Abstract
In this paper we represent a study on the potential use of protein A-tagged gold nanoparticles applied for signal amplification of electrochemical immunosensors. Gold nanoparticles (GNPs) were synthesized by the chemical reduction of tetrachloroauric (III) acid trihydrate using sodium ascorbate, and then tagged with protein A (PrA) via ultracentrifugation. UV-Vis spectroscopy and transmission electron microscopy were used to verify the characteristics of formed GNPs/PrA complex. The analyzed results indicate that GNPs were found spherically, homogeneously, and with an average diameter of about 10 nm. Immunoelectron microscopy was then used to investigate the bioactivity of the GNPs/PrA complex in solution by the effective binding of GNPs to viral particles. Scanning electron and fluorescence microscopies were also used to investigate the distribution and the bioactivity of the GNPs/PrA complex on the surface of the interdigitated sensor. Consequently, this study provided some assumptions of the potential application of protein A-tagged gold nanoparticles for signal amplification of electrochemical immunosensors in virus detection from clinical samples.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In recent decades biosensors/biochips have been envisaged to compensate and complement conventional diagnostic methods due to their easy operation and transport; they require no specific reagent and provide results in a few minutes [1–3]. Among them, immunosensors based on electrochemical detection have the advantage of being highly sensitive, rapid, inexpensive and highly amenable to micro-fabrication, and it is also easy to measure the changes in electrical/electrochemical properties resulting from the antigen–antibody reaction on the surface of the sensor [1, 3]. However, the electrochemical signal obtained from the reaction of antigen–antibody binding is normally very small. Hence, the signal amplification of electrochemical response and noise reduction has become critical for the successful development of an electrochemical immunosensor with a low detection limit and high sensitivity. Recently, the nanosciences and nanotechnologies could help to improve the detection performance of immunosensors in diagnostics due to a better control of the organization of atoms and molecules to create nanostructures with new or improved properties [4, 5]. In these nanostructures gold nanoparticles have revealed excellent biocompatibility, a large surface area, potential carriers, and especially an ability to facilitate the electron transfer between redox proteins and micro-electrodes, leading to amplification of the signal of the electrochemical sensor with or without electron transfer mediators [6, 7]. Furthermore, protein A (PrA) molecules are very heat-stable and bind well to the fragment crystallizable (Fc) region of the immunoglobulin molecules without disturbing antigen binding sites [1, 8], leading to the improvement of the possibility for detecting viral antigens in solution. Hence, the combination of gold nanoparticles and PrA could be ideal for signal amplification of electrochemical immunosensors in direct detection of virus from clinical samples.
2. Experimental
2.1. Reagents
Tetrachloroauric (III) acid trihydrate 99.5% (HAuCl 4.3H 2 O), potassium carbonate (K 2 CO 3) were purchased from Merck. Fluorescein isothiocyanate (FITC)-conjugated mouse anti-human immunoglobulin G (IgG) antibodies (FITC-Ab), bovine serum albumin (BSA), 3-aminopropyl-triethoxy-silane (APTES), glutaraldehyde (GA), sodium ascorbate (C 6 H 7 NaO 6) and polyethylene glycol (PEG) and protein A (PrA) were purchased from Sigma, USA. All other chemicals were of analytical grade.
Inactivated Japanese encephalitis virus (JEV) and human serum antibodies against JEV were provided by the National Institute of Hygiene and Epidemiology (NIHE) of Vietnam.
The interdigitated sensors (10 μm wide and 10 μm gap size) were designed and fabricated at the Hanoi University of Science and Technology (HUST).
2.2. Preparation of PrA tagged GNPs complex
0.5 ml of l% HAuCl 4 and 0.5 ml of 0.1 M K 2 CO 3 were mixed in 12 ml of distilled water at 4 °C. Then 1 ml of 0.7% C 6 H 7 NaO 6 was added quickly into the above solution during stirring. The color would become immediately purple-red. Distilled water was then added to the solution until 50 ml of volume and boiled until the color became red. 1 mg ml -1 of PrA was then mixed with 15 ml of the GNPs solution. After 5 min, 0.15 ml of 0.02% PEG was added to stabilize the complex [9]. GNPs/PrA solution was ultracentrifuged (Airfuge, Beckman Coulter) for 45 min at 70 000 rpm. The supernatant was removed carefully. The pellet was dissolved in the remaining solution and layered over a 45% glycerol gradient. The gradient was centrifuged for 20 min at 20 000 rpm. A solution of GNPs/PrA complex was obtained by collecting the red zone of the gradient. The final product of GNPs/PrA was investigated using the UV-Vis spectrometry (HP 8453 Spectrophotometer) in the wavelength region 300–900 nm and transmission electron microscopy (JEM 1010, JEOL) operating at 80 kV.
