Abstract
The severe corrosion of carbon supports has attracted the development of ceramic-based support materials. Non-precious metal oxides are potential support materials for fuel cells owing to their corrosion resistance under the harsh fuel cell environment. However, they cannot be used as primary support materials because they are not good electric conductors. In this study, we demonstrate that Pd nanoparticles supported on NaOH-functionalized carbon nanodots blended with zirconium dioxide can act as stable and electroactive anode catalysts for alkaline direct alcohol fuel cells (ADAFC). The Pd/fCNDs-ZrO2 electrocatalyst was synthesized by a sonochemical method and characterized by x-ray diffraction (XRD) and transmission electron microscopy (TEM). Cyclic voltammetry (CV) and chronoamperometry (CA) were used to study the electrochemical activity and stability of the Pd/fCNDs-ZrO2 catalyst towards methanol and ethanol oxidation in alkaline media. The observed results revealed that the Pd/fCNDs-ZrO2 catalyst exhibits higher current densities (12.5 mA cm−2 for ethanol and 20.05 mA cm−2 for methanol) and lower poisoning rates compared to the Pd/fCNDs and commercial Pd/C catalysts.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Direct alcohol fuel cells employ small molecules such as methanol and ethanol as fuels. These fuels are eco-friendly because their electrooxidation reactions produce water and a substantially low amount of carbon dioxide gas as by-products [1, 2]. Direct methanol and ethanol fuel cells continue to attract attention due to high power densities (6.09 kW h kg−1 for methanol and 8.01 kW h kg−1 for ethanol) which can be harvested directly from these fuels [2, 3]. Ethanol is more attractive than methanol owing to its non-toxicity and abundance [3]. Despite the toxicological issues associated with methanol, it is much easier to oxidize because each methanol molecule has five bonds only which need to be cleaved during electrooxidation reactions compared to eight bonds in one ethanol molecule [4]. In addition to that, the retail price of methanol is significantly lower than that of ethanol. Owing to appealing advantages of ethanol and methanol there is a need to develop both direct methanol and ethanol fuel cells.
The performance of direct alcohol fuel cells has been investigated in alkaline and acidic environments. Aquoues solutions of NaOH, KOH and NH4OH are the most commonly used alkaline supporting electrolytes whilst H2SO4 and HClO4 solutions are the commonly used acidic electrolytes [2, 5–7]. Supporting electrolytes supply ions which helps to avoid potential drop during electrooxidation reactions. As much as they influence the overall performance of the fuel cell, they also impact negatively on the architecture of the fuel cell. Carbon supports get corroded by the harsh conditions of the fuel cell resulting in catalyst detachment and dissolution [8–10]. It has been demonstrated that alkaline conditions are favourable for alcohol electrooxidation reaction [5, 7]. The challenge associated with alcohol electrooxidation reactions in alkaline conditions is the formation of stable carbonate salts intermediate which precipitates out of the electrolyte solution. The precipitates get deposited on the electrode surface thus preventing fuel molecules from adsorbing on the surface of the catalysts. They also destroy the active layer of the catalyst resulting in poor cell performance [10]. Despite these problems, alcohol electrooxidation kinetic is higher in alkaline environments compared to low pH mediums. High concentrations of OH- ions improves the oxophilicity of the catalyst surface thus enhancing the oxidation reaction. High current densities have been observed in alkaline media using palladium as a catalyst instead of platinum. Palladium costs almost half the price of platinum and it is abundant [11]. This motivates the development of Pd-based catalysts for alkaline direct alcohol fuel cells.
