Abstract
The inhibitive performance of Sapindus on the acidic corrosion of Aluminium was studied using myriad experimental and computational techniques. Sapindus showed 98% of inhibition efficiency at 2000 ppm. Inhibition efficiency of Sapindus was found to be directly proportional to its concentration in the acidic medium. Effect of temperature was also studied and it played a corrosive role by increasing the corrosion rate and decreasing the inhibition efficiency of Sapindus. Thermodynamic parameters of adsorption were derived to enunciate the findings. Mechanism of adsorption was also explored using electrochemical impedance spectroscopy and potentiodynamic polarization (Tafel) studies which displayed a profound physisorption mechanism.
Export citation and abstract BibTeX RIS
1. Introduction
Corrosion of metals is an ever persisting problem since archaeological era and it has shown its drastic effects on metals like Copper [1], Iron [2, 3], Aluminium [4, 5] and their alloys [6–9]. Indigenous effects of corrosion on industrially important metals like Aluminium are enormous thus corrosion mitigation is important process in terms of economic growth, technical development, environment and safety of humans [10]. Out of different methods used to suppress corrosion of metals, addition of chemical species (inhibitors) to aggressive medium is one of the commonly used methods [11]. The Inhibitors consisting of hetero atoms (N, O, S and P) which act as an adsorption centers are considered to be effective corrosion inhibitors under acidic conditions [12]. The interactions between low unoccupied molecular orbitals (LUMO) of metal surface and high occupied molecular orbitals (HOMO) of inhibitor molecules enables the adsorption of inhibitor on the surface of metal is another important characteristic of an effective corrosion inhibitor. This property is mainly dependent on the presence of π- electrons in the structure of inhibitor [13]. Toxicity of chemical inhibitors is a major issue among the researchers of current era hence environmental compatibility of corrosion inhibitors has changed their view point and the immense demand of green/eco-friendly/natural/biocompatible corrosion inhibitors is continuously emerging [14]. Plant extracts pose an interesting alternative to the commercially available corrosion inhibitors. Many plant extracts have been reported in literature, as effective corrosion inhibitors. Some of them include Black Pepper, Flacourtia jangomas, Boerhavia diffusa, Piper guinensis, launa nudicalis, ginger Hibiscus, nyctanthis arbortritis, opuntia and ficus indica yielding an inhibition efficiency of around 90%–98% [15]. The inhibition efficiency of above mentioned plant extracts encourages us to continue this pursuit with an aim to achieve higher efficiency. In our study, Sapindus was selected for the mitigation of acidic corrosion of Aluminium. The etymology of the term Sapindus is from Greek sapo means soap and indicus means from India. The presence of saponins makes Sapindus useful for washing of clothesand as a remedy for treatment of ulcers. Due to its natural origin and its structural resemblance (presence of O, π-electrons and conjugation) in its structure to other corrosion inhibitors it was assumed that Sapindus can serve as potentially good corrosion inhibitor of Aluminium. The chemical structure of Sapindus has been represented in figure 1:
Figure 1. Chemical structure of Sapindus (Reetha).
Download figure:
Standard image High-resolution image2. Experimental
2.1. Material preparation
The specimens of 1 cm2 dimensions were used for weight loss, potentiodynamic polarization measurements and electrochemical impedance studies. These specimens were abraded with different grades of emery papers, washed with acetone and then rinsed with distilled water before inserting them into the test solution. 1 M HCl was used as a corrosive medium for Aluminium. Raw Sapindus fruits (reetha) were purchased from market and were shade dried for 48 h and were grinded to powder [16]. 100 grams of this powder was used in a soxhlet apparatus in order to make aqueous extract of Sapindus. This aqueous extract found was then filtered and was tray dried for 48 h at 318.15 K. The dried powder obtained after drying was preserved in desiccator. The test solutions of different concentrations were made using this dried extract in 1 M HCl as a medium.
