Abstract
Bioactive glasses are able to chemically bond to hard and soft tissues and have been proposed and used for tissue regeneration in several dentistry and medical applications. However, the majority of bioactive glass compositions do not support prolonged or repeated heat treatments, since these procedures often result in uncontrolled crystallization, which usually degrade their mechanical properties and, in most instances, substantially diminish their bioactivity. Therefore, the manufacturing of 3D devices, fibers or scaffolds, which aim to expand the usage of these materials, is a challenging task. To overcome this phenomenon, a new bioactive glass composition was recently developed at the Vitreous Materials Laboratory (LaMaV—UFSCar, Brazil) and licensed to the start-up company VETRA. This new bioactive glass composition shows high stability against crystallization coupled with high bioactivity, which allows the development of bioactive fibers, meshes and other complex 3D shapes. In addition, this bioactive glass has an elevated bioactivity, is bioresorbable and flexible (in fiber form), which makes this glass a potential alternative for soft and hard tissue regeneration. In this article, we discuss this recent development and summarize the latest advances in testing the effectiveness of this new material in in vitro and in vivo tests. To date, the results indicate that this new glass composition presents a larger workability window, which allows the development of numerous medical devices. This feature combined with the high bioactivity of this new glass delivers a promising broad spectrum of applications as a material for tissue engineering.
Export citation and abstract BibTeX RIS
1. Biomaterials
During the 1960s, the first generation of implantable biomaterials was developed for the replacement of damaged or missing tissue in the human body. These biomaterials had suitable physical properties and produced a minimal toxic response in the host [1]. The most desired property of the 'first generation' material was 'inertness' [1]. However, the significant incidence of implant rejections and implant deterioration caused by the formation of a non-adherent fibrous capsule between the material and host tissue were the driving forces for the development and optimization of biomaterial compositions and surface characteristics, since biocompatibility and/or tissue tolerance were no longer sufficient [1, 2]. Thus, in the early 1970s, bioceramics began to be used in certain implant applications and the concept of bioactivity was firstly defined as [sic] 'A bioactive material is one that elicits a specific biological response at the interface of the material which results in the formation of a bond between the tissues and the material' [2, 3]. In this context, the 'second generation' of biomaterials was developed, including new compositions of ceramics, glasses and glass-ceramics [1, 2, 4].
Currently, there is growing interest in the development of 'third-generation' biomaterials, which promote specific cellular responses at the molecular level. In this class of materials, the previously separated properties of bioactivity and bioresorbability converge and seek a better clinical response [1].
1.1. Bioactive glasses
Bioactive glasses were first developed by the late Prof Larry L Hench and collaborators in 1969, and the most bioactive composition, termed Bioglass 45S5, consists of 46.1% SiO2, 24.4% Na2O, 26.9% CaO and 2.6% P2O5 (mol %). This was the first glass to demonstrate the ability to strongly and rapidly bond to bone tissue [4, 5].
Currently, several compositions of bioactive glasses are available, including: silicate, phosphate and borate based glasses [5, 6]. Their physicochemical properties can be tailored to match a target application with the incorporation of several elements, such as Mg, F, K, Ag, Cu, Sr, Zn, etc [6, 7]. Several typical silicate based bioactive glass compositions are presented in table 1.
Table 1. Common silicate based, melt derived bioactive glass compositions (wt.-%) [9, 11].