Stability and cost are the major critical issues hindering the commercialization of direct alcohol fuel cells. To counteract the corrosion of carbon supports by acids, non-precious metal oxides such as titania (TiO2) [12], tungsten trioxide (WO3) [13], cerium oxide (CeO2) [14], aluminium oxide (Al2O3) [15], tin (IV) oxide (SnO2) [16] and molybdenum oxide (MoO3)[3] have been used as secondary supports. Zirconium dioxide nanoparticles have very attractive properties such as huge dielectric constants, fast electron transfer for the enhancement of CO adsorption on the surface of electrocatalyst, high sensitivity and stability under the fuel cell environment [17]. The corrosion of ZrO2 coatings on metals has been investigated by cyclic voltammetry in 1M HCl and 0.4 M NaOH solutions. It was discovered that metals in HCl corroded more than those in NaOH solution [18]. Zirconium oxide offers attractive characteristics for being used as a support material because it is hydrophilic; this property makes it less prone to oxidation under the fuel cell environment especially at higher pH values. Another attractive feature of ZrO2 is its ability to remove CO poisoning species on the surface of the catalyst through the bifunctional mechanism. This was demonstrated by Song et al [19] and Aguilar-Vallejo et al [20] when they investigated methanol oxidation in alkaline media using mesoporous Pd supported on ZrO2 nanoparticles and Pt/ZrO2 catalysts respectively. However, metal oxides have low electrical conductivity, this means they cannot be used as stand-alone support materials. ZrO2 has been used with various support materials such as carbon nanotubes and Vulcan XC-72 as composites support materials. Malolepszy et al [21] demonstrated that Pd-ZrO2 nanoparticles supported on multi-walled carbon nanotubes are resistant to deactivation by poisoning species produced by formic acid fuel cells. Bai et al [22] prepared a Pt–ZrO2/C electrocatalyst by reducing hexachloroplatinic acid and zirconium nitrate in NH4OH solution. Cyclic voltammetry results showed that the Pt–ZrO2/C electrocatalyst yielded a higher current density at lower potentials than the commercial Pt/C (20 wt% Pt, E-TEK) during ethanol electrooxidation in alkaline media. The charge transfer resistance of the Pt– ZrO2/C eletrocatalyst observed from impedance spectra was also smaller than that of Pt/C electrocatalyst indicating enhanced reaction kinetics. Guo et al [23] prepared sulphated zirconia nanoparticles on multi-walled carbon nanotubes as new supports of Pt nanoparticles with the intention to enhance electron conductivity, proton conductivity and the catalytic performance of Pt towards methanol electrooxidation in acidic media. Song et al [24] used a sol-gel method to prepare Pt-ZrO2/CNT electrocatalysts for methanol and ethanol electrooxidation. They concluded that introducing ZrO2 on CNTs using the sol–gel method improves the electroactivity of Pt towards methanol through the bifunctional mechanism between ZrO2 and Pt where ZrO2 provides oxygen which react with the CO intermediates [7, 15, 25, 26]. The promoter also modifies the crystalline structure of Pt resulting in a geometry favouring improved activity [26]. They also reported that the enhanced ethanol oxidation reaction was brought by the combined effect of the oxygen vacancies in ZrO2 and the bifunctional mechanism. Wen et al performed a comparative study on the electrochemical performance and stability of Pd/ZrO2/C, Pd/CeO2/C and Pd/ZrO2-CeO2/C (where is C is Vulcan XC-72) nanocatalysts during alcohol oxidation in alkaline media. They revealed that the Pd/ZrO2/C catalysts is more active and stable than the Pd/CeO2 catalysts. It was further revealed that using ZrO2-CeO2 mixed oxides as support materials further improve the electrocatalytic properties of the Pd catalyst [27].
From the literature review conducted, we found that ZrO2 nanoparticles are usually used in conjunction with carbon nanotubes and Pt nanoparticles for alcohol oxidation in acidic media [3, 22–24]. However, researchers have confirmed that alcohol oxidation is favoured in alkaline environments where palladium performs better platinum [27–29]. For instance, Ma et al [30] proved that Pd/C showed a higher activity toward ethanol oxidation than the Pt/C in both acidic and alkaline media. This is mainly due to the higher oxyphilic characteristics of the Pd/C and the relatively inert nature of the Pd/C on C–C bond cleavage. Furthermore, Pt is more expensive than Pd. This study was devoted to developing a low cost, active and stable Pd/fCNDs-ZrO2 electrocatalyst for ethanol and methanol oxidation in alkaline media. The influence of different alkaline electrolytes (NaOH, KOH and NH4OH) towards alcohol electrooxidation was also investigated. On the other hand, it is known that primary support materials play a vital role in providing suitable electrical conductivity and porosity to boost the flow of electrons within the electrode. From the previous studies involving ZrO2 we noted that carbon nanotubes have been used as primary supports. However, carbon nanotubes have chemically unreactive surfaces which make it difficult to deposit catalyst nanoparticles. For this reason, nanoparticles on CNTs generally have poor dispersion and large particle sizes, this diminishes the electrocatalytic activity. This problem is usually overcomed by acid-functionalizing pristine CNTs [2]. Nonetheless, this method destroys the graphitic structure and reduces the electrical conductivity of the functionalized CNTs. Therefore, there is a need to develop new primary support materials. Carbon nanodots have large surface area, good porosity, easy to functionalise and they can be prepared by various methods in a standard laboratory [31, 32].
