Abstract
Modern agriculture calls for a decrease in pesticide application, particularly in order to decrease the negative impact on the environment. Therefore the development of new active substances and plant protection products (PPP) to minimize the chemical load on ecosystems is a very important problem. Substances based on silver nanoparticles are a promising solution of this problem because of the fact that in correct doses such products significantly increase yields and decrease crop diseases while displaying low toxicity to humans and animals.
In this paper we for the first time propose application of polymeric guanidine compounds with varying chain lengths (from 10 to 130 elementary links) for the design and synthesis of modified silver nanoparticles to be used as the basis of a new generation of PPP. Colloidal solutions of nanocrystalline silver containing 0.5 g l−1 of silver and 0.01–0.4 g l−1 of polyhexamethylene biguanide hydrochloride (PHMB) were obtained by reduction of silver nitrate with sodium borohydride in the presence of PHMB. The field experiment has shown that silver-containing solutions have a positive effect on agronomic properties of potato, wheat and apple. Also the increase in activity of such antioxidant system enzymes as peroxidase and catalase in the tissues of plants treated with nanosilver has been registered.
Export citation and abstract BibTeX RIS
1. Introduction
World production of plant protection products (PPP) to control weeds, plant pests and phytopathogens of over 2 million tons has an unquestionable negative effect on the environment [1]. Often wide application of pesticides leads to the emergence of resistance in pests and pathogens, pesticides bioaccumulation, the decrease in soil biodiversity and nitrogen fixation as well as depletion of the numbers of pollinating insects and the loss of birds' natural habitats [2–6]. In order to eliminate the aforementioned adverse effects and to protect the environment the application rate of PPP per hectare must be decreased. This aim can be reached only by development of new broad-spectrum products.
Development of products based on silver nanoparticles is a very promising approach to the creation of innovative PPP. It has been established that silver compounds including silver nanoparticles possess powerful antiseptic [7–11], immunomodulating [12] and growth-promoting properties [13].
Nevertheless the mechanisms of activation of various biochemical and physiological processes in plants and animals with silver nanoparticles have been studied insufficiently [14]. Biochemical mechanisms of plant growth regulation with silver nanoparticles are based on the ethylene receptors inhibition which leads to IAA growth phytohormone accumulation [15]. Thus, plant growth stimulating properties of silver nanoparticles were confirmed during experiments on various grain crops including Lycopersicon esculentum [16], Arabidopsis [17, 18], Bacopa monnieri [19], Oryza sativa [20, 21].
Biological properties of silver nanoparticles are defined not only by their shape and size but also by the stabilizing agent [22]. The latter in most cases has a major influence on the properties of nanoparticles.
A number of recent research papers describes functional stabilization of silver nanoparticles (i.e. anchoring bioactive substances such as enzymes onto the nanoparticle surface) which results in a dramatic increase of their biological activity [23–26].
Polyhexamethylene biguanide hydrochloride (PHMB) is one of those promising stabilizing substances. PHMB is an efficient antibacterial and fungicidal agent, at the same time it is characterized by high biocompatibility and low toxicity [27].
It has been established that the medium molecular weight/chain length of polymers used for silver nanoparticles stabilization has a pronounced effect on formation [28–30], stability/aggregative stability [31, 32], the shape [33, 34] and the size [35–38] of particles in sols. Therefore a study of the influence of PHMB with various chain lengths on the properties of the silver nanoparticles obtained in its presence is of a particular interest.
This paper describes the influence of PHMB concentration and its average molecular chain length on the properties of silver nanoparticles obtained in its presence. The most promising substance to be used as a PPP has been selected, its field trials have been carried out on potato, wheat and apple to study the substance's growth stimulating properties. Special attention was paid to the variations in the enzymes (peroxidase, catalase and polyphenol oxidase) activity in plant tissues triggered by PHMB-stabilized silver nanoparticles as an alteration in the percentage of these enzymes indicates fungicidal activity of the particles.
2. Materials and methods
2.1. Reagents and materials for AgNPs synthesis
Silver nitrate (Sigma-Aldrich, 99+%), sodium borohydride (Acros Organics, 99%), 20% PHMB hydrochloride aqueous solution with M = 2200 (Arch Biocidals, 98%), 20% PHMB hydrochloride aqueous solution with M = 3300 (Shanghai Terppon Chemical, 98%), 20% PHMB hydrochloride aqueous solution with M = 4400 (Hainan Zhongxin Chemical, 98%), PHMB hydrochloride aqueous solution with M = 28 500 (Shijiazhuang Lemandou Chemicals, 98%) were used without further purification.
2.2. Synthesis of PHMB-stabilized silver nanoparticles colloidal solutions
Colloidal solutions of nanocrystalline silver containing 0.5 g l−1 of silver and 0.01–0.4 g l−1 of PHMB were obtained by reduction of silver nitrate with sodium borohydride in the presence of PHMB. 50 ml of a 0.046 M aqueous solution of silver nitrate were added dropwise to 300 ml of a 0.008–0.333% aqueous solution of PHMB (with varying average molecular weight) with continuous stirring. As a result a white suspension was obtained. The suspension was kept for 15 min. Then an aqueous solution of sodium borohydride (150 ml, 0.062 M) was added dropwise with continuous stirring. After a reducing agent was added the mixture was kept for 1 h to interfuse, its color going from dark brown to dark green, depending on the average molecular weight of the stabilizer. For further analysis a part of the resulting colloidal solutions was rinsed with distilled water several times, isolated by centrifugation (25 000 rpm) and air-dried for 24 h at 50 °С.
