable design decisions across the whole lifecycle. They have also been used to help build collaborative relationships between diverse stakeholders [308, pp 15–17].
This cross-disciplinary approach was explored in the MFF programme and contributed to a roadmap for the Swedish fashion industry. In 'the supply change guidelines and ecodesign action list' Roos et al details one of the three goals for 2030, 'reduce emissions of GHGs from textile use by 50%, and by 2050 be carbon-neutral' [306, p 19]. Roos et al argues how a range of approaches can bring the industry's impacts down by 50% (figure 34).
Figure 34. How a series of changes at different stages of the lifecycle could lower the Swedish fashion industry's emissions by 50%. S Roos, M Larsson, C Jönsson Supply chain guidelines: vision and ecodesign action list. Mistra Future Fashion report. [Online] (available at: http://mistrafuturefashion.com/wp-content/uploads/2019/10/Supply-Chain-Guidelines_S.Roos-Mistra-Future-Fashion-report.pdf) 2019.
Download figure:
Standard image High-resolution imageWRAP's Textile 2030 pathway report presents a similar set of ideas for the UK and also shows that circularity—using recycled fibres and moving to 'sharing economy' business models—could constitute 26% of the savings (figure 35) [307, p 12]. Both note that garments designed to last longer will help. Roos also shows the savings made if we just used our clothes twice as much as we currently do [306, p 11] Changing the way we use energy, and the kinds of energy the industry uses, right across the value chain from factories to homes, will make the biggest difference to the garments footprint.
Figure 35. Carbon footprint reduction scenario 2019–2030. © Data source: Textiles 2030. The Waste and Resource Action Programme www.wrap.org.uk.
Download figure:
Standard image High-resolution imageTable 2 shows other technical and strategic design innovations for cotton and polyester users to aim for. Innovative recycled manmade cellulosics and synthetic biology (e.g. bacteria and mycelium) play an important role in how we might create a more balanced palette of materials [301, pp 56–58, 309]. In dyeing and finishing new efficiencies like dope dyeing are helping to reduce impacts; as well as 'bio-fabricate' approaches, like bacterial colouration [309, p 10].
Table 2. Advances specific to polyester and cotton textiles which are 75% of the current fibre market.
| Fibre type/production phase | Polyester | Cotton |
|---|---|---|
| Agriculture | Recycled and bio-based synthetics replacing use of oil. | Preferred cottons and regenerative organic cotton. |
| Fibre, textile and garment production and finishing | Recycled polyester—dope or vat dyed; air dye processes; design for sequential/tonal transfer printing. | Replace cotton with regenerated cellulose alternatives derived from clothing and foot waste streams; use novel colour processes; limit finishing processes, e.g. digital printing, laser finishing. |
| Cutting waste reduction techniques; tech to address overproduction; parallel remanufacture processes to address fall out; redistributed manufacturing. | ||
| Use phase | Polyester | Cotton |
| Retail | Reduce overstocking; improve sale and resale processes to reduce garment waste through returns. | |
| Laundering | Low launder: design garments to reduce need to wash, stain resistant coatings and low impact cleaning solutions. | |
| Overconsumption and underutilisation | Replace and/or extend use phase: virtual reality clothing experiences. Recirculation/reuse and sharing economy. Digital tech, e.g. block chain, for efficiency and transparency. | |
| Disposal phase | Polyester | Cotton |
| Collection | Improve options: banks (not 'bins') and more doorstep schemes. | |
| Sorting | Automated sorting: | |
| Redistribution | Disassembly and remanufacture: construction threads that enable garment parts to be separated; models for | |
| Materials recycling | Mechanical recycling: | |
| Fibre and molecular recycling | Recycled rPET | Regenerated into Lyocell |
Towards a sustainable and circular industry
Prototypes co-created by designers with other experts can show us how these new materials, processes, product concepts, business models and end-of-life reprocessing approaches can be brought together through systems design, to create both slow and new kinds of fast fashion/textiles, using less energy 46 [310]. These highlight the urgent need for sustainability and circularity challenges to be approached together, through working in challenging new partnerships. Such partnerships are being developed and supported by organisations and programmes such as: WRAP 47 who look at the full supply chain; at the fashion product level, the Institute of Positive Fashion (at the British Fashion Council) developed the Circular Fashion Ecosystem 48 report for the UK and the Ellen MacArthur Foundation has been focusing on jeans through their Make Fashion Circular programme (Jeans Redesign project) which has enabled a rich picture to emerge around one product type 49 ; and in terms of technologies, Accelerating Circularity 50 is focused on digitised system to increase textile resource efficiencies. World Circular Textiles Day 2050 51 , established in 2020, is a global platform that brings all these stakeholders together with the specific intention of sharing insights and charting progress every year on 8 October.
