Abstract
A novel biosensing platform was designed by the functionalizing reduced graphene oxide sheets (rGO) with electroactive copolymer juglone. The composite film showed well-defined, stable electroactivity in a biocompatible buffer medium. Square wave voltammetry is used to record the redox signal for DNA hybridization. Current increase upon hybridization (signal-on) evidenced that short DNA target as well as polymerase chain reaction (PCR), so called 'real sample' products, related to different lineages of Mycobacterium tuberculosis strain. The signal-on reached ∼40% with 1 nM of short DNA (25 mer) target, while PCR product (Africanum, EAI and Beijing strains) produced a current change of ∼20%.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Graphene has attracted considerable interest since its discovery in 2004 [1]. It has been affecting many areas of science and technology due to excellent properties such as high specific surface area, extraordinary electronic properties and electron transport capabilities, unprecedented pliability and impermeability, strong mechanical strength and excellent thermal and electrical conductivities [2]. In the 'graphene family', reduced graphene oxide (rGO) is the most commonly used material (68% of all publications [3, 4]) because of its easy access, scalable synthesis, low cost and simple functionalization with good electrical conductivity.
In this report we designed the signal-on (current increase) system with poly(5-hydroxy-1,4-naphthoquinone-co-5-hydroxy-2-carboxyethyl-1,4-naphthoquinone)/reduced graphene oxide-modified glassy carbon electrode (noted: poly(JUG-co-JUGA)/rGO/GCE) for a label-free and reagentless electrochemical DNA sensor format. In this system, the poly(JUG-co-JUGA) is a bifunctionalized polymer: the quinone and the carboxylic groups act as the transducer between the biomolecule /the electrode and the binding site, respectively [5]. The short DNA probes of Mycobacterium tuberculosis (M. tuberculosis) were grafted on electrode surface via the carboxylic groups of JUGA, and the electrochemical signal-on behavior was due to the cation exchange process of quinone groups with the immobilized redox-active label in neutral aqueous medium [6, 7]. We detected this effect not only with short DNA target but also with PCR products from different lineages (of different geographical origins) of M. tuberculosis including: Africanum, EAI (East-African Indian) and Beijing strains [8, 9].
2. Experimental
2.1. Chemicals and biological
A 5-hydroxy-1,4-naphthoquinone monomer (juglone, JUG), 1-naphtol (1-NAP) were purchased from Fluka. Lithium perchlorate (LiClO4), N'-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS); 2-(N-morpholino)ethanesulfonic acid (MES), epigallocatechin gallate (EGCG), tween 20 and phosphate buffered saline (PBS, pH 7.4) were purchased from Sigma Aldrich. Single-layer graphene oxide (diameter of 1–5 μm, thickness of 0.8–1.2 nm) was purchased from ACS Material LLC (Medford, MA, USA). Acetonitrile (ACN) was supplied by Aldrich (HPLC grade). 5-hydroxy-2-carboxyethyl-1,4-naphthoquinone (juglone acid, JUGA) was synthesized from 5-hydroxy-1,4-naphthoquinone (JUG) and succinic acid, which was previously described in detail [10]. All other reagents used were of analytical grade.
Two types of hybridization were considered on poly(JUG-co-JUGA)/rGO systems: short DNA probe versus DNA (short-chain nucleic acids) target and DNA probe versus real sample of DNA (from PCR products) target. The DNA probe sequences (25 mer) are Amino-Sp19 (5'-TAA-CTG-GCT-TGG-CGC-TGA-TCC-TGG-T-3'-C6-NH2) and Amino-Sp42 (5'-ATG-GTG-GGA-CAT-GGA-CGA-GCG-CGA-C-3'-C12-NH2). For hybridization: complementary 25 base DNA target sequences (short-chain nucleic acids) are Spacer 19 (3'-ATT-GAC-CGA-ACC-GCG-ACT-AGG-ACC-A-5') and Spacer 42 (3'-TAC-CAC-CCT-GTA-CCT-GCT-CGC-GCT-G-5') present in the clustered regularly interspersed palindromic repeats (CRISPR) from M. tuberculosis. The real samples of DNA targets are three different lineages of PCR products from M. tuberculosis as follows: Africanum, EAI (East-African Indian) and Beijing strains [11].