2.3. Characterization of silver nanoparticles
2.3.1. UV–visible spectrophotometry
A double beam spectrophotometer Shimadzu UV-1800 was used to register the absorption spectra in the visible and near-UV ranges.
2.3.2. Dynamic light scattering
Particle size distribution was determined by using a dynamic light scattering spectrometer Zetasizer Nano ZS with an installed 4 mW He–Ne laser with λ = 633 nm (Malvern Instruments Ltd, Great Britain). Zeta potential was measured by determining the velocity of particles in the electric field by means of laser Doppler anemometry on NanoBrook Omni equipment.
2.3.3. Transmission electron microscopy
Microphotographic images of the nanocrystalline silver samples were obtained using a transmission electron microscope LEO 912 AB OMEGA (Carl Zeiss, Germany) with accelerating voltage of 100 kV. The samples were prepared by applying of 1–2 ml of the suspension on a copper mesh with further air-drying. Particle size distribution according to the data obtained by microscopy was calculated using Femtoscan Online v. 2.2.91 software (Advanced Technologies Center, Russia).
2.3.4. X-ray powder diffraction
The x-ray images of the centrifuged colloidal solution of nanocrystalline silver were obtained by means of a Bruker D8 Advance diffractometer (in Bragg-Brentano geometry) using CuKα radiation. Diffraction maximums were identified using the JCPDS database. Сoherent scattering regions of the nanocrystalline silver samples were calculated using the Scherrer formula [39].
2.3.5. IR spectroscopy
The spectra of the powders were registered using an IR-Fourier spectrometer Bruker EQUINOX 55 within the range from 520 to 3700 cm−1 utilizing frustrated total internal reflection. Band assignment was carried out according to [40].
2.3.6. X-ray photoelectron spectroscopy
XPS for this research was carried out using a LAS-3000 (Riber, France) spectrometer equipped with a hemispherical ESCA electron analyser OPX-150. Monochromatic Al Kα x-ray source (1486.6 eV) was used as a source of exciting radiation. Working pressure of 10−10 was maintained. To correct for sample charging all the recorded spectra were referenced to the aliphatic C1s peak at 284.8 eV as an internal standard.
2.3.7. XPS analysis
For spectrum deconvolution it was assumed that the line shapes are Gaussian after the background removal. To obtain images the samples were applied on aluminium substrates in a powdered form.
2.4. Field experiments
Growth stimulating properties of PHMB-stabilized silver nanoparticles were evaluated during field experiments on potato (Solanum tuberosum L., 1753 cv. Meteor), spring wheat (Triticum L. 1753 cv. Triso) and apple (Malus P. Mill., 1754 cv. Lobo and Vishnevoye) plants [41–43].
2.4.1. Design of the experiment on potato
(pС0) Control experiment without silver nanoparticles treatment.
(pA1) Treatment with an Ag-PHMB(15) sample. Preplant treatment of tubers with the product consumption of 50 ml metric ton−1, working solution consumption of 10 l metric ton−1; spraying of plants at a flower bud stage with the product consumption of 50 ml ha−1, tank solution consumption of 300 l ha−1.
(pA2) Treatment with an Ag-PHMB(15) sample. Preplant treatment of tubers with the product consumption of 75 ml metric ton−1, working solution consumption of 10 l metric ton−1; spraying of plants at a flower bud stage with the product consumption of 75 ml ha−1, tank solution consumption of 300 l ha−1.
(pA3) Treatment with an Ag-PHMB(15) sample. Preplant treatment of tubers with the product consumption of 100 ml metric ton−1, working solution consumption of 10 l metric ton−1; spraying of plants at a flower bud stage with the product consumption of 75 ml ha−1, tank solution consumption of 300 l ha−1.
The experiment was repeated 3 times. Randomized block design was maintained, the area of each plot was 42 m2.
The overall yield of tubers and the yield structure were evaluated for each plot, commercial (tubers with a cross-sectional diameter over 50 mm) and non-commercial (tubers with a cross-sectional diameter under 50 mm) fractions were weighed separately.
The following characteristics were measured in the harvested potato tubers: starch content was determined by a weight method [44]; dry matter content was measured according to the method by Shipley [45]; vitamin C content was measured according to the method by Murry [46]; nitrate content was measured by an ion-selective method [47]; cooking qualities were evaluated by the procedure specified by the European Association [48]; blackening of the flesh (raw and cooked); cooking property.
2.4.2. Design of the experiment on wheat
A randomized experiment with four replicates was set up in plots of 100 m2 (25 × 4) considering four treatments:
(wС0) Control experiment without treatment.
(wA1) PHMB stabilized AgNPs. Pre-seeding treatment of seeds with the product consumption of 40 ml metric ton−1, working solution consumption of 10 l metric ton−1; spraying of plants at the end of the tillering stage – beginning of leaf-tube formation with the product consumption of 40 ml ha−1, tank solution consumption of 300 l ha−1;
(wA2) PHMB stabilized AgNPs. Pre-seeding treatment of seeds with the product consumption of 60 ml metric ton−1, working solution consumption of 10 l metric ton−1; spraying of plants at the end of the tillering stage—beginning of leaf-tube formation with the product consumption of 60 ml ha−1, tank solution consumption of 300 l ha−1;
(wA3) PHMB stabilized AgNPs. Pre-seeding treatment of seeds with the product consumption of 80 ml metric ton−1, working solution consumption of 10 l metric ton−1; spraying of plants at the end of the tillering stage—beginning of leaf-tube formation with the product consumption of 80 ml ha−1, tank solution consumption of 300 l ha−1;
During the experiment the seed germination capacity and crop productivity were measured for spring wheat [41].
