Abstract
Cherenkov radiation is the primary source of unwanted light in a scintillator dosimetry system. In this work we compare two techniques for temporally separating Cherenkov radiation from a slow scintillator signal. These techniques are applicable to a pulsed radiation beam. We found that by analysing the rising edge of the light pulse to identify the fast Cherenkov light only removed 74% of the Cherenkov light. By integrating the tail of the signal where only scintillation light is present a more accurate result is achieved. The average of the results of the two methods provides up to a 90% improvement in the accuracy of the relative dose when compared to ionisation chamber, in certain measurements. This work demonstrates an alternative methodology for the removal of Cherenkov light using signal analysis, while preserving all the scintillation light signal and minimising the bulk of the experimental equipment.
Export citation and abstract BibTeX RIS
This article was updated on 05 July 2021 to add permission lines to the figures.
1. Introduction
In an optical dosimetry system, Cherenkov radiation is generated throughout the components exposed to radiation and is the primary source of unwanted optical signal [1]. Cherenkov radiation is generated when a charged particle travels faster than the local speed of light [2]. In dosimetry for radiotherapy, where the region of interest is usually tissue, primary electrons (i.e. in electron therapy) or secondary electrons (in x-ray therapy) with a kinetic energy greater than 175 keV will generate Cherenkov radiation.
Scintillator dosimetry uses a scintillating material to generate light, which is guided by optical fibre to a photodetector [1, 3]. The signal of interest is the scintillation light (which is generated only in the scintillator) while Cherenkov light (generated not only in the scintillator but also the optical fibre) contaminates the total light signal reaching the photodetector. Scintillator dosimeters are a relatively inexpensive dosimetry tool, easy to fabricate and water equivalent. They have been applied to external beam therapies [4, 5], brachytherapy [6] and prostate cancer therapy [7] so the removal of Cherenkov radiation from the light signal is an important field of investigation to ensure the accuracy of this dosimetry method.
The simplest method for removing this unwanted light is to use a parallel fibre without any scintillator to measure only the Cherenkov signal, and to subtract this from the signal containing both the scintillator and Cherenkov light, leaving only the scintillator signal [1, 3]. This method is simple and accurate, and is considered to be the 'gold standard' in the field. However there are some limitations to the subtraction method. The assumption is that the dose delivered to both fibres is equal, which may not be the case in beam with a high dose gradient. Further, two photodetection devices must be used in parallel, increasing the bulk of experimental equipment and introducing cross-calibration challenges [4].
A number of other methods for the removal of Cherenkov light has been investigated. Using an air-core fibre (rather than plastic) removes all Cherenkov light generated within the fibre [8, 9]. This method allows an array of scintillator dosimeters to be practical [10]. Air-core fibres however attenuate much more than plastic core fibres and are inflexible, limiting possible applications. Filtration of various wavelengths can remove a significant portion of the Cherenkov light, but at the expense of scintillation light as well (due to the wavelength overlap) [11–13]. Spectral analysis has been shown to remove most of the Cherenkov light by measuring narrow wavelength bands and using a mathematical model to determine the scintillation and Cherenkov light contributions [14, 15]. This requires measurement of the scintillation and Cherenkov spectra for the dosimeter. A much larger amount of Cherenkov light is removed using this method than with filtration [16–18].
The difference in timing properties of the Cherenkov and scintillation light allows discrimination in a pulsed beam. Once the beam is off, the Cherenkov stops nearly immediately while the excited scintillation molecules undergoes much slower decay [19]. The authors of this study claim to remove 99.9% of Cherenkov light at the expense of 44% of the scintillation light by using a scintillator with a long decay time (257 ± 6ns). They fired a 450 ns electron beam pulse from a linear accelerator (LINAC) and integrated the detector response over a 5 ns window after 700 ns from the rising edge of the pulse. The pulse duration was chosen to be 450 ns to prevent saturation of the scintillator light output. For longer pulse durations this method is not valid as the tail is no longer proportional to the total dose delivered once the signal saturates. Work has also been done using ruby-based fibre optic dosimeters where a decay time of 3 ms with narrow emission spectra allows a combination of time-delayed gated integration and filtration to bypass the Cherenkov generated in the fibre [20].
