Abstract
Extrusion-based bioprinting is a promising technology for the fabrication of complex three-dimensional (3D) tissue-engineered constructs. To further improve the printing accuracy and provide mechanical support during the printing process, hydrogel-based support bath materials have been developed. However, the gel structure of some support bath materials can be compromised when exposed to certain bioink crosslinking cues, hence their compatibility with bioinks can be limited. In this study, a xanthan gum-based composite support material compatible with multiple crosslinking mechanisms is developed. Different support bath materials can have different underlying polymeric structures, for example, particulate suspensions and polymer solution with varying supramolecular structure) and these properties are governed by a variety of different intermolecular interactions. However, common rheological behavior can be expected because they have similar demonstrated performance and functionality. To provide a detailed exploration/identification of the common rheological properties expressed by different support bath materials from a unified perspective, benchmark support bath materials from previous studies were prepared. A comparative rheological study revealed both the structural and shear behavior characteristics shared by support bath materials, including yield stress, gel complex moduli, shear-thinning behavior, and self-healing properties. Gel structural stability and functionality of support materials were tested in the presence of various crosslinking stimuli, confirming the versatility of the xanthan-based support material. We further investigated the effect of support materials and the diameter of extrusion needles on the printability of bioinks to demonstrate the improvement in bioink printability and structural integrity. Cytotoxicity and cell encapsulation viability tests were carried out to confirm the cell compatibility of the xanthan gum-based support bath material. We propose and demonstrate the versatility and compatibility of the novel support bath material and provide detailed new insight into the essential properties and behavior of these materials that serve as a guide for further development of support bath-based 3D bioprinting.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Additive manufacturing as a newly developed technology has enabled the flexible, precise fabrication of customized, complex structures and is compatible with a wide range of materials, including metals, polymers, ceramics and soft materials [1]. As a form of additive manufacturing technique specifically developed for tissue engineering, extrusion-based three-dimensional (3D) bioprinting has attracted significant attention and interest over recent years as a promising approach for the generation of bio-constructs with improved fabrication resolution, structural complexity, cell/biomolecule deposition accuracy and ultimately enhanced functionality [2, 3]. Extrusion-based 3D bioprinting generally utilizes soft, bio-mimicking hydrogel-based materials, often referred to as 'bioink carrier materials', for their superior biocompatibility and cell interactive capacity [4]. During the printing process, these materials are forced through an extrusion nozzle, driven by a pneumatic piston or mechanical screw, forming strands that are deposited onto a substrate. To facilitate the extrusion process, bioinks that exhibit shear-thinning, fluid-like behaviors during extrusion are preferred. Due to the relatively weak mechanical strength and fluidic characteristics of the hydrogels, strands printed with these materials tend to suffer from a range of irregularities including over-spreading, distortion, and strand inconsistency [5], which ultimately leads to compromised printing accuracy and structural integrity of the printed constructs. While the deposited bioinks can be crosslinked via physical and/or chemical processes, forming a rigid, self-supporting gel network after extrusion, the time needed for such crosslinking reactions and subsequent gelation of the deposited bioinks may vary. For crosslinking processes that occur in a shorter timeframe [6], the deposited bioink and the structure can be better preserved. However, for crosslinking processes that are more time-consuming, defects such as filament drying, strand/construct shrinkage and collapse [7] can occur, resulting in compromised printing outcomes.
Formation of support structures for constructs with complex geometric shapes, overhanging elements and/or delicate internal features using soft, extrudable hydrogels is impractical, as they tend to suffer from similar printing defects as extruded bioinks for their similar rheological and mechanical properties [8]. These drawbacks impose limitations to the printing accuracy, attainable structural complexity, as well as scalability in extrusion-based bioprinting.
To tackle these challenges, an elegant approach has been developed, in which extrusion bioprinting takes place in an immersive printing medium that provides mechanical support throughout the printing process [9]. Fully encapsulated and supported by the surrounding soft gel during the 3D building-up process, the dispensed bioinks can then be exposed to crosslinking stimuli which are either supplemented in the support medium (e.g. Ca2+ ions for crosslinking alginate) or applied externally (temperature, UV irradiation), forming a stabilized gel structure and scaffold.
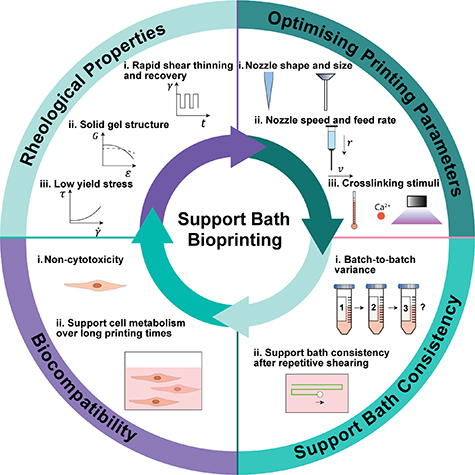
While some polymeric solution [10] and hydrogel systems have been developed as support baths for extrusion-based bioprinting with their respective advantages and drawbacks as summarized in table 1, there are several outstanding challenges and technical gaps remaining. First, the preparation of hydrogel support materials can be time-consuming and technically complicated. One way of preparing supportive gel media involves mechanical fragmentation that breaks pre-crosslinked gel into particles, followed by further processing such as mixing with filler materials, centrifugation, and purification before use [11–13]. The size distribution of gel particles in such suspension has been found vital to the performance of the support bath material, and the requirement of refined control over the particle size distribution adds additional complexity to the preparation process. Second, similar to hydrogel-based bioinks, hydrogel-based support materials are usually crosslinked using mechanisms such as temperature-induced phase transition [14], ionic crosslinking [15], and pH-responsive gelation [16]. The extent of crosslinking requires delicate control, as both insufficient or excessive crosslinking in the hydrogels can undermine the functionality of support baths. Since most bioink hydrogels share similar crosslinking mechanisms with support bath hydrogels, the application of crosslinking stimuli during or after the printing process can cause excessive gelation or disruption of gel structure in the support bath, rendering the latter dysfunctional. While such conflicts in crosslinking mechanisms can be utilized as a mechanism to release the printed constructs from support baths [5], they also impose restrictions on the selection of bioinks, only allowing for the ones that are compatible with the support medium material. As a result, it has been challenging to obtain a support bath material that can be prepared with ease consistently, while being able to remain functional in response to the commonly used bioink crosslinking stimuli and therefore be compatible with a variety of bioinks. Last, while some recent studies have provided characterization of the rheological properties of support bath materials, there remains a need for a comparative assessment to fully identify the essential rheological characteristics of functional support bath materials [17]. The general requirements for support bath bioprinting requirements are shown in figure 1.
Figure 1. Essential aspects and characteristics for support bath materials that enable functionality for 3D bioprinting in support bath materials.
Download figure:
Standard image High-resolution imageTable 1. Preparation, advantages, and disadvantages of commonly used support bath materials.
| Materials | Preparation | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Gelatin slurry | Mechanical fragmentation of physical gel, or controlled coacervation to obtain gel particles | Non-cytotoxic, simple removal mechanism by increasing temperature | Incompatible with printing at physiological temperature, batch-to-batch variance depending on the control in particle size and distribution | [11, 18] |
| Pluronic® F-127 | Accurate control of temperature, 21 °C−25 °C depending on the concentration | Optical and UV transparency for monitoring of the printing process and UV crosslinking of bioinks | Relatively high yield stress, filler material required when recovery of support bath is slow | [19] |
| Carbopol® | Dissolve in water, adjust pH value to 5–6 to induce gelation | Provides accurate printing outcomes, optical transparency for monitoring the printing process, easy preparation | pH-responsive, some variants of Carbopol are sensitive to the presence of divalent salts, potential long-term effects on cells | [9, 20] |
| Alginate | Controlled ionic crosslinking, followed by mechanical fragmentation into gel particles | Stable at physiological temperature, refined printing fidelity achievable | Requires control over particle size distribution, properties of the support bath can be susceptible to Ca2+ ions, relies on enzymatic reaction to release printed structures | [13] |
| Agarose | Mechanical stirring and fragmentation of physical gel | Improved thermal stability compared to gelatin slurry | Batch-to-batch variance, as support bath properties dependent on stirring and blending time | [12, 21] |
In this study, we present a versatile, cell-friendly, easy-to-prepare xanthan gum-gelatin composite support bath material which is compatible with multiple crosslinking mechanisms commonly used in 3D bioprinting. Xanthan gum has been recognized as a polymer that can endure harsh working conditions in heavy industry, including heat, acidic/basic and saline environments, without losing its chemical stability and functionality [22], and has also drawn interest in 3D bioprinting for its shear-thinning nature and other rheological properties that can facilitate the extrusion process [23, 24]. Based on its chemical and functional stability, we propose the compatibility of xanthan gum with multiple crosslinking mechanisms as a support bath material at lower concentrations. Unlike many hydrogel-based support bath materials whose favorable rheological properties rely on closely packed microgel particles and require delicate size distribution control, xanthan gum-gelatin composites are comprised of a blend of dissolved polymer chains that can be easily prepared with batch-to-batch consistency and good cytocompatibility. To provide detailed insight into the common rheological characteristics possessed by different support bath materials, we prepared and reproduced a variety of support bath materials as benchmark materials along with our xanthan-gelatin composites. We then conducted a comparative study of the rheological characteristics of support bath materials based on comprehensive rheological profiling that investigated both the structural information of the gel structures with a set of oscillation rheology tests and the dynamic shear behaviors of the support bath materials with rotation rheology tests that were designed to mimic the shear conditions in support bath 3D bioprinting. We identified the key rheological properties of support bath materials and their contribution to the functionality via the comparative study. To evaluate the versatility of the support bath materials and to investigate their compatibility with different bioink crosslinking stimuli, we then applied multiple crosslinking stimuli commonly used in 3D bioprinting to the support bath materials and monitored if the structural and functional stability of the support bath materials were affected. To investigate the effect of various printing parameters on the printability of bioinks in support baths, two bioinks that utilize different crosslinking mechanisms were printed both on tissue culture plastic surfaces and in xanthan support bath materials, and the printability of the bioinks printed with different parameters both on polystyrene substrates and in xanthan gum support bath was examined and compared. To further examine and demonstrate the functionality of the xanthan-gelatin support bath in the generation of volumetric structures, we test-printed a variety of 3D structures in the support bath. Finally, we conducted both cell encapsulation viability tests and serial dilution cytotoxicity tests to demonstrate and validate the cytocompatibility and non-toxicity of our xanthan-gelatin support material.