2.2. Weight loss method
Weight loss method was employed to evaluate corrosion rate, inhibition efficiency and surface coverage of Sapindus because of its simplicity and accuracy. This technique involves the specimen to be tested for its weight loss by using weighing balance of sensitivity of ±0.01 mg. Aluminum coupons having the following composition: 0.35% Fe, 0.25% Si, 0.05% Cu, 0.05% Zn, 0.03% Mg, 0.03% Mn, 0.03% Ti and 99.21% being Al were used to perform the tests. The specimen (metal) is treated with acid and variety of concentrations of the inhibitors and the weight loss is seen in the specimen before it is immersed in the acid solution and after immersion for some specific time. The concentration of inhibitor in this technique is taken in mgL-1. By using this technique the corrosion rate, inhibition efficiency as well as surface coverage were calculated by using equations (1)–(3) respectively.
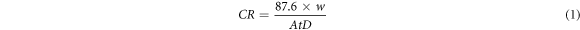
Here
CR = Corrosion rate in mpy
W (mg) = the Aluminium weight loss
A = the coupon area (cm2)
t = the time of exposure
D = the Aluminium density (g/cm3)
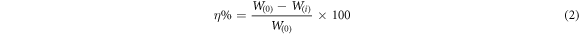
Here
η = Inhibition efficiency
W(i) = average weight loss with the inhibitor
W(0) = average weight loss without the inhibitor
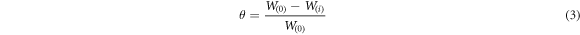
2.3. Electrochemical measurements
Electrochemical experiments were done using three electrode cells. The three electrodes are saturated calomel electrode, platinum counter electrode, which was attached with the luggin capillary. Luggin capillary acted as the reference electrode. The Luggin capillary tip was kept near to working electrode, to minimize the ohmic contribution. To embed the working electrode Teflon holder was used having the epoxy resin area of 0.785 cm2.
The potential of potentiodynamic polarization curves started from potential of −250 mV to potential of +250 mV with respect to open circuit potential at a scan rate of started rate of 0.5 mV s−1. Inhibition efficiency ηp (%) is written as:
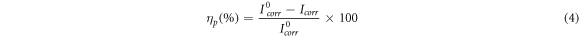
Where
I°corr = Standard corrosion current density of blank.
Icorr = Corrosion current density of inhibitor.
2.4. Potentiodynamic polarization
This technique was used in order to know the values of corrosion potential (Ecorr), current densities which are denoted by (Icorr), anodic tafel slopes and cathodic tafel slopes (βa and βc), and surface coverage (θ) and inhibition efficiency as functions of inhibitor concentration was measured from the curves. The equation (4) mentioned above was used for calculation of the corrosion current [17].
2.5. Electrochemical impedance spectroscopy
The measurement of EIS was done by the help of corrosion potential (E corr) with frequency from 100 000 to 0.1 Hz. The result of EIS were obtained by utilizing electrical equivalent circuit system (figure 2) obtained along with the charge transfer resistance which is donated by Rct value. In this method the IE % is calculated by the formula:
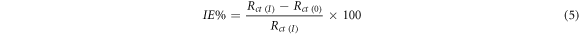
Here Rct (0) = charge resistance transfer of Aluminium without inhibitor
Figure 2. Equivalent circuit model for the studied system.
Download figure:
Standard image High-resolution imageRct (I) = charge resistance transfer of Aluminium having the inhibitor.
2.6. Surface adsorption and morphology analysis
The optimum concentration of extracts was calculated by using Gamry instrument 600 AC. Voltametry was used to understand the nature of Aluminium surface adsorption. Bode plot method (figures 3(d) and 4(c)) used to identified the maximum frequency of sample. The final and initial range of potential was set at 1 V to −1 V with voltage at 0.01. The evaluation of surface morphology of Aluminium specimen is done by scanning electron microscope [18].
Figure 3. (a) Arrhenius plot for solution containing Sapindus in 1 M HCl, (b) Graph between 1000/T versus log Cr, (c) Graph between 1000/T versus ln Cr/T.
Download figure:
Standard image High-resolution imageFigure 4. (a) Tafel polarization curves, (b) Nyquist plot, (c) Bode plot (Log Freq. (Hz) versus (−) Phase(deg)) and (d) Bode plot (Log freq (Hz) versus LogZ (Ohm/cm2)) for Aluminium in 1 M HCl in the presence of various concentrations of Sapindus at 298 K.
