Abstract
This study endeavors to investigate the progression, research focal points, and budding trends in the realm of skin bioprinting over the past decade from a structural and temporal dynamics standpoint. Scholarly articles on skin bioprinting were obtained from WoSCC. A series of bibliometric tools comprising R software, CiteSpace, HistCite, and an alluvial generator were employed to discern historical characteristics, evolution of active topics, and upcoming tendencies in the area of skin bioprinting. Over the past decade, there has been a consistent rise in research interest in skin bioprinting, accompanied by an extensive array of meaningful scientific collaborations. Concurrently, diverse dynamic topics have emerged during various periods, as substantiated by an aggregate of 22 disciplines, 74 keywords, and 187 references demonstrating citation bursts. Four burgeoning research subfields were discerned through keyword clustering—namely, #3 'in situ bioprinting', #6 'vascular', #7 'xanthan gum', and #8 'collagen hydrogels'. The keyword alluvial map reveals that Module 1, including 'transplantation' etc, has primarily dominated the research module over the previous decade, maintaining enduring relevance despite annual shifts in keyword focus. Additionally, we mapped out the top six key modules from 2023 being 'silk fibroin nanofiber', 'system', 'ionic liquid', 'mechanism', and 'foot ulcer'. Three recent research subdivisions were identified via timeline visualization of references, particularly Clusters #0 'wound healing', #4 'situ mineralization', and #5 '3D bioprinter'. Insights derived from bibliometric analyses illustrate present conditions and trends in skin bioprinting research, potentially aiding researchers in pinpointing central themes and pioneering novel investigative approaches in this field.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The wound healing cascade initiates with hemostasis and inflammation, characterized by the recruitment of blood platelets and immune cells to regulate blood loss and pathogen elimination [1]. The primary immune cells contribute to chemokine and growth factor secretion, attracting cells and steering the healing process towards its subsequent phase: proliferation. This phase comprises a sequence of events including granulation tissue development (provisional extracellular matrix (ECM) formation), angiogenesis (blood vessel formation), and re-epithelialization (epidermal skin layer formation), culminating in wound contraction. This phase is orchestrated through intercommunication among various cells, primarily macrophages, fibroblasts, endothelial cells, and keratinocytes. Concluding the cascade is the remodeling event where the previously formed matrix slowly transmutes into either a functional skin or semi/non-functional scar tissue [1]. In cases of non-healing wounds, such as diabetic wounds, the healing process is impeded at the initial phase and progressively becomes chronic due to healing failure [2]. Obstruction can occur during any of the four stages depending on the type of chronic wound, be it pressure sores, venous ulcers, or diabetic wounds. With extensive burn wounds, natural healing is compromised due to considerable loss of skin tissue or failure in developing a provisional ECM matrix consequential to tissue necrosis [3, 4]. Hence, clinical interventions are indispensable for healing such wounds.
Modern strategies for skin wound repair encompass a range of techniques, including autologous skin transplantation, utilization of artificial skin substitutes, and cutting-edge three-dimensional (3D) bioprinting technologies [5–8]. Although autologous skin grafts are widely hailed as the 'gold standard' in managing severe wounds due to their unique adaptability and self-regeneration abilities, they are inherently limited by the scarcity of suitable donor sites, complicating the reconstruction of extensive skin damage [9]. Recently, tissue-engineered skin substitutes (TESSs) have shown considerable promise in treating full-thickness skin defects [10]. Tissue engineering leverages a combination of cells, biomolecules, and biomaterials, providing an alternative to conventional skin grafts. Currently, a broad range of commercial TESSs are available, each utilizing different technologies and cell sources. Typically, these substitutes are composed of natural, synthetic, or hybrid dermal and epidermal elements [11]. However, critical issues associated with commercial skin substitutes include risks of rejection due to the incorporation of allogeneic and xenogeneic materials [12–14], insufficient neovascularization at the wound site [15], high costs that can limit their clinical utility [16], as well as very specific clinical indications accompanied by contraindications and side effects. Furthermore, these skin substitutes have undergone only limited characterization, lacking the histological architecture and structure that mimic human skin. Each advancement in human science and technology arises from a preceding dilemma. The introduction of 3D printing with living cells and biomaterials has ushered in novel opportunities for tissue engineering and regenerative medicine [17], broadening their clinical applications and making strides toward personalized therapies [18, 19]. This innovative approach employs computer-designed models to construct biocompatible 3D structures that closely emulate natural systems. It facilitates the creation of intricate 3D tissue constructs by deploying programmed models and dispensing bio-ink, which comprising biomaterials, biomolecules, and cells, through the controlled motion of a motorized stage [20, 21]. The attributes of these bio-inks, including their printability, biocompatibility, and physical strength, directly impact the quality of the final printed product [21]. To improve stability and structural integrity, the printed constructs can be crosslinked post-bioprinting. Unlike traditional skin regeneration methods, 3D bioprinted dermal replacements offer several advantages in terms of automation, standardization, and precision integration of living cells, growth factors, and other biomolecules. Consequently, this technique has the potential to facilitate high-throughput production [21].