For the first time, we report the use of functionalized carbon nanodots as support materials for Pd-ZrO2 nanoparticles. Carbon nanodots can be synthesized by bottom up (physical) and top down (chemical) methods [33]. The top-down methods involve treating materials such as graphite powder or multi-walled carbon nanotubes (MWCNTs) with acids or bases. Bottom-up techniques employ external energy such as ultrasonication, microwave pyrolysis, mechanical crushing and hydrothermal treatment of carbon rich molecules such as starch. Carbon nanodots have been synthesizing by pyrolysing oats and it was observed that CNDs prepared by this approach are amorphous and unstable under the harsh fuel cell environment [2, 13, 23]. The crystallinity or amorphous nature of CND depends on the starting material and synthesis method. A review prepared by Li et al highlighted that CNDs produced by the electrochemical treatment of MWCNTs exhibit the same nanocrystalline graphitic structure as pristine MWCNTs [34]. Amorphous CND are normally produced by chemical methods such as pyrolysis or hydrothermal treatment of carbonaceous materials such as carbohydrates [35]. In this study, functionalized carbon nanodots (fCNDs) were prepared by combining both physical and chemical methods. Carbon nanodots were functionalized under mild conditions to avoid structural defects and maintain the graphitic structure of MWCNT. Multi-walled carbon nanotubes were chosen as starting materials owing to their nano structure, good porosity and outstanding electrical conductivity.
It has been demonstrated that the effectiveness of direct alcohol fuel cell catalysts depends greatly on the synthesis method. Malolepszy et al [21] demonstrated that PdZrO2/f-MWCNTs nanocatalysts can be synthesized by a microwave-assisted polyol method. In this study, the Pd/fCNDs-ZrO2 catalyst was prepared by a simple sonochemical method. Sonochemistry is the application of ultrasound to chemical reactions. Ultrasound is used because of its effects in reactions. It produces activated metal nanoparticles through velocity interparticle collisions. With sonochemistry, it is possible to control surface morphology and particle size by regulating the velocity of the ultrasound bath [36].
The prepared electrocatalyst was physico-chemically characterized by TEM and XRD. The electrocatalytic activity of the Pd/fCNDs-ZrO2 catalyst towards methanol and ethanol oxidation was studied in alkaline using aquoues solutions of KOH, NaOH and NH4OH as supporting electrolytes at room temperature. Electrochemical characterization techniques (cyclic voltammetry and chronoamperometry) were performed to study the catalytic performance and poisoning tolerance by the Pd/fCNDs-ZrO2 catalyst. A synthesized Pd/fCNDs electrocatalyst and a commercial Pd/C electrocatalyst were used as benchmark standards.
2. Materials and methods
2.1. Chemicals
Multi-walled carbon nanotubes, Nafion solution 10 wt%, Absolute ethanol > 99.9%, Methanol > 99.9%, Anhydrous PdCl2, Pd/C (10% Pd) commercial standard, NaOH and KOH pellets were bought from Merck and used as received without purification or modification. Calcined ZrO2 nanoparticles (∼10 nm) were purchased from BDH Chemical (England).
2.2. Synthesis of functionalized carbon nanodots (fCNDs)
Carbon nanodots were prepared and functionalized according to a method developed in this study. In our procedure, 5 g of multi-walled carbon nanotubes were pulverized for 10 min. The fine powder was sieved using a 100 nm mesh, dispersed in deionised water and centrifuged five times at 7500 rpm for 5 min to remove bigger particles. The recovered carbon nanodots were dried in an oven at 60 °C for 12 h. To functionalize the carbon nanodots, dry CNDs were refluxed in 0.1 M NaOH solution at 90 °C for 12 h. The black solids were washed with deionized water until a pH of 7.8 was reached and were dried in an oven at 60 °C for 12 h.