Download figure:
Standard image High-resolution image2.6.1. Scanning electron microscopy (SEM) and AFM
The SEM study can be done by LEO435 VPinstrument at an accelerating voltage of 5 KV and 500 × magnifications [19]. The analysis of AFM can be done by using the NT-MDT multimode AFM, Russia controlled by solver scanning probe microscope controller. The single beam cantilever with resonance frequency in the range of 240–255 kHz in semi –contact mode with corresponding spring constant of 11.5 Nm−1 containing NOVA program was used for image interpretation. The scanning area of AFM is 10 μm × 10 μm [20].
2.7. Quantum chemical calculations
Gaussian software was used to determine HOMO, LUMO and dipole moment of the inhibitors on the basis density function theory method. The computational methodology of quantum chemistry was being used in order to obtain the structure and electronic parameters. The tendency of an inhibitor to donate electrons to acceptor molecule is shown by the value of EHOMO and to accept electrons is given by ELUMO. The reactivity of an inhibitor towards metal is determined by the energy gap [21].
2.8. Monte Carlo simulations
These studies were performed by using reliable DMol3 module implemented in the high-performance software (Materials Studio version 6.0). For the whole simulation procedure the compass force field was used to optimize the structure of all component of the system of interest. The simulations were carried out in the simulation boxes (42 Å × 42 Å × 55 Å) having α = 90.00; β = 90.00 and γ = 90.00 with periodic boundary condition in order to simulate a representative part of an interface devoid of any arbitrary boundary effects. The Al (111) planes were next enlarged to a (10*10) super cell. After that, a vacuum slab was built above the surface to convert the system to 3D periodicity. The optimized inhibitor using the Forcite code was then added near the surface of Al (111) and a Monte Carlo simulation annealing procedure was carried out [22].
3. Result and discussion
3.1. Weight loss method
Values of corrosion rate as reported in table 1 signified that corrosion rate was inversely proportional to Sapindus concentration inacidic medium. Such behavior was observed due to the higher adsorption of inhibitor on the surface of metal. The concentration of inhibitor was varied from 400 to 4000 mg L−1 and highest inhibition was attained at 2000 mg L−1 after which inhibition efficiency did not considerably vary. Therefore, the other variations like temperature variation and electrochemical studies were performed at an inhibitor concentration of 2000 mg L−1.
Table 1. Weight Loss Parameters of Aluminium in 1 M HCl in different concentration of Sapindus at 298.15 K.
| Concentration (mg/L) | Initial weight | Final weight | Weight loss | % wt loss | η% |
|---|---|---|---|---|---|
| 400 | 0.3152 | 0.2726 | 0.0426 | 13.5152 | 83.0075 |
| 800 | 0.3128 | 0.2793 | 0.0335 | 10.7097 | 86.6374 |
| 1000 | 0.2930 | 0.2778 | 0.0152 | 5.1877 | 93.9369 |
| 1500 | 0.2970 | 0.2839 | 0.0131 | 4.4107 | 94.7746 |
| 1600 | 0.2959 | 0.2904 | 0.0055 | 1.8587 | 97.8061 |
| 1800 | 0.2713 | 0.2666 | 0.0047 | 1.7323 | 98.1252 |
| 2000 | 0.2750 | 0.2710 | 0.0040 | 1.4545 | 98.4044 |
| 3000 | 0.2931 | 0.2899 | 0.0032 | 1.0917 | 98.7235 |
| 4000 | 0.2871 | 0.2866 | 0.0005 | 0.1741 | 99.8005 |
| 0 | 0.3670 | 0.1163 | 0.2507 | 68.3106 | 0 |
3.2. Effect of temperature
Weight loss measurement of Aluminium was carried out at temperature ranging from 293.15 to 308.15 K in the presence and absence of standard concentration (2000 mg L−1) of inhibitor (Sapindus). The results of temperature variation have been represented in table 2.