2.2. Electropolymerization of graphene-poly(JUG-co-JUGA) composite
Graphene oxide (GO) was transformed to a reduced graphene oxide (rGO) using green tea polyphenol epigallocatechin gallate (EGCG) [12, 13]. Briefly, GO aqueous solution (1 mg mL−1) was prepared by ultrasonication for 30 min, and then, EGCG (300 wt% relative to GO) was added to the GO dispersion. The mixture was heated to 80 °C under stirring for 8 h. The resulting slurry was filtered and washed by distillated water with a nylon membrane (0.22 μm) to obtain rGO as a black precipitate.
Glassy carbon (GC) working electrodes (BAS Inc.; diameter of 3 mm, area of 0.07 cm2) were thoroughly polished to a mirror finish using a slurry containing 0.3 μm α-Al2O3 power on a soft cloth and rinsed thoroughly with ethanol and then sonicated in doubly distilled water for 3 min to remove possible contaminants. Next, 5 μL of 0.5 mg mL−1 rGO solution suspended in water was dropped on polished GC electrodes and allowed to evaporate at room temperature. Then, rGO/GC electrodes were immersed into a solution containing 50 mM JUG + 3.75 mM JUGA + 0.1 mM 1-NAP in acetonitrile, and electropolymerization was carried out by potential cycling in the range of 0.4–1.1 V versus saturated calomel reference electrode (SCE) and a platinum grid counter electrode for 20 cycles at 50 mV s−1. The electrosynthesis of poly(JUG-co-JUGA) on rGO/GC electrode has been described elsewhere [12]. Electrodes were then washed with acetonitrile and ethanol to remove adsorbed monomers, before obtaining conducting polymer JUGA-co-JUG coated rGO-modified GC electrodes [poly(JUGA-co-JUG)/rGO/GC].
2.3. DNA probe grafting and hybridization
Probe grafting: poly(JUG-co-JUGA)/rGO/GC electrodes were incubated in a solution containing 0.1 μM of probe, 0.1 M MES buffer with 150 mM EDC and 300 mM NHS at 37 °C, overnight. NH2-modified DNA targets were covalently attached to the exposed COOH-groups of the JUGA film by EDC and NHS. After incubation, the electrodes were washed with PBS containing 0.05% Tween 20 and incubated again in PBS at 37 °C for 1 hr to release physisorbed DNA probes.
Target hybridization: for DNA (short-chain nucleic acids) target, 1 nM of target DNA (spacer 19 and 42) were used. For PCR fragments, 5 μL of the PCR products diluted in 995 μL of PBS was first denatured at 95 °C, then slowly cooled to 55 °C and the probe-modified bioelectrodes were placed into the aliquot of hybridization solution containing the target DNA at 45 °C for 2 h. After hybridization, electrodes were rinsed thoroughly with 0.05% Tween 20 in PBS. Finally, the electrodes were immersed in PBS for 1 hr at 37 °C in order to remove non-hybridized targets.
2.4. Electrochemical detection
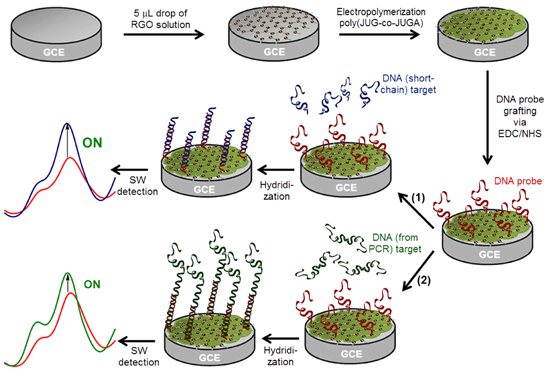
Hybridization was detected by recording the modification of the quinone electroactivity using square wave voltammetry (SWV) measurements on PGSTAT30 Autolab (GPES 4.9 software). A conventional 20 mL three-electrode cell was used, consisting of platinum grid counter electrode, saturated calomel reference electrode (SCE) and a modified-GC working electrode. The following parameters were used: pulse height 50 mV, pulse width 50 ms, scan increment 2 mV, frequency 12.5 Hz, potential range (−1.0 V to −0.1 V). The SWV scans were recorded in PBS medium, bubbled with argon for 20 min before the measurement. The stepwise schematic of fabrication procedure and signal-on detection of DNA biosensor is shown in figure 1.