In a previous work, to allow for arbitrary pulse durations we used a scintillator with a slow bulk rise time (approximately 1 μs) to discriminate between the fast rising edge of the Cherenkov light signal, and the slow rising edge of the scintillation signal [21]. The integral of the total signal without the Cherenkov component yields the scintillator light contribution. This method assumes that the Cherenkov signal remains constant once it initially peaks (an assumption that relies on the LINAC beam pulse intensity remaining constant across the pulse). However it was found that the Cherenkov signal increases slowly after this in the duration of the pulse. We were able to achieve 74% removal of the Cherenkov signal at the expense of only 1.5% scintillation signal. These single probe Cherenkov techniques have the advantage of being more compact than background subtraction, and do not compromise sensitivity by removing any of the scintillator light signal with filtering. In this work we couple this method with an analysis of the tail of the signal, to give a more accurate dosimetry method.
2. Materials and methods
2.1. Data collection
The scintillator fibre optic dosimeter used in this work is the same as in a previously published work [21]. It used BC-444 plastic scintillator as the radiation conversion material (purchased from Saint-Gobain Crystals). A cylindrical section of scintillator with diameter 2mm and thickness 500 μm was optically coupled to a 10 m length of Eska CK-40 plastic optical fibre (1 mm core diameter). The scintillator was coated in BC-620 reflective paint to improve the light capture of the probe. A diagram of the probe is shown in figure 1. The geometry of scintillation light that can undergo total internal reflection within the fibre gives a sensitive volume of 0.393 mm3. When aligned with the fibre axis perpendicular to the beam (edge-on mode) a one-dimensional spatial resolution of 500 μm can be achieved laterally across the beam. Aligning the fibre axis parallel to the beam gives a resolution of 1 mm. For the data presented in this work the probe was scanned with the fibre axis and scanning direction parallel, and so the effective spatial resolution was 1 mm.
Figure 1. Diagram of the scintillator fibre optic dosimeter probe (not to scale). [22] John Wiley & Sons. © International Union of Crystallography.
Download figure:
Standard image High-resolution imageA Varian LA1-EX LINAC treatment beam was used to test the dosimetry method. The LINAC was operated in 6 MV mode, 600 MU/min, 10 × 10 cm2 field size. The source to surface distance was kept at 100 cm. A lateral scan of the beam was done at 15 mm depth in a water tank, and a depth dose scan was done from surface to 200 mm depth. The beam pulses for 3.5 μs with a 2.7 ms peak-to-peak pulse separation. The LINAC also has a trigger pulse allowing synchronisation of acquisition over multiple pulses.
Photodetection was performed with a RCA-4526 photomultiplier tube (PMT) with negative bias and readout with a digital oscilloscope. The oscilloscope sampled at 1 GHz for 10 μs (to cover an entire beam pulse and background) and collected an average of 128 pulses at each position (giving a minimum acquire time of 350 ms per position) to reduce noise in the signal. A single averaged waveform was saved for each measurement position. This was repeated three times per position to assess the repeatability of the measurement and analysis. The same data set used in a previous work [21] is analysed here to compare the analysis methodology.
A Scanditronix/Wellhofer Compact Chamber CC13 was used to measure the beam profile and depth dose responses and compare to the scintillator dosimeter. It has a sensitive volume of 130 mm3 (approximately 300 times larger than the scintillator dosimeter) and a one-dimensional spatial resolution of 3 mm.
2.2. Analysis methods
In the previous work we analysed the rising edge of the scintillator signal and temporally separated the Cherenkov signal from the scintillator signal [21]. The main limitation of this work was the varying beam intensity during the pulse, which reduced the accuracy of determining the Cherenkov contribution. For this reason we investigated how using the tail of the scintillation signal (once the beam pulse has ended) can improve accuracy. We used the same data set to analyse the tail of the net light signal and compare to the results acquired with the rising edge method. Figure 2 shows an example of a scintillator and Cherenkov signal pair. Note that at after 6500 ns, when the beam has stopped, the Cherenkov signal drops off very quickly but the scintillator signal decays much more slowly. The difference in the signal here is purely scintillator signal and so integrating the light output in a window in this region will give an accurate relative dose reading.
Figure 2. An example pulse signal measuring the net light output (blue), the Cherenkov output (red), mesaured with a background reference probe, and the (calculated) scintillator only signal (yellow).
Download figure:
Standard image High-resolution imageThe moment in time when the Cherenkov signal was determined to vanish was found using the derivative of the signal, and finding the maximum (which corresponds to the inverted signal rising the fastest, seen at approximately 6500 ns in figure 2) and using this as a reference point. The signal was integrated from this point onwards, relative to the background after both signals vanish (using the average of the last 100 points of the dataset). The data used to calculate the derivative was smoothed using a moving average with a window size of 21 points. This reduced the noise in the signal enough to reliably determine the point where the signal was changing the fastest.
