ABSTRACT
CO2 is one of the most abundant components of ices in the interstellar medium; however, its formation mechanism has not been clearly identified. Here we report an experimental observation of an Eley–Rideal-type reaction on a water ice surface, where CO gas molecules react by direct collisions with surface OH radicals, made by photodissociation of H2O molecules, to produce CO2 ice on the surface. The discovery of this source of CO2 provides a new mechanism to explain the high relative abundance of CO2 ice in space.
Export citation and abstract BibTeX RIS
1. INTRODUCTION
The high abundance of CO2 in ices in the interstellar medium (ISM) greatly exceeds that in the gas phase (van Dishoeck et al. 1996). This suggests that a solid phase CO2 synthesis process is probably involved, but the CO2 formation mechanism has not been identified. Recent observations indicate that CO2 (ice) formation is linked to H2O formation (Boogert et al. 2004). Theoretical and laboratory studies suggest an efficient CO + OH → CO2 + H reaction in CO/H2O mixed ices due to its low activation energy barrier (Garrod & Pauly 2011; Ioppolo et al. 2011; Noble et al. 2011; Oba et al. 2010; Ruffle & Herbst 2001a; Zins et al. 2011); however, the limited mobility of CO and OH species at low temperatures inhibits the reaction probability. Carbon monoxide is the second most abundant gas species after hydrogen in the ISM (Tielens 2005). It may well be the parent molecule for the synthesis of CO2, as well as for many organic molecules in space (Ehrenfreund & Charnley 2000). Here we report an efficient surface reaction pathway for gas phase CO conversion to solid CO2 on amorphous solid water (ASW) films or crystalline water ice films in the temperature range 71–82 K, activated by ice absorption of Lyα (10.2 eV) radiation. A direct Eley–Rideal (E-R) process (Eley 1948; Eley & Rideal 1940; Rideal 1938) that does not involve prior energetic accommodation of CO by the ice surface is observed, when the incident CO molecules interact with surface-bound OH radicals that are produced by water photodissociation. The surface reaction is kinetically separate from the previously studied solid phase CO+OH reaction. This finding of a significant new process for CO2 production will influence kinetic models involving CO(g) consumption and CO2(ice) formation in molecular clouds (Ruffle & Herbst 2001a; Whittet et al. 2010) or in the atmospheres of icy moons in the solar system (Teolis et al. 2010). More generally, this result suggests that similar E-R surface reactions on ice films which contain surface-bound radical species are likely to occur for other gas molecules in space.
Photochemical processes are known to drive molecular synthesis in the ISM, especially inside thin ice films condensed on dust grains (Gerakines et al. 1996; Ruffle & Herbst 2001b; van Dishoeck et al. 2006). During photodissociation of mixed CO/H2O ices, hydroxyl radicals are produced that can interact with CO. This reaction has been studied in the laboratory by hydrogenation of CO/O2 binary ices (Ioppolo et al. 2011; Noble et al. 2011) and by CO reaction with non-energetic OH radicals (Oba et al. 2010; Zins et al. 2011). In contrast to these studies of bulk ices containing condensed CO molecules, here we report that gas phase CO has a remarkable reactivity with photochemically generated OH radicals on the surface of H2O ice. This finding reveals a significant new kinetic process for CO2(ice) formation in space.
The classical E-R mechanism for a surface reaction involves, in its simplest form, the direct collision of an incoming species from the gas phase with a surface-bound species to produce a product molecule and was originally proposed as the mechanism for a metal-catalyzed surface reaction (Rideal 1938). This type of surface reaction occurs with reactive incoming species such as atomic H (Cheng et al. 1992). In space, it is proposed that molecular H2 formation by H+H recombination in high temperature ISM regions occurs efficiently through the E-R mechanism (Lemaire et al. 2010). A modification of the classical direct E-R reaction involves an incoming species which partially accommodates on the surface as a mobile precursor and, in its subsequent diffusive motion, encounters an immobile reactive partner which then participates with the mobile species in a chemical reaction (Harris & Kasemo 1981). Such a process should exhibit temperature dependence due to the decreasing lifetime and steady-state coverage of the mobile species as temperature is raised. We present kinetic evidence that the CO+OH (on water ice) reaction occurs by a direct E-R process not involving CO surface mobility.