2.4.3. Design of the experiment on apple
(aС0) Control experiment without treatment.
(aA1) PHMB stabilized AgNPs. Spraying of plants was carried out twice: at the pink bud stage and at the post-fruit set ('walnut') stage with the product consumption of 150 ml ha−1, tank solution consumption of 1000 l ha−1.
(aA2) PHMB stabilized AgNPs. Spraying of plants: at the pink bud stage and at the post-fruit set ('walnut') stage with the product consumption of 200 ml ha−1, tank solution consumption of 1000 l ha−1.
(aA3) PHMB stabilized AgNPs. Spraying of plants: at the pink bud stage and at the post-fruit set ('walnut') stage with the product consumption of 250 ml ha−1, tank solution consumption of 1000 l ha−1.
Number of plants in each replication was 3. Number of sample trees was 9. The experiment was repeated 3 times.
During the experiment the following characteristics were measured: biometric parameters [41], yield, ascorbic acid and sugar content in the fruits [49], degustation evaluation, evaluation of the market and consumer qualities of the fruits.
2.5. Biochemical studies
2.5.1. Determining activity of polyphenol oxidase
The activity of polyphenol oxidase was determined via a common spectrophotometric technique measuring changes of optical density of the reaction products formed during oxidation of catechol (1,2-dihydroxybenzene) within a certain period of time [50].
2.5.2. Determining activity of peroxidase
The activity of peroxidase was determined by means of a common spectrophotometric technique, based on the assessment of the velocity of chemical reaction of benzidine (4,4'-diaminobiphenyl) oxidation in the presence of hydrogen peroxide and peroxidase [51].
2.5.3. Determining activity of catalase
The activity of catalase was determined by means of the spectrophotometric technique, as in the assessment of the velocity of the chemical reaction of decomposition of hydrogen peroxide with catalase [52].
2.6. Toxicity studies
Acute and chronic toxicity of the single-time intragastrically administered and long-term enterally administered aqueous dispersion of PHMB stabilized AgNPs was studied on white non-linear mice. All studies were carried out in Scientific Institution Research Center of Federal Medical and Biological Agency of Russia according to 'OECD Guidelines for the Testing of Chemicals, section 4: Health Effects' and 'Guideline for hygienic estimation of novel pesticides, № 4263-87 (Russia).
2.7. Bioaccumulation studies
The content of silver in tissues or organs of plants and laboratory animals was determined by means of inductively coupled plasma—atomic emission spectrometry using a Varian 720-ES ICP-AES spectrometer (Agilent Technologies, USA). Samples of biomaterial were decomposed with a mixture of saturated HCl and HNO3 3:1 v/v and then analyzed. All samples of biomaterial were prepared after crop harvesting.
2.8. Statistical analysis
Standard dispersions were calculated for the obtained data (n = 3 or 4). Analysis of statistically significant deviations of the processed data was carried out by means of the one-way ANOVA test with further application of Tukey test at the significance level 0.05. In some cases the least-significant difference was also provided (multiple t-test without α-correction).
3. Results and discussion
3.1. Synthesis of PHMB-stabilized silver nanoparticles
Silver reduction with sodium borohydride is one of the most applicable methods for silver nanoparticles manufacturing both in homogeneous and heterogeneous systems. Sodium borohydride, unlike any of other reducing agent (including citric and ascorbic acids, polyphenols and carbohydrates), possesses high reactivity; it is easier to use compared to the reduction with gaseous hydrogen or physical methods and it is also characterized by comparatively low toxicity. Simplicity of the methods allows to upscale this technique of PHMB-stabilized silver nanoparticles production to the industrial level.
Silver reduction with sodium borohydride proceeds according to the following scheme:
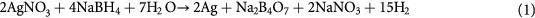
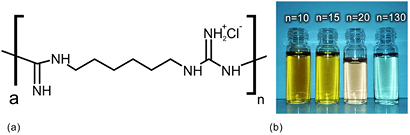
Silver reduction with sodium borohydride in the presence of PHMB (figure 1(a)) with a varying degree of polymerization resulted in formation of variously colored sols (figure 1(b)); these sols were stable for over 18 months. The sample names of colloidal solutions of silver nanoparticles stabilized with PHMB with various degrees of concentration and polymerization are listed in table 1.