| Glass Composition | SiO2 | Na2O | CaO | P2O5 | MgO | K2O | B2O3 | CaF2 |
|---|---|---|---|---|---|---|---|---|
| 45S5 | 45.0 | 24.5 | 24.5 | 6.0 | 0 | 0 | 0 | 0 |
| 13-93 | 53.0 | 6 | 20 | 4.0 | 5.0 | 12.0 | 0 | 0 |
| S53P4 | 53.0 | 23 | 20 | 4.0 | 0 | 0 | 0 | 0 |
| 1-98 | 53.0 | 6.0 | 22.0 | 2.0 | 5.0 | 11.0 | 1.0 | 0 |
| 13-93B | 0 | 5.5 | 18.5 | 3.7 | 4.6 | 11.1 | 56.6 | 0 |
| 58S | 58.2 | 0 | 32.6 | 9.2 | 0 | 0 | 0 | 0 |
| 45S5F | 45.0 | 24.5 | 12.25 | 6.0 | 0 | 0 | 0 | 12.25 |
Numerous investigations demonstrated the osteoconductive and osteoinductive nature of bioactive glasses [4, 5, 8] and their classical applications, which involve bone grafting in orthopaedics and dental procedures and small bone implants [4, 5, 8]. Nonetheless, recent successful outcomes refer to the application of bioactive glasses in soft tissue regeneration [9, 10].
1.1.1. Mechanisms of action
Several authors have reported that bioactive glasses, especially Bioglass 45S5, can rapidly bond to bone, but these types of bioactive materials can also stimulate bone growth away from the bone–implant interface [5, 8].The mechanism for bone bonding is mainly attributed to the formation of a hydroxycarbonate apatite (HCA) layer on the surface of the bioactive glass after its implantation, due to glass dissolution in the aqueous medium [3, 5, 8]. HCA is similar to bone mineral phase and its formation promotes bone bonding, since it can interact with collagen fibrils and later integrate with the host bone tissue [4, 5].
These leaching reactions, which occur with bioactive glasses in contact with water, culminate in the formation of an HCA layer and are well established in the literature as a five-stage reaction sequence [5, 8]. Briefly, in stage I, alkali and alkali earth ions are released from the glass into the fluid and are replaced by H+ or H3O+ ions in the glass structure. This reaction increases the local pH, resulting in the rupture of Si–O–Si bonds. Then, in stage II, silicon is released into the fluid in the form of silanol groups (Si(OH)4). In stage III, the silanols condense, forming a polymerized silica gel layer on the surface of the glass. Subsequently, in stage IV, calcium and phosphate ions that had diffused from the glass or from the medium form an amorphous calcium phosphate layer over the silica gel. Following these reactions, in stage V, the amorphous calcium phosphate layer incorporates the carbonate species and crystallizes into HCA [8].
Recently, several reports have shown that the key phenomenon in bone bonding for highly bioactive glasses (Class A biomaterials) is their ability to release ionic dissolution products or biologically active ions at controlled rates, particularly in critical concentrations that can stimulate cell proliferation and differentiation [4, 12–14]. Molecular biology studies have shown that within a few hours of exposure to Bioglass 45S5 extracts, several families of genes were activated in human primary osteoblasts, which include the genes that encode nuclear transcription factors and potent growth factors [4, 12–15]. These findings indicate that Class A bioactive glasses enhance new bone formation (osteogenesis) by direct control over the expression of genes that regulate cell proliferation [4, 12–15].
1.1.2. Clinical use of bioactive glasses
Certain bioactive glasses and bioactive glass-ceramics compositions have been used in clinical practice in powder form for over 20 years [4, 16]. Bioglass 45S5 has been used in more than one million patients as a bone grafting material on orthopaedic and dental applications [5, 16].
Oral applications of bioactive glasses have been the most clinically relevant up to now. NovaMin® (Glaxo Smith Kline, UK) is a Bioglass 45S5 fine powder incorporated toothpaste that can mineralize exposed dentin microtubules, thereby reducing or ceasing dental hypersensitivity [5, 16].
According to Hench L L [4], the first particulate bioglass, NovaBone®- PerioGlas®, was indicated for oral and periodontal bone defects and cleared for sale in the US In 1993, the original indication for this product was to restore bone loss resulting from periodontal disease in infra-bony defects. Later, in 1996, additional indications were cleared by the FDA, including tooth extraction site and alveolar ridge augmentation procedures [4].
Because of the success of bioglass in the US market, this material was also introduced into the European market in 1999. The product was indicated and approved for general non-load bearing orthopaedic bone grafting applications [4, 5, 16].