2. Materials and methods
2.1. Materials, chemicals, and cells
For the preparation of bioinks and hydrogel-based support baths, sodium alginate, xanthan gum, and Pluronic® F-127 were purchased from Sigma-Aldrich Pty Ltd (Australia). Carbopol® Ultre-Z 20 was purchased from Lubrizol (United States). For in vitro cell culture, L929 (NCTC clone 929) mouse fibroblasts (ATCC-CCL-1) were purchased from ATCC (United States). Dulbecco's modified eagle medium (DMEM), fetal bovine serum (FBS), and Trypsin/EDTA were purchased from Thermo Fisher Australia Pty Ltd (Australia). For cell characterization including live/dead imaging and cytotoxicity assay, Calcein AM and propidium iodide (PI) were purchased from Sigma-Aldrich Pty Ltd (Australia). MTS assay reagent was purchased from Promega (United States).
2.2. Synthesis and characterization of gelatin methacryloyl (GelMA)
A one-pot approach was used for the synthesis of GelMA. Twelve grams of gelatin (Type A) in powder form was added to 120 ml of 0.1 M sodium carbonate/bicarbonate buffer to form a 10% w/v. Solution using vigorous stirring at a temperature of 50 °C. Methacrylic anhydride (MAA) was then added to the solution in a drop-wise manner until the ratio of MAA and gelatin was 0.1 ml g−1 . The pH of the solution was adjusted to a value of approximately 8.9–9.0 using 5 M solutions of either sodium hydroxide or hydrochloride acid. The solution was kept at a temperature of 50 and stirred until the reaction was complete. Periodically, the pH of the solution was measured and adjusted back to 9.0 every 30 min if necessary. After 3 h, the reaction was stopped by adjusting the pH to a value of 7.4 using a 5 M HCl solution added dropwise. The resulting solution was then transferred to dialysis bags (14 kDa, Sigma-Aldrich) and dialyzed against purified water (MilliQ™) at 50 °C for 10 d. The MilliQ water was changed every 24 h. After dialysis, the solution was transferred to 50 ml Falcon tubes and freeze-dried for 7 d. The resulting GelMA was stored in the dark at 4 °C for future use. For characterization of the GelMA synthesis product, GelMA and gelatin samples were prepared by dissolving 10 mg of each material in 0.5 ml D2O at a concentration of 2% w/v. The dissolved samples were then transferred to NMR tubes for analysis. The 1H NMR spectra of samples were obtained using a Bruker AVANCE 400 MHz spectrometer. Analysis of the sample was carried out at 25, with a total 32 scans for each spectrum and delay of 2 s between each scan. The degree of substitution of GelMA was determined using both the data obtained from NMR spectroscopy and a 2,4,6-trinitrobenzene sulfonic acid (TNBS) assay. The decrease in the integral of the lysine methylene proton signal from GelMA in comparison to the integral of the lysine methylene proton signal from an unmodified gelatin sample was used to calculate the degree of substitution as shown below:
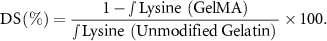
Samples of GelMA and gelatin were dissolved in sodium bicarbonate buffer at a concentration of 1.6 mg ml−1. A TNBS solution at 0.01% w/v was prepared in sodium bicarbonate buffer (pH = 8.5). The TNBS solution (0.5 ml, 0.01% w/v) was added to 0.5 ml of gelatin/GelMA samples in a 48 well plate and incubated at 37 °C for 3 h. A plate reader (Multiskan photometric plate reader, Thermo Fisher) was then used to obtain the fluorescence intensity from each of the sample at a wavelength of 400 nm. The degree of substitution of GelMA was obtained by comparison to a calibration curve generated with glycine and calculated accordingly.
2.3. Preparation and characterization of granulated gelatin slurry support bath media
For the preparation of gelatin slurry, 11.25 g of gelatin (Type A, Bloom number 300, Sigma-Aldrich) was dissolved in MilliQ™ water at 37 °C to give 250 ml of a 4.5% w/v gelatin solution. The solution was stored at 4 °C overnight to allow gelation. The fully gelled gelatin was added to a food blender along with 250 ml of chilled phosphate buffered saline (PBS) and blended at pulse speeds for 2 min. The resultant suspension was transferred to Falcon tubes and repetitively washed with chilled PBS solution, followed by centrifugation at 3000 rpm for 5 min to remove the foam and air bubbles until a homogeneous gelatin slurry was formed. The suspension was then centrifuged at 3000 rpm for 5 min, and the supernatant solution was removed. The gelatin slurry obtained was characterized using a Nikon Eclipse optical microscope with a 20x objective for analysis of the gel particle size distribution.
2.4. Preparation of Carbopol® microgel support bath media
Carbopol® UltreZ 20 (5 mg) was dissolved in 50 ml of MilliQ™ water to form a 0.1% (w/v) solution. The pH of the solution was then adjusted with 5 M NaOH solution to a value of 6, to achieve an adequate gel. At pH values below 6 the material was a low viscosity liquid. The resultant hydrogel was then transferred to a 50 ml Falcon tube and centrifuged at 3000 rpm for 5 min to remove air bubbles.
2.5. Preparation of Pluronic® hydrogel support bath hydrogel
Pluronic® F-127 was dissolved in MilliQ™ water to obtain a solution with a concentration of 25% w/v. Given the thermo-responsive gelling behavior of Pluronic® polymers, the mixture was stored at 4 °C to prevent gelation and precipitation of the Pluronic® polymer during the dissolution process. The mixture was stirred constantly with a magnetic stirrer at 300 rpm to facilitate the complete dissolution of Pluronic® polymers. The fully dissolved 25% Pluronic® F-127 solution was centrifuged at 2000 rpm to remove trapped air and placed at room temperature for stabilization for at least 2 h before experiments to ensure the temperature of the hydrogel was at room temperature.
2.6. Preparation of xanthan gum—gelatin composite support bath hydrogel
Gelatin and anhydrous calcium chloride were added to MilliQ™ water and heated at 37 °C to form a 0.25% w/v gelatin solution with a calcium ion concentration of either 15 or 50 mM. Once the gelatin particles were fully dissolved, xanthan gum powder (Sigma-Aldrich) was gradually dissolved in the warm gelatin solution whilst being stirred using a magnetic stirring bead at 600 rpm, to give final concentrations of 0.50, 0.75 and 1.0% xanthan gum. The composite hydrogel was then mixed with vigorous stirring at 1400 rpm for 3 h until no xanthan aggregates were observed in the solution. The solution obtained was then transferred into Falcon tubes and centrifuged at 3000 rpm for 10 min to remove trapped air bubbles. To ensure the support bath material had reached homogeneity and had an equilibrium structure, the previously centrifuged support bath material was placed on bottle/tube rollers and rolled at 15 rpm for 12 h at 4 °C before use.
