Abstract
Due to the strong 'lip–lip' interactions between neighboring nanosheets layers, the scalable exfoliated fabrication of few-layer-thick boron nitride nanosheets (BNNSs) from the bulk hexagonal boron nitride (h-BN) is more challenging than these graphene nanosheets. In this work, few-layer (generally less than 10 layers) BNNSs were efficiently synthesized through a simple ball milling technique with the 2-furoic acid (FA) as a modifier. The BNNSs as-prepared with a average thickness of about 2.0 nm and an extremely high production yield (~98%) as well as unprecedentedly high dispersed concentration (~35 mg ml−1) in water. The stable aqueous dispersions of BNNSs can be directly used to fabricate ultralight aerogels with a low density of 2.0 mg cm−3 and thermally conductive film with a highly thermal conductivity of 25.2 W m−1 K−1. This approach provided us with an environmentally friendly agent with high efficiency for few-layer BNNSs synthesis and demonstrated promising applications for ultralight aerogels and heat dissipation in electronic components.
Export citation and abstract BibTeX RIS
Introduction
As the isoelectric analog of graphene, BNNSs has many unrivalled properties, such as highly resistant to oxidation (stable up to 840 °C under atmospheric conditions) [1], outstanding resistance corrosion [2], superior thermal (thermal conductivity ~1000 W m−1 K−1) [3], and mechanical properties (Young's modulus ~1 TPa, yield strength 35 GPa) [4], excellent chemical stability (stable up to 1000 °C in air atmosphere) [5], ultra-low density (2.1 g cm−3) [6], as well as intriguing electronic property [7]. Therefore, it would enable particularly applications of BNNSs in fabrication of electronic packaging [8] and high power electronics [9], 3D aerogels [3], hydrogels [10], even improve the thermal and mechanical properties of polymeric composites [11]. For all these applications, the fabrication of BNNSs with atom-thick is a prerequisite to take full advantage of its advanced mechanical and thermal properties [11, 12].
Up to date, several strategies have been developed for fabricating few-layered BNNSs from h-BN: chemical vapor deposition (CVD) [13], mechanical-assisted exfoliation [14], and unzipping of h-BN [15]. The CVD approach has many advantages in preparing large-area and highly crystalline atomic layers BNNSs. However, the employments of explosive or toxic reagents, as well as the tedious multiple-step chemical reaction are inevitable during the process [16]. In addition, it is more difficult to obtain few-layer BNNSs with low defect density than that with graphene due to the strong 'lip–lip' interaction between neighboring nanosheets layers [17], and such BNNSs are prone to irreversible aggregation after drying or concentration after dispersed in solutions. Due to its extremely poor solubility in traditional solvents, BNNS is difficult to be uniformly incorporated into hydrogel networks or polymer composites. Recently, mechanical exfoliation like ultrasonic agitation, mechanical shearing or ball-milling has been found to be an alternative approach to scalable fabricates solubility BNNSs by introducing hydrophilic functional groups (e.g. –OH or –NH2) onto their basal plane or edges. Fu et al [18] reported the fabrication of hydroxyl functionalization BNNSs by a facile liquid exfoliation under the assistance of molten sodium hydroxide, and the production yield of few-layered BNNSs was up to 19%. Kim et al [19] reported a simple method for the exfoliation of bulk-layered h-BN in pure water by merely controlling the temperature. However, the concentration of the BNNSs dispersions was typically below 0.018 mg ml−1, even after long periods of intense ultrasonication. As a result, it still remains a large challenge to obtain high concentration BNNSs dispersions for sonication-assisted liquid exfoliation methods and such approach require a large amount of appropriate solvents. Unlike the solution approaches, ball-milling treatments provide an efficient exfoliation method of h-BN to few-layered BNNSs with high yield and desired functionalities. Several modifiers have been applied for the ball-milling preparation of functional BNNSs, e.g. sodium hydroxide, urea, and polymers [20, 21]. However, the relatively small size of BNNS as-synthesized through these approaches, which limits them many practical applications in thermal devices.