The latest bioactive glass devices on the market, Bigran® (Biomet 3i) and BonAlive®, are available as granules and plates. Both are manufactured from S53P4 bioactive glass by Vivoxid Ltd (Turku, Finland) [4].
While bioactive glass sales currently extend to numerous countries, its distribution is still limited to particulate and bulk form, mainly because of the narrow processing range or low workability of these types of glasses. Section 1.1.3 describes this relation in more detail.
1.1.3. Workability
Any liquid can, in principle, be transformed into a glass if the applied cooling rate is sufficiently fast to avert crystallization [17, 18]. However, the ability or easiness of a substance to vitrify (to form a glass) depends on its chemical composition. Several oxides, such as SiO2, B2O3, GeO2 and P2O5, are known to be good 'glass-formers'. Whereas, oxides such as sodium, calcium and potassium, create smaller structural units in the vitreous matrix thus making glass formation more challenging.
Compared to traditional glass compositions bioactive glasses have higher amounts of network modifiers and considerably lower amounts of network formers. As presented in table 1, the majority of bioactive glasses are made of sodium oxide, calcium oxide, phosphorous pentoxide and have considerably less silicon dioxide (45–53%) than most commercial (non-bioactive) silicate glasses (70–80%). The reduced silicon dioxide content significantly decreases the glass stability against crystallization and facilitates devitrification during working processes at temperatures ranging between the glass transformation range and the liquidus [11].
Thus, manufacturing any complex shapes, such as 3D scaffolds or fibers, with these types of glasses is a challenging task, since the energy provided from the heating during working processes is enough to induce the breaking of the glass network bonds facilitating atomic arrangement into crystalline structures [11].
Therefore, there is currently a growing effort to improve the workability of bioactive glasses by diminishing their crystallization tendency. With a larger working range, bioactive glasses could significantly enlarge their spectrum of application. If bioactive glass fiber meshes, spherical granules and/or coated metal prostheses were available, these biomaterials could easily satisfy the requirements of various clinical applications.
However, increasing workability frequently leads to diminishing glass bioactivity since they are antagonistic properties [11, 17, 19]. Several research groups have investigated the compositional dependence of glass bioactivity. For instance, Brink et al conducted an extensive study by analyzing up to 40 different bioactive glass compositions relating their crystallization tendency, viscosity and biological activity [19, 20]. Some compositions presented a fair balance between workability and bioactivity, which enabled the production of fibers. Their studies have verified that the forming properties of these bioactive glasses were improved when the content of silica was increased from 45% to 53–56 (mol %) [17, 19, 20]. Nonetheless, without completely inhibiting the bioactivity of these glasses, this amount of silica considerably diminished their reactivity compared to Bioglass 45S5.
Therefore, highly reactive or 'Class A' bioactive glasses tend to easily devitrify (crystallize) and their working range is typically narrow [11, 19, 21]. Additionally, their high 'fragility' (extremely high temperature versus viscosity dependence) hinders their manufacturability using conventional methods since the curve described in their viscosity-temperature dependence is too steep for most glass processing machines [21].
The viscosity-temperature dependence is one of the most important factors associated with manufacturing and forming of glass. This dependence is crucial for determining which forming operations can be used for producing different glass articles. Usually, fiber drawing or other manufacturing processes are performed with 'strong' melts, that is, a glass that shows moderate changes in viscosity as a function of temperature [11, 22]. However, bioactive glasses commonly have low silica content and form fragile melts, thus showing a marked increase in viscosity with decreasing temperature [11, 23]. Such behavior renders bioactive glass fiber production infeasible when using the simplest and most used process methods, the down drawing.
1.1.3.1. Bioactive fiber drawing
During fiber manufacturing, for any selected technique, a carefully controlled isothermal condition should be maintained [11]. Fiber drawing is usually performed at viscosity values between 102 and 104 dPa · s and this viscosity will occur at different temperatures depending on the glass composition [22].