2.7. Rheological profiling of support bath materials
For all rheological characterization, an Anton-Paar MCR 702e rheometer with both a parallel plates (25 mm, 0.250 mm gap) and a cone-plate (plate diameter 25 mm, cone angle 0.4°) system was used. For rheological characterization of gelatin slurry, the parallel plate system was used in accordance with the particle size (SI, figure S1). The yield stress of support bath materials was characterized by applying shear rates from high to low values (1000–0.0001 s−1) and recording the shear stress of the sample. The linear viscoelastic region (LVE) of samples was obtained by monitoring the elastic modulus component of the complex shear modulus during the application of a shear amplitude sweep (0.1%–1000%). Flow curves for the gels were obtained by measuring the viscosity as a function of shear rate (0.001–1000 s−1) where the zero-shear viscosity was extrapolated from the flow curve. The storage and loss modulus components of the complex shear modulus of the gels were determined as a function of frequency by applying a 0.1% shear strain at frequencies ranging from 0.01 to 10 Hz. For repetitive shear/recovery characterization, samples were subjected to alternating shear rates of 0.01 and 10 s−1 for 7 cycles where each shear rate was maintained for 10 s. All rheological tests were conducted at 23  to assess the rheological properties of the sample materials in an ambient environment, where bioprinting processes are typically carried out (n = 3).
to assess the rheological properties of the sample materials in an ambient environment, where bioprinting processes are typically carried out (n = 3).
2.8. Compatibility tests of support bath media hydrogels subjected to different crosslinking cues
Xanthan-gelatin support material samples (prepared as summarized above) containing a wide range of calcium ion concentrations (0, 15, 30, 45, 60, 75 mM) were prepared by mixing corresponding amounts of calcium chloride powder with the support bath composite gel. For Carbopol® polymers, calcium ion concentrations were achieved by adding varying volumes of 100 mM CaCl2 solution to the hydrogel. The pH of the support bath composite was adjusted with 5 M hydrochloride acid and 5 M sodium hydroxide to achieve final pH values of 5, 6, 7, and 8. The effect of temperature on support bath materials by varying the temperature between 4 °C–37 °C at a rate of 1  (0.016 per second). Rheological measurements at constant-shear test (0.1% strain, 90 s) were then carried out to detect any change in storage and loss moduli for all samples when exposed to different physical/chemical factors.
(0.016 per second). Rheological measurements at constant-shear test (0.1% strain, 90 s) were then carried out to detect any change in storage and loss moduli for all samples when exposed to different physical/chemical factors.
The attenuation of UV light intensity was measured at 365 nm using a UV intensity radiometer (Cole-Parmer, United States) and an Omnicure Series 1500 UV-visible light source with a 365 nm filter in a dark room. Varying volumes of xanthan-gelatin support materials of different concentrations were transferred in a UV transparent 24 well-plate (1, 2, and 3 ml per well). The light source was set 20 mm above the surface of the support bath and the UV radiometer was placed beneath the multi-well plate. The reading for different samples was compared to the UV intensity measured through an empty well on the same plate from an identical light source position to determine the level of attenuation of UV light intensity by the materials in the support bath.
2.9. 3D bioprinting, printability tests, and removal of structures from support bath materials
Bioprinting of different hydrogels and samples was carried out using a GeSim Bioscaffolder 3.2 multi-channel extrusion-based bioprinter (GeSim GmbH, Germany). The printability of bioinks was determined by measuring the width, and consistency of the strands as well as the size and shape of the pores in the designed and printed scaffolds. For the printability test, general-purpose dispensing needles with an inner diameter of 150, 250, and 410 μm (gauge 30, 25, 22, EFD Nordson) were used, and a 3% GelMA bioink and a 4% sodium alginate bioink were printed independently. A Nikon Eclipse optical microscope was used for the imaging of the bioprinted strands and scaffolds. For the demonstration of bioprinting of different volumetric and 3D structures, 150 μm internal diameter (30 gauge) general-purpose dispense needles and a 4% sodium alginate bioink were used. To remove printed structures from xanthan-gelatin composite support bath, warm PBS solution supplemented with 15 mM CaCl2 was added to the support bath after the printing process for dilution of the support bath material. The support bath material was then gently aspirated.
2.10. Cell viability and cytotoxicity tests
Murine fibroblast cells (L929) were cultured in DMEM supplemented with 10% FBS and 5% penicillin/streptomycin as described in section 3.1. Cells were harvested and seeded in a 96-well plate at a seeding density of 5000 cells per well. Xanthan gum solutions with concentrations of 0.0625, 0.125 and 0.25% were added to wells containing attached monolayers of L929 cells, incubated for 30 and 60 min and washed thrice with sterile PBS buffer solution. Timepoints of 30 and 60 min were chosen to simulate the typical exposure time of cells to support bath materials during 3D bioprinting. For the MTS assay, cell culture media in 96 well-plate was first replaced with 100 μl fresh media, MTS reagent (Cell Titer 96 assay, Promega) was then thawed in a water bath at 37 °C and 20 μl of reagent was added to each well, forming a 120 μl mixture of reagent and cell culture media. The cells were then placed in the cell incubator and incubated for 45 min. After incubation, 100 μl of media/MTS reagent mixture from each well was transferred to a new 96-well plate, and the multi-well plate was placed in a plate reader (Thermo Fisher) for characterization by UV-Vis spectroscopy at 490 nm wavelength. Assuming identical cell metabolic activity, cell viability was calculated based on the optical density measured for each sample when compared to the optical density obtained from control groups after subtracting background values from the media and the substrate.
For live/dead staining imaging, L929 cells were harvested and encapsulated in 2% alginate. The alginate samples were then crosslinked with 15 mM CaCl2 solution, and subsequently immersed in 3 ml of 1% xanthan-gelatin support bath in a 12-well plate for 30 and 60 min. To investigate any potential impacts of the bioprinting process on cell viability, an additional group of two- layer 5 mm  5 mm scaffolds comprising L929 cells encapsulated in 2% alginate was printed into the support bath using a 150 μm internal diameter needle at a pressure of 120 kPa. A live/dead assay was then carried out on the cells within the hydrogel, both bioprinted and non-printed, to characterize the viability of the cells after exposure to xanthan-gelatin composites using an Olympus FV 3000 confocal microscope.
5 mm scaffolds comprising L929 cells encapsulated in 2% alginate was printed into the support bath using a 150 μm internal diameter needle at a pressure of 120 kPa. A live/dead assay was then carried out on the cells within the hydrogel, both bioprinted and non-printed, to characterize the viability of the cells after exposure to xanthan-gelatin composites using an Olympus FV 3000 confocal microscope.
2.11. Statistical analysis
Statistical analysis of data was conducted with GraphPad Prism 9.0 (GraphPad, United Kingdom). Normality and equal variance were first tested for each group of data. For parametric data, two-way ANOVA tests were performed. Statistical significance was identified for p-values < 0.05, noted as * for 0.01 < p < 0.05, and ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001.
3. Results and discussion
3.1. Comprehensive rheological profiling of support bath materials
As illustrated in figure 2, during the bioprinting process, support bath materials are subjected to a range of forces, and evolution of the polymeric structure occurs. When subjected to the shear stress exerted by the moving printing nozzle, the support bath material is sheared and upon imposition of a critical level of shear stress (the yield stress), yielding and subsequent flow of the support bath material occurs. This yielding of material leads to a reorganization of the polymeric structure of the support bath in the immediate vicinity of the printing nozzle. As these materials are typically shear thinning, local fluidization of the support bath material occurs as a result of such reorganization in the polymeric structure of the support bath material. As the printing nozzle travels away from its previous position, self-healing of the gel structure occurs rapidly, resulting in the restoration of higher viscosity values as well as other gel properties, and encapsulation of the deposited bioinks. For accurate and precise printing of 3D structures in support baths, the deposited bioinks need to be supported and locked in at a given location and remain undisturbed during the remaining printing process. Therefore, it is important that the support bath provides resistance against buoyancy and dampens any mechanical stimuli that occur during the printing process such as vibration. On the other hand, excessive resistance to shear (higher yield stress, high zero shear viscosity) could lead to increased resistance against nozzle movement and slow refilling of crevices formed by the needle in the support bath during printing.
Figure 2. Schematic of support bath assisted 3D bioprinting, changes in properties that support bath material undergo during the bioprinting process, and crosslinking mechanisms.