Figure 1. Schematic of fabrication procedure and signal-on detection of DNA biosensor based on poly(JUG-co-JUGA)/rGO/GC electrode with (1) DNA (short-chain nucleic acids) target and (2) real sample of DNA (from PCR) target.
Download figure:
Standard image High-resolution image3. Results and discussion
3.1. Electroactivity of poly(JUG-co-JUGA)/rGO modified GCEs
The electroactivity of poly(JUG-co-JUGA) and poly(JUG-co-JUGA)/rGO film onto GC electrodes was investigated in deoxygenated PBS by cyclic voltammetry technique. The cyclic voltammograms are presented in figure 2.
Figure 2. Electroactivity of (A) poly(JUG-co-JUGA) and (B) poly(JUG-co-JUGA)/rGO-modified GC electrode in PBS deaerated solutions. Scan rate of 50 mV s−1.
Download figure:
Standard image High-resolution imageThe observed peaks are due to electroactivity of quinone groups in PBS electrolyte solution. Under the same experimental conditions, as seen from figure 2(B), current response for poly(JUG-co-JUGA)/rGO electrode was much larger and more stabilized than that of pure poly(JUG-co-JUGA) electrode. If the poly(JUG-co-JUGA) electrodes need more than 50 scans to stabilize the signal [14], so poly(JUG-co-JUGA)/rGO composite electrodes need less than 20 scans to do it. It can be supposed that the electron transfer rate and the number of active sites of poly(JUG-co-JUGA) film was remarkably enhanced owing to high electro-active surface area, excellent electrical conductivity and electronic transport properties of rGO layer. Moreover, the effect of scan rates (from 10 to 400 mV s−1) on the peak currents at poly(JUG-co-JUGA)/rGO/GC electrode was investigated (figures not shown). The anodic (Ipa) and cathodic (Ipc) peak currents increased linearly along with the square root of scan rate (ν1/2) and suggested an expected diffusion-controlled process.
3.2. Probe grafting
In this work two different probes were studied: Amino-Sp19 and Amino-Sp42 with 25 bases (short DNA probes). Figure 3 shows the square wave voltammograms (SWVs) of poly(JUG-co-JUGA)/rGO/GC electrode before (line a) and after Sp19 (line b) and Sp42 (line c) probe grafting. As can be seen from this figure, the signal current decrease about −50% ± 5% (signal-off), following general equation:
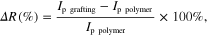
where Ip grafting, Ip polymer are the peaks' height after and before probe grafting, respectively. The decrease in SWV peak currents is attributed to steric hindrance generated by immobilization of the DNA probe strand on the surface.
Figure 3. SWVs in PBS degased with Ar of (line a) poly(JUG-co-JUGA)/rGO-modified electrode and (line b) Amino-Sp19, (line c) Amino-Sp42 probes are grafted.
Download figure:
Standard image High-resolution image3.3. Detection of DNA
After grafting short DNA probes (Sp19 and Sp42) onto poly(JUG-co-JUGA)/rGO/GC electrodes, the bioelectrodes were incubated with different targets as described in subsection 2.3. The height of the SWV peak was used to calculate the relative current change (ΔI/I) before and after hybridization, using the following equation:
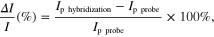
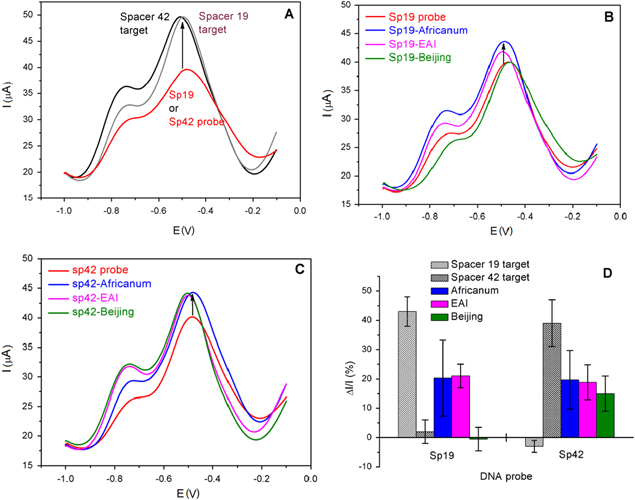
where Ip hybridization and Ip probe are the height of the SWV peak before and after hybridization, respectively. Figure 4 shows the SWVs of the bio-electrode before and after hybridization. Figure 4(D) presents the current change before and after hybridization (ΔI/I) of short DNA Sp19 and Sp42 probe with different targets.