Table 1. Sample codes, concentration, average particles size (according to TEM) of silver nanoparticles and the average polymerization degree of PHMB used for stabilization; in each case silver concentration amounted to 0.5 g · l−1 (500 ppm).
| Sample code | PHMB average polymerization degree | PHMB, g · l−1 | Ag:PHMB | Average particle size according to TEM, nm |
|---|---|---|---|---|
| Ag-PHMB5(10) | 10 | 0.05 | 200:1 | 5 ± 1 |
| Ag-PHMB(15) | 15 | 0.01 | 1500:1 | 6 ± 1 |
| Ag-PHMB5(15) | 15 | 0.05 | 300:1 | 5 ± 1 |
| Ag-PHMB40(15) | 15 | 0.4 | 40:1 | 4 ± 2 |
| Ag-PHMB(20) | 20 | 0.01 | 2000:1 | 5 ± 1 |
| Ag-PHMB5(20) | 20 | 0.05 | 400:1 | 5 ± 1 |
| Ag-PHMB(130) | 130 | 0.01 | 13000:1 | 15 ± 1 |
| Ag-PHMB5(130) | 130 | 0.05 | 2600:1 | 13 ± 1 |
| Ag-РHMB40(130) | 130 | 0.4 | 300:1 | 11 ± 4 |
Figure 1. (a) Structural formula of PHMB and (b) image of colloidal solutions of silver nanoparticles stabilized with PHMB with varying degrees of polymerization n.
Download figure:
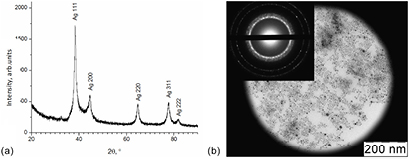
Standard image High-resolution imageIn each case during the reduction of silver nitrate the formation of crystalline silver nanoparticles was observed that was verified by the data obtained from RFA and electron diffraction. In figure 2(a) an x-ray photograph of an Ag-PHMB5(20) sample that is typical for all obtained samples can be observed. Silver phase formation (PDF 004-0783) can be observed in the figure 2. It has to be noted that a halo is present at 40° 2θ which is likely to correspond to the amorphous polymer. An electron diffraction pattern of the Ag-PHMB5(20) sample is presented in figure 2(b); it correlates with the data obtained from the x-ray phase analysis. Measuring of the first four rings of the diffraction pattern allowed us to determine the corresponding interplanar spaces d (2.34 Å, 2.01 Å, 1.43 Å, 1.22 Å). Their closeness to those of metallic silver (2.36 Å, 2.04 Å, 1.44 Å, 1.23 Å) indicates that the obtained nanoparticles have the same crystalline structure as bulk silver [53]. It should be noted that a wide ring (d = 2–2.3 Å) is observed in the diffraction pattern which is likely to correspond to diffraction on amorphous PHMB attached to the particles' surfaces.
Figure 2. (a) X-ray photograph and (b) electron microdiffraction pattern of the Ag-PHMB(20) sample.
Download figure:
Standard image High-resolution imageGiven that PHMB is a polycationic polymer containing imine nitrogen in its chain it can effectively stabilize silver nanoparticles by formation of a strong coordination bond Ag-N [54].
Presence of PHMB macromolecules on the surface of AgNPs is confirmed by the data obtained from XPS, namely by the presence of bands with a specific structure in C1s and N1s spectra (figure 3). Deconvolution analysis showed two C1s lines at 285 eV (carbon in CH2-groups) and at 287 eV (carbon in C =N-groups). N1s band contains three lines with energy peaks at 402, 403 and 403.5 eV that corresponds to the three various types of nitrogen compounds in PHMB molecules.
Figure 3. (a) C 1s, (b) Ag 3d and (c) N 1s photoelectron spectra for an Ag-PHMB5(15) sample.
Download figure:
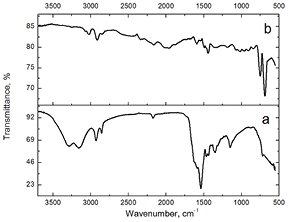
Standard image High-resolution imageCoordination of PHMB with AgNPs is indicated by the IR-spectroscopy results for the Ag-PHMB5(15) sample, presented in figure 4. Thus 3294 and 3157 cm−1 stretching vibration bands of N–H groups of PHMB(15) shift to 3073 and 3023 cm−1 correspondingly. Similar shift for the N–H amino groups stretching vibrations is observed in the presence of octadecylamine coordination on the AgNPs surfaces indicating formation of a strong coordination bond [55]. Disappearance of the C=N stretching vibration band with the peak at 1539 cm−1 also indicates the imine groups coordination with the AgNPs. Two narrow bands with peaks at 686 and 753 cm−1 appear in the IR spectrum of Ag-PHMB5(15) compared with the polymer spectrum corresponding to N–H bending. High intensity of the bands may correlate with participation of these groups in hydrogen bonds [40].
Figure 4. IR absorption spectrum for (a) PHMB(15) and (b) Ag-PHMB5(15) sample.
Download figure:
Standard image High-resolution imageAverage size of the AgNPs according to TEM was from 5 to 15 nm (table 1). The mean size and the size distribution of particles depend on the chain length of stabilizing PHMB and its concentration.
3.2. Effect of the PHMB concentration
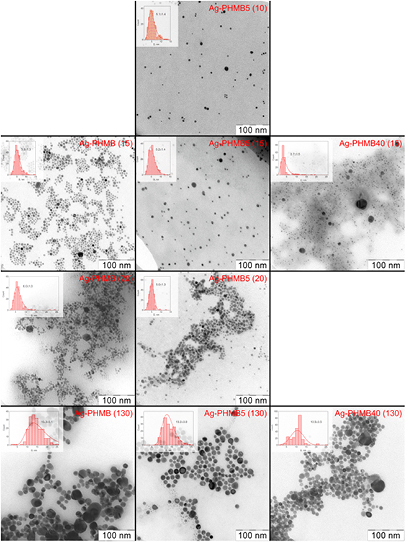
Figure 5 (the second line) represents microphotographs of the AgNPs obtained in the presence of 0.1, 0.5 and 4 g l−1 solutions of PHMB(15). At the low PHMB concentration a small amount of polycrystalline aggregates (~ 20–25 nm) is formed along with the major fraction of particles (~ 6 nm). Increase in the PHMB concentration up to 4 g l−1 provides a means to reduce the aggregates fraction as well as the average particle size from 6 to 4 nm. Evidently it happens because the polymer adsorption by the AgNPs blocks both their growth and aggregation.