Download figure:
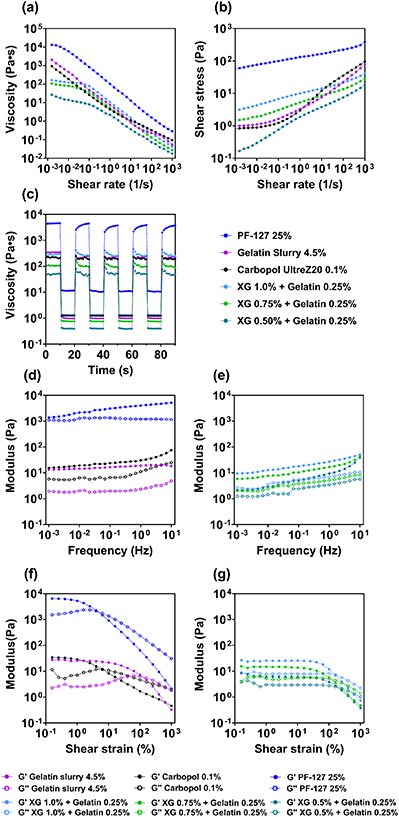
Standard image High-resolution imageSummarized in figures 3(a)–(c) and table 2 are the dynamic shear behaviors of both the benchmark materials and the xanthan-gelatin support bath under shear conditions that mimicked the actual printing process. A more detailed presentation of data for the different rheological tests presented in figure 3, with standard deviations, can be found in the supplementary information (SI, figures S2–S6).
Figure 3. Rheological characterization of benchmark support bath materials and xanthan gum-based support bath at various concentrations. (a) Viscosity as a function of shear rate measured for support bath materials. (b) Shear stress of support bath materials as a function of shear rate. (c) Viscoelastic recovery of support bath materials over five cycles. (d) Shear modulus as a function of oscillatory frequency obtained for the benchmark and (e) xanthan-gelatin support bath materials. (f) Shear modulus as a function of strain % obtained for the benchmark and (g) xanthan-gelatin support bath materials.
Download figure:
Standard image High-resolution imageTable 2. Rheological characteristics of support bath materials and Herschel–Bulkley model fitting parameters.
| Materials | Yield stress (Pa) | Zero shear viscosity (Pa s) | Consistency index (k) | Flow behavior index (n) | Recovery of viscosity after shear (%) |
|---|---|---|---|---|---|
| Gelatin slurry 4.5% | 1.27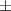 0.05 0.05 | 2040 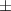 120 120 | 1.65 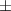 0.1 0.1 | 0.56 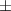 0.01 0.01 | 71.4 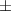 2.8 2.8 |
| Pluronic® F-127 25% | 48.4 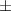 17 17 | 13 300 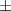 4000 4000 | 72.6 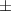 25 25 | 0.20 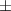 0.03 0.03 | 96.9 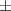 17.7 17.7 |
| Carbopol® 0.1% | 0.79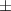 0.03 0.03 | 931 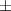 380 380 | 4.0 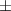 1.5 1.5 | 0.46 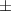 0.02 0.02 | 89. 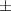 7.7 7.7 |
| Xanthan-gelatin 1% | 2.9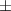 1.2 1.2 | 164 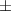 54 54 | 6.0 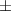 0.4 0.4 | 0.250 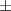 0.001 0.001 | 79.6 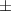 5.4 5.4 |
| Xanthan-gelatin 0.75% | 1.3 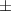 0.1 0.1 | 108 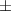 6 6 | 3.4 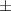 0.4 0.4 | 0.290 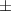 0.005 0.005 | 91.9 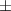 9.1 9.1 |
| Xanthan-gelatin 0.50% | 0.18 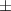 0.1 0.1 | 26.6 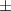 18.8 18.8 | 1.4 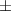 0.1 0.1 | 0.360 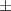 0.007 0.007 | 94.6 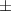 17.5 17.5 |
For the characterized support bath materials, a Herschel–Bulkley model can be used to describe their dynamic rheological characteristics (SI, figure S7, table 2):
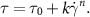
Aside from the Herschel–Bulkley model (SI, figure S7), a Carreau-Yasuda model was also applied to describe the flow data obtained for the support bath materials (SI, figure S8 and table S1). However, the fitting of the data obtained was found to be poor in comparison to the Herschel–Bulkley model, primarily because plateaus in viscosity at low and high shear rates for most of the materials were not very apparent.
Measurements of the shear stress versus shear rate properties of commonly used support bath materials, presented in figure 3(a), show that the yield stress of 0.1% Carbopol® polymers was approximately 0.79 Pa, while the yield stress of gelatin slurry and Pluronic® F-127 were found to be 1.27 and 48 Pa respectively, as can be determined from extrapolation of the shear stress recorded at extremely low shear rate, back to zero shear rate. For the xanthan-gelatin-based support bath, the yield stress was found to be approximately 2.9 Pa, 1.3 Pa and 0.18 Pa respectively for 1 %, 0.75% and 0.50% xanthan gum concentration, close to that found in the benchmark materials. With such low yield stress values (i.e. from 0.18 Pa to 2.98 Pa), the support bath materials investigated would be anticipated to yield easily when subjected to the shear stress exerted by the printing needle, allowing easy initiation of the printing process and smooth needle movement. Meanwhile, the observed yield stress values are expected to be sufficient for the encapsulation and mechanical support of deposited bioink filaments and scaffolds against gravity, buoyancy, vibration, and other mechanical stimuli, preventing undesirable movement and displacement of the construct during and after the printing process. Such a low yet distinct yield stress value is one of the key characteristics leading to the functionality of support bath materials, and in some studies, mechanical fragmentation has been utilized as an effective approach to decrease the shear yield stress of hydrogels to make them suitable as support materials. For example, the yield stress of a 4.5% gelatin sample was observed to be approximately 1.27 Pa after the 4.5% w/v monolithic gelatin gel was blended into a suspension of microgel particles, giving a gelatin suspension that enabled smooth and accurate nozzle movement while providing sufficient support for accurate 3D bioprinting of soft hydrogels. As a comparison, the yield stress of a non-blended, monolithic gelatin hydrogel at 23  is in the range of 102 Pa (SI, figure S1) Similar approaches have been adopted for the preparation of other crosslinked hydrogels such as sodium alginate [25] and agarose [12], as these materials tend to form rigid gel structures upon crosslinking, giving unfavorable flow behavior similar to monolithic gelatin.
is in the range of 102 Pa (SI, figure S1) Similar approaches have been adopted for the preparation of other crosslinked hydrogels such as sodium alginate [25] and agarose [12], as these materials tend to form rigid gel structures upon crosslinking, giving unfavorable flow behavior similar to monolithic gelatin.
It can be seen from the flow curves obtained for support bath materials, presented in figure 3(a), that the viscosity decreased rapidly as the applied shear rate increased, confirming the shear-thinning behavior of support bath materials investigated. For the gelatin slurry, xanthan-gelatin composite support bath material and Carbopol® polymer gels, the observed viscosity reduced from values of 103–10−1 Pa s as the shear rate was increased from 10−3 to 103 s−1, while the viscosity of Pluronic® F-127 hydrogel was found to decrease from higher values of 104 Pa s to 100 Pa s over the same shear rate range. Thus, in a typical bioprinting scenario, the support bath material in the close vicinity to the moving printing needle would undergo rapid shear-thinning, leading to fluidization of the material in the support bath along the path of printing, facilitating the movement of the printing needle. Rapid shear-thinning of the support bath material in the region surrounding the printing needle creates a high shear rate regime that is confined to the close vicinity of the printing needle, ensuring that the rest of the support bath remains undisturbed during the printing process, as suggested by particle velocimetry study on Carbopol® support bath materials [9]. As the printing nozzle traces along the defined pattern during 3D bioprinting, the support bath material is subjected to shear and forced out of its original position temporarily, leading to the formation of crevices as indicated in figure 2. It can be deduced from a simplified laminar flow model, as presented in section 3.3 and in table 3, that the shear rate experienced by the support bath materials as a result of the movement of an extrusion needle is typically within the range of 101–102 s−1, depending on the specific needle diameter and printing speed [9]. The localized, rapid shear-thinning of the support bath materials at high shear rates effectively turns the material in the vicinity of the printing nozzle into low-viscosity liquids that not only facilitate nozzle movement but also minimize the size of crevices created by the translational nozzle motion.