2. EXPERIMENTAL
The apparatus has been described previously in detail (Yuan & Yates 2013). Briefly, the apparatus is a baked high vacuum system with a base pressure of ∼10−8 torr, pumped by turbomolecular and ion pumps. Figure 1 shows an experiment which demonstrates the photochemically induced reactivity toward CO(g) of a 20 nm thick ASW film. The H2O film is deposited at 76 K on a 0.3 cm2 disk of pressed KBr powder held on a tungsten grid in the high vacuum stainless steel cell of 1.9 × 10−3 m3 volume (Yuan & Yates 2013). In addition, crystalline ice films were made by heating deposited ASW to 170 K until the phase transition was completed and then cooling to 76 K for similar studies. Lyα radiation (2.7 × 1018 photons m−2 s−1) (Rajappan et al. 2010, 2011), passing through a LiF window and an internal aperture, is incident at 45° to the ice film. Transmission IR spectroscopy at a 45° incidence angle to the ice surface is performed during the irradiation to observe the formation of condensed CO2 product. A quadrupole mass spectrometer senses the pressure of flowing CO(g) before, during and after irradiation. A mixture of C16O and C18O is employed with a mole fraction, XC18O. We measured the C18O (m/z = 30) behavior and converted it to total CO pressure using measurements of XC18O. The CO pressure is established over the ice film using a measured CO pumping speed of 2 × 10−4 m3 s−1 (Yuan & Yates 2014). When the UV light is admitted, CO begins to be consumed immediately and a steady-state CO pressure is reached in about 300 s. When the UV light is extinguished, the CO pressure rises back to the original value in about 300 s. This behavior is observed at many CO pressures and the experiment closely resembles the well-known King–Wells method used in surface science to measure adsorption kinetics (King & Wells 1974). The change of CO pressure (ΔPCO) is found to be similar (±20%) with both thick and thin ASW films, as well as on a crystalline ice film.
Figure 1. (a) CO(g) consumption during experiments at various CO pressures at 76 K. The curve is determined by measurements of C18O behavior and then converted to total CO pressure using the fraction of C18O. Typically XC18O is in the range 10%–80%. (b) Schematic of CO(g) consumption measurement procedure on irradiated ASW.
Download figure:
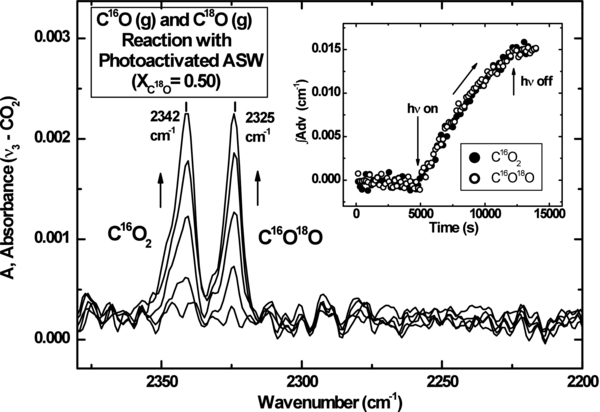
Standard image High-resolution imageFigure 2 shows the photochemical production of condensed CO2 isotopomers during the experiment where XC18O = XC16O = 0.50 ± 0.05. The strong ν3 modes for C16O2 and C18O16O are observed. Their frequencies are close to the typical frequencies of pure CO2 isotopomer ices (Broekhuizen et al. 2006; Escribano et al. 2013; Falk 1987), showing that the solid CO2 product is formed. It is seen that the two CO2 absorption bands grow monotonically. Plots of their integrated absorbance over time display the same slope, indicating that any 18O isotope effect for CO is negligible.
Figure 2. IR spectra of C16O2 and C16O18O isotopomer ices produced during C16O(g) and C18O(g) reaction on irradiated ASW. The inset shows the growth of integrated absorbance of C16O2 and C16O18O isotopomers vs. time.
Download figure:
Standard image High-resolution image3. RESULTS AND DISCUSSION
3.1. Evidence for the Eley–Rideal-type Reaction
The rate of CO(g) consumption, RCO (molecules m−2 s−1), can be found by measuring the change of CO pressure (ΔPCO) during UV irradiation. The ΔPCO is directly proportional to RCO in a rapidly pumped system (Yates 1985), as shown in Equation (1):