Acknowledgments
Centre for Circular Design (CCD): Sanne Visser, UAL PhD researcher. Mistra Future Fashion (MFF) Programme: all partners and especially Sandra Roos and Gustav Sandin, Research Institutes of Sweden (RISE). MFF was funded by the Swedish Government via Mistra, the Foundation for Strategic Environmental Research, to bring about a significant change in the Swedish fashion industry leading to sustainable development in the industry and wider society. Thanks also to all the generous partners on the Trash-2-Cash project, hosted by RISE (Horizon 2020 research and innovation programme under Grant Agreement No. 646226).
Quantifying sustainability
27. Life cycle assessment (LCA) of materials
Göran Finnveden1,2 and Anna Björklund1
1 KTH Royal Institute of Technology, Department of Sustainable Development, Environmental Sciences and Engineering, Stockholm, Sweden
2 Luxembourg Institute of Science and Technology, Environmental Sustainability and Circularity, Belvaux, Luxembourg
Status
As evidenced by this paper, the necessity to reduce emissions contributing to global warming and other environmental impacts is a major driving force for materials development. In order to ensure that new materials and products actually lead to reduced environmental burdens, the environmental impacts must be assessed. This should preferably be done in a life cycle perspective to make sure that environmental problems are not just shifted from one location to another, or from one type of problem to another. LCA is a method for assessing the potential environmental impacts of a product from 'cradle to grave', i.e. from raw material extraction, via production and use to waste management [311]. An LCA is performed in four phases: goal and scope definition where also system boundaries are defined, life cycle inventory analysis where inputs and outputs to and from the system are calculated, life cycle impact assessment (LCIA) where the potential environmental impacts of the inputs and outputs are assessed, and Interpretation where conclusions are drawn. Ideally the system boundaries should be defined so that the inputs are resources from the environment and the outputs are emissions to the environment. In a comprehensive LCIA, three broad areas of impacts should be considered: natural resources, ecological impacts and human health impacts. These broad categories can be further divided into more specific impact categories such as climate change, human toxicity, mineral resources use, fossil energy use, and land transformation [311]. An LCA can be made for a single product, where the aim can be to identify the most important environmental impacts or parts of the life cycle. LCAs are often made in comparative assessments. In such cases the products need to fulfil similar functions in order to allow for a fair comparison. An important part of the goal and scope definition is therefore to define the functional unit, which is a quantitative measure of the function(s) the product fulfils, and which is the reference value for the inventory analysis and the LCIA. A distinction can be made between attributional LCA, which is defined by its focus on describing the environmentally relevant physical flows to and from a life cycle and its subsystems [312], and consequential LCA, which is defined by its aim to describe how environmentally relevant flows will change in response to possible decisions [313]. An ISO standard for LCA [314] provides a common framework, terminology and some methodological guidance.
Current and future challenges
Since LCA is primarily a method for assessing potential environmental impacts of products (including both goods and services), it may not always be straightforward how to apply it for materials. One common approach is to do what is often called a 'cradle-to-gate' analysis [312], i.e. to include raw material extraction and production up to the point where the material leaves the production gate, but not include further production, use and waste management stages. Such studies can be used for comparing different raw materials or production methods for the same material. This is possible since environmental impacts occurring after the materials have been produced can be assumed to be equal and therefore can be disregarded in a comparative study. It can however not be used for comparing different alternative materials that behave differently during the use and waste management stages. One example of this is the assessment of new so-called self-reinforced, or single polymer, composite material that can be used for structural weight minimisation of vehicles. In order to compare its potential environmental impacts to that of other light-weight materials, it is not enough to make the comparison based on cradle-to-gate data, see figure 36 [315].