Table 3. Key common rheological properties of support bath materials and an estimated desirable range of the properties that enable support bath functionality.
| Yield strain (%) | Yield stress (Pa) | Storage moduli (G', Pa)/damping factor (tanδ) | Consistency index (k) | Flow behavior index (n) | Recovery of viscosity after shear (%) |
|---|---|---|---|---|---|
| 1–100 | 0.1–10 | 10–100/0.1–0.5 | 1–10 | 0.20–0.60 | 70–100 |
During the printing process, the extrusion needle moves past and revisits certain X and Y positions repetitively, creating multiple crevices in the same area at different depths as illustrated in figure 2. The crevices in the support bath gels formed during the printing process need to be rapidly re-filled, ready for subsequent shearing on the next pass. These furrows can be filled by the flow of the fluidized support bath material back into its original position, or by a secondary liquid filler material when the original viscosity is too high [19]. Considering the motion of the extrusion needle, rapid shear-thinning and localized low viscosity of support bath hydrogel materials occur at the front of the needle as it is moving, while recovery in the gel structure and increased values of properties such as the viscosity occur simultaneously as the needle travels away from the deposited bioink strand (figure 2). This situation was simulated via rheological measurements by monitoring the viscosity of support bath materials as they were subjected to alternating high (102 s−1) and low shear rates (10−2 s−1) in repetitive cycles, as shown by the data presented in figure 3(c). The recovery of the support bath materials in response to repetitive shear loading is defined by the percentage recovery of the viscosity of each material 10 s after the high shear rate loading was removed, compared to the initial viscosity of the material, as shown in table 2. It was observed that for xanthan-gelatin composite materials, 0.1% Carbopol® and the gelatin slurry, the viscosity decreased rapidly from approximately 102 Pa s to 100 Pa s as the high shear rate was applied, indicating the occurrence of rapid shear-thinning and localized fluidization when support bath materials are subjected to high shear rate caused by the moving printing needle. Upon removal of the high shear rate as the printing needle moves away from the region, the viscosity of the materials started to increase back to their pre-sheared level in a short period of time, as shown by the vertical increase in viscosity. The process of rapid shear-thinning and viscosity recovery was found to be reversible without causing shear-related changes to the structure of the support bath materials, as the viscosity of the mentioned materials was able to recover back to the initial level after multiple shear-unload cycles. For the 25% Pluronic® F-127 gel, the viscosity was also found to decrease rapidly from 103 Pa s to 101 Pa s upon application of high shear rate loading. However, it can be observed that the recovery process of support bath materials is time-dependent, and only partial recovery of the viscosity value was observed in support bath materials, while full recovery of the viscosity was not achieved in a time period of 10 s. As summarized in table 2, around 70%–90% recovery in viscosity value was observed 10 s after the removal of shear in support bath materials. The similarities and differences in flow behaviors observed in the support bath materials stem from the similarities and differences in their polymeric structure and molecular interactions under shear conditions. The yield stress-enabled flow and the shear-thinning properties of microgel particle-based materials such as gelatin slurry and Carbopol® can be attributed to the deformation and subsequent sliding of jammed particles above a critical shear stress level, while in polymeric solutions such as the xanthan gum-gelatin composite, flow is initiated by the sliding and re-arrangement of entangled, flexible polymeric chains in response to shear. The recovery of support bath materials within a certain timeframe is dependent on the capacity of materials to rearrange their molecular or particulate structure after the application and removal of shear.
The flow behavior of the support bath materials presented above exhibits similarities in terms of shear-thinning, zero shear viscosity, and self-healing in support bath materials, which may further indicate some very general similarities in viscoelastic properties. This was explored via measurement of the complex shear modulus and its components as a function of amplitude and frequency using oscillatory rheometry techniques. Here the storage and loss components of the shear modulus of both the benchmark materials and the support bath materials in their respective LVE were characterized over a wide range of frequencies, i.e. from 10−2 to 101 Hz, representing a large range of velocities of the shearing plate in the rheometer, which roughly translates into a wide range of shear rates (figure 3(d)). As shown in figures 3(d) and (e), as a function of amplitude, the storage modulus of 0.1% Carbopol® polymer, gelatin slurry and xanthan-gelatin composite support baths measured in LVE region were found to be approximately 101 Pa, indicating the presence of a relatively weak gel structure in these materials. On the other hand, for the Pluronic® F-127 gel, the storage modulus value obtained was almost three orders of magnitude higher (i.e. almost 104 Pa), indicating stronger interactions between the polymers in the gel. Where the storage and loss modulus components were measured as a function of frequency (figures 3(f) and (g)), the values obtained for 0.1% Carbopol® polymer, gelatin slurry and xanthan-gelatin composites were found to be in the range of 10° to 101 Pa over a wide range of frequency from 10−3 to 101 Hz, and with very limited change in moduli as the shear frequency was increased. The stable shear complex moduli and limited frequency dependency are clear evidence of the presence of stable, viscoelastic structures within these materials. Such structures enable the overall gel-like behavior of the support materials at low shear loading while allowing the localized transition from viscoelastic gel to liquid-like, very weak gel at high shear rates. The observation of low yield stress and shear-thinning behavior in our xanthan-gelatin support bath material can be attributed to the homogenous, soft, and flexible networks for low xanthan gum concentrations as indicated by experiments where the amplitude and frequency sweep were varied, as discussed above. The formation of such a relatively weak yet stable structure in xanthan gum hydrogels originates from longer-term reorganization and subsequent stabilization of xanthan gum molecular chains and their networks [26]. In the case of particulate suspensions such as Carbopol® acrylate co-polymers and gelatin slurries, such weak structures are considered to originate from a shear-induced jamming transition in closely packed microgel particle suspensions [27, 28].
From the experiment results and empirical model fitting parameters obtained from all support bath materials in the comparative study, a quantitative estimation of the desirable range of the key rheological properties of support bath materials can be summarized, as presented in table 3. A shear yield strain of 1%–100% is found to be suitable for the swift yielding of the support bath material surrounding the bioink dispensing needle, while a yield stress within the range of 0.1–10 Pa has been demonstrated to be sufficient for mechanical support without exerting excessive resistance for printing nozzle movement. On the viscoelastic properties of the support bath materials, a storage modulus within the range of 10−100 Pa with a damping factor between 0.1–0.5 can result in a balanced viscoelastic composition, enabling a soft solid-like gel due to the storage moduli. Meanwhile, excessive gel rigidity can be avoided, and gel fluidity and flexibility can be maintained as a result of a balanced loss modulus (hence damping factor) and fluid-like structure. For the flow behavior and the Herschel–Bulkley empirical model fitting, a consistency index between 1–10 and a flow behavior index between 0.2–0.6 appeared sufficient for the shear thinning of support bath materials that enables localized fluidization of the support bath and smooth printing nozzle movement. After the shear is removed, a prompt recovery in support bath viscosity takes place, and a 70%—100% recovery in viscosity is required for timely encapsulation and mechanical support of the deposited bioinks. Apart from the support bath materials characterized in this study, the rheological properties of a variety of support bath materials with different crosslinking mechanisms are also found within the desirable range given in table 3, including Laponite® nanoclay particulate suspension [5], oil-based silica nanoparticles suspension [29], gellan gum-based support gels [30], and a κ-carrageenan sub-microgel medium [31]. Therefore, this standard can be applied to different support bath materials regardless of their crosslinking mechanisms, underlying particulate or supramolecular structures, and types of intermolecular interactions, hence serving as both a guide for the development of potential support bath materials and a quantitative standard for assessing the functionality of different support bath materials.
3.2. Compatibility tests of support bath hydrogels in the presence of crosslinking cues
As discussed in section 3.1, one critical feature that enables embedded 3D bioprinting is the stable, weak gel structures present in support bath media. This structure can be easily disturbed by physical and chemical factors such as changes in temperature [11] and pH [20], enzymatic reactions [13] and the presence of ions [32], causing a rapid loss of structure of the support medium and subsequent loss of function. While these factors can be used as a strategy to release printed structures [33, 34], they also impose a limitation on the printing of bioinks that utilize these methods to induce crosslinking reactions. As illustrated in figure 2(b), by utilizing a support bath material that is compatible with multiple crosslinking mechanisms, it is potentially possible to 3D bioprint structures using multiple types of bioinks, e.g. ionic crosslinking bioinks, photo-crosslinking bioinks, thermally crosslinking bioinks, and potentially pH-sensitive bioinks, by depositing and supporting these bioinks within the support bath material. The deposited bioinks could then be crosslinked by applying different crosslinking cues either within or through the support bath, without changing the properties of the support bath. After completion of the crosslinking steps, the encapsulated structure can then be safely released.
Experiments were carried out investigating the storage moduli of xanthan-gelatin and Carbopol® support media supplemented with a wide range of calcium ion concentrations, the data obtained from which is presented in figure 4(a). It can be seen that the storage moduli of xanthan-gelatin composites remained stable in the presence of calcium ions at concentrations varying from 15 to 75 mM, thus rendering it suitable for the use of encapsulating bioinks that rely on ionic crosslinking using Ca2+ ions (e.g. sodium alginate-based bioinks). In contrast, however, the storage modulus of 0.1% Carbopol® gels was found to change significantly under the same conditions, where the presence of calcium ions was found to cause aggregation and subsequent precipitation of the Carbopol® polymer at all concentrations tested, resulting in a sharp decrease in the storage modulus measured. This indicates that the gel structure of Carbopol® was affected significantly in the presence of divalent ions, which directly affected the functionality of Carbopol® as support bath material for use with bioinks which require divalent ion crosslinking for stabilization of the construct shape. This was presumably due to the bridging of the acrylic acid residues by Ca2+ ions in the support material causing aggregation and precipitation. Similarly, more extensive crosslinking may occur in sodium alginate-based support bath materials in the presence of increased divalent ions, affecting the functionality of the material [13].