2.3. Synthesis of Pd/fCNDs-ZrO2 electrocatalyst
Firstly, fCNDs and calcined ZrO2 nanoparticles (in the ratio 4:1) were mechanically crushed together and dispersed in 50:50 ethanol/water solution. The black suspension was agitated in an ultrasound bath for an hour. The resulting black solids were recovered by filtration, dried in the oven at 60 °C for 12 h before they were calcined in a furnace at 300 °C for 2 h. The contents were cooled down at room temperature to obtain a fCNDs-ZrO2 composite. Pd nanoparticles were deposited onto the composite support by a sonochemical-assisted borohydride reduction method. In a typical procedure, to prepare 5% Pd loading, 42 mg of PdCl2 were mixed with 500 mg of fCNDs-ZrO2. The mixture was dispersed in 0.1 M borohydride solution and agitated in an ultrasound bath at room temperature for an hour. The temperature of the ultrasound bath was then increased to 90 °C and the mixture was treated for 3 h. The Pd/fCNDs-ZrO2 electrocatalyst was recovered by filtration and dried in an oven at 60 °C for 12 h. Pd/fCNDs electrocatalyst was prepared under the same conditions using fCNDs as a support material instead of fCNDs-ZrO2.
2.4. Physico-chemical characterization of the nanoparticles
Surface morphology of the material was examined using a transmission electron microscope (TEM) JEOL JEM 2100. XRD patterns were obtained from a Philips X'PertPro x-ray Diffractometer using Cu Kα radiation with the following conditions: λ = 0.154443nm, 40 kV and 40 mA. Raman spectra were recorded at room temperature using a Thermo Scientific DXR2 Smart Raman equipped with a 455 nm laser source. Palladium loading on fCNDs-ZrO2 was determined using ICP-OES (iCAP 6500 Duo, Thermo Scientific, UK). ICP-OES calibration standards ranging between 1ppm and 100 ppm were prepared from PdCl2 (the palladium precursor). Catalyst samples for ICP-OES analysis were produced by etching 25 mg of each electrocatalyst in 4 ml of solution of HCl/HNO3 (1:3) mixture. The solution was diluted with double distilled water to make a total volume of 50 ml. The suspended solids were removed by filtration prior to analysis.
2.5. Electrochemical characterization
To prepare the working electrodes, dry electrocatalysts (10 mg) were dispersed in 2.5 ml solution of ethanol containing 10 μl of 10% wt Nafion solution®. The suspensions were sonicated for 10 min resulting a homogeneous black ink. Each of the homogenous ink solutions (2 μl) were transferred onto the cavity of a clean 3 mm diameter glassy carbon (GC) electrode. The modified GC electrode was dried under vacuum at room temperature in the absence of oxygen. Electrochemical measurements were recorded on a Dropsens potentiostat (μSTAT8000, Switzerland). In a three electrode set-up, a silver/silver chloride (Ag/AgCl saturated with 3M KCl solution) reference electrode was used in conjunction with a platinum wire counter electrode. The commercial 10%wt Pd/C and pre-synthesized Pd/fCNDs electrocatalyst were treated in a similar way. Oxygen was eliminated from the electrolytes by purging in argon gas for thirty minutes before conducting the experiments at ambient temperature.