Figure 36. Life cycle comparison of light-weight materials in vehicle design. Reprinted from [315], Copyright (2015), with permission from Elsevier.
Download figure:
Standard image High-resolution imageAnother aspect that should be included in this case is the weight of the alternative material as included in a component in the vehicle, since this will influence the amount of fuel needed during vehicle operation. Finally, end-of-life processes will differ depending on possibilities for recycling, which is expected to be better for single-polymer composites compared to conventional composite materials. This example illustrates the importance of scenarios for the use and waste management stages when a full material LCA is performed [316]. The example also illustrates that comparisons are often made between new and already established alternatives. This can be challenging because the established materials have had time to optimise production processes and lots of data are available, whereas for emerging technologies and new materials there may only be limited data available. The example also illustrates that there can be different types of emissions and resources used for different materials. For a comprehensive assessment it is therefore important that all relevant impact categories are included.
Advances in science and technology to meet challenges
In early stages of materials development, possibilities to influence life cycle impacts through design are still large. On the other hand, only laboratory or pilot scale data may be available, which typically are not representative for the environmental impacts from a full-scale implementation. Methods for upscaling data may therefore be useful, including process simulation tools, stoichiometric relationships, scaling factors, molecular structure models, and use of proxy data [317, 318]. The use of such methods is however still rare and there is a need for further developing and evaluating such methods. It is also known that costs and environmental performance of the production of products and materials typically decrease when the market becomes more mature. Learning curves that describe the performance as a function of cumulative production can therefore be a useful tool for estimating environmental impacts of emerging technologies [319]. Data for background processes, such as energy and transportation systems, are necessary for LCAs and they will change with time. Future scenarios for these processes are therefore important when evaluating emerging technologies. An example of integrating such future background scenarios in LCA is presented in Joyce and Björklund [320]; showing how the widely used LCA database Ecoinvent can be adjusted in a systematic manner to accommodate future scenarios. A special aspect of environmental assessments of new materials is that future applications of the new materials may be largely unknown. This will make it difficult to define the functional unit and also to estimate data for the use phase of the life cycle. Currently used LCIA methods can need further development to be relevant for new materials. It is however also possible to use life cycle thinking approaches to do preliminary assessments in early stages. These can provide actionable environmental information in the research process and in doing so it can help steer technology development towards overall improved environmental performance [321]. Such methods can include semi-quantitative 'red-flag' assessments, highlighting areas of concern, to guide further research as well as streamlined assessments, possibly using upscaling methods as soon as quantitative data becomes available.
Concluding remarks
The threat of climate change and other environmental challenges requires a transformation [322]. New materials and emerging technologies are needed but they must be evaluated from a sustainability perspective. LCAs can be used for comparing alternatives, identifying advantages and disadvantages with different alternatives and therefore guide the further development. It can however normally not conclusively show that one material is environmentally preferably to another [323]. This is because there are usually assumptions and value choices that are necessary which can be challenged. Even if an LCA can show that one material is in some ways preferable, the question whether it is good enough has not been answered. In order to do that an assessment against absolute sustainability criteria has to be done [322].
Acknowledgment
Funding from Formas is gratefully acknowledged.
Towards a material circular economy
28. Critical materials recycling
Gavin D J Harper1,2,3,3, Allan Walton1,2,3,4 and Paul A Anderson1,2,3,4
1 Birmingham Centre for Strategic Elements and Critical Materials, University of Birmingham, Birmingham, United Kingdom
2 The UKRI Interdisciplinary Circular Economy Centre for Technology Metals (Met4Tech), United Kingdom
3 The EPSRC Critical Elements and Materials Network (CREAM), United Kingdom
4 The ReLiB Project, The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot, United Kingdom
Status
Recycling can be considered as both solving a waste-management problem, whilst creating new resource opportunities [324]. There are many positive benefits to recycling. Recycling has potential to aid in reducing GHG emissions, avoid accumulation of waste in the environment, decrease dependence on limited resources, and recover economic value from wastes [325]. An idealised scenario would be a closed-loop circular economy, however, there are physical limitations and practical obstacles to this. With many materials, recycling results in a downgrading of materials properties. Contamination and processes negatively impact intrinsic properties of the material [325].