Here, we provide a high-yield and one-step ball milling process strategies with the assistance of FA to scalable synthesize few-layer (generally less than 10 layers) hydroxylated BNNSs via the synergetic effect of chemical peeling and mechanical shear forces. The BNNSs as-produced possesses an extremely high production yield (~98%) and exhibited outstanding aqueous compatibility with a concentration of 35 mg ml−1, which can be stored in the form of a concentrated slurry for months without the risk of re-stacking. As expected, the as-exfoliated BNNSs dispersion can be directly used for fabricating ultra-light aerogels and thermally conductive film, thus avoiding the use of copious quantities of organic solvents and lowering the manufacturing cost.
Results and discussion
Synthesis and characterization of BNNS
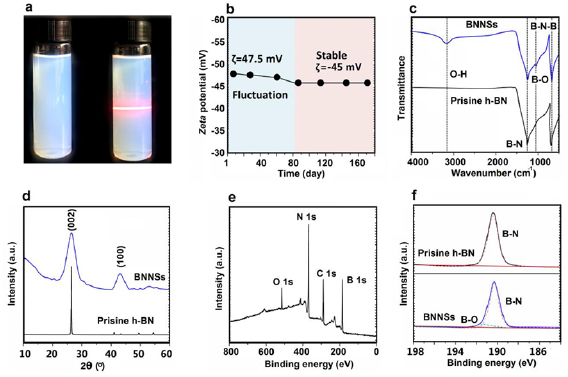
The efficiently synthesis of few-layer BNNSs through a direct ball milling route from bulk h-BN was successfully achieved in our work. After washing by water to remove unreacted FA, the hydroxyl functionalized BNNSs exhibited excellent aqueous compatibility can be readily dispersed in aqueous solution to form stable solutions (figure 1(a)) without any sonication treatment (figure S1 (stacks.iop.org/TDM/5/045015/mmedia)). It is well known that it is very challenging to obtain a stable h-BN aqueous solution without incorporation surfactants. We added BNNSs into water (pH = 8–9) at a high concentration of 35 mg ml−1, found that there was no sediments were observed in the colloidal solution after storage for 1 year under ambient condition (figure S2). As shown in figure 1(a), the BNNSs water solution with a concentration of 3.0 mg ml−1 exhibits a homogenously dispersive constitution, even after storage for 1 year, without any precipitate, a Tyndall effect is clearly observed in the photo. Zeta potential (ζ) is commonly used as an indicator for the stability of nanomaterials. As shown in figure 1(b), the BNNSs solution possesses a maximum ζ value of −47.5 mV at the beginning, while it is noteworthy that the ζ value stays constant of −45.0 mV after 80 d, indicting a strong electrostatic repulsive interaction between BNNSs. This excellent aqueous compatibility of BNNSs can mainly be attributed to the existence of hydroxyl groups (–OH) as confirmed by the followed characterizations. We firstly used FT-IR to probe the variation of structure of BNNSs, in comparison with the spectrum of the bulk h-BN (figure 1(c)). From the FT-IR spectra, both h-BN and BNNSs exhibit two main broad absorptions at about 1350 cm−1 and 800 cm−1 [22], which is assigned to the characteristics of in-plane B–N stretching vibration (E1u mode) [23] and the out-of-plane B–N–B bending vibration (A2u mode) [24], respectively. The BNNSs shows the additional shoulder peaks at 3200 cm−1 and 1185 cm−1 [25], corresponding to the –OH stretching vibration and B–O deformation [26]. XRD was further used to investigate the structural changes of BNNSs. As shown in figure 1(d), the h-BN and BNNSs exhibit two strong diffraction peaks at ~26.2° and ~42.8°, which can be assigned to arising from (0 0 2) and (1 0 0) planes [15], respectively. However, the intensity and width of the (0 0 2) and (1 0 0) diffraction peaks of BNNSs tend to become weaker and wider compared to h-BN, which give a direct evidence for the existence of ultra-thin nanosheets in the BNNSs samples [27]. The XPS spectra also was carried out to further investigate the composition and the functional groups in BNNSs. We calibrated the binding energy according reference to the C 1s energy as 284.5 eV in the XPS spectra. In the XPS wide spectrum (figure 1(e)), the observed peaks at 189.5 and 395.5 eV are assigned to the B 1s and the N 1s of BNNSs, respectively. In figure S3, the peak at 532.3 eV is attributed to the O 1s of –OH in BNNSs. Figure 1(f) displays the high-resolution B–N peak at 190.0 eV (B1s) of h-BN and BNNSs. Compared to the bulk h-BN, BNNSs demonstrates another B–O peak at 191.1 eV, which might be attributed to the present of –OH in the BNNSs [28, 29].