3. Results and discussion
A transmission electron microscope was used to study the morphology of the synthesized materials. Figure 1 shows the TEM micrographs of fCNDs and Pd/fCNDs-ZrO2 electrocatalyst with corresponding particle size histograms. The TEM micrograph of fCNDs (figure 1(a)) shows that the nanodots exhibit oval shapes and are mono dispersed. The sizes of the fuctionalized carbon nanodots range between 2 nm and 7 nm. The image is not showing any tubular shapes indicating that multi-walled carbon nanotubes were successfully pulverized to produce carbon nanodots. Lattice fringes with distances of 0.253 nm and 0.303 nm which correspond to the C(002) and C(100) graphic facets were observed. These results are in agreement with those reported in literature [37, 38]. From the TEM image shown in figure 1(b) it is observed that metal nanoparticles (black spots) were successfully deposited on the composite support and they have sizes ranging between 1nm and 4 nm. This is confirmed by the presence of lattice fringes of both Pd (0.227 nm) and ZrO2 (0.297 nm). Other studies have reported that the alcohol reduction method produces nanocatalysts with the smallest particles sizes [6, 18], however, this study demonstrates that small catalyst nanoparticles can be prepared by a sonochemical method using borohydride as a reducing agent. Ultrasound prevents the nucleation growth of nanoparticles, this result in electrocatalysts nanoparticles with very small sizes. Literature states that increasing the speed of the ultrasound bath retards the growth of the nanoparticles thus resulting in very small particle sizes [33]. The proposed method is attractive because it does not require sophisticated equipment and harsh conditions. Elemental mappings presented in figure 1(c) were done to reveal the distribution of the nanoparticles in the composite electrocatalyst. The carbon map is denser compared to the map of Zr, O and Pd because C as the primary support contributes a higher percentage towards the composition of the composite catalyst. The palladium map show that the nanoparticles were uniformly distributed in the electrocatalyst. The maps for Zr and O confirm the presence of Zr and O in the electrocatalyst.
Figure 1. (a) TEM image for CNDs, (b) TEM image for fCNDs, (c) TEM image for the Pd/fCNDs-ZrO2 electrocatalyst, (e) Elemental maps for the Pd/fCNDs-ZrO2 electrocatalyst.
Download figure:
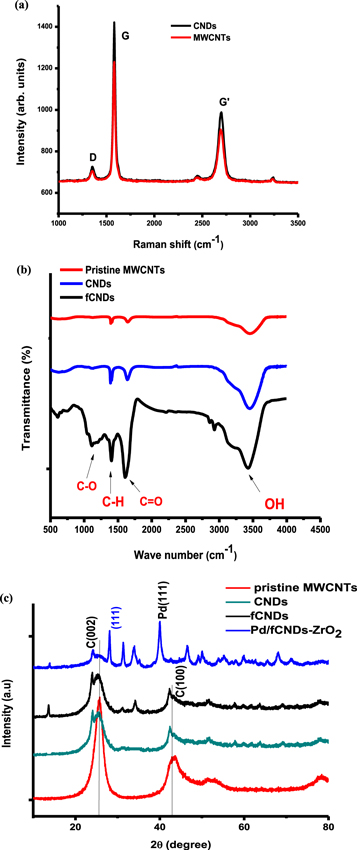
Standard image High-resolution imageRaman analysis was done to determine the vibrational modes of MWCNTs and CNDs prior to functionalisation. The G-band is a characteristic feature of the graphitic layers and corresponds to the tangential vibration of carbon atoms. Another characteristic feature of carbon materials is the presence of the D-band which corresponds to the vibration of defective graphitic structures [36–44]. The IG/ID ratio is often used to measure the quality of a sample, however, other researchers have argued that this method cannot be used as a standardized evaluation of sample quality because the intensity of D-band is dependent on the wavelength of the laser source [41]. Figure 2(a) shows the Raman spectra for MWCNT and unfunctionalised CNDs. The G(graphite), D(disorder) bands and their second-order harmonic (G' band) clearly appear at 1577 cm−1, 1350 cm−1 and 2696 cm−1 respectively. The position of our Raman peaks is in good agreement with other studies and theoretical calculations for single walled nanotubes suggesting that the vibration structure of MWCNT is quite similar to that of single walled nanotubes [39, 42]. The similarities in the Raman spectra of MWCNT and CNDs indicates that pulverizing does not destroy the graphitic structure of MWCNT.
Figure 2. (a) Raman spectra for CNDs and MWCNTs, (b) FTIR spectra of fCNDs and pristine MWCNTs, (c) XRD patterns of fCNDs, pristine MWCNTs and Pd/fCNDs-ZrO2.