2.3. Immunogold electron microscopy
A droplet of 20 ml JEV sample was placed on a clean parafilm surface. The collodion-carbon coated grid was put on top of a sample droplet for 10 min to pick up the sample. The grid was washed with 2–3 droplets of phosphate buffered saline (PBS) and then allowed to float on a droplet of PBS 2% bovine serum albumin (BSA) for 30 min to block non-specific antigenic sites. The grid was then reacted with the human serum antibodies against JEV, diluted in PBS/BSA 0.5%. After careful washes with PBS/BSA 0.2%, the grid was subsequently incubated for 30 min with the GNPs/PrA complex (diluted 1 : 60 in 0.5% BSA/PBS). The grid was then thoroughly washed with a solution of 0.1% ammonium acetate, and negative staining was carried out using a 1% aqueous solution of uranyl acetate [10]. After negative staining, the grids were dried slowly before observation with transmission electron microscopy (TEM, JEM1010, JEOL, Japan).
2.4. Scanning electron and fluorescence microscopies
The interdigitated electrode was silanized by APTES [8], and incubated with the GNPs/PrA complex (diluted 1 : 60 in 0.5% BSA/PBS) for 45 min. Then, it was washed carefully in 0.2% BSA/PBS and subsequently incubated in 1 mg ml -1 human serum containing antibodies against JEV for 1 h. The unsaturated and non-specific binding sites on the surface were blocked with 2% BSA in PBS for 30 min, washed five times with PBS before the last incubation with FITC-Ab (diluted 1 : 50 in PBS, pH 7.0) for 30 min, washed carefully with PBS and air-dried before investigation with fluorescence microscopy (Eclipse 90i, Nikon, Japan). Another electrode was coated by a very thin layer of Pt before observation with scanning electron microscopy (S-4800, Hitachi, Japan). All immobilization steps were performed at room temperature.
3. Results and discussion
3.1. Morphology of synthesized GNPs
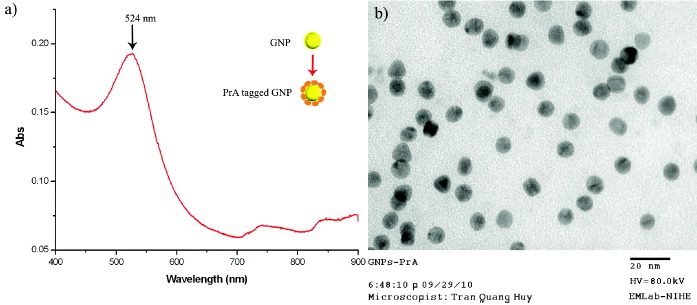
Figure 1(a) shows UV–Vis absorption spectrum of GNPs/PrA complex. The surface plasmon resonance (SPR) peak of GNPs/PrA complex was found at the wavelength of 524 nm and indicated the formation of the GNPs. GNPs were obtained by the reduction from a gold salt using sodium ascorbate. This process occurred through the electrons transfer from the hydroxyl groups of the sodium ascorbate to the Au 3+ ions leading to the formation of Au [11, 12]. Figure 1(b) shows the morphology and size distribution of PrA-tagged GNPs in the colloidal solution. GNPs are mainly spherical, and the mean size is about 10 nm. In this study PrA molecules were used as stabilizer of GNPs, they also helped to protect GNPs from aggregation and precipitation over time, so these particles seem to have a mono-dispersed and non-clustered distribution. The result showed that PrA revealed itself to be a good stabilizer in comparison with other stabilizers such as polymers and micelles [11, 12].
Figure 1 (a) UV–Vis absorption spectrum and (b) TEM image of PrA-tagged GNPs synthesized.
3.2. Bioactivity of GNPs/PrA in solution
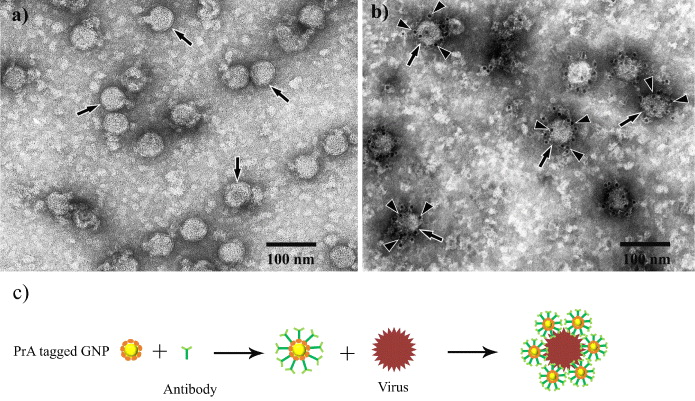
Figure 2(a) shows the TEM image of JEV particles (arrows) before the immunogold labeling reaction, this image revealed that the JEV particles have spherical shapes and about 45 nm in diameter (arrows). Figure 2(b) shows JEV particles after the immunogold labeling reaction, all viral particles are localized by many GNPs (head arrows). This result demonstrated that the presence of viruses in the samples could be detected easily by PrA-tagged GNPs. Moreover, viral particles have been modified from the non-conductivity to the state of conductivity without the influence of biological nature. The formation of JEV particles localized by GNPs is illustrated clearly in figure 2(c).
Figure 2 (a) TEM image of JEV particles before (arrows), and (b) after reaction with GNPs/PrA complex (arrow heads), (c) illustration of the formation between GNP, antibody and viral particle.