According to Wallenberger et al [22], the temperature selected during fiber drawing should be preferentially above the liquidus temperature to provide a comfortable margin for fiber spinning or drawing while avoiding the formation of crystals during processing. To minimize the risk of devitrification during fiber pulling operations, the optimum drawing temperature must be approximately 40–100 °C above the liquidus temperature. However, for the great majority of bioactive glass compositions, the optimum fiber drawing viscosity is at temperatures below the liquidus, which leads to unavoidable crystallization. This hinders the manufacturing of bioactive glass fibers via simple fiber drawing processes, such as down drawing from the melt [24]. This phenomenon and the fact that bioactive glasses tend to be fragile melts (due to their low content of glass-forming oxides) demand more complex fiber manufacturing processes, such as updrawing from supercooled melts or the rapid quenching of fibers [22].
The advantages linked to fiber handling and multiple anticipated applications for this type of biomaterial have encouraged many research groups to study the improvement of bioactive glasses workability to obtain reactive glass fibers. To date, the attainment of hand-drawn [25–27], melt-spun [28–31], drawn from preforms [32] and electrospun [33–35] bioactive glass fibers have been reported. Some studies have incorporated chopped bioactive glass fibers into polymer matrix aiming the improvement of their mechanical and biological properties [32, 36–38]. Bioactive glass fibers have been manufactured not only from silicate based bioactive glasses but also from phosphate-based glasses [32, 39, 40] and by using the sol-gel method [41, 42]. However, with all the above mentioned approaches, the continuous fibers manufacturing with a tight diameter control for long periods of time is still not possible. Typically, small fiber bundles or fiber entanglements are obtained, as can be observed in figure 1 which presents fibers manufactured by Brink et al [43] using the bioactive glass 20–92. This happens because glass crystallization during fiber drawing results in the filament breakage, which prevents the manufacturing of continuous fibers [44]. In order to improve the processing of bioactive glasses, targeting the development of compositions that can be heated, sintered or drawn into fibers without crystallization, new formulations need to be investigated and their temperature and biological behavior characterized.
Figure 1. Bioactive glass fibers from 20 to 92 glass manufactured by Brink et al [43]. (Reproduced with permission of Elsevier.)
Download figure:
Standard image High-resolution imageAs the technical problems related to bioactive glass fibers manufacturing are being solved, the possibilities for the creation of new medical products with bioactive fibers are numerous. Bioactive glass fibers have been successfully used for bone grafting [34, 43] and regeneration of soft tissues, such as in chronic skin wounds and nerve repair [25, 45].
2. Development of the novel bioactive glass composition
To meet a better balance between seemingly incompatible properties, such as glass stability and bioactivity, researchers at the Vitreous Materials Laboratory (LaMaV—Federal University of São Carlos—Brazil) have developed a new highly reactive glass composition (NBG).
This newly developed glass composition belongs to the system SiO2–Na2O–K2O–CaO–MgO–P2O5 (and other minor additives) and presents a larger workability range while maintaining a high bioactivity.
Using this glass composition, it was possible to design various types of devices aiming to improve biointeraction and also the handling of the biomaterial during their implantation. Figure 2 presents several devices that were developed using the NBG fibers, such as highly porous scaffolds, nerve guide conduits and flexible meshes for tissue engineering applications.
Figure 2. Several devices developed from the NBG fibers. (A) and (B) Bioactive glass meshes (credit: Clever R Chinaglia); (C) highly porous scaffolds [47] (reproduced with permission of Springer) and (D) a double-layered nerve guide conduit [48]. (Reproduced with permission of Wiley.)
Download figure:
Standard image High-resolution imageThese devices were evaluated regarding their capability to stimulate cell proliferation and tissue regeneration of hard and soft tissues [46, 47].
Sections 2.1 and 2.2 summarize the in vitro and in vivo tests conducted with the NBG in bulk and fiber forms.
2.1. In vitro studies
2.1.1. Acellular tests
The in vitro tests were performed with the simulated body fluid (SBF) solution, whose composition is shown in table 2, according to the method proposed by Kokubo et al [49, 50] using the NBG bulk and fiber samples. These tests aim to verify the rate of HCA layer formation and the in vitro bioactivity of glass compositions. Fourier transform infrared spectroscopy (FTIR) analysis, x-ray diffraction (XRD) and scanning electron microscopy (SEM) results showed that the NBG is highly reactive and bioactive.