Download figure:
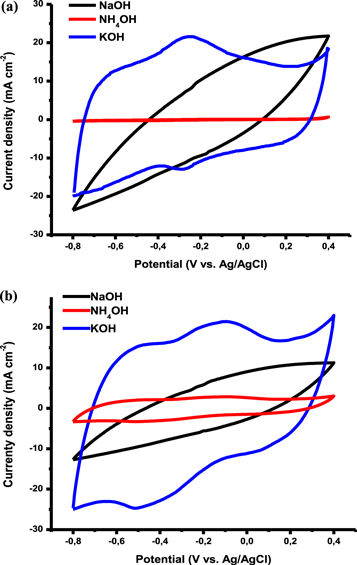
Standard image High-resolution imageFigure 2(b) shows the FTIR spectrum of functionalized carbon nanodots. The carbon nanodots were functionalized to facilitate interaction with ZrO2 and attachment of Pd nanoparticles through oxygen-containing functional groups. FTIR study was done to confirm the presence of oxygen-containing functional groups in fCNDs. An intense peak at 1606 cm−1 corresponding to the carbonyl (C=O) moeity was observed in the fCNDs spectrum. This peak is absent in the spectrum of pristine MWCNT. Another peak was observed at about 1089 cm−1 which is due to the (C–O) stretching mode of the COOH functional group. The broad peak at 3400 cm−1 due to OH stretching from water is present in both spectra. This is expected because the fCNDs were washed with water during the synthesis procedure. The presence of this peak in the spectrum of pristine MWCNT might be an indication of the presence of moisture in commercial MWCNT.
XRD was done to study the crystalline phases of the synthesized materials and presence of surface defects. Figure 2(c) shows the XRD patterns for pristine MWCNTs, fCNDs and Pd/fCNDs-ZrO2 electrocatalyst. A peak at 2ϴ = 25 degrees is present in both MWCNT and fCNDs patterns. This peak corresponds to graphitic carbon C (002). The peak is not shifted or altered indicating that functionalizing the carbon nanodots did not destroy the graphitic structure. It was also observed that the C (100) and C (110) peaks are quite small in the fCNDs pattern indicating that functionalizing carbon nanodots with NaOH destroys other phases resulting in a purer crystalline graphitic carbon. The pattern for Pd/fCNDs-ZrO2 shows a strong diffraction peak at 2ϴ = 40 degrees which corresponds to the Pd (111). A peak corresponding to C (002) at 2ϴ = 25 degree is also observed in the Pd/fCNDs-ZrO2 pattern. Diffraction peaks 2ϴ = 26, 30, 35 and 48 degrees were assigned to ZrO2 facets with orientations (110), (111), (002) and (211) respectively according JCPDS: 37:1484. Peaks observed at 2ϴ = 42 and 68 degrees are due to the presence of Pd (200) and Pd (220) facets respectively. It should be pointed out that the C (002) peak in Pd/fCNDs-ZrO2 is deformed due to the interaction between carbon and ZrO2. XRD is a very useful analytical technique for investigating the crystalline phases present in a material. In this study it was observed that functionalizing carbon nanodots with NaOH does not destroy the crystalline graphitic structure of carbon. The graphitic structure of carbon must be preserved as it is necessary in maintaining the conductivity of the support material [2]. From the XRD pattern shown in figure 2(c) it can be seen from the sharp C (002) diffraction peak that fCNDs produced from MWCNT are crystalline in nature. From the XRD pattern of fCNDs and Pd/fCNDs-ZrO2 a small peak is observed at 2ϴ = 17 degrees which could be due to the presence of sodium (Na) as the materials were functionalized using NaOH solution. This peak is absent from the XRD patterns of pristine MWCNT and unfunctioalised CNDs. The crystal sizes of the materials were calculated using Debye–Scherer equation;
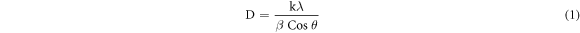
where D is the crystal size, 0.9 is the shape factor,β is the peak width at half peak height (radians),λ the wavelength of the x-ray and θ is the angle of reflection.
The average crystal sizes of the nanoparticles are summarized in table 1. It is noted that the crystal sizes of CNDs and fCNDs is smaller than that of MWCNT.