Since Lee et al seminal proposition in 2009 advocating for the use of 3D printing methodologies in devising skin regeneration scaffolds [22], the subsequent decade has witnessed an explosion of scholarly contributions deliberating on bioprinting applications for skin ailments and traumas, as per the Web of Science (WoS) database. These academic dispatches span numerous journals and traverse various domains including medicine, materials science, and immunology. However, undertaking a comprehensive review and analysis of such a voluminous body of literature to grasp the evolutionary trajectory of bioprinting for skin ailments or injuries, and extrapolate research trends, constitutes a daunting, labor-intensive endeavor. Moreover, subjective judgments of researchers are susceptible to influences from their own experiences, memory constraints, and literature accessibility, thereby potentially introducing biases when crafting a historical narrative of development within this field [23]. As opposed to conventional reviews grounded in individual perspectives, bibliometric reviews based on shifts in scholarly productivity can provide a more objective and comprehensive landscape of the historical terrain, research hotspots, and prospective trajectories within a discipline [24–26]. An assortment of bibliometric tools, including R studio (The scholar's best friend: research trends in dog cognitive and behavioral studies. Animal Cognition), CiteSpace [27], CitNetExplorer [28], VOSviewer [29], and HistCite [30], have been employed in scientometrics to delineate the academic landscape of various fields. Although there are many related analysis software, each has its advantages. Normally, an excellent and complete bibliometric article needs multiple software tools for completion. In our investigation, we deployed CiteSpace (version 5.8 R3), R software (versions 4.2.1), HistCite Pro2.1, and the alluvial generator to analyze the bibliographic corpus related to bioprinting for skin diseases or traumas. Among them, CiteSpace is the most commonly used software in bibliometrics, and the majority of analyses in this article were completed using CiteSpace, as detailed in the Method section. R software is one of the most professional statistical software; the publication-related (annual publications, national publications, author publications, institutional publications, journal publications) and cooperation-related charting and statistics were completed using it. HistCite Pro is a powerful tool for analyzing literature citations, primarily handling literature citation-related work. The alluvial generator is a relatively niche software, mainly used for creating the keywords alluvial map in the article.
Our study aimed to: (1) encapsulate the historical features of scholarly literature concerning bioprinting for skin diseases or injuries; (2) underscore publications that have rendered pivotal contributions to the discipline; (3) discern prevailing research themes within the domain; and (4) unveil nascent trends poised to shape future inquiry.
2. Method
2.1. Data collection
WoS serves as a comprehensive repository for information across diverse fields such as the hard sciences, social sciences, arts, and humanities. Additionally, it functions as an international citation database for renowned publishers worldwide. To conduct our literature review, we utilized the following index keywords on the WoS Core Collection database: 'bio-printing or bioprinting' and 'skin', within the timeframe of 2010–2023. This search strategy yielded a total of 622 documents, which were subjected to a bibliometric analysis that included 327 research articles, 200 review articles, and 24 meeting abstracts.