where SCO is the measured pumping speed of CO(g), A is the irradiated sample area, kB is the Boltzmann constant, and Tg is the CO gas temperature. At any CO pressure, the CO consumption rate during irradiation is proportional to the accumulated surface OH coverage, NOH. When the reaction reaches steady state, the surface-bound OH production rate is equal to its consumption rate, including the OH reaction rate with CO(g), and the OH recombination rate with H or OH. Molecular dynamics studies show a large fraction of photo-produced H desorbs from the ice surface because of its weak binding energy (Andersson & van Dishoeck 2008), leaving an OH-enriched surface. We therefore postulate that the OH + OH → H2O2 → H2O + O reaction is the major process that consumes the excess OH (Oba et al. 2011). After the interruption of the irradiation, the OH species remaining on the surface continue to be consumed by CO(g) and by the recombination reactions, resulting in a decrease of RCO. An empirical fit with second-order kinetics gives  from the data in six measurements at steady state, corresponding to a fractional surface OH coverage of
from the data in six measurements at steady state, corresponding to a fractional surface OH coverage of  = 0.05 ML (monolayer) at steady state.
= 0.05 ML (monolayer) at steady state.
The incident flux FCO of CO(g) on the ice surface is calculated from the kinetic theory of gases using Equation (2):

where  is the total pressure of CO(g) before irradiation, and m is the mass of the CO molecule. Therefore, the cross section, σCO, for CO(g) reaction can be obtained as d[CO]/dt = FCO·σCO·NOH = RCO. The reaction cross section, σCO, is found to be 6 × 10−20 m2, which is of the order of a geometrical molecular cross section, consistent with a direct E-R-type process.
is the total pressure of CO(g) before irradiation, and m is the mass of the CO molecule. Therefore, the cross section, σCO, for CO(g) reaction can be obtained as d[CO]/dt = FCO·σCO·NOH = RCO. The reaction cross section, σCO, is found to be 6 × 10−20 m2, which is of the order of a geometrical molecular cross section, consistent with a direct E-R-type process.
When CO molecules strike the ice surface, their average residence time can be calculated from Equation (3):