Table 1. Average crystal sizes for the synthesised nanomaterials calculated from XRD data.
| Sample | ZrO2 | Pd in Pd/CNDs-ZrO2 | MWCNT | CNDs | fCNDs |
|---|---|---|---|---|---|
| Average crystal size | 8.57 nm | 2.52 nm | 3.46 nm | 2.13 nm | 2.13 nm |
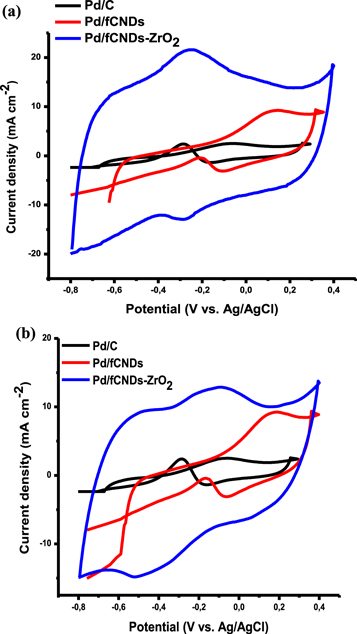
The electrooxidation of methanol and ethanol was studied in the presence of NaOH, KOH and NH4OH as supporting electrolytes to investigate the influence of different electrolytes on electrooxidation reactions. A supporting electrolyte is a solution whose ions are electrochemically inactive in the range of applied potentials being studied, and its ionic strength is usually much greater than the strength of the electroactive analyte dissolved in it. Supporting electrolytes are used to increase the conductivity of the solution, to avoid potential drop during the analysis, and to maintain a constant flow of ions within the cell [2]. NaOH, KOH and NH4OH solutions with concentrations of 0.1 M were used as electrolytes during the electrooxidation of ethanol and methanol. The results are presented in figure 3. No oxidation peaks were observed during methanol and ethanol electrooxidation in NaOH and NH4OH electrolytes. The lowest current densities yielded during ethanol and methanol oxidation were observed with the NH4OH electrolyte. It was observed that both methanol and ethanol produce high current densities when oxidized in KOH electrolyte. We expected NaOH and KOH electrolytes to behave in a similar manner since they are both strong bases which dissociate completely in water to produce ions. The superior performance of KOH electrolyte could be due to that fact the solubility of group 1 hydroxides increases down the group, so at similar conditions 0.1 M KOH solution produces more free ions than 0.1 M NaOH. On the other hand, we expected NH4OH electrolyte to yield the lowest current density because NH4OH does not dissociate completely in water to release free ions. Further electrooxidation reactions of ethanol and methanol on Pd/fCNDs-ZrO2 electrocatalyst were then studied in the presence of 0.1 M KOH supporting electrolyte.
Figure 3. (a) CV curves for Pd/fCNDs-ZrO2 electrocatalyst towards methanol (CH3OH) in NaOH, NH4OH and KOH electrolytes; (b) CV curves for Pd/fCNDs-ZrO2 electrocatalyst towards ethanol (CH3CH2OH) in NaOH, NH4OH and KOH electrolytes.
Download figure:
Standard image High-resolution imageCyclic voltammetry tests for methanol and ethanol electrooxidation reactions were performed at a scan rate of 20 mVs−1. Cyclic voltammograms for alcohol electrooxidation reactions are characterized by signal peaks in the forward and reverse scans [1]. The peak observed from the forward scan is attributed to the alcohol electrooxidation reaction whereas the peak from the reverse scan is attributed to the removal of intermediates formed from the forward scan. A good electrocatalyst improves the efficiency of the electrooxidation reaction thereby yielding high current density in forward scan. The ratio of the current produced in the forward scan (If) to the current produced in the reverse scan (Ib) is used to measure the degree of oxophilicity. A higher degree of oxophilicity indicates improved electroactivity of the electrocatalyst [1, 2, 45]. Figure 4 shows the cyclic voltammograms observed during the electrooxidation of methanol and ethanol on the Pd/fCNDs-ZrO2 electrocatalyst. From the CV curves it can be seen that the synthesized Pd/fCNDs-ZrO2 electrocatalyst yields the highest current density compared to the commercial Pd/C and Pd/fCNDs electrocatalyst. For the Pd/fCNDs-ZrO2 electrocatalyst it can be clearly seen that the ratio of the forward scan to the reverse scan is greater than that of other catalysts suggesting that incorporating ZrO2 improves tolerance to poison. Both methanol and ethanol yielded high current densities when oxidized on the Pd/fCNDs-ZrO2 electrocatalyst compared to the Pd/fCNDs and Pd/C electrocatalyst. It should be stated that the synthesized electrocatalyst had 4.82% Pd loading (according to ICP-OES results) and it performed better than the benchmark standards with 10% Pd loading.