Table 2. Effect of Temperature on the weight loss parameters of Aluminium in 2000 mg L−1 of Sapindus in 1 M HCl.
| Temperature | Initial weight | Final weight | Weight loss | η% | Inhibition efficiency | |
|---|---|---|---|---|---|---|
| 293 | Blank | 0.3343 | 0.1099 | 0.2244 | 67.1253 | 99.1532 |
| Inhibitor | 0.3005 | 0.2986 | 0.0019 | 0.6322 | ||
| 298 | Blank | 0.3231 | 0.1023 | 0.2208 | 68.3379 | 98.3242 |
| Inhibitor | 0.3476 | 0.3439 | 0.0037 | 1.0644 | ||
| 303 | Blank | 0.3071 | 0.0905 | 0.2165 | 70.5177 | 88.9176 |
| Inhibitor | 0.2960 | 0.2720 | 0.024 | 8.1081 | ||
| 308 | Blank | 0.2977 | 0.0726 | 0.2250 | 75.5962 | 86.3141 |
| Inhibitor | 0.2878 | 0.2570 | 0.0308 | 10.7018 | ||
| 318 | Blank | 0.2881 | 0.0461 | 0.242 | 83.9982 | 69.8347 |
| Inhibitor | 0.2638 | 0.1908 | 0.073 | 27.6724 |
3.3. Thermodynamics of adsorption
Adsorption isotherm signified the mechanism of adsorption of inhibitor molecule on the surface of metal. The surface coverage of different concentration of inhibitor at different temperatures enabled to get the isotherm plot. Equation (7) was utilized to calculate Kads using Langmuir isotherm, similarly equation was for Freundlich isotherm
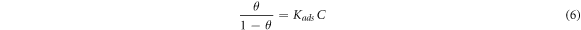
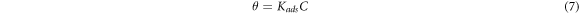
Here C is the concentration and θ is the surface coverage and Kads is the equilibrium constant.
Langmuir absorption was the method used to check the adsorption behavior of Sapindus molecule on the surface of metal of Aluminium. Figure 3(a) represents the (Arrhenius Plot)graph between the log C/θ versus log C. The slope of this graph was found to be 0.999 25 (=1). This signified that the Langmuir desorption isotherm was obeyed for the adsorption of Sapindus on the surface of Aluminium.
The activation energy was calculated using Arrhenius equation (9) from the slope of the plot between Log Cr and 1000/T (figure 3(b)):
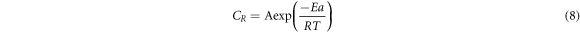
Where, A is the pre-exponential factor, T is the temperature, R is the gas constant, and Ea is the activation energy.
Table 3 represents the value of activation energy for inhibitor as well as for blank and due to the physical barrier created by the adsorbed inhibitor molecules for charge and mass transfer, the value of the activation energy is high for inhibitor as compared to the value of activation energy for blank. To calculate the enthalpy of activation Erying equation (10) was used. Figure 3(c) represents the plot between ln Cr/T versus 1/T and the values of slope (−ΔH/R) and intercept  of this plot was used to calculate the values of ΔH and ΔS.
of this plot was used to calculate the values of ΔH and ΔS.
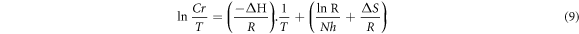
Here,
Table 3. Values of activation energy of blank and Sapindus immersed in 1 M HCl.
| S.No. | Inhibitor | Slope | Ea(KJmol−1) |
|---|---|---|---|
| 1. | Blank | −0.12853 | 2.4609 |
| 2. | Sapindus | −6.1371 | 117.4927 |
ΔH = enthalpy of activation.
ΔS = entropy of activation.
R = gas constant.
N = Avogadro's number.
h = plank's constant.
Table 4 gives the values of derived activation parameters. The relation between the standard free energy of adsorption ΔG0ads and Kads is given by the equation:
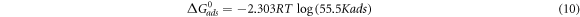
Here the 55.5 value is the molar concentration of water in the solution and from the values of ΔG0ads and ΔH0ads value of ΔS0ads was calculated using equation (12):
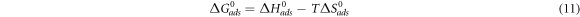
Table 4. Values of corrosion rate and Kads.
| S.No. | Inhibitor | concentration | θ | CR | Kads |
|---|---|---|---|---|---|
| 1. | Sapindus | 2000 ppm | 0.98 | 0.015 | 1.26 |
The value of ΔG0ads for Sapindus is −10.181 KJ mol−1. The negative value tells the spontaneity of the adsorption as well as the stability of adsorbed layer which is made by the Sapindus on the metal surface. The ΔG0ads value confirmed the Physisorption mechanism followed by Sapindus for inhibition of acidic corrosion of Aluminium in 1 M HCl. The calculated values of ΔG0ads suggest a strong interaction between the inhibitor and the surface of Aluminium [23]. The dissolution of Aluminium is endothermic in nature due to the positive value of ΔH0ads and the higher positive value in presence of inhibitor than in blank is an evidence of slower dissolution of metal in presence of inhibitor [24]. The disordering increases which is the driving force for inhibitor adsorption on Aluminium surface due to the positive value of ΔS0ads. The calculated values for standard enthalpy of adsorption ΔH0ads and standard entropyof adsorption ΔS0ads has been reported in table 5.