where ν is the desorption frequency factor ≈1012 s−1, Edes is the CO desorption energy from the H2O ice surface, and T is the ice temperature. The CO desorption energy from H2O ice has been investigated experimentally and theoretically (Al-Halabi et al. 2004a, 2004b; Collings et al. 2003). Because the CO coverage is low in our experimental conditions, we use Edes = 0.125 eV here (Karssemeijer et al. 2014). Therefore at T = 76 K, the residence time for CO(g) on the H2O ice surface is ∼2 × 10−4 s. The fractional coverage of CO adsorbed on the ASW–H2O ice surface at a given CO(g) pressure is θCO = (FCO × ts)/NH2O, where NH2O ≈ 9 × 1018 m−2 is the surface density of H2O adsorption sites based on the density (0.94 g cm−3; Jenniskens & Blake 1994) of ASW. Under our experimental conditions, the CO fractional coverage is only θCO = 1 × 10−6 ML, i.e. five orders of magnitude smaller than the steady-state fractional OH coverage. This low coverage is consistent with the absence of the adsorbed CO IR absorption band. Thus, it is improbable under the conditions of the experiment for the reaction to occur by a mechanism where CO adsorbs on the surface and translates to an OH species.
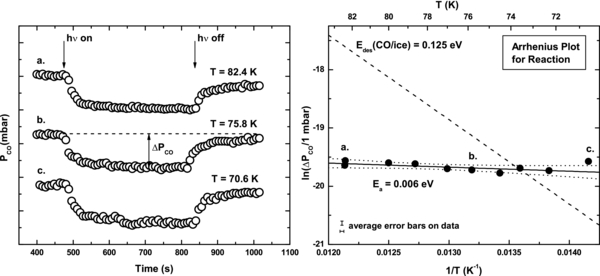
Figure 3 shows the temperature dependence of the reaction rate. As temperature decreases the CO consumption rate remains constant. This is additional evidence to support the E-R reaction mechanism. The measured activation energy of 0.006 eV, indicative of a zero activation energy within experimental error, implies that activated diffusion processes for CO is not kinetically significant at 76 K for the CO+OH reaction. The formation of the stable intermediate compound HOCO at unobservable concentrations is probably involved (Arasa et al. 2013; Ioppolo et al. 2011).
Figure 3. Temperature effect on CO(g) consumption rate between 70.6 K and 82.4 K. CO consumption is displayed for measurements at three temperatures. An Arrhenius plot of the data yields an activation energy of 0.006 eV. Ninety-five percent confidence intervals (dotted lines) on the weighted data show that the activation energy is zero within experimental error. The slope of Edes (CO/ice) = 0.125 eV is shown for comparison, and represents the expected temperature effect if the CO surface lifetime is rate controlling in the mobile precursor mechanism.
Download figure:
Standard image High-resolution imageIt should be pointed out that in the ISM, competitive reactions may influence the overall results found here. The OH+OH reaction, the H+H reaction, the CO+H and CO+O reactions (Ioppolo et al. 2011; Noble et al. 2011; Oba et al. 2010; Roser et al. 2001) may interfere with the CO+OH reaction investigated here.
3.2. Advantage of the Method
The use of a mass spectrometer to observe the rate of processes occurring at an ice surface provides a highly sensitive kinetic method. Measurements of the CO reaction rates, such as those shown here, have a sensitivity of the order of 10−7 ML s−1 or ∼1012 molecules m−2 s−1, whereas measurements of the photochemical rate of production of a molecule in the ice bulk, using the widely applied IR measurement method, have a sensitivity of only ∼1017 molecules m−2 s−1 if a strong vibrational mode is measured (Yuan & Yates 2013).
4. KINETIC MODELING
A kinetic model was used to fit the data of Figure 1 as shown in Figure 4. The model considers the flux of incident light, the cross section for H2O photodissociation, and the incoming flux of CO(g) in the system of known pumping speed to calculate the solid curve which is superimposed on the data for one measurement. It is found that the rate of the OH+OH reaction on the irradiated ice is mainly responsible for the shape of the PCO curve, in agreement with the observation in Figure 1 that these curves are only weakly dependent on  . A schematic for the reaction of photo-produced OH with CO(g) via a direct E-R process is shown in the inset of Figure 4, along with the proposed second order recombination of the OH species.
. A schematic for the reaction of photo-produced OH with CO(g) via a direct E-R process is shown in the inset of Figure 4, along with the proposed second order recombination of the OH species.
Figure 4. Modeled CO(g) consumption rate on irradiated ASW and its comparison to experimental data. The inset shows the schematic of the surface processes on the ice surface.
Download figure:
Standard image High-resolution imageTo study the kinetics of the CO consumption process, CO(g) + OH → CO2(ice)+H, we divide the experimental CO pressure curves (as shown in Figures 1, 3, and 4) into two processes. Process I describes the CO pressure decay to steady state during irradiation and Process II describes the CO pressure recovery after irradiation ends.
As mentioned earlier, the CO(g) consumption rate, RCO, is proportional to the initial CO pressure and the surface OH coverage, NOH:
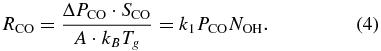
Because of the observed weak dependence of RCO on CO pressure, PCO, and the zero activation barrier, we propose that the CO(g)+OH reaction only consumes a small portion of OH species on the surface. The majority of surface OH species are consumed by the competitive OH+OH second-order recombination process. This assumption is confirmed by the following analysis.
In Process II, we assume the OH consumption rate is simply determined by the CO(g)+OH reaction rate and by the OH+OH recombination rate, despite other complex processes that could occur in an irradiated water ice:

