Abstract
Size-controllable monodisperse CdSe nanocrystals with different organic capping were prepared based on the hot-injection method. The effective separation of nucleation and growth was achieved by rapidly mixing two highly reactive precursors. As a contrast, we prepared CdSe/CdS nanocrystals (NCs) successfully based on the selective ion layer adsorption and reaction (SILAR) technique. This inorganic capping obtained higher photoluminescence quantum yield (PLQY) of 59.3% compared with organic capping of 40.8%. Furthermore, the CdSe-epoxy resin (EP) composites were prepared by adopting a flexible ex situ method, and showed excellent stability in the ambient environment for one year. So the composites with both high PLQY of nanocrystals and excellent stability are very promising to device application.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The colloidal semiconductor nanocrystals (NCs), also known as quantum dots (QDs), have attracted particular attention due to their controllable size-dependent optical and electronic properties, narrow and intense fluorescence emission and immunity from photobleaching [1, 2]. The CdSe NCs, with band-edge photoluminescence (PL) emission ranged from 450 nm to 700 nm, have been widely used to fabricate solar cells [3] and visible light emitting diodes (LEDs) [4]. Over the past ten years, a series of methods have been used to synthesize CdSe NCs with uniform size distribution and narrow band-edge emission [5, 6]. However, as-prepared NCs exhibit low photoluminescence quantum yield (PLQY) and their PL is extremely sensitive to the local environment [7]. The passivation of the NCs surface with organic ligand can improve PLQY, but the ligand can desorb from the surface of NCs, resulting in bad stability. Compared with organic passivation, the inorganic passivation by forming core/shell nanostructure can improve both the PLQY and environmental stability. In particular, Bawendi et al [8] recently developed a new method to prepare CdSe/CdS NCs to achieve suppressing blinking, and the ensemble PL photodarkening induced by blinking under long time excitation is eliminated.
Even so, many applications based on NC solids need more stable optical properties. Incorporating NCs into polymer materials can not only enable special optical properties but also improve the stability of NCs. At the same time, these composites provide novel manufacturing properties, which allow the achievement of patterning manufacture [2]. In the past years, many LED applications based on these NCs-epoxy polymer composites have emerged [9–11], which combine the high PLQY of NCs, and good stability against UV radiation and heating of epoxy polymer. Based on this excellent property of composites, different single color as well as white light LEDs were fabricated, promising to achieve industrialization.
Even though the QDs preparation is very mature, the preservation of QDs is still a problem because nanometers size QDs have lots of exposed atoms on their surface, which are quite easily oxidized in air. In this paper, based on the necessity of long-term preservation of QDs, the QDs-epoxy resin composites were prepared by adopting a flexible ex situ method, and this composite greatly improved the optical stability in ambient environment for one year. We report the detailed preparation method and emergent novel optical properties of CdSe-TOP NCs, CdSe-ODA-TOPO NCs, CdSe/CdS NCs, and CdSe-EP composites. In particular, we adopted a flexible ex situ method to get CdSe-EP composites. Achieving wonderful dispersion of NCs in the epoxy resin is the key point of this application. Due to the organophobic surface of NCs and intrinsic hydrophobic property of epoxy resin, the homogeneity mixture can be got with the help of organic solvent. In particular, many emergent optical phenomena such as size-dependent Stokes shift in CdSe NCs are explained in detail, and the reason for the improved photoluminescence quantum yield and stability are also analyzed. This route can be convenient to control the mixing proportion of two components to achieve customized propertiesem, which paves the way for device application.
2. Experimental
2.1. Synthesis of CdSe-TOP and CdSe-ODA-TOPO NCs
CdSe NCs were prepared based on previous methods [6, 12]. Typically, CdO (0.06 g) and oleic acid (1.8 ml) were added into 4.5 ml of 1-octadecene (ODE) in a 3-neck round-bottom flask, and the mixture was heated to 240 °C for 30 min to obtain clear light yellow cadmium oleate precursor solution. Then the reagent was cooled down to room temperature. For CdSe-ODA-TOPO NCs, 0.3866 g of trioctylphosphine (TOPO) and 1.16 g of octadecylamine (ODA) were added into cadmium oleate precursor solution, and then the reagent was heated to 280 °C until the TOPO and ODA were totally dissolved. Next, a solution of Se (0.47 mmol) dissolved in 1.4 ml of TOP was swiftly injected into the reagent at 280 °C, and the reaction temperature was cooled to 250 °C for different growth time. Then the reaction was quenched with a water bath. Finally, the NCs were separated by addition of methanol/acetone and centrifugation at 4800 rpm for 20 min. All syntheses were performed under argon flow in order to get an air-free atmosphere.