Table 5. Thermodynamic parameter obtained for Aluminium in 1 m HCl in the absence and presence of Sapindus.
| S.No. | Inhibitor | ΔHads | ΔSads |
|---|---|---|---|
| 1. | Blank | 0.034 | — |
| 2. | Sapindus | 49.91 | 343.35 |
4. Electrochemical studies
4.1. Potentiodynamic polarization studies
Polarization curves of the Aluminium in 1 M HCl solution in the presence and absence of Sapindus with different concentrations were used to determine various electrochemical parameter like corrosion potential (Ecorr), corrosion current densities (Icorr), and tafel slopes (figure 4(a)). Icorr or corrosion current densities are used to calculate the inhibition efficiency. The tafel plot demonstrated that the increase in inhibitor concentration leads to the decrease in the corrosion rate densities. This suggested that reduction of electrochemical rate due to the formation of barrier layer over the surface of Aluminium. Cathodic and anodic curves are too observed to fluctuate towards lesser current densities in the presence of inhibitor. This can be presumed that the inhibition here occurs by blocking of active sites on surface of metal. The surface coverage (θ) was calculated by:
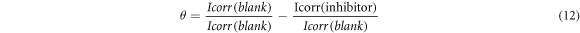
Here Icorr(blank) is the corrosion current density of without inhibitor and Icorr (inhibitor) is the corrosion current density with inhibitor. If anodic slope (ba) > cathodic slope (bc), it suggests that anodic disintegration process is more preferred and inhibitor will go about as an anodic type of inhibitor and If cathodic slope (bc) > anodic slope (ba), then cathodic disintegration process is more preferred and inhibitor will go about as a cathodic type of inhibitor [25, 26]. In the present studies, the values of Ecorr remain almost constant, indicating the mixed type of inhibitor. It is clear from the table 6 that both cathodic and anodic slopes are affected in presence of inhibitor, it can therefore be assumed that Sapindus gets adsorbed by inhibiting the corrosion of Al in HCl solution by precipitation of chloride salt on both cathodic and anodic sites of metal oxide surfaces.
Table 6. Corrosion parameters of Aluminium in 1MHCl in the presence of Sapindus at 298.15 K.
| S.No. | Concentration (mg/L) | Icorr × 10−3 | IE | (−)Ecorr | Ba(mV/dec) | Bc(mV/dec) |
|---|---|---|---|---|---|---|
| 1. | 1000 | 2.02 | 93.03 | 0.74 | 4.48 | 5.01 |
| 2. | 2000 | 1.37 | 95.28 | 0.78 | 6.94 | 22.20 |
| 3. | 3000 | 0.61 | 97.89 | 0.77 | 6.99 | 8.09 |
| 4. | 4000 | 0.05 | 99.82 | 0.74 | 7.21 | 9.46 |
| blank | 29.00 | — | 0.70 | 4.49 | 4.51 |
4.2. Electrochemical impedance spectroscopy (EIS)
The Aluminium metal corrosion from the HCl is explored by the EIS at 298.15 K after immersion of 1 h. By EIS we can calculate the double fold layer capacitance (cdl) and charge transfer resistance value (Rct) depicted in figures 4(b)–(d). By measuring the width of semicircle and the two fold layer capacitance, calculation of charge transfer resistance. The equation (14)was used to calculate that:
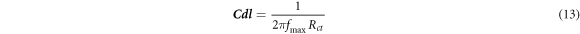
Here, fmax is the frequency where imaginary part of impedance i.e., Z' has maximum magnitude.