Figure 4. Comparison of rheological characteristics of support bath materials under different crosslinking conditions. (a) Storage/loss components of the complex shear modulus of xanthan gum–gelatin composite and Carbopol® at different calcium ion concentrations. (b) Storage and loss components of the complex shear modulus of xanthan gum–gelatin composite and Carbopol® at various pH values. (c) Evolution of the storage component of the complex shear moduli of xanthan gum—gelatin composite of various concentrations over a temperature range from 4 to 37  . (d) Measured UV light intensity at 365 nm after attenuated by xanthan gum—gelatin composite material of various depth and thickness.
. (d) Measured UV light intensity at 365 nm after attenuated by xanthan gum—gelatin composite material of various depth and thickness.
Download figure:
Standard image High-resolution imageVariation of pH is a commonly used approach, leading to both the formation and degradation of gel structures [35]. It can be seen in figure 4(b) that the storage and loss component of the shear moduli of the xanthan-gelatin composites were stable within the pH range of 5–8, a pH range that allows the crosslinking of pH-sensitive bioinks such as chitosan and, in some cases, pH-responsive decellularized ECM. In contrast, we found that the pH of Carbopol® materials needs to be adjusted to a value of 6 (figure 4(b)) in order to induce gelation, increasing the storage modulus to a value that enables Carbopol® to be used as a support bath material, a finding which was also observed in earlier research that utilized different variants of Carbopol® as a support bath material [32]. Thus, the effectiveness of Carbopol® as a support bath material for pH-sensitive bioinks may be compromised if the bioink used needs to be printed and crosslinked in an environment with a pH less than 6.
Apart from pH and divalent salt contents, changes in temperature are also a common method both for crosslinking and the disruption of a solid gel structure. As shown in figure 4(c), measurement of the storage modulus of support bath candidate materials as a function of temperature showed significant variation, presumably due to changes in gel structures as a function of temperature, thus influencing their rheological and mechanical properties strongly. A significant increase in storage modulus, over four degrees of magnitude, was observed for a 25% w/v Pluronic® F-127 gel as the temperature was increased from 14 to 18 °C, resulting in a final storage modulus of approximately 104 Pa (figure 4(c)) at higher temperatures. This strong temperature dependency makes it very difficult to control the degree of formation of micelle and other supramolecular structures in the gel, maintaining the consistent gel structure (G' = 101–102 Pa) of Pluronic® F-127 as a support material without rigorous temperature control during the bioprinting process. It can be also expected that the bioprinting of cell-laden bioinks at physiological temperatures (i.e. 37  ) would cause local stiffening of the Pluronic® support material in the vicinity of the printing nozzle, making accurate bioprinting very challenging or potentially impossible in such situations.
) would cause local stiffening of the Pluronic® support material in the vicinity of the printing nozzle, making accurate bioprinting very challenging or potentially impossible in such situations.
The structural characteristics of gelatin slurries were found to be relatively stable at low to ambient temperatures. However, a decrease in the storage modulus from 101 Pa to 10−1 Pa was observed at 27 °C as the gelatin gel particles underwent a solid-liquid transition (figure 4(c)). In addition, the structural stability of gelatin hydrogel slurry at room temperature can be compromised over an extended period of time, giving some limitations to the working time at ambient temperature. During this time, the gelatin particles in a slurry may undergo changes in size and size distribution due to the influence of temperature, both of which could affect bioink printing outcomes [18]. On the other hand, it can be readily observed in figure 4(c) that the storage modulus of the xanthan gum–gelatin support media remained stable at values of 70, 50 and 10 Pa, for concentrations of 1 %, 0.75% and 0.5% respectively, as the temperature was varied from 4 to 37 °C, confirming the presence of a thermally stable, soft gel structure in these samples. The structural stability of xanthan gum at room and physiological temperatures, as well as over a broad range of pH and divalent ion concentrations can be largely attributed to its polymer chain flexibility and the stabilization effects of the helical chain conformations resulting from hydrogen bonding networks [36, 37].
Previous studies using xanthan gum have shown that the polymer does not actively respond to UV stimuli without chemical modification [24], and it has been shown that the absorbance of UV light by xanthan gum occurs primarily when the UV wavelength is <330 nm [33]. Here, we found that attenuation of 365 nm UV radiation did occur to a degree in solutions of varying depth and xanthan gum concentration and that the attenuation was consistent with the Lambert-Beer law (figure 4(d)). For example, the UV intensity was found to be attenuated from 50 mW cm−2 to 15 mW cm−2, i.e. approximately 30% of the input intensity value, after travelling through a 2 mm thick 1% xanthan gum support material. However, UV at these lower intensities has been shown to be sufficient for the UV-induced crosslinking of commonly used hydrogel materials (e.g. Tyrosine-based light-crosslinking decellularized ECM, GelMA) [6, 34] and has been found to be highly compatible with the presence of cells, inducing minimal cytotoxicity [24]. Furthermore, the linearity of UV light attenuation in xanthan gum solutions both as a function of depth and concentration used for support bath applications, also makes it possible to fine-tune the exposure intensity of UV radiation during printing processes by changing parameters such as support bath depth, polymer concentration and UV input intensity. For the use of visible light (400–500 nm)-induced crosslinking with specific photo-initiators [38], these are wavelengths where xanthan gum gels exhibit much lower absorbance so any attenuation will be much reduced [39].
3.3. Investigation of the printability of bioinks in support bath materials
The printability of bioinks is one of the major characteristics used for the assessment of their overall performance. The term primarily describes the extent to which bioinks can be printed with the correct two-dimensional (2D) and 3D geometric accuracy (as designed), consistency and controllability [40]. It has been widely acknowledged that the rheological characteristics and crosslinking mechanisms of some hydrogel-based bioinks can affect the printing outcomes in an unfavorable way, leading to defects such as strand irregularities and discontinuities, distortion and shrinkage of geometric features of scaffolds [41]. The printability of extruded bioinks is commonly characterized by features such as strand width [40], strand uniformity [42], strand circularity [43] and the pore area ratio [44]. While these features are primarily governed by the rheological properties of bioinks, printing parameters such as nozzle size and shape, pneumatic pressure and feed rate as well as the print speed also have a significant impact on the observed printability outcomes [45]. In this study, we primarily investigated the effect of nozzle diameter and support bath materials on the printability of both GelMA and sodium alginate bioinks. The degree of modification calculated using the NMR spectra was found to be approximately 97 %, which matches the result found using the TNBS colorimetric assay. To minimize the potential error introduced by other printing parameters, pre-optimization of printing parameters such as pneumatic pressure, print speed, and feed rate was carried out prior to the printability test (summarized in table 4). The shear rate of support bath materials induced by the movement of printing nozzles can be approximated using a two-plate shear model:
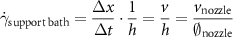
Table 4. Summary of range of printing parameters used in printability study.
| Bioink materials | Nozzle diameter (μm) | Printing pressure (kPa) | Nozzle speed (mm s−1) | Approx. shear ratein support bath (1/s) | Feed rate (mm s−1) |
|---|---|---|---|---|---|
| GelMA | 150 | 80–100 | 3–7.5 | 20–50 | 2.5–4 |
| 250 | 40–60 | 2–5 | 8–20 | 3–4 | |
| 410 | 15–25 | 2–5 | 5–12.5 | 2–3 | |
| Alginate | 150 | 120–150 | 2–7.5 | 13.3–50 | 4–4.5 |
| 250 | 50–65 | 2–4 | 8–16 | 2–3 | |
| 410 | 25–35 | 2–4 | 5–10 | 2–2.5 |
where the shear rate can be derived from the proportion of the velocity of the nozzle movement and the diameter of the nozzle, as summarized in table 4. The calculated shear rates were found to be comparable of that measured and calculated from a silicone support bath using particle imaging velocimetry [46]. By comparing the calculated shear rates and the flow curves in figure 3(a), the effective viscosity in the vicinity of the printing nozzle can be determined to be within the range of 10°–101 Pa s.