Table 2. Ion concentration (mM) in SBF-K9 and human blood plasma [49].
| Ion | Na+ | K+ | Mg2+ | Ca2+ | Cl− | 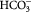 |
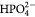 |
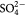 |
pH |
|---|---|---|---|---|---|---|---|---|---|
| SBF-K9 | 142.0 | 5.0 | 1.5 | 2.5 | 147.8 | 4.2 | 1.0 | 0.5 | 7.25 |
| Human Blood Plasma | 142.0 | 5.0 | 1.5 | 2.5 | 103.0 | 27.0 | 1.0 | 0.5 | 7.3 |
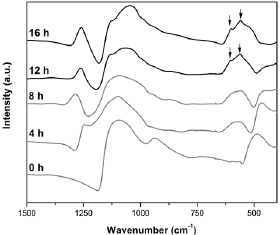
Infrared spectroscopy analysis showed HCA layer formation in only 12 h for the bulk samples (figure 3) and 4 h for the glass fiber samples (figure 4). These results were determined by the presence of the two main peaks related to HCA formation at approximately 610 and 560 cm−1 in FTIR spectra (indicated by black arrows in figures 3 and 4). In addition, the appearance of the shoulder at 959 cm−1 may be attributed to C–O vibration modes in  and to P–O–P bonding and the weak peaks around 860 cm−1 and 1400–1515 cm−1 show the carbonate bands evidencing the formation of crystalline HCA [8]. According to the literature and our own tests using the same SBF solution, the most bioactive glass known so far is Bioglass 45S5, which takes 6–8 h to form the HCA layer [8, 51]. The results for the NBG indicate that it is a highly bioactive glass, since the stages linked to HCA formation occur within a few hours. The high reactivity of the fibers followed the same trend as the in vitro bioactivity of glass monolithic samples (figure 4), forming the HCA layer faster (4 h) due to a higher surface area. This trend was also reported by Arstila et al [52] for other bioactive glasses. In their studies, the glasses that were classified as fast reacting in bulk form, were also found to react rapidly as fibers [52].
and to P–O–P bonding and the weak peaks around 860 cm−1 and 1400–1515 cm−1 show the carbonate bands evidencing the formation of crystalline HCA [8]. According to the literature and our own tests using the same SBF solution, the most bioactive glass known so far is Bioglass 45S5, which takes 6–8 h to form the HCA layer [8, 51]. The results for the NBG indicate that it is a highly bioactive glass, since the stages linked to HCA formation occur within a few hours. The high reactivity of the fibers followed the same trend as the in vitro bioactivity of glass monolithic samples (figure 4), forming the HCA layer faster (4 h) due to a higher surface area. This trend was also reported by Arstila et al [52] for other bioactive glasses. In their studies, the glasses that were classified as fast reacting in bulk form, were also found to react rapidly as fibers [52].
Figure 3. Infrared spectroscopy (FTIR) for the NBG bulk samples after SBF soaking from 0 to 16 h (main HCA peaks are depicted by black arrows).
Download figure:
Standard image High-resolution imageFigure 4. Infrared spectroscopy (FTIR) for the NBG fiber samples (with 25 µm diameter) after soaking in SBF solution from 4 to 16 h (main HCA peaks are depicted by black arrows).
Download figure:
Standard image High-resolution imageAs observed in figure 5, distinct reaction layers were detected based on the SEM images of the cross-section of the NBG fiber after 4 h of immersion in the SBF solution. This type of behavior was also reported for hand-drawn fibers of Bioglass 45S5 and S53P4, compositions which are known to show a high degree of bioactivity for both in vivo and in vitro analysis [52]. The formation of a silica-rich layer and a calcium-phosphate-rich layer were related by Arstila et al to fast reacting glasses that could be successfully used in biomedical applications [52]. As soaking time increased, the HCA layer formed on the NBG fiber surface thickened and its known globular structure became even more evident (figure 5).