Figure 1. (a) BNNSs water solution with a concentration of 3 mg ml−1 before (left) and after (right) storage for 1 year under ambient conditions. (b) Zeta potential of BNNSs water solution as a function of storage time. (c) Fourier transform infrared spectra, (d) XRD spectra, and (e) XPS survey scan of BNNSs, (f) B1s narrow XPS scan of h-BN powder of h-BN and BNNSs.
Download figure:
Standard image High-resolution imageWe used SEM, AFM, and TEM to observe the lateral size and the number of layers of BNNSs as-exfoliated. Under SEM inspection (figure 2(a)), the BNNSs show typical wrinkles and fine flexible features. As observed in AFM (figures 2(b) and S4), the thickness of the nanosheets ranging from 0.5–2.3 nm, which confirms the single- and few-layer states of BNNSs based on the thickness of a single-layered h-BN is about 0.4–0.5 nm [3]. SEM and AFM statistical results (figures 2(c) and (d)) demonstrate that the BNNSs as-obtained with about 4% more than 5 layers and approximately 18% is single layer, the average thickness and lateral size of BNNSs is ~2.0 nm and ~1.8 µm, respectively. TEM images (figures 2(e) and S5) exhibit typical single- and few-layer BNNSs with the typical six-fold symmetry selected-area electron diffraction (SAED) pattern [8], which implies the hexagonal lattices of the BNNSs were not suffer damage during the milling processes. As measured by high resolution TEM over 100 BNNSs (figures 2(f) and S5), about 16% of nanosheets are single layer and approximately 90% are no more than five layers, which are agreement with the AFM SEM and AFM measurement results. Our method can efficiently convert bulk h-BN into few-layered BNNSs at a production yield of about 98% (figure 2(g)) and make full use of all the starting h-BN, which is superior to those in other exfoliation techniques [4, 3, 8, 10, 11, 15, 18, 21, 37, 39–41]. Figure 2(h) shows the mass-produced BNNSs aqueous solutions with a concentration of 5 mg·ml−1. It is note that although after a long storage period of 1 year none precipitates are observed on the bottom of the solutions.
Figure 2. (a) The SEM image of BNNSs on Si/SiO2 substrate. (b) Tapping mode AFM image and height profile of BNNSs. The distribution of the thickness (c) and lateral size (d) of BNNSs. (e) Low-resolution and corresponding selected-area diffraction (SAED) patterns, (f) high-resolution TEM images of BNNSs. (g) Comparison of produced yield of prepared strategy with other exfoliation strategies. (h) Mass produced BNNSs aqueous solution with a concentration of 5 mg ml−1. Scale bar: (a) 2 µm; (b) 2 µm; (e) 0.5 µm; (f) 3 nm.