Figure 4. (a) CV curves for the electrooxidation of 1 M methanol in 0.1 M KOH electrolyte on Pd/fCNDs-ZrO2 electrocatalyst at −0.2V, (b) CV curves for the electrooxidation of 1M ethanol in 0.1 M KOH electrolyte on Pd/fCNDs-ZrO2 electrocatalyst at −0.1V.
Download figure:
Standard image High-resolution imageChronoamperometry (CA) tests were conducted to study the poisoning rates of the catalysts by CO-like intermediates produced during alcohol electrooxidation. Figures 5(a) and (b) shows the chronoamperometry curves observed during alcohol electrooxidation reactions. A sharp decrease is observed for all the catalysts at the start of the reaction due to rapid adsorption of alcohol molecules and formation of CO-like intermediates on the electrode surface. A pseudo-steady state is reached after 500 s. As the reactions progress it is observed that the Pd/CNDs-ZrO2 electrocatalyst retains the highest current density compared to the Pd/C and Pd/fCNDs electrocatalysts. After The CA curves for both methanol ethanol electrooxidation on Pd/fCNDs and Pd/fCNDs-ZrO2 electrocatalyst do not lie straight for the entire period of 2000 s indicating that oxidation reactions produce intermediates which adsorb on the surface of the electrocatalysts. Nonetheless, the Pd/fCNDs-ZrO2 electrocatalyst is capable of continuously oxidizing the poisoning intermediates and retains the highest current density compared to the other two catalysts.
Figure 5. (a) CA curves for 1.0 M methanol electrooxidation on Pd/fCNDs-ZrO2 electrocatalyst in 0.1 M KOH electrolyte, (b) CA curves for 1.0 M ethanol electrooxidation on Pd/fCNDs-ZrO2 electrocatalyst in 0.1 M KOH electrolyte.
Download figure:
Standard image High-resolution imageChronoamperometry curves for ethanol electrooxidation show a continuous current drop for the entire period of 2000 s owing to the continuous production of CO intermediates which adsorb strongly onto the electrocatalyst surface. It is also noted that the fCNDs retains more current that the Pd/C catalyst. This could be brought by the large surface area of fCNDs. The ratio current retained by the Pd/fCNDs-ZrO2 electrocatalyst is about 1.85 times as that of the Pd/fCNDs. This observation indicates that the synthesized electrocatalyst is capable of overcoming the poisoning by CO intermediates. The tolerance to poisoning is brought by the ZrO2 promoter which provides oxygen that react with the CO intermediates.
4. Conclusions
In conclusion, functionalized carbon nanodots were successfully prepared by combining physical (pulverizing) and chemical (NaOH treatment) synthesis methods. XRD results proved that functionalizing carbon nanodots with NaOH preserves the graphitic structure and reduces other amorphous phases of carbon. An electroactive Pd/fCNDs-ZrO2 electrocatalyst was successfully synthesized by a simple sonochemical method. Cyclic voltammetry results demonstrated that the Pd/fCNDs-ZrO2 electrocatalyst is superior over the Pd/fCNDs and Pd/C electrocatalysts during methanol and ethanol electrooxidation reactions. Lower poisoning rates were observed with the Pd/fCNDs-ZrO2 electrocatalyst during methanol and ethanol electrooxidation reactions. This study proves that ZrO2 is a potential secondary support material/promoter for alkaline direct alcohol fuel cells.
Acknowledgments
The authors would like to thank the National Research Foundation of South Africa grant numbers (112812 and TTK-15071-0125-019) for financial support.