2.2. Tools for bibliometric analysis
2.2.1. R software
We depicted the annual worldwide publication volume in the field of 'bioprinting for skin' using a bar chart and used the logistic regression model to assess the growth trend in this sector. We accounted for the number of productions and/or citations from countries, institutions, authors, and journals as these individually reflect productivity and influence [31]. Research collaborations among countries, institutions, and authors were analyzed as scientific research necessitates extensive collaboration, and investigating such partnerships can illuminate the research status of a specific scientific field. R software was employed to distinguish the merged network through color-coded nodes and edges, where identical colors denote shared collaborative relationships. Larger nodes suggest broader collaboration, while thicker edges imply closer cooperation between the linked entities.
2.2.2. CiteSpace
The co-occurrence networks were comprised of 'Elkhorn coral' in varying hues, where the thickness signifies the frequency of co-occurrences within a specific year. A red ring in a designated year indicates a citation burst, marking an increase in citations for that year. The purple ring represents the degree of betweenness centrality among nodes. A node with a high degree of betweenness centrality plays a crucial role as it serves as a bridging link between other nodes.
2.2.2.1. The co-occurrence networks
The co-occurrence networks were composed of 'Elkhorn coral' in various hues, where the thickness signifies the count of co-occurrences within a specific year. A red ring marking a particular year represents a citation burst, indicating a surge in citations for that year. The purple ring symbolizes the degree of betweenness centrality among nodes. A node distinguished by elevated betweenness centrality is notable as it serves as an interconnecting bridge from one node to another.
2.2.2.2. Burst detection
Jon Kleinberg proposes that a sequence of documents, like emails or articles, typically concentrates on a particular topic for a specific period, which eventually subsides along a defined timeline. These temporal thematic shifts can be detected using specialized text data mining algorithms and identified as 'bursts of activity' [32]. Chen, drawing from Kleinberg's algorithm, characterized citation bursts as indicators of active topics [33]. Citation bursts represent the detection of a burst event, which could span multiple years or just one year. CiteSpace enables the identification of citation bursts across disciplines, keywords, and references [34, 35]. The emergence of a citation burst indicates a correlation between a discipline, keyword, or reference and a rise in citations. In essence, it suggests that the respective discipline, keyword, or reference has elicited substantial attention within its scientific community.
2.2.2.3. The cluster analysis
CiteSpace provides three clustering algorithms, anchored in titles, abstracts, and keywords, to amalgamate publications into conceptual clusters with unique research characteristics [36]. The cluster mapping, influenced by the slice configurations, mirrors alterations in concept clusters across diverse time periods. Additionally, timeline mapping can effectively illustrate the emergence, decline, and interconnections of a cluster. Regarding the procedural specifics, data was imported into CiteSpace, 'Time Slicing' was configured to encompass the period from 2010 to 2023, and the value was set to '1'.
2.2.3. Hiscite
Each scholarly article serves as a beacon in the obscurity, where every citation acts as fuel intensifying its radiance. The more citations an article garners, the more conspicuous and influential it becomes. Utilizing HistCite Pro 2.1 software's ability to graphically elucidate this numerical interrelation allows us to pinpoint and discern the most cardinal literature and highly-cited works readily. This is accomplished by employing both the total local citation score (LCS) and the total global citation score (GCS) to assess the articles. LCS refers to the frequency of a study's citation within the software, whereas GCS denotes the citation frequency within the WOSCC database. We incorporated data from 622 papers into HistCite Pro 2.1, established 'Limit' at 30, maintained other settings in their default state, and opted for 'Make Graph'. This procedure empowered us to chart the foundational body of knowledge in the bioprinting for skin field and swiftly recognize the pivotal literature.
2.2.4. The alluvial generator
Alluvial flow diagrams are crafted to illuminate temporal patterns within a dynamic network [36]. The segregation and convergence of thematic sequences can be perceived as numerous streams fluidly coursing over time. CiteSpace was utilized to generate an alluvial map, constructing a series of individual networks based on co-occurring keywords. These networks were subsequently loaded into the alluvial generator following their export from CiteSpace. Each keyword is regarded as a node, with nodes grouped on each individual time slice, and every cluster treated as a module. Nodes are segmented and amalgamated across varied time slices to form new modules, while the most recent module is formed by intersecting prior nodes. The most enduring nodes in the imported network are accentuated by color-coding the flows they initiate.