Figure 4. SWVs of the poly(JUG-co-JUGA)/rGO/GC bio-electrode before and after hybridization: (A) short probe versus short target sequence; (B) short Sp19 probe and (C) short Sp42 probe versus real sample PCR products: Africanum; Beijing and EAI. (D) ΔI/I of the height of the SWV peak.
Download figure:
Standard image High-resolution imageTaking into account the modeling of the steric hindrance of DNA strands, the hybridization between probe and target DNA leads to current increase of the redox signal (signal-on), induced by the quinone group embedded in JUG film. This effect was well studied and reported previously [6, 15]. In this study, after incubation with full complementary target, short probe Sp19 with target spacer 19 and probe Sp42 with target spacer 42, the height of the SWV current peak increases about 43% ± 5% and 40% ± 8%, respectively (figure 4(A)), whereas the negligible current change occurs with non-complementary (short probe Sp19 with target spacer 42 and probe Sp42 with target spacer 19). These results show the specific hybridization of the short M. tuberculosis DNA sequences.
As a next step, we have used the same probes (Sp19 and Sp42) to investigate their hybridization with PCR products of different lineages including Africanum; Beijing and EAI strains. Figure 4(B) (with probe Sp19) shows the increase in SWVs peak currents with ΔI/I = 20.3% ± 13% and ΔI/I = 21% ± 4% after hybridization between Sp19 probe with PCR product from Africanum (blue line) and EAI (magenta line), respectively. In particular, it can be observed from this figure that the PCR product from Beijing strain, the current response (green line) is not significantly changed (ΔI/I = −0.53% ± 4%). It is in accordance with the report of Sebban et al [16] confirming that this strain of PCR does not possess any complementary base with the probe sequence. Similarly, after hybridization of Africanum, EAI and Beijing strains with Sp42 probe, the increase in the peak current is insignificantly observed: ΔI/I = 19.7% ± 10% (blue line), ΔI/I = 18.8% ± 6% (magenta line) and ΔI/I = 15% ± 6% (green line), respectively. It can be logically expected that hybridization between the short DNA probes with targets is more selective and thus triggers significant SWVs current change (about 40%), while the PCR products, containing much longer DNA sequences compared to those of the probes induced only about 20% current increase. Indeed, only a part of the DNA target (from PCR products) is hybridized and therefore rigid, while most of the target is not hybridized, thus coiled. Thus, the SWVs peak current changes are lower because of the greater steric hindrance onto electrode surface [6].
4. Conclusions
The electrosynthesis of poly(JUG-co-JUGA)/rGO composite was designed for Mycobacterium tuberculosis DNA sensor. The short oligonucleotides targets and long, real sample PCR products were detected by signal-on in the label-free and reagentless electrochemical detection. It was found that reduced graphene oxide improved the electrocatalytic activity and voltammetric response of copolymer poly(JUG-co-JUGA). Poly(JUG-co-JUGA)/rGO based DNA sensors worked stably. Understandably, due to the steric hindrance effect, the response upon the hybridization of short Sp19 and 42 probes with real sample PCR products (Africanum, EAI and Beijing strains) was much less compared with the short DNA target. However, the obtained results are promising for 'point-of-care' Mycobacterium tuberculosis DNA sensors.
Acknowledgments
This research was financially supported by VAST-CNRS joint project (grant VAST.HTQT.PHAP.02/2012-2013). The authors also would like to thank Professor MC Pham and Professor B Piro (ITODYS, University Paris-Diderot, France) for their invaluable discussion on the biodetection measurements.