Figure 5. TEM microphotographs and the particle size distribution of the PHMB stabilized AgNPs samples.
Download figure:
Standard image High-resolution imageData obtained from the electron microscope studies correspond well with the results obtained from the x-ray phase analysis and presented in figure 6.
Figure 6. X-ray diffractogram of (a) Ag-PHMB(15) and (b) Ag-PHMB5(15) samples.
Download figure:
Standard image High-resolution image3.3. Effect of the PHMB polymerization degree
According to TEM (table 1) the variations in the PHMB polymerization degree n from 10 to 20 have almost no effect on the nanoparticles' size, while the increase in the polymerization degree up to n = 130 results in the sharp rise of the nanoparticles' size in sols from 4–6 nm to 13–14 nm. It has been established that the molecular weight of stabilizing polymers (polyethylene glycol, polyvinylpyrrolidone, polyethyeneimine, natural polymers, chitosan) is a strong influence on formation [28–30], aggregative stability [31, 32], the shape [33, 34] and the size [35–38] of AgNPs. Thus in the case of polyvinylpyrrolidone the particle size enlargement along with the polymer chain elongation is generally associated with the increase of the specific sites of a macromolecule (free amide I groups), on which silver ions are deposited [36, 37]. PHMB also has an ability to coordinate silver ions through the secondary nitrogen [56]. From reduction of these complexes silver atoms may appear which later form clusters and nanoparticles. If we assume that initially a nanoparticle is formed out of atoms of metal coordinated by a single polymer molecule, then a particle size ought to depend linearly on the polymerization degree. However, a twofold increase in n from 10 to 20 results in no significant particle enlargement.
Particle size increase observed along with the enlargement of the stabilizing polymer molecules might be connected to the decrease in the nanoparticle stabilization efficiency because of reduction of the average number of macromolecules per each silver particle. Yet this assumption fails to square with the data as the sizes of the silver particles obtained at the same Ag:PHMB mole ratio for PHMB(15) and PHMB(130) differ twofold (table 1).
TEM data analysis indicates formation of large spherical cavities inside the particles (figure 5, bottom line). Apparently, AgNPs grow on the specific sites of polymer globules which get encapsulated inside the metallic shell and, as a result, large particles of the core–shell type are formed.
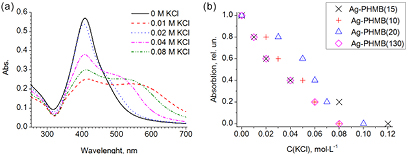
Absorption spectrum characteristic for the obtained AgNPs samples is presented in figure 7. A wide band with a peak near 390–405 nm is connected to the plasmon absorption of silver nanoparticles.
Figure 7. Effect of various concentrations of potassium chloride on the stability of silver nanoparticles coated with PHMB with various polymerization degrees.
Download figure:
Standard image High-resolution imagePHMB has proven to be an exceptionally good stabilizer allowing obtaining of highly concentrated dispersions of silver nanoparticles (silver density up to 5 g l−1) dilution resistant at least up to a silver density of 0.02 mg l−1. Nevertheless, PHMB-stabilized dispersions lost their aggregative stability in the presence of a fractional amount of chloride ion (figure 7(a)). With the increase in the ionic strength of the solution the ζ-potential of the nanoparticles falls below the dispersion stability level (±30 mV) leading to aggregation and sedimentation of the sol particles (table 2). Along with the increase in the stabilizing polymer chain length zeta potential of the particles reaches its peak value at n = 15 (table 2) while their resistance to the ionic strength remains unchanged (figure 7(b)). This phenomenon may be due to the increase in steric hindrances to aggregation because of the polymer molecular chain elongation which adds to the particles' stability when they avoid coagulation even when the ζ-potential is below the threshold level of 30 mV.
Table 2. Values of the destabilizing concentration of chloride anions and ζ-potential for PHMB-stabilized nanoparticles.
| Stabilizer | Number of sruct. units n | Mr(mean) g · mol | ζ-potential, mV | Destabilizing concentration of KCl, М |
|---|---|---|---|---|
| PHMB(10) | 10 | 2200 | +39.1 ± 1.5 | 0.079 M |
| PHMB(15) | 15 | 3300 | +46.4 ± 1.2 | 0.104 M |
| PHMB(20) | 20 | 4400 | +28.2 ± 1.3 | 0.096 M |
| PHMB(130) | 130 | 28500 | +17.2 ± 1.0 | 0.075 М |
According to table 3 silver nanoparticles stabilized with PHMB(15) possess the highest ζ-potential, that is why a sample based on this solution was selected for further study of its growth-stimulating and enzymatic activity because of its resistance to aggregation.