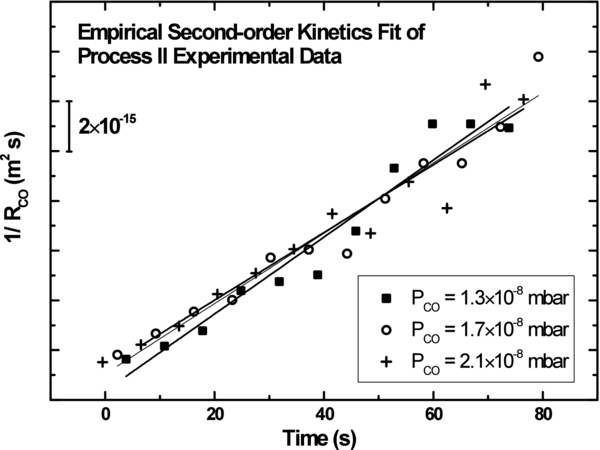
Because the CO(g)+OH reaction rate is relatively slow, the OH consumption rate is approximately a second-order kinetic process in NOH. The empirical second-order kinetic fits of 1/RCO versus time from the experimental data are consistent for a range of pressures, as seen in Figure 5. We use this simplified second-order kinetics assumption in our further modeling.
Figure 5. Empirical second-order kinetic fits of Process II experimental data.
Download figure:
Standard image High-resolution imageBy integrating Equation (5),
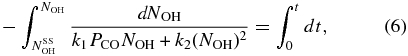
where  is the OH coverage during irradiation at steady state, we get
is the OH coverage during irradiation at steady state, we get

In Process I, the steady-state OH consumption rate is equilibrated with its production rate by irradiation. The kinetic equation is

where Fhv is the flux of photons, σH2O = 1 × 10−21 m2 is the Lyα photodissociation cross section of H2O ice (van Dishoeck et al. 2006), and NH2O is the surface density of H2O molecules. Here we assume that only the first layer of water ice contributes to the surface OH production. Integrating Equation (8)

we get

where  .
.
At steady state,

Here we assume a steady-state surface OH concentration,  , so that the rate constants k1 and k2 can be calculated from Equations (4) and (11). These rate constants are used in Equations (7) and (10) to model the curves to best fit the experimental data.
, so that the rate constants k1 and k2 can be calculated from Equations (4) and (11). These rate constants are used in Equations (7) and (10) to model the curves to best fit the experimental data.
The value of  can be estimated from the empirical second-order kinetics fit in Figure 5, and then be slightly adjusted to best fit the experimental data. An optimum
can be estimated from the empirical second-order kinetics fit in Figure 5, and then be slightly adjusted to best fit the experimental data. An optimum  is obtained from the data of six measurements. The fitting result is shown in Figure 4 for one experiment. The modeled curve for Process II fits the experimental data well, while the curve for Process I has a small deviation from the data when PCO approaches the steady state. This may be rationalized by postulating a small contribution from slow diffusion of OH from the underlying layers of the water ice (Andersson & van Dishoeck 2008). Since the Lyα light penetrates into the ice, the diffusing OH species produced in the bulk may contribute to the surface OH concentration, causing a slightly increasing CO consumption rate as one moves to longer times in the plateau region of Figures 1 and 4.
is obtained from the data of six measurements. The fitting result is shown in Figure 4 for one experiment. The modeled curve for Process II fits the experimental data well, while the curve for Process I has a small deviation from the data when PCO approaches the steady state. This may be rationalized by postulating a small contribution from slow diffusion of OH from the underlying layers of the water ice (Andersson & van Dishoeck 2008). Since the Lyα light penetrates into the ice, the diffusing OH species produced in the bulk may contribute to the surface OH concentration, causing a slightly increasing CO consumption rate as one moves to longer times in the plateau region of Figures 1 and 4.
With the calculated values of k1 and k2, the OH consumption rate by the CO(g)+OH reaction and by the OH+OH recombination are 3% and 97% of the total OH production rate by irradiation at steady state, respectively. Therefore our previous assumptions that the CO(g)+OH reaction rate is relatively small and that the OH consumption rate can be simplified as a second-order kinetic process are reasonable.
5. CONCLUSIONS
In summary we have observed the reaction between photochemically produced surface-bound OH species and incident CO(g) molecules at water ice surfaces between 71 and 82 K. The reaction is found to occur by a direct Eley-Rideal mechanism where a CO(g) molecule collides and reacts directly with a surface OH species to produce CO2(ice). This observation may explain the origin of some of the CO2(ice) observed in the ISM as well as the CO consumption in the atmospheres of icy moons. Our experiments demonstrate a new process to convert CO(g) to CO2 by reaction with surface OH species on astronomical ices.
C.Y. acknowledges full fellowship support from the Department of Chemistry, University of Virginia. I.R.C. acknowledges funding from a Fulbright Science and Innovation Graduate Award. We also acknowledge helpful conversations with Professor Eric Herbst and very helpful comments from the reviewer.