3. Result
3.1. The historical features of the literature on bioprinting for skin diseases or injuries
3.1.1. Global publication trends
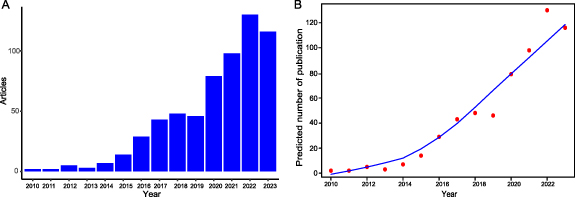
As delineated in figure 1(A), annual research outputs have been plotted. The genesis of utilizing 3D printing technology as a provider for skin substitutes was first proposed in 2009 [22], and the subsequent year saw the publication of two articles focused on bioprinting for skin ailments or injuries. Between 2010 to 2015, the volume of published works exhibited relative stability. However, there was a slight increment observed in the publication volume from 2015 to 2019 when compared to previous years. Remarkably, post-2019, the publication volume witnessed an exponential surge. As per our projections for 2023, we anticipate an accumulation of over 150 published articles. The escalating count of annually published articles underscores the heightened scholarly interest in bioprinting for skin diseases or injuries. Additionally, according to the logistic regression model (figure 1(B)), this field is currently undergoing a swift acceleration in publication growth.
Figure 1. Publication trends in the field of bioprinting strategies for skin diseases and injuries during 2010 and 2023. (A) Annual publication volume and (B) curves of growth trends.
Download figure:
Standard image High-resolution image3.1.2. Distribution of publications
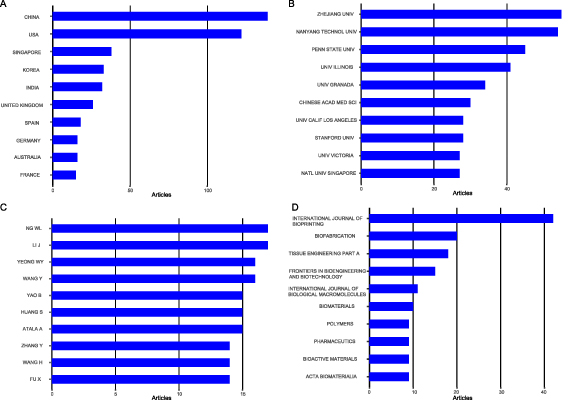
Contributions to this research domain originate from 48 countries and regions, with China leading the count of articles (139, 22.34% overall), followed by the United States (122, 19.61%), Singapore (38, 6.11%), Korea (33, 5.31%), and India (32, 5.14%) as depicted in figure 2(A). In terms of citation statistics, studies from the United States top the list with 11 835 citations, followed by China (3393), Singapore (1968), Sweden (1119), and Germany (1067). Supplementary table 1 enlists the publication and citation counts for each of the 48 participating countries. In total, a consortium of 1004 institutions have contributed to this field of study. As illustrated in figure 2(B), Zhejiang University stands dominant with the majority of publications (55, 8.84%), succeeded by Nanyang Technological University (54, 8.68%), Pennsylvania State University (45, 7.23%), University of Illinois (41, 6.59%), and University of Granada (34, 5.47%). Supplementary table 2 enumerates the number of publications produced by each of the top 50 participating institutions. Authors exhibiting higher publication counts, citation rates, and H-index scores are typically more influential within their field. Figure 2(C) showcases the top 10 authors in bioprinting for skin diseases or injuries based on their publication counts. Supplementary table 3 provides additional insights into the publication count, H-index, and citation count of the top 50 authors, ranked by their publication count. Renowned contributors include NG WL (17 publications, H Index 14, total citations 1143), Li J (17 publications, H Index 9, total citations 474), Yeong WY (16 publications, H Index 14, total citations 1143), Wang Y (16 publications, H Index 9, total citations 235), and Atala A (15 publications, H Index 9, total citations 5167). Likewise, figure 2(D) presents the top 10 journals within the field of bioprinting for skin diseases or injuries according to their publication volume. Supplementary table 4 offers a comprehensive view of the publication count, H-index, and citation count for the top 50 journals, sorted by their publication count. The leading journals include International Journal of Bioprinting (42 publications, H Index 11, total citations 523), Biofabrication (20 publications, H Index 13, total citations 1339), Tissue Engineering Part A (18 publications, H Index 2, total citations 176), Frontiers in Bioengineering and Biotechnology (15 publications, H Index 7, total citations 194), and International Journal of Biological Macromolecules (11 publications, H Index 8, total citations 333). These details provide valuable insights for researchers considering submission.