Also the corrosion inhibition efficiency was calculated by the below equation:
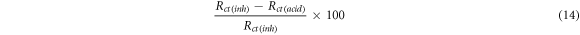
Here
Rct(inh) = charge transfer resistance exhibited by Aluminium in the presence of Sapindus in 1 M HCl,
Rct(acid) = charge transfer resistance exhibited by Aluminium in the absence of Sapindus in 1 M HCl, From table 7 it is clearly seen that as the concentration is increasing charge transfer resistance is also increasing and this will help to decrease the corrosion current for aluminium in 1 M HCl [27].
Table 7. Impedance parameters for the corrosion of Aluminium in 1 M HCl in the presence of Sapindus.
| S.No. | Conc.(mg/L) | Rct (Inh.) | Rct(blank) | fmax(Inh.) | fmax(blank) | IE | c blank(μF) | C inhi |
|---|---|---|---|---|---|---|---|---|
| 1. | 1000 | 50.24 | 3.87 | 46.50 | 1.83 | 92.30 | 22520.48 | 68.16 |
| 2. | 2000 | 77.22 | 3.87 | 37.50 | 1.83 | 94.99 | 22520.48 | 54.99 |
| 3. | 3000 | 112.12 | 3.87 | 21.20 | 1.83 | 96.55 | 22 520.48 | 66.99 |
| 4. | 4000 | 130.32 | 3.87 | 14.30 | 1.83 | 97.03 | 22 520.48 | 85.44 |
5. Quantum chemical results
Optimized structure of lowest energy of conformer was used to calculate the quantum chemical parameters and to have insight into the reactivity of Sapindus. Frontier molecular theory was applied to interpret the results of quantum chemical studies. According to this theory reactivity, stability and interactions of a molecule solely depends upon the energy gap between the HOMO and LUMO of the interacting species. Larger the value of ∆E signifies the low reactivity, easy polarizability and high stability of interacting molecules. The molecules with large ∆E value are easily adsorbed on the surface of metal and acts as an effective corrosion inhibitor. Results of Quantum chemical study of Sapindus (table 8 and figure 5) revealed that it is an effective corrosion inhibitor and forms a adsorption layer on Aluminium which in turns protects Aluminium from corrosion in aggressive acidic medium.
Table 8. Calculated quantum chemical parameters for Sapindus.
| Inhibitor | EHOMO (eV) | ELUMO(eV) | ∆E(eV) |
|---|---|---|---|
| Sapindus | −5.44 | −1.21 | 4.23 |
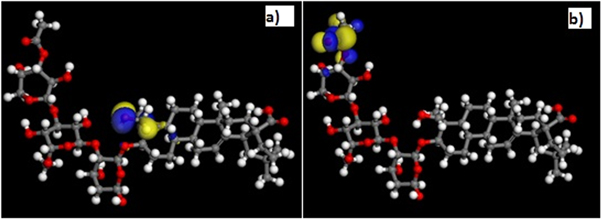
Figure 5. (a) Highest occupied molecular orbital (HOMO) and (b) Lowest unoccupied molecular orbital (LUMO) for Sapindus.
Download figure:
Standard image High-resolution imageThe HOMO and LUMO electron density distributions of studied compound showed the presence of O and π electrons that could offer electrons to the metal surface to form a coordinate type of bond. The distribution of HOMO electron density suggests that the π electrons are likely to participate in donating electrons to the appropriate vacant orbitals of the Al for effective corrosion inhibition. The LUMO electron density suggests that the compound is capable of accepting electrons from the appropriate occupied orbitals of the Al atom during the donor–acceptor interactions.
6. Monte carlo stimulation
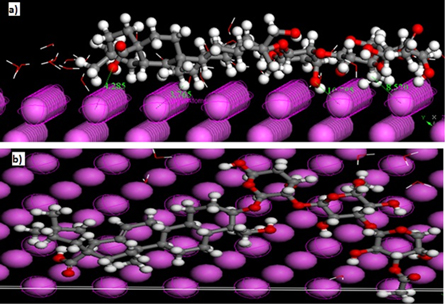
Monte Carlo studies revealed that adsorption energy of the Sapindus was far higher than that of water molecules thereby, suggesting the formation of protective layer. Figures 6(a) and (b) represents side and top view of most stable low energy configuration of Al(111)/50H2O system respectively, whereas results of MonteCarlo stimulation studied have been represented in table 9. It is very evident from table 9 which the adsorption energies of the inhibitors on Al surface within the presence of water expanded. It is by and large recognized that the essential phenomenon of corrosion inhibition of Aluminium is by adsorption. So the adsorption energycan furnish us with immediate information about the efficiency of inhibitors. In all cases, the adsorption energy of the Sapindus was far higher than that of water molecules. This shows the likelihood of steady substitution of water molecules from the Aluminium surface bringing about the formation of a protective layer which thereby inhibits corrosion on the surface of Aluminium.