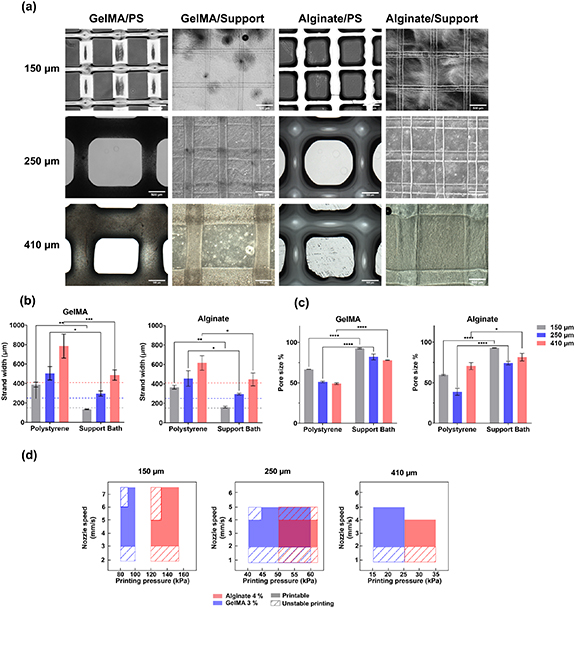
Optimization of the printing parameters and the printer setup in a way that is specific for different bioinks can help mitigate defects and inaccuracies in printed constructs. However, for the bioprinting of free-standing bioinks onto substrates such as tissue culture plastic, the overall printability can still be limited. This is mainly due to the rheological behavior of the bioinks used and the interaction of the bioink with the substrate surface interaction can affect the outcome of the printed structure when no spatial constraint on the deposited bioink strands are present [47]. As illustrated in figure 5(a), defects such as strand fusion and spreading were observed for both sodium alginate and GelMA bioinks when printed on polystyrene substrates. The spreading of bioinks on the substrate can be primarily attributed to a combination of hydrogel wetting of the substrate as well as their moderately liquid-like rheological behavior [48]. Strands in the lower layer of the cross-hatched structure printed using both sodium alginate and GelMA exhibited expanded strands with an apparent diameter that was nearly 200% of the nozzle diameter (150 μm), with an average width of 370 and 380 μm respectively (figure 5(b)), as they both exhibited relatively low viscosity and shear modulus (SI, figure S9) Strand expansion can also be observed for larger nozzles, with a strand width of over 500 and 800 μm observed for nozzles with inner diameters of 250 and 410 μm. Strand expansion and fusion with adjacent strands also led to distortion of the overall shape of the scaffold, where the designed square-shaped pores in scaffolds were observed to be elongated translationally and the corners of the pores were found to be significantly rounded instead of being sharp and accurate at an angle of 90 degrees in the design. Changes in the area of the pores in the scaffolds was also observed and the average pore area of scaffolds printed using both bioink materials was found to be approximately 60% of the pore size originally designed when printed with 150 μm nozzles (figure 5(c)). Distortion and shrinkage in the shape and size of pores within scaffolds were also found when printed with larger nozzles, with an average size of only 50% of the designed value. These defects can cause significant errors in scenarios such as the bioprinting of volumetric constructs with delicate internal channels, tubular structures for perfusion, and/or drug delivery purposes, where the geometric profile of the internal features in scaffolds can have a decisive effect on the performance and functionality of the system [49].
Figure 5. Comparison of the printability of sodium alginate and GelMA bioinks printed on polystyrene substrate and printed in the xanthan gum hydrogel-based support bath using nozzle with different diameters. (a) Optical images of bioink strands printed on polystyrene substrate and in xanthan gum support bath. (b) Comparison of the strand width printed on polystyrene substrate and in support bath. (c) Area size of the pores printed in scaffolds as a percentage of designed value. (d) Printing parameter window for alginate and GelMA bioink using different printing nozzles.
Download figure:
Standard image High-resolution imageA general drawback of a 2D printing environment such as printing on a planar substrate is the lack of effective, direct spatial confinement over the shape and size of the printed bioink strands. After deposition on a substrate, the shape and size of strands are dependent on both the rheological properties of the bioink and the wetting of the substrate by the bioink as well as the fusion of individual strands through mutual wetting. On the other hand, the characteristics of strands printed in a support bath material are primarily determined by the shape and size of the crevice created by shearing the support bath medium as the dispensing tip travels in the 3D hydrogel environment. The shape and size of these crevices, when printed in a support bath medium with appropriate rheological properties, are precisely the size of the external diameter of the printing nozzle [50]. When strands were printed in the xanthan-gelatin support bath materials, post-shear spreading of the bioink strands was significantly inhibited by the surrounding gel environment for both sodium alginate and GelMA bioinks (figure 5(a)). Expansion of both alginate and GelMA bioink strands, when printed within the xanthan-gelatin support bath, was found to be well confined, giving rise to smooth and uniform strands as well as improved printing accuracy, with the average strand width being 152 and 145 μm for alginate and GelMA bioinks respectively (figure 5(a)), which was in accordance with the internal nozzle dimensions. In addition, undesirable strand fusion was significantly reduced for the sodium alginate bioink as the Ca2+ ions contained within the support material aided rapid crosslinking of the strands, in situ, occurring as the strands were printed. The prompt crosslinking prevented printed strands from fusing into each other. For bioinks that require UV exposure or a change in temperature for crosslinking to occur (i.e. GelMA), the shape of the printed strands was shown to be retained due to the mechanical support and physical constraints exerted by the support bath medium, while it was still possible to mitigate strand fusion via application of UV-induced crosslinking after each layer was printed. In summary, for both sodium alginate and GelMA, reduction of post-shear flow and inhibition of strand fusion was achieved when both bioinks were printed in a support bath medium. Aside from improved strand width accuracy as discussed previously, this also led to circumvention of distortion of the pore shape, i.e. the shape of pores for both alginate and GelMA scaffolds printed in the support bath materials were sharp and no strand fusion was apparent. As a result, an improvement in pore area accuracy was also achieved, with square pores post-printing and the actual pore area was determined to be over 90% of the design area (figure 5(c)). The same effect was validated with both 250 and 410 μm printing nozzles, the pore area was found to be 80% and 78% of the designed value when printed in support bath materials.
It is worth noting from data presented in figure 5 that the printing outcomes for both bioinks printed with 250 and 410 μm nozzles were less optimal than 150 μm nozzles, despite major improvement over printing on a polystyrene substrate. This finding can be attributed to the relatively low shear rate exerted on support bath hydrogels when printing with larger printing nozzles. Thus, localized fluidization induced by shear-thinning of the support bath material may be insufficient for the creation of well-formed crevices for the smooth deposition of bioink. The choice of printing needles is considered to be a critical factor for successful support bath bioprinting and in some studies, specially designed thin nozzles were used for optimal printing outcomes [9]. While higher nozzle movement speeds may be applied to compensate for the relatively low shear rate exerted on the support materials during printing, a higher pressure would be needed to increase the bioink feed rate accordingly, which can be difficult for materials with higher viscosities and less ideal flow behaviors, resulting in unstable printing results As shown in figure 5(d), a balance between printing pressure and printing nozzle is required for creating consistent shear rate and matching feed rate of bioink in the sheared crevice. For nozzle moving speed of lower than 3 mm s−1 for 150 μm and 2 mm s−1 for 250 and 410 μm nozzles, the shear rates created by the nozzle movement can only induce limited shear-thinning effect, leading to unstable printing conditions. On the other hand, nozzle speed higher than 5–6 mm s−1 requires increased bioink feed rate, which translate into higher printing pressure for printing consistency.
3.4. Multi-layered printing of 3D structures in xanthan-gelatin support bath
In a similar manner to the defects observed when printing strands in the absence of mechanical constraints, scaffolds obtained by free-standing bioprinting of hydrogels are also prone to distortion, collapse and bulging if printed without spatial confinement and mechanical support. Thus, 2D printing errors and defects can build up as the printing process proceeds and result in structural instability. Mechanical support and confinement are also considered vital for the fabrication of bio-mimicking structures with delicate features and specific shapes and geometric characteristics [51]. Presented in figure 6(a) is the time-lapse 3D bioprinting process of a volumetric cross-hatch designed scaffold. It can be noticed that both the location and the shape of deposited filaments in each layer during the build-up process were supported and secured after deposition. A close observation of the deposited filaments in a similar volumetric cross-hatch structure is presented in figure 6(b), where distinct and undistorted filaments were deposited into the support bath. The printed volumetric scaffold was then successfully released from the support bath material by dilution and subsequent aspiration. To demonstrate the mechanical support and spatial confinement effect of the xanthan-gelatin support material, a multi-layered cross-hatch structure was printed and kept in the support bath for more than 48 h as shown in figure 6(c). No further strand expansion and scaffold dislocation was observed over this period of time. This stability enables the application of various crosslinking mechanisms during or after the printing process, allowing for extended printing times without the concerns of scaffold deformation and warpage due to insufficient crosslinking or scaffold dehydration and shrinkage or cell death. Xanthan-gelatin support material can also be supplemented with cell culture media for the construction of cell-laden scaffolds. We further examined the functionality of the xanthan-gelatin support material by printing multiple volumetric structures with relatively complex geometric shapes, as illustrated in figures 6(d)–(f). A blood vessel-mimicking bifurcated structure was successfully printed in the xanthan-gelatin support bath as shown in figure 6(d). The freestanding printing of such a bifurcated structure can be challenging without mechanical support due to the complex shape of the construct and the softness of the hydrogel bioink, as also demonstrated in other vascular-related bioprinting studies [11]. The biological functionality of bioprinted, cell-laden volumetric structure requires an accurate fabrication process that conforms to the intended design and architecture. The scaffolds presented in figures 6(b) and (c) demonstrate the precise construction of delicate internal pores and channels that remain intact and stable for more than 48 h, thus illustrating their potential to support cell metabolism, viability, and ultimately functionality in volumetric, tissue constructs by allowing the transport of biomolecules, nutrients, and oxygen for metabolic activities. For some volumetric tissue constructs where their functionality originates from the delicate structures that can be difficult to print, such as the bifurcated vascular graft shown in figure 6(d), support bath bioprinting has provided a promising solution for consistent, high-precision fabrication of bio-functional constructs.