Figure 2. Distribution of publications. (A) The top 10 fruitful countries; (B) the top 10 fruitful institutions; (C) the top 10 fruitful authors; (D) the top 10 fruitful journals.
Download figure:
Standard image High-resolution image3.1.3. Scientific cooperation
Figure 3 visualizes a considerable number of nodes and abundant connections, signifying an extensive scientific collaboration across three dimensions: countries, institutions, and authors. The national collaboration network is composed of 45 nodes which form 11 distinctive clusters, each represented by different colors. The orange cluster emerges prominently, comprising 23 nodes and dominated by nations such as the United States, China, Iran, the United Kingdom, and Korea (figure 3(A)). The node size represents the centralization degree of collaborations, while the edge thickness symbolizes the strength of these collaborations. The institutional collaboration network consists of 43 nodes segregated into 8 clusters. Key nodes include the Research Center for Tissue Repair and Regeneration from the largest orange cluster, Nanyang Technological University from the blue cluster, PLA Medical College from the orange cluster, Harvard University from the red cluster, and Chinese Academy of Medical Sciences from the orange cluster, listed based on their sizes (figure 3(B)). Additionally, the author collaboration map, illustrated in figure 3(C), encompasses 45 nodes grouped into 6 clusters. Notably, Fu X, Wang Y, Yao B, Huang S, and Li Z display the highest count of scientific collaborations among researchers. All of them are part of the green cluster.
Figure 3. Scientific cooperation networks. (A) Scientific cooperation network of countries; (B) Scientific cooperation network of institutions; (C) Scientific cooperation network of authors.
Download figure:
Standard image High-resolution image3.1.4. The key aspects in the field of bioprinting for skin diseases or injuries
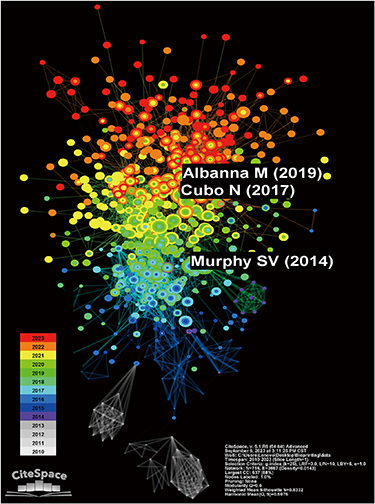
The co-citation map visualizes the interconnectedness of literature in the field of bio-printing for skin diseases or injuries over the past 14 years (figure 4). The network consists of a total of 716 nodes and 3667 links, indicating extensive connections between research papers in this domain. Similar to an Elkhorn coral, during the initial phase (2010–2013), the gray-marked nodes diverged into two smaller clusters, resembling nourishing coral cups that sustain the field's growth. In the middle phase (2014–2017), the presence of blue and purple nodes gradually increased and expanded, laying a solid foundation for rapid advancements. From 2018 onwards, there was further proliferation of green, yellow, and red nodes throughout the literature, spreading outward. Although distinctive clusters have not yet formed, it is anticipated that with further progress in the field, various research branches will gradually emerge.
Figure 4. The citation co-occurrence network. The color bar from bottom (white) to top (red) indicates the year from 2010 to 2023.
Download figure:
Standard image High-resolution imageOf particular note, Murphy and Atala [37], Cubo et al [38], and Albanna et al [8] hold significant positions in the field of bio-printing research on skin diseases or injuries due to their notably higher co-citation frequencies of 88, 87, and 84, respectively. Supplementary table 5 displays the top 30 co-cited works. Additionally, table 1 highlights the 30 most locally cited articles in this field, including their associated global citation frequencies. The three most locally cited articles are Murphy and Atala [37], Koch et al [39], and Michael et al [40], with respective local citation frequencies of 227, 115, and 113. By considering co-citation, local citation, and global citation metrics, we can swiftly identify the pivotal and influential literature within this field.