2.2. Synthesis of CdSe/CdS core/shell NCs
The CdSe/CdS core/shell NCs was prepared based on Mulvaney's method [7]. 0.4 μmol of CdSe NCs in chloroform synthesized above was added to a 3-neck round-bottom flask along with a mixture of ODA/ODE to produce a 40 μM of CdSe NCs solution. Then the solution was heated to 70 °C to remove chloroform. Next, the calculated amount of Cd-OA-ODE precursor was firstly injected into reaction solution at 230 °C for 15 min to passivate CdSe NCs. Then specific amounts of S-ODE and Cd-OA-ODE precursor were injected successively to achieve a different CdS monolayer. The specific added amount of both precursors is calculated by the equation in the supporting information. Finally, the reaction was processed at 200 °C for 1 h to improve the crystal. The wash and centrifugation process were the same with CdSe NCs.
2.3. Preparation of CdSe/EP composite
Firstly, EP700A and EP700B were mixed with the mass ratio of 1:1. Then, a certain amount of CdSe NCs in chloroform was added under vigorous stirring. The mixture was injected into the mould after it reached homogeneity. Finally, the mould was transferred to a vacuum drying chamber at 90 °C for 5 h so that the epoxy resin could cure.
Transmission electron microscope (TEM) characterization was performed on a JEOL JEM-2100HR operating at 200 kV. Optical absorption data were acquired with a Jasco V-570 UV–Vis spectrophotometer. Photoluminescence spectrums were done using Gilden Photonic Sens-9000.
3. Results and discussion
Figure 1(a) shows the evolution of absorption profile with increasing growth time of synthesized CdSe-TOP NCs. From it, the first absorption peak is relatively sharp, which indicates a relatively narrow size distribution. With increasing growth time, the absorption peak appears as a red-shift indicating a progressive increase in the particle size of CdSe-TOP NCs. The corresponding value is quite consistent with previous reports [6, 12]. Moreover, according to Peng's fitting functions [13] between the wavelength of the first absorption peak and particle size, we can get the corresponding particle sizes as 2.85 nm, 3.12 nm, 3.46 nm and 3.78 nm, respectively. Figure 1(b) presents the evolution of corresponding PL spectrum. The Stokes shift gradually decreases with increased size of CdSe NCs, which also has been observed in other semiconductor QDs such as CdTe [14], and ZnO [15]. This size-dependent Stokes shift in semiconductor QDs has been theoretically analyzed by Bawendi [16]. When CdSe QDs are excited above the band-edge absorption, the resulting full luminescence Stokes shift is called the nonresonant Stokes shift, which is connected with the band-edge exciton fine structure. The difference in energy between the upper state and the dark exciton ground state for small size QDs is larger than the big one, which accounts for the magnitude of the Stokes shift. This large Stokes shift of semiconductor QDs effectively avoids reabsorption, which will be helpful to calculate PLQY accurately.
Figure 1. (a) Optical absorption spectrum and (b) PL spectrum of CdSe-TOP NCs with different growth time: (a) 20 s, (b) 30 s, (c) 60 s, (d) 120 s. The absorption peak is 540 nm, 554 nm, 568 nm and 579 nm, respectively, and the corresponding PL peak is 574 nm, 584 nm, 593 nm and 595 nm, respectively.