Before recycling processes, waste must be sorted and segregated. A wide variety of sorting and segregation technologies for pre-treatment of waste prior to recycling exist [326]. Some of these processes rely on the direct sorting of the physical properties of materials e.g. screening, froth flotation, air separation, jigging, cyclonic separation, electrostatic separation, magnetic and magnetic density separation, triboelectric separation and eddy current separation [326], whilst others are smart relying on machine intelligence and automation in combination with sensors for indirect sorting—e.g. optical based, spectral based, NIR, MIR, VIS, x-ray, etc [326]. Different combinations of processes are able to deal with varying degrees of materials diversity [326] in input waste streams. In some cases, recycling efficiency is affected by fundamental physical processes, like the thermodynamics of separation [327]. A significant factor constraining the efficiencies of processes, and the quality of the product of recycling, is the degree to which waste can be pre-sorted. For low value products, this can be costly, and time, labour and energy intensive [325].
In other cases, it is the implementation of technologies—whether at the product stage, in terms of product design and a lack of design for recycling, or the state of the art of recycling technologies as they stand. Recycling is often seen as an end-of-pipe solution [328], whereas in the future, it may make more sense to design products with the end-of-life in mind, with easy recycling considered from the start [328]. Additionally, there are other factors such as social behaviour [327], which constrain the effectiveness of recycling processes.
Current and future challenges
In waste management, final options are often conceived as a hierarchy (shown in figure 37). Recycling is preferred to energy recovery, and this in turn is preferred over disposal, however, reuse is considered more optimal than recycling, and prevention of waste overrides all. However, within recycling, it should be considered that there are a range of different technologies, some of which are preferable to others.
Figure 37. A hierarchy of waste management, with an expanded hierarchy of recycling options. Reprinted by permission from Springer Nature Customer Service Centre GmbH: [Springer Nature] [Nature] [324], (2019).
Download figure:
Standard image High-resolution imageCurrent research challenges for recycling, are manifold—how to increase the recovery rates of current recycling processes, new techniques to recycle materials that cannot currently be recycled, developing better processes for sorting and segregation of feedstocks [324], more selective processes to deal with mixed feedstocks [325] and cleaner, low-energy processes for recycling [325]. Composite products and materials present special challenges, where materials are intimately mixed together [325]. In some cases, clean separation of materials can result in significant cost-savings [328] compared to using material which has been mixed-together through pre-treatment by shredding [328].
Automation is seen as a significant tool in enabling smarter recycling processes [328].
In the case of metals, the end-of-life recovery rate for the most common 'base metals' (iron, copper, zinc, etc) is very high, above 50% in the main [327] although, there are many technology metals for whom recycling rates are very low. Technology metals are used, often in small amounts for specific technology applications—the colour emitting elements of LEDs and displays, high-strength magnets, the materials in battery storage applications, the active materials in electronic devices and thin-film solar panels etc. For these speciality technology critical metals, recovery of the materials for recycling is both economically and technologically challenging and so their recycling rate is often low [327]. Precious metals like gold and platinum group metals are used in many high-technology applications, and because of their high value and ease of separation, there are established processes for recovering them [327, 329].
Some recycling issues that can be solved at the product design stage [328]. Technology products are becoming more complicated over time and employing a wider range of materials [329]—making recycling difficult. This is shown in figure 38.
Figure 38. Elements employed in technologies as complexity progresses over time. Redrawn from [329]. © University of Birmingham, Birmingham Centre for Strategic Elements and EPSRC Critical Materials and Critical Elements and Materials (CrEAM) Network 2021.
Download figure:
Standard image High-resolution imageAdvances in science and technology to meet challenges
The complexity of high technology products, and intimate mixture of many different materials in close proximity means that current approaches to liberating materials are not well suited to the efficient recycling of these more complex products—and successful recovery of critical materials. One area of concern in high tech products, is the use of adhesives and binders as a joining method. This frustrates recycling and disassembly. Development of 'reversible adhesives' [330] or even substituting adhesives with other joining methods could significantly enable disassembly and recovery of active materials [328]. Designing complex technology products for disassembly will facilitate a greater shift towards a circular economy [328].