In fact, the immunogold labeling reaction has been used widely in the field of electron microscopy for localization of antigen sites of infected cell cultures and/or virus detection as well [10], and it would be a great idea to apply the PrA/GNPs complex for signal amplification of electrochemical immunosensors in pathogen detection. As mentioned, the signals of all electrochemical immunosensors are normally very week [1], whereas GNPs have good electrical properties. This could help to improve the electron transfer between the active center of redox enzymes and electrodes [6, 7]. Consequently, the detection signal of immunosensors could be improved significantly.
3.3. Distribution and bioactivity of GNPs/PrA on the surface of the electrodes
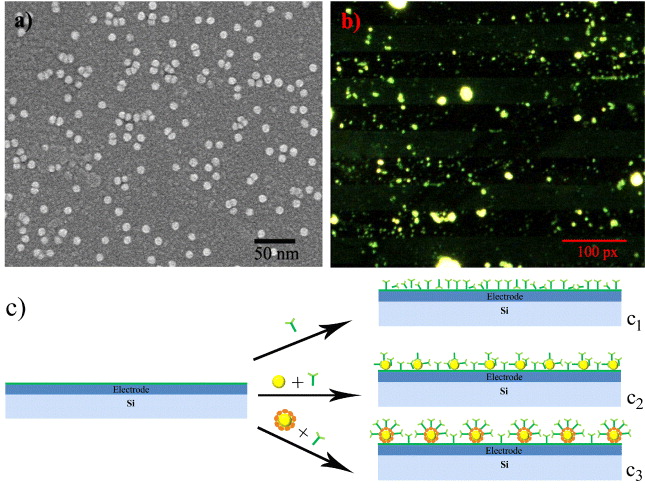
Scanning electron microscopy (SEM) was used to investigate the distribution of GNPs/PrA on the surface of the microelectrodes for development of electrochemical immunosensors. Figure 3(a) shows the SEM image of GNPs located firmly and homogeneously on the surface of the electrode without aggregation. This would be significant for the development of electrochemical immunosensors, because a large number of GNPs could cover the entire surface of the sensor, resulting in the increase of the surface area of electrode with viral particles in the solution. GNPs could play a crucial role in the improvement of the electrochemical signal biosensing process as well as electron transfer 'electron wires', immobilization platform and electrocatalyst [7]. With their special ability to facilitate the direct transfer of electrons between redox proteins and micro-electrodes, GNPs have been applied widely in the development of electrochemical immunosensors in pathogen detection [6, 13].
Figure 3 (a) SEM image of GNPs on the surface of the electrode, (b) fluorescence image of interdigitated electrode treated with GNPs/PrA and human serum antibody against JEV, (c) models of electrodes modified with antibody (c 1), GNPs and antibody (c 2), GNPs/PrA and antibody (c 3).
To verify the biological activity of GNPs/PrA complex on the surface of the electrodes, human serum antibodies against JEV were used to incubate with the microelectrodes immobilized by GNPs/PrA, followed by FITC-Ab. Figure 3(b) shows the fluorescence image of the surface of the interdigitated electrodes after incubation. It shows that a density of green fluorescent spots could be observed clearly on the surface of interdigitated electrodes, whereas they were not seen on the surface using BSA instead of human serum antibodies (not shown). In these experiments, PrA-tagged on the surface of GNPs could bind with high affinity to the FC region of human IgG molecules without influencing their antigen binding sites [1, 8]. This means that PrA could remain and orientate well IgG antibodies with the antigen binding sites outwards from the GNPs' surface. Moreover, a large surface area of GNPs could also lead to a high possibility of capturing viral antigens in solution. This leads to significant improvement of antigen–antibody reaction on the sensor surface as well as electrochemical signals. Figure 3(c) illustrates three models of electrochemical immunosensors using the direct immobilization of antibody on the electrode (figure 3, c 1), modified with GNPs (figure 3, c 2), and treated with GNPs/PrA (figure 3, c 3). Consequently, the combination of GNPs and PrA could lead to the detection of viral antigens and electron transfer becoming easier, resulting in signal amplification of electrochemical immunosensors in virus detection.
4. Conclusion
In this study we described a simple preparation of protein A-tagged gold nanoparticles (GNPs/PrA) complex based on the reduction of tetrachloroauric (III) acid trihydrate using sodium ascorbate. GNPs were mainly spherical, and the mean size was about 10 nm. The GNPs/PrA complex has a good bioactivity in solution, demonstrated by the high possibility to detect and effectively bind to JEV particles. For the development of electrochemical immunosensors, SEM revealed mono-dispersed and non-clustered distribution of GNPs/PrA on the surface of the electrode. Fluorescence microscopic investigation also revealed the effective bioactivity of GNPs/PrA complex on interdigitated electrodes. This study showed a great perspective of GNPs/PrA towards the application for signal amplification of electrochemical immunosensors in virus detection.
Acknowledgments
This work was financially supported by Vietnam's National Foundation for Science and Technology Development (NAFOSTED), project code 106.16.181.09.