The light output power (in units of energy per time) in the tail is given for t > T by [21]:
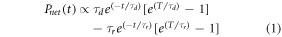
Where T is the beam pulse duration. The rise time τr and decay time τd of BC-444 is 19.5 ns and 285 ns respectively. Integrating the tail gives:
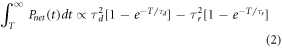
We can see from equation (2) that the tail integral is a function of the beam cut-off time T. However this is only significant for beam pulse durations that are close to the rise and decay times (the closer to the decay time the more significant) and for a beam duration of 1.314 μs the difference between the tail integral and the limiting integral (as  the integral approaches
the integral approaches  ) are within 1% of each other. As such for typical beam durations (in the multiple microsecond range) the tail integral is approximately independent of this duration, and only depends on the scintillator timing properties and the beam intensity. By measuring the instantaneous voltage across the PMT we can measure the intensity of light emitted, as the PMT voltage is proportional to the light power intensity falling on the photocathode.
) are within 1% of each other. As such for typical beam durations (in the multiple microsecond range) the tail integral is approximately independent of this duration, and only depends on the scintillator timing properties and the beam intensity. By measuring the instantaneous voltage across the PMT we can measure the intensity of light emitted, as the PMT voltage is proportional to the light power intensity falling on the photocathode.
To combine both the methods used on this dataset, the average of each result at each position or depth were averaged with each other to give a third method for measuring the relative dose.
To measure how well the scintillator results matched the ionisation chamber (IC) results the average sum of the the relative squared difference percentages (S) was used on data points:
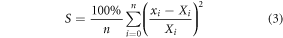
where there are n data points, xi is the scintillator data point, and Xi is the corresponding IC data point.
Four measurements were taken at each position to assess the repeatability of the method and quantify the uncertainty. Uncertainties in the measurements at each position or depth were calculated using the 95% confidence interval (2 × standard deviation) of the group of repeated measurements. The standard deviation s was calculated using:
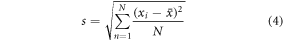
Here xi
is each relative dose calculation at the position, and  is the average of these values. Uncertainties in the results for the average of the rising edge and tail signals were calculated by combining uncertainties in quadrature:
is the average of these values. Uncertainties in the results for the average of the rising edge and tail signals were calculated by combining uncertainties in quadrature:
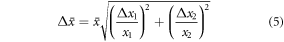
These uncertainty values are shown in figures 3 and 4. The repeatability of the ionisation chamber data is within 0.5% inside the beam, and up to 2% outside the beam.
Figure 3. Beam profile result for the three analysis methods, compared with ionisation chamber.
Download figure:
Standard image High-resolution image3. Results and discussion
The combined set of results for the beam profile is presented in figure 3 and the depth dose in figure 4. The rising edge results overestimate the dose, and the tail results underestimate the dose. The average of these lies very close to the ionisation chamber results. To quantify this, the S values for the two experiments and three analysis methods are presented in table 1. The beam profile results improved by 87% and the depth dose by 90% when we used the average. It should be noted that the comparison of the depth dose was only done for depths 15 mm and greater where ionisation chamber data was collected. The largest variations in the scintillator signal was less than 15 mm depth and is not reflected in the results in table 1.
Figure 4. Depth dose results for the three analysis methods. The entire dataset is shown in (a), while the surface dose is shown in (b).
Download figure:
Standard image High-resolution imageTable 1. The S values (calculated with equation (3)) for the different data sets presented.
| Measurement | Rising edge (%) | Tail (%) | Average (%) |
|---|---|---|---|
| Beam profile | 17.1 | 16.9 | 2.20 |
| Depth dose | 0.506 | 0.914 | 0.050 |
Table 2 quantifies the uncertainties in the scintillator results in the beam profiles and depth dose. The uncertainties are averaged over the different regions of interest (the centre, penumbra and outside the beam for the profiles, and depths separated by 20 mm depth). The tail method has lower variations between individual measurements than the rising edge method. In all cases in the depth dose response, at higher depths the results are more consistent.