Table 3. Yield and biochemical indicators of the tuber quality in potato cv. Meteor.
| Version of experiment | Gross yield (increase compared to the baseline), metric ton ha−1 | Food grade yield, metric ton ha−1 | Dry matter, % | Starch, % | Vitamin C, mg per 100 g of tubers | Nitrates, mg per kg of tubers |
|---|---|---|---|---|---|---|
| pС0 | 30.2 | 7.6 | 19.7 | 14.0 | 15.4 | 59 |
| pA1 | 33.0 (9.3%) | 12.8 | 18.4 | 12.7 | 15.5 | 52 |
| pA2 | 35.1 (16.2%) | 14.6 | 18.0 | 12.3 | 14.8 | 52 |
| pA3 | 35.9 (18.9%) | 15.0 | 18.3 | 12.5 | 15.0 | 53 |
| LSD05 | 1.6 | 1.6 | 0.7 | 0.6 | 1.5 | 15 |
3.4. Plant growth and development stimulation of potato, wheat and apple
In experiments with potato the pre-planting treatment of tubers (50, 75 and 100 ml metric ton−1) followed by the foliar application (50, 75 and 100 mg ha−1) of Ag-PHMB(15) resulted in a considerable increase in the overall potato productivity and the food and seed grades of the tuber yield (table 3). The maximum additional yield was registered in the cases of pA2 and pA3 treatment and amounted to 4.9–5.7 metric ton ha−1 or 16–19 %; the seed potato yield was by 13.2 thousand tubers (4.5%) higher than the baseline value. We observed an increase in the food grade yields after each treatment with Ag-PHMB(15), this effect was dose-dependent and amounted to 68–100% compared to the baseline value (figure 8). After the treatment with various doses of Ag-PHMB(15) the weight of seed tubers remained stable (63–64 g) while the food grade tubers became larger weighing up to 118–126 g compared to 63 g and 82 g respectively.
Figure 8. Potato plants (a) (pC0) without treatment and (b) (pA3) pre-planting treatment of tubers with 100 ml/ton of Ag-PHMB(15), spraying of plants at a flower bud stage with 75 ml ha−1 of Ag-PHMB(15).
Download figure:
Standard image High-resolution imageTreatment of spring wheat seeds with Ag-PHMB(15) increased their germination energy by 1.7–4.9% over control (table 4) while the field germination rate increased by 9.2%, number of seedlings from the treated seeds was above control values by 11.1–11.4 %, on the average.
Table 4. Yield and productivity indicators of spring wheat.
| Version of experiment | Germination energy, % | Germination rate, % | Number of fertile stems, pieces m−2 | Ear grain content, pcs | 100 grains weigh, g | Yield, dt ha−1 |
|---|---|---|---|---|---|---|
| pС0 | 79.6 | 85.5 | 358 | 19.0 | 35.5 | 23.3 |
| pA1 | 81.3 | 89.2 | 364 | 19.5 | 35.2 | 24.0 |
| pA2 | 83.7 | 92.4 | 376 | 20.6 | 36.0 | 25.8 |
| pA3 | 84.5 | 94.7 | 393 | 21.1 | 36.6 | 27.2 |
| LSD05 | — | — | — | — | — | 2.38 |
Positive effect from treatment with the colloidal solution of Ag-PHMB(15) was registered in the variants where the spring wheat seeds and plants received 60 ml and 80 ml of the solution: the number of fertile stems increased by 18 and 35 pcs m−2, number of grains per ear increased to 20.6 and 21.1 grains, the weight of 100 grains increased by 0.5–1.1 g, respectively. Treatment with 40 ml produced results equal to those of the control experiment. Significant yield increase in the cases when the spring wheat was treated with 80 ml and 60 ml of Ag-PHMB(15) amounted to 3.9 dt ha−1 and 2.5 dt ha−1, respectively at LSD05 = 2.38 dt ha−1. The minimum dose of Ag-PHMB(15) resulted in no significant increase in the spring wheat yield.
It should be noted that in the case of the plants treated with Ag-PHMB(15) phenological growth stages were hastened by 1–4 days compared to the control (figure 9). Early and vigorous tillering indicates favorable growth conditions and is important for obtaining highly productive plants.
Figure 9. (a) Tillering stage (sets of 8 plants) and (b) ear stage (sets of 10 plants) of the spring wheat depending on the dose of Ag-PHMB(15): (wC0) without treatment, (wA1) treatment with 40 mg l−1 of Ag-PHMB(15), (wA2) treatment with 60 mg l−1 of Ag-PHMB(15) and (wA3) treatment with 80 mg l−1 of Ag-PHMB(15).
Download figure:
Standard image High-resolution imageStudy of foliage treatment of apple trees (cv. Lobo and Vishnevoye) with Ag-PHMB(15) has exposed the positive effect of the preparation which is manifested in slowing down the plants' vegetative growth and the improvement of their productivity (table 5). When the dose of the agent was increased from 150 ml ha−1 to 200–250 ml ha−1 the vegetative development indicators improved in both cultivars, at the same time productivity of cultivar Lobo decreased. The highest productivity rates for apple cultivar Vishnevoye were registered in the case of the 200 ml ha−1 treatment dose.