An example of a material that is used in high-tech products but is difficult to recycle is 'rare earth magnets' [332]. When shredded, in typical waste electrical and electronic equipment (WEEE) shredders, the magnet material sticks to recycling equipment, causing premature failure, and the material oxidises—losing its unique value in the process. More sophisticated post-processing techniques exist for recovering the magnetic material—however, as an example of the challenges that remain, extracting the magnets from products efficiently is an example of the sort of research gap that exists [333].
With growing demand for technology-critical metals around the world, nations are alert to the opportunity that could come from valorising the secondary material contained in end-of-life products [329] however, scientific and technological challenges remain to unlock that value.
The digital industrialisation and use of robotic technologies for sorting and separation also has a significant role to play in recycling [324, 333]. Data can aid advanced recycling processes significantly [333]. Data driven recycling can aid in the disassembly and materials segregation of complex technology products [333]. This is important as in the case of municipal solid waste, it reduces the burden on the final user for sorting and segregation [326] and reduces the need for compliance with good waste management behaviours. Regulation is encouraging the use of data to track critical materials through the supply chain and through all stages of the circular economy. The new EU battery regulations mandate the use of 'digital passports' for batteries, and such an approach could be transferable to other critical materials and technology product [334]. Furthermore, some technologies may be impossible to recycle efficiently economically, without significant automation [324]. Automation also has a role to play in reducing menial and sometimes dangerous labour in the recycling process [324, 333] and improving the system efficiency of recycling processes [333].
Concluding remarks
Closed-loop 'total recycling' is argued to be impossible due to the second law of thermodynamics [335]. There will always be a trade-off between effort and energy expended in order to recover materials from a diffuse state in end-of-life products. Whilst energy-intensive, if it can be sourced from clean renewables, materials can be conserved in a sustainable way that is compatible with environmental goals [335].
Considering the end-of-life at the genesis of new products, will reduce the significant environmental burden of end-of-life products and aid in not only recycling, but also—preferably—remanufacture and reuse; conserving energy, value and resources.
It should be noted that many recycling challenges are not all scientific or technical in nature—many of the problems around collection rates are societal in nature. Development of appropriate legislation to incentivise both recycling and the optimisation of products for end-of-life treatment will also help facilitate this shift [327].
Acknowledgments
The authors would like to thank The Faraday Institution ReLiB Project Grant Numbers FIRG005 and FIRG006, the UKRI Interdisciplinary Circular Economy Centre for Technology Metals (Met4Tech) Grant No. EP/V011855/1 and the EPSRC Critical Elements and Materials Network (CREAM) EP/R020140/1 for providing financial assistance for this research.
Data availability statement
No new data were created or analysed in this study.
Footnotes
- 37
Tensile modulus of ca. 5.4 GPa based on Plastcom SLOVALEN obtained from www.matweb.com.
- 39
- 40
Tim Ingold develops at length how materials, design and thinking processes are intimately bonded in Ingold [287].
- 41
If the concept of evolution can somehow be applied to minerals (see Hazen op cit), we want to believe that co-evolutionary factors should apply to them as well, for example 'aesthetic mate choice', as described by Richard O Prum in his book: Prum [290]. In this particular case, stones' aesthetic features would be the seducing factor towards humans who would in return start reorganizing minerals into architecture.
- 42
Caillois [288].
- 43
MFF research programme was based in Sweden and ran between 2011 and 2019. It created collaborative approaches between design researchers, material scientists, lifecycle assessment experts, business and user behaviour researchers and brands. http://mistrafuturefashion.com/.
- 44
The Trash-2-Cash project (2015–2018), www.trash2cashproject.eu/.
- 45
- 46
Circular design speeds, www.circulardesignspeeds.com/.
- 47
WRAP, https://wrap.org.uk/.
- 48
Institute of Positive Fashion, www.britishfashioncouncil.co.uk/Institute-of-Positive-Fashion.
- 49
Ellen MacArthur Foundation, https://ellenmacarthurfoundation.org/.
- 50
Accelerating Circularity, www.acceleratingcircularity.org/.
- 51
World Circular Textiles Day (WCTD), https://worldcirculartextilesday.com/.