Download figure:
Standard image High-resolution imagePossible mechanism of exfoliation and functionalization of BNNSs
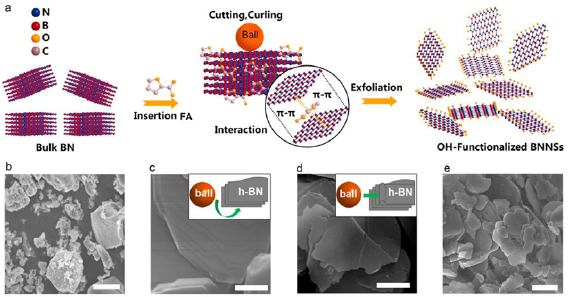
In this work, we present a facile FA-assisted ball-milling exfoliation route for the preparation of single- and few-layered BNNSs through a combination of large agate balls (5 mm diameter) and small agate balls (0.5 mm diameter). According to the previously structural characteristics and analysis, we give the following possible mechanism for the synthesis of functionalized BNNSs (figure 3(a)). We selected ball milling as an exfoliated technique due to it is a well-established industrial that can easily scale up. Generally, a efficient ball milling exfoliation can only be achieved under a suitable milling condition [30, 42–44]. In order to minimize the damage to the in-plane crystal structure, we choose a reasonable combination of large agate balls and small agate balls for milling. Firstly, the large bulk h-BN (figures S6(a) and (b)) particles were cleaved into small ones (figures 3(b) and S6(c)) by large agate balls through the high-speed crash. After then, the small balls detach small h-BN particles into many thin BNNSs (figure 3(c)) through the strong shear force; the relatively large number of the small balls makes the milling more efficient. The cleavage intermediate process was investigated by SEM observation. As shown in figure 3(d), the laminated particles were cleaved into thin sheets from the h-BN edges by the sliding balls. Subsequently, the rolling balls on the surface of h-BN peel off the BNNSs from the top of the thin particles (figure 3(e)). In order to fabricate BNNSs with high yield, the FA was used as the modifier. During the exfoliation process, the inter-plane bonds of h-BN were opened via strong π–π interactions (figure 3(a)) from the aromatic groups contained of FA. Meanwhile, the FA molecular absorbed on the surface of h-BN to achieve in situ covalent modification by pressure forces and avoid restacking of exfoliated BNNSs during milling. Moreover, as a lubricant, FA can effectively prevent the welding effect and reduce the damage of BNNSs structures. Under the efficient shear force, the conversion yield of BNNSs from h-BN was up to ~98% (figure S7) and without any post-treatment for separation (figure S1). And it is note that the yield of BNNS including the weight of OH groups.
Figure 3. (a) Possible mechanism for enhanced ball-milling synthesis of BNNSs by FA. (b)–(e) SEM image of BNNSs. Scale bar: (b) 1 µm; (c) 0.2 µm; (d) 0.2 µm; (e) 1 µm.
Download figure:
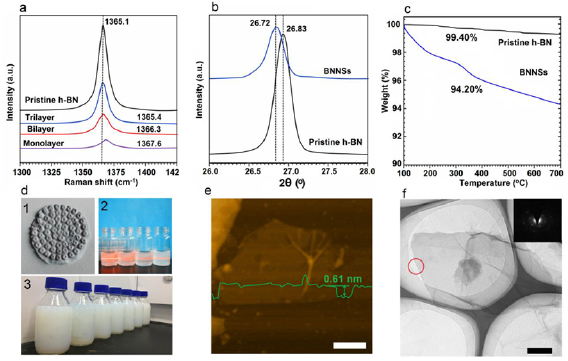
Standard image High-resolution imageFor the case of nanomatrials, Raman spectroscopy has proven to be an indispensible tool for identifying single- and few-layered BNNSs. Similar to the G peak in graphene-based materials, h-BN also shows a Raman characteristic absorption due to the E2g phonon. Therefore, we used Raman spectra to characterize the mono-, bi- and tri-layered BNNSs through a green laser at λ = 514.5 nm. Figure 4(a) shows the Raman characteristic G peak at ~1365 cm−1 of BNNSs and h-BN. In general, once a Raman spectrometer is calibrated for a given substrate, this can be exploited to distinguish between mono-, bi- and thicker h-BN layers. It can be seen from figure 4(a), the G peak becomes progressively