Table 5. Yield and biochemical quality indicators of apples, cultivars Lobo and Vishnevoye.
| Version of experiment | Vishnevoye | Lobo | ||||
|---|---|---|---|---|---|---|
| Apple yield, dt ha−1 | Sugars content, % | Ascorbic acid (Vitamin C), mg/100 g | Apple yield, dt ha−1 | Sugars content, % | Ascorbic acid (Vitamin C), % | |
| aC0 | 21.0 | 12.1 | 8.8 | 280.9 | 10.2 | 8.8 |
| aA1 | 22.7 | 13.9 | 10.6 | 315.5 | 8.9 | 10.6 |
| aA2 | 25.0 | 13.5 | 8.8 | 285.4 | 10.5 | 7.0 |
| aA3 | 24.6 | 12.3 | 9.7 | 291.3 | 12.1 | 10.6 |
| LSD05 | 1.9 | 1.1 | 1.3 | 7.5 | 1.2 | 0.9 |
Foliage treatment of apple trees cultivar Lobo with Ag-PHMB(15) in larger doses results in an increase in bud primordia formation by 74.8 %. Foliage treatment of apple plants cultivars Lobo and Vishnevoye with Ag-PHMB(15) lead to almost no significant increase in Vitamin C and sugars content of fruits.
Thus, the PHMB-stabilized silver nanoparticles have a considerable growth-stimulating effect on the studied agricultural plants. This effect can be explained in terms of the biological activity of silver nanoparticles. Silver has the most pronounced effect on physiological mechanisms regulated by the ethylene plant hormone [57]. Often the observed biological influence of low and medium doses of silver ions is explained by the activity of an inhibitor of the ethylene response [58]. For example, silver salts have a stimulating effect on the stem, root and leaves growth, root branching, early flowering, also these compounds prevent leaf abscission and blossom withering. A number of other effects traditionally associated with the inhibition of the ethylene biosynthesis or a decrease in the plant sensitivity to ethylene are observed, in particular the stimulation of the transport of auxins, an increase in production of biogenic polyamines [58], which accelerate cell proliferation.
Such activity of silver can be attributed to the ability of Ag+ ions to displace copper ions (I) with the resembling structure from the ethylene receptor ETR1 localized in the cell membrane [59]. Silver ions interfere with the normal activity of the ethylene receptor and prevent it from transmitting signals to the cell [59].
3.5. Influence of silver nanoparticles on the activity of enzymes in plant tissues (potato, wheat, apple)
Phytopathogenic microorganisms have a strong impact on biochemical processes in plants. In the course of evolution plants have developed various systems allowing them to resist diseases. Mechanisms of plant immunity vary greatly. Production of reactive oxygen intermediates is one of the most common responses to the phytopathogens' influence [60]. Interaction between a pathogen and a host plant results in a serious alteration in the functional status of the plant that is reflected, first of all, in the activity of enzymes [60].
Peroxidase is one of such enzymes. It is a two-component enzyme, an iron containing protein. The enzyme is localized in the cell wall, mitochondria, chloroplasts, cell nucleus, cytoplasm and other organelles. Peroxidase is highly sensitive to the environmental influence on the plants, moreover, the plant's response to biotic stressors resembles the response to the abiotic ones [61].
Figure 10(a) represents the experimental data on peroxidase activity in potato, wheat and apple. Its activity increased in every case. The highest degree of increase was registered in apple leaves after treatment with Ag. It may be connected to the development of the hypersensitive reaction due to the oxidative stress. This suggestion is supported by the observation of marginal necrosis on apple leaves on a number of occasions (figure 11).
Figure 10. Peroxidase activity in healthy agricultural plants of potato, wheat and apple (a) and in infested potato plants (b).
Download figure:
Standard image High-resolution imageFigure 11. Marginal necrosis on the apple leaves after treatment with the preparation.
Download figure:
Standard image High-resolution imageAlong with the aforementioned the peroxidase content can increase in response to the invasion by pathogens and the infection contamination of the plant tissues [61]. Quinones and products of their condensation resulting from oxidation with peroxidase possess fungitoxic and bactericidal properties and serve as a chemical barrier during necrotic surface formation. This biochemical response can be used as an indicator of the plant's resistance because these processes often occur simultaneously. Nevertheless, the increased peroxidase activity does not always correlate with resistance and does not always induce it. Some infections cause reduction in the peroxidase activity.
Figure 10(b) represents the activity of peroxidase in potato infested with late blight and Alternaria blight. The study showed that as a whole, activation dynamics of this enzyme is higher in plants treated with Ag-PHMB(15) than in control plants. Also, the peroxidase activity in the infested plants treated with Ag-PHMB(15) appears to be higher compared to non-infested plants treated with the same dispersion. This observation indicates an increase in one of the main non-specific defense reactions and, consequently, in non-specific resistance of the treated plants.
As in the case of peroxidase, treatment with Ag-PHMB(15) influenced catalase activity, especially in the affected plants. Catalase activity in the leaves increased to some degree (figure 12(a)), particularly in the apple plants, which, similarly to the case with peroxidase, indicates high sensitivity of the plant to the preparation. Catalase activity in the potato leaves slightly decreased under the influence of late blight and Alternaria blight (figure 12(b)).
Figure 12. Catalase activity in the healthy agricultural plants of potato, wheat and apple (a) and in the infested potato plants (b).