Figure 6. (a) The most stable low energy configuration for the adsorption of Sapindus on Al(111)/50H2O system obtained using the Monte Carlo simulation, (a) side view and (b) Top view.
Download figure:
Standard image High-resolution imageTable 9. MonteCarlo simulation Results of corrosion inhibitors interaction on Al(111) in aqueous phase (Kcal/mol).
| Type of inhibitors | Total energy kJ mol−1 | Adsorption energy kJ mol−1 | Rigid adsorption energy | Deformation energy | sapindus: dEad/dNi | H2O: dEad/dNi |
|---|---|---|---|---|---|---|
| Al(111)/Sapindul/50 H2O | −503.19 | −519.10 | −534.17 | 15.07 | −130.80 | −1.74 |
7. Scanning electron microscopy (LEO435 VP)
The surface morphologies of plain Aluminium surface and aluminium in 1 M HCl without and with 2000 mg L−1 of Sapindus have been exhibited in figure 7. It is clear that the surface of plain aluminium before inundation in 1 M HCl solution displays a smooth surface. The Aluminium coupon inundated in 1 M HCl solution without the inhibitors demonstrated a profoundly rough and disintegrated surface due to corrosion, and the aluminium coupon immersed in a solution of 2000 mg L−1 in 1 M HCl shows visibly smooth surface with much less pores and cavities. This affirms the Sapindus shapes a defensive covering of Sapindus extract on the Aluminium surface thus, inhibiting corrosion.
Figure 7. (a) SEM images of polished Aluminium, (b) Aluminium immersed in 1 M HClwithout inhibitor and (c) Aluminium immersed in 1 M HClsaturated with Sapindus extract.
Download figure:
Standard image High-resolution image8. Atomic force microscopy (NT-MDT-INTEGRA)
In atomic force microscopy we predicted the morphology of the metal with or without Sapindus (figure 8). It is clear from the roughness values in table 10 that Sapindus forms a protecting covering on the surface of Aluminium, thus inhibits the acidic corrosion of Aluminium.
Figure 8. (a) AFM images of polished Aluminium, (b) Aluminium immersed in 1 M HClwithout inhibitor and (c) Aluminium immersed in 1 M HClsaturated with Sapindus extract.
Download figure:
Standard image High-resolution imageTable 10. Roughness values of pure, blank, inhibitor Aluminium with 1 M HCl.
| Sr.No. | Pure | Blank | Inhibitor |
|---|---|---|---|
| Roughness (Ra) | 141.54 nm | 827.89 nm | 147.90 nm |
9. Conclusions
Sapindus behaved as an excellent inhibitor of acidic corrosion for Aluminium with efficiency of 98% in 1 M HCl at 2000 mg L−1. As per Potentiodynamic polarization studies Sapindus was found to be a mixed type inhibitor which follows Langmuir adsorption isotherm. The EIS study revealed that Sapindus formprotective film on Aluminium surface. Theexperimental results showed that the studied Sapindus adsorbs spontaneouslyon Aluminiumsurface and confirmsto the Langmuir adsorption sotherm. Formation of a protective layer on the surface of Aluminium by Sapindus in acidic medium was the main reason for the inhibition of corrosion of Aluminium. Potentiodynamic polarization confirmed the formation of protective layer as the adsorption energy of Sapindus was higher than that of water molecules. Morphology (SEM and AFM) and computational studies (Quantum chemical and Monte carlo) also confirmed the same and proved Sapindus a green corrosion inhibitor.
Acknowledgments
We are grateful to Shree Ashok Mittal, Honorable Chancellor, Lovely ProfessionalUniversity, Punjab, India for providing facilities to carry out the aforementioned research. We also thank Professor Gurmeet Singh, Delhi University for providing EIS facility.