Table 2. The uncertainties in the results, as a percent of the normalised response maximum. The rising edge and tail values were calculated with equation (5) and combined together for the average with equation (5). The centre of the beam is just the single uncertainty of that point. The uncertainties in the three points in each side of the penumbra (6 total) and four outside the field 8 total) are averaged and shown. The depth dose uncertainties are separated by 20 mm depth.
| Average uncertainty | Rising edge (%) | Tail (%) | Average (%) |
|---|---|---|---|
| Centre | 11.3 | 4.02 | 11.95 |
| Penumbra | 5.21 | 2.43 | 5.90 |
| Outside | 2.83 | 1.57 | 3.31 |
Depth dose ( 20 mm) 20 mm) | 7.64 | 4.61 | 9.32 |
| Depth dose (> 20 mm) | 4.37 | 3.55 | 5.75 |
Shown in figure 5 is the difference between the scintillator results and the IC results. There is a consistent over-response at ±4.8 cm and under-response at ±5.2 cm. This can be explained as the effect of using two different spatial resolution dosimeters. In the lateral direction, with both dosimeters mounted in edge-on mode, the scintillator dosimeter has a resolution of 1 mm, while the IC has 3 mm. This will cause a blurring of the penumbra which will be larger as the dosimeter size gets larger. Hence, the scintillator will appear to under-respond on the outer edge of the penumbra, and over-respond on the inner edge, when compared to the IC data. This effect results in the sharp rises and drops in the comparative response, as seen in figure 5(a). There appears to be a slight lack of symmetry about the centre of the field in figure 5(a). This is most likely the effect of a small difference between the position of data sampling and the physical centre of the radiation field, creating a lateral offset.
Figure 5. The difference between the normalised results and ionisation chamber. (a) shows the beam profile results and (b) shows the depth dose. The x axis are not to scale in order to more clearly present the figures. For legend, refer to figures 3 or 4.
Download figure:
Standard image High-resolution imageFigure 5(b) shows that the differences between the scintillator results and IC results is quite small. The main differences is the over-response of the rising edge data and under-response of the tail data, consistent with the results in figure 5(a). The average of the two agrees with the IC data at all depths greater than 15 mm, although the error bars are quite large. The large error bars in all the data is primarily sources from the noise in the individual waveforms Despite averaging over many beam pulses, the noise is still large, especially in regions with low dose. There are several ways to reduce the noise, each with limitation. The scintillator sensitive volume can be increased, with signal increasing in proportion. This will however reduce the spatial resolution of the probe. By decreasing fibre length, the attenuation of light can be reduced, however the 10 m of fibre currently used is the minimum possible to reach from the beam treatment area to outside the radiation bunker. We are, however, investigating performing the data measurement inside the bunker, swapping the need to transport light to transporting a digital signal, which is much easier. Limitations of this are primarily radiation damage or interference with the electronics used. Finally, the simplest way to improve the signal to noise ratio is to average over more beam pulses. This is simple to implement however can result in extended experimental times.
As was discussed in detail in the previous work, the rising edge method only removes approximately 74% of the Cherenkov light in the signal, and so the total dose response is overestimated [21]. There appears to be a small increase in the PMT baseline post-pulse after the signal tail. The average of this baseline was subtracted from the tail values pre-integration to account for this. However if the higher response is due to low intensity long-decay scintillator signal, then by removing it we are slightly underestimating the total scintillator light output. This could explain the slight under-response we are seeing in the tail results in figure 5.
The uncertainties in the tail method are much smaller than the rising edge method, which indicates that the tail signal is much more consistent than the rest of the light pulse. The relatively large uncertainty in the averaged profile and depth dose are mainly due to the high uncertainty in the rising edge data. An advantage of combining both sets of results together is that it can be applied to beam pulses of different durations to get accurate relative dosimetry comparisons. Were just the tail method to be used, there would be no way to discern between pulses of different duration. However, by including the rising edge analysis, while less accurate, the relevant timing information can be found. From this the relative dose from the tail method can be scaled to allow different dose rates and beam pulses to be compared to each other.
4. Conclusions
Using a combination of the rising edge analysis and tail analysis has shown a significant improvement in the accuracy of the relative dose measurement. While the tail analysis has a similar deviation from the IC as the rising edge method, the average of the two improve the accuracy by up to 90% in the depth dose response. The variations between separate measurements of the same data is much smaller when using the tail method than the rising edge, indicating a greater reliability.
This work demonstrates that Cherenkov light can be separated from scintillator light using algorithmic means. The advantages of this is that the signal strength is not compromised by filtration (allowing smaller sensitive volumes to be practically implemented) as well as reducing the bulk of the equipment used by using a single probe and photodetector.
Acknowledgments
This project was supported by UOW's Global Challenges Program. This research has been conducted with the support of the Australian Government Research Training Program Scholarship.