Download figure:
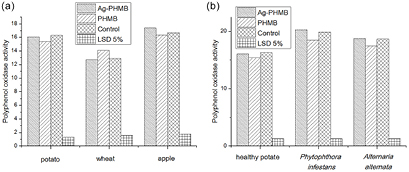
Standard image High-resolution imageAlteration in the polyphenol oxidase activity under the influence of preparations containing silver (figure 13) was not as pronounced as in the case of other enzymes. We observed no significant difference between the variants.
Figure 13. Polyphenol oxidase activity in the healthy agricultural plants of potato, wheat and apple (a) and in the infested potato plants (b).
Download figure:
Standard image High-resolution image3.6. Accumulation of silver nanoparticles in plant tissues
Bioaccumulation of silver nanoparticles in plant tissues after the spraying treatment of potato, wheat and apple trees with the Ag-PHMB(15) dispersion was studied after harvesting; silver content in the potato tubers, wheat grain, apple fruits, leaves and branches (table 6) was determined. After the spraying treatment silver was usually found to accumulate in the leaves and, less often, in the branches. When the dose of the agent was increased from 150 ml ha−1 to 200–250 ml ha−1 the silver content increased respectively. No significant migration of silver in edible parts of the plants was observed. Silver content in the harvested crops did not exceed 0.02 ppm, which is safe enough for human consumption. For example, the US EPA drinking water alert limits of silver is 0.1 ppm [62].
Table 6. Bioaccumulation of silver in the dried plant tissues after treatment with the Ag-PHMB(15) dispersion.
| Version of experiment | pC0 | pA1 | pA2 | pA3 | LSD05 |
|---|---|---|---|---|---|
| Silver content in tubers, ppm | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 |
| Version of experiment | wC0 | wA1 | wA2 | wA3 | LSD05 |
| Silver content in grain, ppm | <0.01 | <0.01 | <0.01 | 0.01 | 0.01 |
| aC0 | aA1 | aA2 | aA3 | LSD05 | |
|---|---|---|---|---|---|
| Silver content in apple fruits, ppm | <0.01 | <0.01 | 0.01 | 0.02 | 0.01 |
| Silver content in leaves, ppm | <0.01 | 1.3 | 1.6 | 2.2 | 0.3 |
| Silver content in branches, ppm | <0.01 | 0.1 | 0.1 | 0.3 | 0.02 |
The Ag-PHMB(15) dispersion was registered as a PPP in Russia and some other countries. As a part of the registration procedure the safety of PHMB-stabilized silver nanoparticles was estimated; Ag-PHMB(15) was characterized by low acute and chronic toxicity when intragastrically administered to laboratory animals (white non-linear mice). The value of DL50 of Ag-PHMB(15) in the case of single-time intragastric administration exceeds 10 ml kg−1 of body weight. According to the classification DL50 the Ag-PHMB(15) dispersion is referred to as a low hazard substance when intragastrically administered to laboratory animals (mice). Also long-term 28 days enteral administration in doses of 2 ml kg−1 of body weight did not lead to a damaging effect on the performance status of the mice. Thus, the Ag-PHMB(15) dispersion may be considered safe enough to be used as a plant protection agent.
4. Conclusions
It was found that an increase in concentration results in a decrease in the average size of nanoparticles, presumably due to blocking and aggregation, and to silver particles' growth during polymer adsorption on their surfaces. Variation of the polymerization degree of PHMB within the range n = 10–20 has almost no influence on the nanoparticle size, while the increase in n up to 130 results in sharp enlargement of nanoparticles in sols from 4–6 nm to 13–14 nm. The most stable colloidal solution of silver nanoparticles was obtained in the presence of PHMB at concentration of 0.5 g l−1 and with an average degree of polymerization n = 15.
In general, treatment with the obtained PHMB-stabilized silver nanoparticles improved the development of the experimental cultures. For potato we recorded an increase in the gross yield by 9.3–18.9 %, the yield of food grade tubers increased by 5.2–7.4 tons ha−1 while the nitrates content was reduced by 5–7 mg kg−1. Treatment of wheat with nanosilver preparations increased the germinating capacity by 10%, earlier phenological stages were observed in comparison to the control, a significant yield increase amounted to 3.9 dt ha−1 and 2.5 dt ha−1 in the cases when spring wheat was treated with 80 ml and 60 ml of Ag-PHMB(15), respectively. Foliage treatment of apple trees (cv. Lobo and Vishnevoye) with Ag-PHMB(15) revealed that the preparation has a positive effect which is manifested in slowing down the plants' vegetative growth and the improvement of their productivity. The highest productivity of apple cultivar Vishnevoye was recorded when the plants were sprayed with 200 ml ha−1 of the preparation, for apple cultivar Lobo the highest productivity was recorded after the treatment with 150 ml ha−1 of the preparation.
Nanosilver enhanced the activity of the antioxidant system enzymes, namely peroxidase and catalase. The highest increase in peroxidase activity was registered in apple leaves. It may be connected to the development of the hypersensitive reaction due to the oxidative stress. This suggestion is supported by observation of marginal necrosis on the apple leaves on a number of occasions.
PHMB-stabilized nanosilver was found to usually accumulate in the leaves after the foliar treatment. No significant migration of silver in edible parts of the plants was observed.
Thus, this research showed that the use of silver-containing preparations for growth stimulation is a successful agricultural method improving the resistance of the treated plants to fungal diseases and increasing the agricultural productivity.
Acknowledgments
This work was partially supported by the RFBR grant (Project No 17-44-680001) regarding potato growth stimulation research.