Download figure:
Standard image High-resolution imageIn order to investigate the effect of organic and inorganic capping on PLQY of CdSe NCs, we prepared CdSe-ODA-TOPO NCs and CdSe/CdS NCs. Compared the high-resolution TEM (HRTEM) images in figures 2(a) and (b), we can get the particle size of 3.75 nm and 6.05 nm. Based on Mulvaney's calibration curve between the core/shell dimensions and their optical response [7], we can determine that the thickness of CdS shell is 1.15 nm, which corresponds to 3 monolayer (ML). Besides, the lattice spacing in both HRTEM images is 0.35 nm, corresponding to the (002) plane of the wurtzite structure. Due to the 3.9% lattice mismatch in CdSe/CdS NCs, it is hard to identify the boundary. Figure 2(c) presents the TEM images of CdSe/CdS-3ML NCs in a large scale, and the particle size distribution analysis is shown in figure S1. The mean size is 6.01 nm and standard deviation is 10%, which indicates their uniform size distribution. The selected area electron diffraction (SAED) of CdSe/CdS-3ML NCs is shown in figure 2(d), which further confirms the wurtzite crystal phase of the core/shell NCs, and proves that CdS shell growth proceeds in an epitaxial manner. The energy dispersive spectrometer (EDS) analysis performed on a selected area of a few hundred of CdSe/CdS-3ML NCs is shown in figure S2. The relative atomic percentage of Cd, Se, and S is 2.2: 1: 1.2, which is consistent with the fact that the thickness of CdSe and CdS layers is nearly equal for CdSe/CdS-3ML NCs.
Figure 2. (a) HRTEM images of CdSe/CdS-3ML NCs, (b) HRTEM images of CdSe-ODA-TOPO NCs, (c) TEM image of CdSe/CdS-3ML NCs in a large scale, (d) SAED of CdSe/CdS-3ML NCs.
Download figure:
Standard image High-resolution imageFigure 3 shows the absorption and PL spectrum of CdSe-TOP, CdSe-ODA-TOPO and CdSe/CdS-3ML. The rhodamine B was used as a reference standard, and when it was excited at 514 nm in water, the PLQY was 31% [17]. The calculation formula can be written as
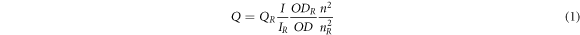
where Q is the quantum yield, I is the integrated intensity, n is the refractive index, OD is the optical density. The subscript R refers to the reference rhodamine B. To avoid reabsorption effects, all solution was diluted making the optical density below 0.1 at the excitation wavelength, which is shown in figures 3(a) and S3. The PLQY of three samples is 40.8%, 49.7%, and 59.3%, respectively. Compared with CdSe-TOP, the CdSe-ODA-TOPO has larger PLQY, because ODA as amines can make cadmium oleate bear significantly high temperatures, which is required for the formation of CdSe nanocrystals with high structural quality [18]. Besides, the TOPO is a good cosolvent to depress the solidification rate and further increase the reaction temperature [19]. No doubt this high crystallization quality produces increased PLQY. Furthermore, CdSe/CdS NCs have the highest PLQY among the three samples. For type I band edge alignment, both electrons and holes in CdSe/CdS NCs are confined to the core by the potential barrier of the shell [20], which increases the PL emission. At the same time, this confinement makes partial delocalization of the electronic wavefunctions into the CdS shell, which results in a red-shift of both absorption and PL peak.
Figure 3. (a) Optical absorption spectrum and (b) PL spectrum of CdSe-TOP, CdSe-ODA-TOPO and CdSe/CdS-3ML were measured for calculating PLQY. The absorption peak is 550 nm, 546 nm, and 602 nm, respectively, and the corresponding PL peak is 583 nm, 571 nm, and 627 nm, respectively. The excitation wavelength was chosen at 496 nm for three samples, and all of the optical density is below 0.1.