Figure 5. SEM image of a cross-section of the NBG fiber showing the formation of reaction layers and the HCA layer after 4 h soaked in SBF (A) and the NBG fiber soaked for 24 h in the same solution (B).
Download figure:
Standard image High-resolution image2.1.2. Cellular tests
Gabbai et al [46] conducted cell viability and genotoxicity tests with fibroblast and osteoblast cell lines (L929 and OSTEO-1 respectively) to explore the NBG fibers biocompatibility in vitro.
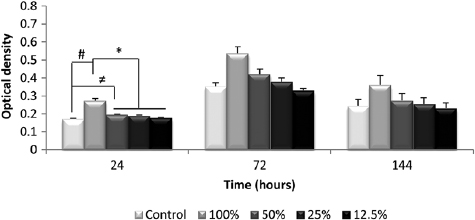
The MTT assays (viability tests) were conducted using extract solutions obtained from incubating the NBG fibers in supplemented DMEM at 37 °C for 7 d. Extracts with concentrations ranging from 12.5 to 100% were used and the cells were incubated for 24, 72 and 144 h. The results showed a significant increase in cell proliferation rate for both cell lines after exposure to the NBG extracts, particularly at 50 and 100% concentrations. Figure 6 presents fibroblast proliferation after the selected experimental periods for all concentrations of the NBG fiber extracts. After only 24 h, the samples in contact with the '100% extract' showed a significant increase in cell quantification [46].
Figure 6. Proliferation of the fibroblast cell line in solutions containing various concentrations of the NBG fibers extracts (100%, 50%, 25% and 12.5%) at different culture times (24, 72 and 144 h); #p ⩽ 0.05 versus CG; ≠p ⩽ 0.05 versus CG; *p ⩽ 0.05 versus 50%, 25% and 12.5% [46]. (Reproduced with permission of Wiley.)
Download figure:
Standard image High-resolution imageIn this study, Gabbai et al also conducted genotoxicity studies to evaluate the potential damage to the fibroblastic and osteoblastic DNA, which were both exposed to the extracts obtained from the NBG fibers. The NBG potential genotoxicity was evaluated through an electrophoresis test in a single-cell gel, i.e. a comet assay. The results indicated no significant differences (p > 0.05) between groups (including the control group), which demonstrated that the NBG fibers did not induce DNA strand breaks in all cell lines for any evaluated period [46].
2.2. In vivo studies
2.2.1. Biocompatibility
In vivo biocompatibility of the NBG fibers in scaffold form was analyzed via subcutaneous implantation [46]. In this study, 30 male Wistar rats underwent surgery, and after 15, 30 and 60 d post implantation, both qualitative and quantitative histopathological analyses were conducted.
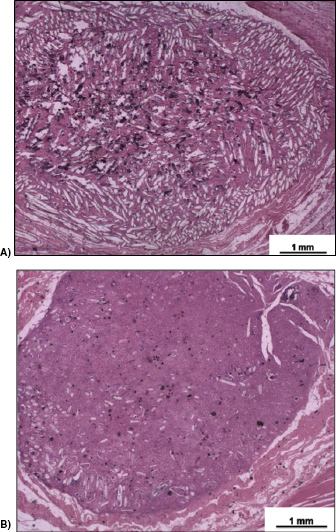
The results showed that the NBG fibers had a favorable effect on soft tissue responses in terms of capsule thickness, capsule quality and interface quality. Histopathological analysis (figure 7) showed that the implantation of the fibrous glass scaffold produced almost no body reaction and after the final experimental period of 60 d the material degraded and was almost completely bioabsorped.
Figure 7. Representative histological subcutaneous implants at two experimental periods: 15 (A) and 60 (B) d; magnification = ×12.5. Haematoxylin–eosin staining. (Adapted from [46].) (Reproduced with permission of Wiley.)