weaker with the decease of layer, for the mono-layered BNNSs, and its intensity is ~40 times smaller than that of pristine h-BN under the same measurement conditions. Besides, the peak was shifted upwards in monolayer (1367.6 cm−1), bilayer (1366.3 cm−1), and trilayer (1365.4 cm−1) with respect to the position of pristine h-BN (1365.1 cm−1), indicating the thin features of exfoliated h-BN [31, 32]. We use XRD to further characterize the crystalline structure of the BNNSs as-exfoliated. As revealed in figure 4(b), the intensity of the (0 0 2) peak of the exfoliated BNNSs significantly decreases and two-theta peak slightly downshifts from 26.83° to 26.72°, corresponding to the inter-planar distance of sheets increased in 3.3 Å to 3.4 Å. Furthermore, the intensity of other diffraction peaks ((1 0 0), (1 0 1), (1 0 2), and (0 0 4)) in figure 1(d) also decreased after exfoliation for h-BN, indicting the formation of ultrathin h-BN nanosheets. In addition, we also performed TGA measures to estimate the amount of the oxygen-containing functional groups under N2 atmosphere (figure 4(c)). The weight loss of BNNSs and h-BN at 100 °C–700 °C are 5.80% and 0.60%, which indicate about 5.20 wt% content of -OH was grafted in BNNSs.
Figure 4. (a) Raman spectra recorded of h-BN and BNNSs. (b) XRD spectra recorded of h-BN and BNNSs. (c) TGA plots of h-BN and BNNSs. (d) 1-photo of the white BNNSs paste on PTFE membranes. 2-Typical BNNSs dispersions and their Tyndall effects (laser light shone from left); the solvents of the solution from left to right are IPA, ethanol, DMF, and water; 3-mass produced BNNSs DMF solutions with a concentration of 5 mg ml−1. (e) Tapping mode AFM image and height profile of DMF dissolved BNNSs. (f) TEM image of DMF dissolved single-layered BNNSs on substrate. Scale bar: (f) 500 nm; (g) 200 nm.
Download figure:
Standard image High-resolution imageUnlike the h-BN precursor, the BNNSs treated by FA with highly soluble or dispersible in water and traditional polar organic solvents based on the grafted –OH groups in the edges B and N sites of nanosheets. The BNNSs possesses good aqueous compatibility close to FA before washing. We obtained a white BNNSs paste after removing free FA as shown in figure 4((d)(1)). BNNSs are readily dispersible in aqueous solution with an extremely high concentrations and good stability as mentioned before. The BNNSs paste is also dispersible in some polar solvents like IPA, ethanol, DMF, but not dispersible in non-polar solvents like toluene. Figure 4((d)(2)) exhibits the BNNSs solutions dispersed in IPA, ethanol, DMF, and water with a concentration of 1 mg ml−1 after centrifuged at 2000 rpm for 30 min, which clear shows that these colloidal dispersions show the Tyndall effects when illuminated with a red laser. The result indicates that these dispersions also possess similarly high concentrations and good stability in different polar solvents. Figure 4((d)(3)) shows the mass produced DMF solutions of exfoliated BNNSs, with concentration of 5 mg ml−1. It is note that although after a long storage period no precipitates are observed on the bottom of the solutions, suggesting the good stability of exfoliated BNNSs. Most of BNNSs are dispersed in a mono- and few-layered states in DMF, as confirmed by AFM and TEM measurements (figures 4(e) and (f)).