Download figure:
Standard image High-resolution imageIn order to identify the stability of CdSe NCs after inorganic capping, we compare the PL intensity and peak position of CdSe and CdSe/CdS NCs, as shown in figure 4. The NCs solution was exposed under inert atmosphere to exclude the effect of oxidation. The CdSe NCs have a blue-shift peak two months later, while the CdSe/CdS NCs have no shift after the same period, which indicates that the CdSe/CdS NCs have a stronger PL stability. The blue-shift of the CdSe PL peak is attributed to decreased NCs size. The specific changing process about semiconductor NCs in solution during inert atmosphere storage was discussed in detail by Yu [21]. They thought that the ligands such as TOP and oleic acid could dynamically interact on the NCs surface through desorption and adsorption. Meanwhile, the oleic acid ligands and the corresponding bonded surface Cd atoms could be lost and form Cd-oleate in solution, which causing the inward atoms to be exposed. This process could happen more and more, which decreases the size of NCs. Compared with organic capping of CdSe-TOP, the inorganic capping CdSe/CdS can slow down the dissolving process above, resulting in high stability of CdSe/CdS NCs.
Figure 4. The PL stability comparison of original and two months later for (a) CdSe, (b) CdSe/CdS.
Download figure:
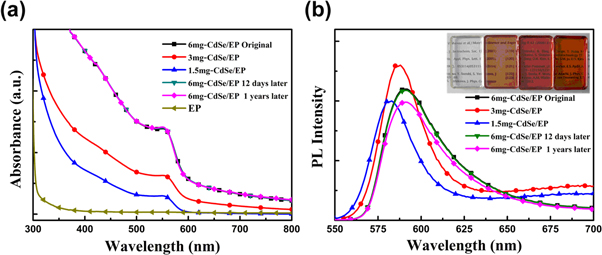
Standard image High-resolution imageFurthermore, we prepared the CdSe/EP composite with the same volume but different amounts of CdSe NCs. Figure 5 shows the absorption and PL spectrum of the composite. Compared with pure EP, all of the CdSe/EP composites have an absorption peak at 550 nm. With the increasing amounts of CdSe NCs, the composites have increased absorption intensity. However, the position of PL peak is shifted toward longer wavelengths, and the intensity of PL peak firstly increases and then decreases. This phenomenon can be explained based on the dipole-dipole coupling interaction between NCs [22]. As the amount of CdSe NCs increases, the spacing between NCs decreases, which results in strong coupling interaction. This is similar to our previous report that short-chain ligand exchange can greatly increase electronic coupling between NCs [23]. The dipole-dipole coupling is radiative electronic coupling, which makes the wavefunction delocalization [24], resulting in the red-shift of PL peak. Besides, compared with original CdSe/EP composite, the composite stored in the air for 12 days still keeps unchanged absorption and PL spectrum. After one year, the composite has a little reduced PL intensity of 5%. This proves that the NCs/EP composite has a wonderful stability in ambient environment. So this composite combines the high PLQY of CdSe NCs and excellent stability, which is very promising for LED application.
Figure 5. (a) Optical absorption spectrum and (b) PL spectrum of CdSe-EP composite with different amounts of CdSe-TOP NCs. At the same time, the absorption and PL stability test are also shown. All of the absorption peak position are 550 nm, while the intensity and position of PL peak have a dependence on the amounts of CdSe-TOP NCs.
Download figure:
Standard image High-resolution image4. Conclusions
We prepared CdSe NCs with different capping using the hot-injection method. The results indicate that CdSe-ODA-TOPO NCs have larger PLQY in contrast to original CdSe-TOP NCs due to higher crystallization quality, and CdSe/CdS have the highest PLQY among the three samples, because both electrons and holes are strongly confined to the core by the potential barrier of the shell. Furthermore, we prepared CdSe/EP composites, combining the large PLQY of inorganic semiconductor NCs, and good stability against UV radiation and heating of organic epoxy resin. It was proved that CdSe/EP composites had a wonderful stability in ambient environment for one year, which is very promising for device application.
Acknowledgments
The authors gratefully acknowledge financial supports from Natural Science Foundation of China (Grants No. 61307041, 61174007 and 61472227). Natural Science Foundation of Shandong Province, (Grants No. ZR2013AQ013, ZR2013AL014), the Science and Technology Project of University of Shandong Province, China (Grant No. J12LJ01), the PhD Start-up Fund of Shandong Institute of Business and Technology (Grant No. BS201418), the prospective research fund for young and middle-aged scientists of Shandong Province (Grant No. BS2010DX022).