Download figure:
Standard image High-resolution image2.2.2. Bone regeneration
Gabbai et al also investigated the capability of the NBG fibrous scaffolds in regenerating rat bone tibial defects [47]. In this study 60 male Wistar rats were divided into two groups: the control group, in which the 3 mm bone defects received no filler, and the biomaterial group (BG), in which the bone defects were filled with the NBG fibrous scaffolds. After 15, 30 and 60 d post-surgery histopathological, morphometry, immunohistochemistry analysis and mechanical tests were performed.
The results indicated that the biomaterial stimulated bone repair due to the NBG scaffold bioactive properties and also improved the mechanical properties of the tibial callus at day 15 after surgery [47].
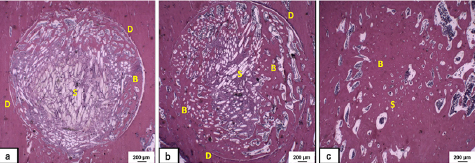
Histological analysis revealed that the degradation of the bioactive glass fibers increased with the implantation time and were concurrently substituted by granulation tissue and newly formed bone (figure 8). According to the literature, this is a key factor for bone regeneration, since resorption of the biomaterial and liberation of space are necessary steps for new tissue ingrowth [47, 53].
Figure 8. Representative histological sections of tibial defects treated with the NBG fiber scaffolds at 3 experimental periods: 15 d (a), 30 d (b), and 60 d (c). Fibers of the porous scaffold (S), bone formation (B), and defect line (D) are indicated in the sections. Bar represents 200 µm. Haematoxylin–eosin staining. Magnification = ×12.5 [47]. (Reproduced with permission of Springer.)
Download figure:
Standard image High-resolution imageThe immunohistochemical analysis indicated that the novel biomaterial enhanced the expression of RUNX-2 and RANK-L immunofactors [47]. RUNX-2 is mainly expressed in osteoblasts and it is required for the differentiation of mesenchymal progenitors towards osteoblast cell lineages [54]. In addition, it is well known that RUNX-2 is fundamental for the upregulation of other osteoblastic markers, such as osteocalcin, osteopontin and alkaline phosphatase [47], which also may have influenced bone formation and deposition. Moreover, RANK-L is known as a key factor for the differentiation and activation of osteoclasts activating bone resorption and remodeling, which are relevant steps for a successful bone healing process [47, 55].
2.2.3. Skin wound regeneration
To evaluate the potential of the NBG for soft tissue regeneration a pilot study was conducted. NBG fibers meshes were implanted in third degree burn wounds in male Wistar rats (n = 5). As soon as the burn wound was created, a 3 × 6 cm bioactive fiber mesh (similar as presented in figure 2(B)) was applied to the lesion. For this study the control group did not receive any treatment. After 7 d post-surgery, clinical, histopathological and immunohistochemistry analyses were performed.
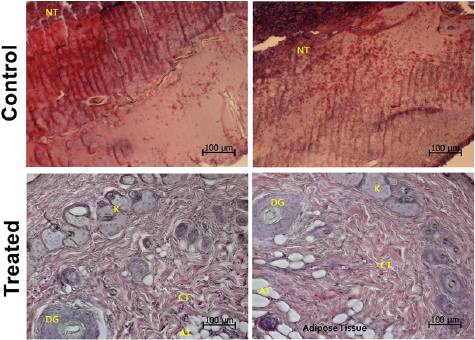
In figure 9, the histopathological analysis showed that the NBG fibers enhanced the rate of soft tissue regeneration and stimulated wound healing. Compared to the control group, where a significant amount of necrotic tissue was still present after 7 d post-surgery, the treated group exhibited structures linked to a normal skin tissue, including the presence of fibroblasts and keratinocytes, connective tissue and sweat glands ducts (figure 9).
Figure 9. Representative histological sections of the burned skin wounds for the control and the treated group (NBG fiber meshes) at the final experimental period. Keratinocytes (K), connective tissue (CT), adipose tissue (AT), necrotic tissue (NT) and sweat glands ducts (DG) are indicated in the sections. Magnification = ×200. Bar represents 100 µm. (Haematoxylin–eosin staining).