BNNSs-based ultralight aerogels and thermally conductive films
The outstanding solubility of BNNSs endows them superior solution processability and application in many functional materials [33–36]. As typical applications, we fabricated 3D ultra-light elastic aerogels and 2D transparent thermal conductive filmsfrom BNNSs. A 3D BNNSs aerogel (figure 5(a)) was prepared through a simple cryodrying technology of BNNSs dispersion; the BNNSs aerogel has an ultralow density of ~2.0 mg cm−3 as demonstrated by placing it on the petal, and with a highly porous structure as confirmed by SEM (figure 5(b)). As shown in figure 5(c), the BNNSs aerogel still remains intact after being compressed by a weight 2000 times its own even after 1000 cycles of 80% compression (figure S8), indicating it highly elastic characterize. In addition, we found that BNNSs aerogel shows a highly compact and ordered structure, similar to previous other graphene-based aerogels obtainted graphene, graphene oxide, and fibers. In view of the preparation and superior properties, BNNSs aerogel exhibits with many promising applications in macroscopic materials like ultralight materials and gels in insulation.
Figure 5. Macroscopic assembled materials of BNNSs. (a) Photo of an ultralight weight BNNSs aerogel with a density of 2.0 mg cm−3 placed on pistil of a flower, SEM images (b), and elastic property of BNNSs aerogel (c). (d) Photo of the freestanding BNNSs flexible paper. (e) Thermal diffusivity and thermal conductivity of BNNS. (f) top-view and (g) side-view SEM images of BNNSs film. Scale bar: (b) 2 µm; (f) 2 µm; (g) 200 nm.
Download figure:
Standard image High-resolution imageBNNSs films were fabricated through vacuum-assisted filtration techniques of BNNSs dispersions. After peeled off from the filter, our BNNSs film exhibits transparent and flexible characterizes (figure 5(d)). The thickness (10 nm ~100 µm) and optical transparency of BNNSs films can be simply dictated by adjusting the concentrations of BNNSs dispersions. The optical transmittances of BNNSs films were further characterized by the UV–vis spectra. From figure S9, all the BNNSs films as-obtained retaining high optical transmittance of 70.5%–94.5% at visible range above 550 nm. After de-hydroxylation treatment by annealing at 500 °C, we tested the thermal diffusivity and corresponding thermal conductivity of BNNSs film with a density of 2.3 g cm−3 at room temperature (figure 5(e)). It is note that the thermal conductivity of BNNSs films was up to 25.2 W m−1 K−1 along the in-plane direction, which is much higher than the most of previous reports based on BN-based materials [17, 21, 22, 24, 26, 37, 45, 46]. The superior heat dissipation performance of BNNSs films is attributing to the well-aligned lamellar structure (figures 5(f) and (g)) and high enough aspect ratio (lateral size/thickness, 991) of single- and few-layered nanosheets. As a result, the excellent thermal conductivity makes BNNs film a promising candidate as a high performance heat spreader in power-converter or integrated circuit packaging [38].
Conclusions
In conclusion, we provide a simple ball-milling technique for the efficient preparation of few-layered (mostly less than 10 layers) BNNSs from h-BN employs. The BNNSs as-synthesized possesses an average thickness of approximately 2.0 nm and lateral size of about 1.8 µm. Under the assistance of FA, the few-layered BNNSs achieved an unprecedented production yield of ~98% and an extreme concentration of 35 mg ml−1 in water. Such stable BNNSs dispersion can easily be used to fabricate 3D ultralight aerogels and ultra-thin 2D thermal conductive films. As a typical example, the thermal conductivity of a BNNSs film as-prepared with a density of 2.3 g cm−3 was up to 25.2 W m−1 K−1, this endows a potential application for BNNSs at high temperature as heat dissipation in electronic components.
Acknowledgments
The research is financially supported by the Ningbo International Cooperation Project (Grant no. 2017D10022).
Associated content
Supporting information
The 'Experimental section', additional optical photos, AFM, TEM, SEM, transmittance and stress–strain curves of BNNSs films can be available free of charge on the supporting information.
Notes
The authors declare no competing financial interest.