Figure 6. Various three-dimensional constructs printed in xanthan-gelatin support bath. (a) Printing process of a volumetric cross-hatch construct in xanthan gum support bath. (b) Volumetric cross-hatch structure after printing and removal from support bath. (c) Micrographs of strands printed in xanthan gum support bath after 0 h and 48 h. (d) A bifurcation vessel-like structure printed in a xanthan gum support bath. (e) Printing process of a three-dimensional MONASH letter-shaped structure. (f) Side view showing the vertical MONASH letter patterns printed in xanthan gum support bath.
Download figure:
Standard image High-resolution image3.5. Investigation of the cytotoxicity of xanthan-gelatin support bath materials
Two different experiments, namely an MTS cytotoxicity test, and a live/dead cell viability test were conducted to investigate the relative contribution of the bioink carrier and the xanthan-gelatin support bath material to any cytotoxicity separately. The cytotoxicity of the support bath material was characterized using cells that were directly exposed to the xanthan gum support bath at various concentrations, followed by an MTS assay to measure viability. To examine any contribution to cytotoxicity related the bioink carrier, cells were mixed with hydrogel precursors, crosslinked, and submerged in the xanthan gum-based support bath without being exposed to shear stress associated with the extrusion process, which could confound the results.
The cytotoxicity of xanthan gum as a cell carrier material has been investigated in some studies and demonstrated to be non-toxic [24, 52]. However, in this study we focused on the potential impact of xanthan-gelatin composite materials on cells after short-term exposure. The timeframe used simulates actual bioprinting scenarios where cell-laden bioinks are typically only deposited within the support gel bath for up to 60 min. Cells encapsulated in 2% alginate were immersed in 3 ml of 1% xanthan-gelatin support bath in a 12-well plate for 30 and 60 min. A live/dead assay was then performed on the cells within the hydrogel to characterize the viability of the cells after exposure to xanthan-gelatin composites. As illustrated in figure 7(a), no apparent changes in cell viability were found in samples exposed to xanthan-gelatin composite materials compared to untreated samples. Specifically, the viability of the cells was found to be 93% and 92% for treated samples with exposure times of 30 and 60 min respectively, while the cell viability observed for the untreated control groups was found to be 93% and 96% (figure 7(a)). Cell viability appeared to be unaffected by the printing process compared to the unprinted bulk gels and other controls as demonstrated in the bioprinted group, where scaffolds comprising fibroblasts in 2% alginate were printed into the xanthan gum-gelatin support bath showed viability of 95 %. Therefore, this experiment demonstrated that short-term, indirect exposure of cells to 1% xanthan-gelatin composites had little impact on the viability of cells encapsulated within a bioink material such as alginate.
Figure 7. Viability and cytotoxicity testing for L929 cells encapsulated in xanthan-gelatin support bath. (a) Live/dead staining of L929 fibroblasts encapsulated in 2% alginate both as non-printed bulk gel and bioprinted scaffolds in 3 ml 1% xanthan-gelatin support bath for 30/60 min and cell viability obtained from live/dead staining. (b) L929 cell morphology after direct exposure to diluted xanthan-gelatin support bath at different depths and signal readings of MTS assay on L929 cells.
Download figure:
Standard image High-resolution imageTo further examine the potential impact of xanthan-gelatin composite on cell metabolism and viability during printing processes, cells were also directly exposed to diluted xanthan-gelatin composite material solutions of 0.25 %, 0.125% and 0.0625% at volumes of 160 and 320 μl (simulating a 5 and 10 mm- depth support bath) for 30 and 60 min in 96 well plate. The xanthan-gelatin composites were then removed by aspiration, and the cell morphology was examined with optical microscopy accompanied by subsequent viability characterization using an MTS assay. No apparent changes in cell morphologies were discovered when in direct contact with diluted xanthan-gelatin support bath materials (figure 7(b)). While no apparent changes in cell morphology were observed when in direct contact with diluted xanthan-gelatin support bath materials (figure 7(b)), a slight variance in cell spread area and aspect ratio can be noticed, both in xanthan gum-gelatin hydrogel treated samples and in control group samples. This is most likely due to a slight over-expansion of L929 fibroblasts on the tissue culture substrate. Cells treated with 160 μl xanthan-gelatin composites at 0.0625 %, 0.125% and 0.25% showed viability of 95 %, 98% and 96% for exposure time of 30 min and 98 %, 99 %, 96% at 60 min. When directly exposed to xanthan-gelatin composite, it is possible that the aspiration of a larger volume of viscous support bath hydrogels led to some cell detachment from the substrate during aspiration, leading to a loss of cells within the well of up to 10%–15% (figure 7(b)), as suggested by MTS readings of cells treated with 320 μl 0.25% xanthan-gelatin composites. Such a phenomenon was not observed in further diluted xanthan-gelatin composites (0.125 %, 0.0625 %) or at the same concentration of xanthan-gelatin composite at a lower volume (160 μl). (Figure 7(b)).
4. Conclusions
Generation of accurate, stable constructs using soft hydrogels with 3D bioprinting is challenging due to the lack of mechanical support and compromised printability of bioinks due to their less-than-ideal flow behavior. To facilitate the deposition of bioinks and provide mechanically supportive environments for the printing of complex and delicate structures, assistive approaches such as printing within a support gel bath have been developed. However, the materials commonly used as support baths tend to be compatible with only a limited range of crosslinking mechanisms and can be difficult to prepare and store, thereby suffering from batch-to-batch variability. In this study, we presented a versatile, cell-friendly and easy-to-make support bath medium utilizing a composite of xanthan gum and gelatin. Comprehensive rheological property profiling was carried out on xanthan-gelatin composites and other benchmark materials to investigate the key rheological properties necessary for functional support bath hydrogels. In the comparative rheological study of benchmark materials and xanthan gum–gelatin composite, set of key common rheological characteristics were identified across the materials examined. These common properties constitute the basis of the functionality of support bath hydrogels, including a relatively low yield stress, significant shear thinning properties, a relatively weak yet stable supramolecular structure, rapid reorganization and structural recovery resulting in higher viscosity values. Quantification of the desirable range for each of the rheological properties characterized in the study was provided based on our analysis of rheological characteristics, giving clear guidance and a benchmark for future development of support bath materials. Xanthan-gelatin composite support bath materials have been shown to retain their structural stability when exposed to different physical and chemical cues that may lead to crosslinking or disturbance of the gel structure of other support bath materials, demonstrating the versatility of this system and compatibility with most commonly used hydrogel crosslinking mechanisms. Bioprinting carried out with multiple bioinks in xanthan-gelatin support baths has shown that the printability of some bioinks with less-ideal printability on polystyrene substrate can be improved significantly. Both the undesirable expansion and fusion of bioink strands can be inhibited in xanthan-gelatin support baths due to superior mechanical confinement and support. Such support enabled the smooth printing of large and complex scaffolds such as bifurcated tubular structures without interruption for crosslinking. It was also possible to dispense a crosslinking agent in the support bath for simultaneous in situ crosslinking of deposited bioinks. Any short-term cytotoxicity of the xanthan-gelatin support bath was examined by both direct exposure of cells to the support bath materials and to encapsulated cells. The composite support bath material was shown to be non-cytotoxic at different concentrations over the short times tested (i.e. 30 and 60 min). This timeframe corresponds to the typical times required for bioprinting processes. This study presents a versatile, novel solution that works well with a range of bioinks that utilize different crosslinking mechanisms, while providing mechanical support and substantial improvement in printing accuracy, potentially enabling multi-material bioprinting using different crosslinking mechanisms simultaneously. In addition, a detailed rheological profiling and comparative study showed new insights into the viscoelastic properties and flow behavior of various support bath materials, guiding further development of new support bath materials for soft material 3D bioprinting.
Acknowledgments
G L acknowledges the support from Monash Graduate Scholarship (MGS) and Monash International Postgraduate Research Scholarship (MIPRS).
Data availability statement
The data cannot be made publicly available upon publication because no suitable repository exists for hosting data in this field of study. The data that support the findings of this study are available upon reasonable request from the authors.
Conflict of interest
The authors declare that they have no competing interests.
Supplementary data (1.1 MB PDF)
Video of printing process (42. MB MP4)