Download figure:
Standard image High-resolution imageThese findings corroborate with previous studies conducted by numerous authors who have applied bioactive glasses in several forms, such as particles, in various types of skin wounds, as presented by Miguez-Pacheco et al [9]. Day and Jung demonstrated a successful outcome in a small-scale human study treating chronic wounds with the application of a cotton-candy like bioactive 13-93B nanofibers (of composition 5.5% Na2O, 11.1% K2O, 4.6% MgO, 18.5% CaO, 3.7% P2O5 and 56.6% B2O3—wt.%), with diameters ranging from 300 nm to 5 µm. In addition to the accelerated rate of tissue regeneration of chronic wounds in diabetic patients, a decrease in scar tissue formation was also found [45, 56].
Lin et al also reported that the application of an ointment with 45S5 bioactive glass particles in full-thickness skin wounds promoted an accelerated healing in both healthy and diabetic rats. The wounds were almost completely healed after 16 d [57].
To evaluate the presence of VEGF and TGF-β growth factors in the novel bioactive glass treated wounds, immunohistochemical staining was performed and the results are shown in figure 10. Positive staining for both factors were detected in all the tissue samples (brownish areas); however, a more intense VEGF immunostaining was detected in the treated group. For TGF-β no statistical difference was observed between the treated and the control groups. A similar phenomenon was also reported by Lin et al with similar and other growth factors [57].
Figure 10. Immunohistochemistry of VEGF and TGF-β at 7 d post-surgery for: (A) control group—VEGF; (B) control group—TGF-β; (C) Treated group with the NBG fiber meshes—VEGF; and (D) Treated group with the NBG fiber meshes—TGF-β. Magnification = ×400. Bar represents 100 µm.
Download figure:
Standard image High-resolution image3. Bioactive glasses scalable manufacturing
A range of bioactive glass compositions in particulate form are widespread in current clinical use. However, according to some authors, seems that their improvements in clinical performance do not warrant the substantial investments required to obtain regulatory approval (such as FDA and CE) and upscale the manufacturing processes to a commercial scale for the current available forms [58]. During clinical practice, medical or dentistry professionals may require scaffolds, complex 3D shapes or machinable and easy handling implantable devices for the regeneration of complex lesions or defects. To date, there is no large-scale production of these bioactive glasses derived devices, mainly because the original Bioglass 45S5 crystallizes during heating treatments, which hinders the sintering processes and fiber drawing.
Although, improvements have been made throughout the years concerning the processing window of bioactive glasses by tailoring their compositions and developing formulations, such as 13–93 and 1–98, these glasses still present several disadvantages regarding their glass stability, inhibiting an up-scaled production of complex devices. Furthermore, their diminished crystallization tendency frequently represents a decreased bioactivity compared to Bioglass 45S5.
The NBG is a composition that presents a more suitable balance between bioactivity and workability, which allows the fabrication of continuous glass fiber for long periods of time via simple and large scale processing techniques, including downdrawing. The development of this NBG has led to the manufacturing of various devices such as membranes for skin wound regeneration, nerve guide conduits [48] and high porous scaffolds [46, 47].
The range of new possibilities originating from this new bioactive glass composition was the driving force for the foundation of a spin-off company from LaMaV-UFSCar, named VETRA. This start-up company intends to link academic developments to industry, bringing new technologies to the market and to encourage academic-industrial collaborations to fill the gap between research, development, up scaling manufacturing and commercialization.
4. Conclusions
The developed NBG is a composition that has demonstrated an elevated bioactivity though in vitro and in vivo tests. It is also bioresorbable and allows one to develop numerous medical devices due to its wide workability, such as flexible continuous fibers, porous sintered bodies and 3D complex shapes. All these features make this NBG a potential alternative for soft and hard tissue regeneration in various clinical applications.
Acknowledgments
The authors wish to thank the São Paulo Research Foundation (FAPESP -Brazil) for the following research grants, Process 2011/22937-9 and Process 2013/07793-6 (CeRTEV), for the financial support of this study.