Table 1. The information of the top 30 literature sorted by LCS citations.
| Document information | Article | Local Citations | Global Citations |
|---|---|---|---|
| Cui et al 2017 Adv. Healthcare Mater. | 3D bioprinting for organ regeneration [41] | 227 | 3961 |
| Koch 2012 Biotechnol. Bioeng. | Skin tissue generation by laser cell printing [39] | 115 | 378 |
| Michael et al 2013 PLoS One | Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice [40] | 113 | 365 |
| Cubo et al 2017 Biofabrication | 3D bioprinting of functional human skin: production and in vivo analysis [38] | 99 | 277 |
| Mandrycky et al 2016 Biotechnol. Adv. | 3D bioprinting for engineering complex tissues [20] | 78 | 885 |
| Ng et al 2018 Biofabrication | Proof-of-concept: 3D bioprinting of pigmented human skin constructs [42] | 76 | 133 |
| Pourchet et al 2017 Adv. Healthc. Mater. | Human skin 3D bioprinting using scaffold‐free approach [43] | 62 | 188 |
| Gudapati et al 2016 Biomaterials | A comprehensive review on droplet-based bioprinting: Past, present and future [44] | 61 | 440 |
| Min 2018 Exp. Dermatol. | Bioprinting of biomimetic skin containing melanocytes [45] | 60 | 105 |
| Markstedt et al 2015 Biomacromolecules | 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications [46] | 58 | 952 |
| NG et al 2016 Trends Biotechnol. | Skin bioprinting: impending reality or fantasy? [47] | 55 | 170 |
| Vijayavenkataraman et al 2016 Biofabrication | 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes [48] | 53 | 149 |
| Huang et al 2016 Acta Biomater. | 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration [49] | 51 | 109 |
| HE et al 2018 Burns Trauma | Bioprinting of skin constructs for wound healing [19] | 43 | 128 |
| Matai et al 2020 Biomaterials | Progress in 3D bioprinting technology for tissue/organ regenerative engineering [17] | 38 | 416 |
| Ng et al 2016 Int J Bioprint. | Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering [50] | 37 | 177 |
| Augustine R, 2018 Prog Biomater | Skin bioprinting: a novel approach for creating artificial skin from synthetic and natural building blocks [51] | 37 | 88 |
| Lee 2014 Biomaterials | Creating perfused functional vascular channels using 3D bio-printing technology [52] | 34 | 325 |
| Varkey 201 Burns Trauma | Skin bioprinting: the future of burn wound reconstruction? [53] | 32 | 65 |
| Chouhan 2019 Biomaterials | Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances [54] | 27 | 254 |
| Lee 2014 Cell Mol. Bioeng. | Generation of multi-scale vascular network system within 3D hydrogel using 3D bio-printing technology [55] | 26 | 233 |
| Singh 2020 Acta Biomater. | In situ bioprinting–bioprinting from benchside to bedside? [56] | 26 | 111 |
| Bajaj et al 2014 Annu. Rev. Biomed. Eng. | 3D biofabrication strategies for tissue engineering and regenerative medicine [57] | 25 | 423 |
| Ozbolat et al 2016 Drug Discovery Today | Application areas of 3D bioprinting [58] | 23 | 181 |
| Shi et al 2018 Biomed. Mater. | Tyrosinase-doped bioink for 3D bioprinting of living skin constructs [59] | 21 | 68 |
| Zhang et al 2017 Ann. Biomed. Eng. | 3D bioprinting for tissue and organ fabrication [60] | 20 | 339 |
| Liu et al 2020 Biofabrication | A biofabricated vascularized skin model of atopic dermatitis for preclinical studies [61] | 20 | 45 |
| Pedde et al 2017 Adv. Mater. | Emerging biofabrication strategies for engineering complex tissue constructs [62] | 19 | 250 |
| Cheng et al 2020 Biofabrication | Handheld instrument for wound-conformal delivery of skin precursor sheets improves healing in full-thickness burns [63] | 18 | 49 |
| Aljohani et al 2018 Int. J. Biol. Macromol. | Bioprinting and its applications in tissue engineering and regenerative medicine [64] | 17 | 177 |
3.2. Dynamics of prevalent research themes
3.2.1. Surge in academic disciplines
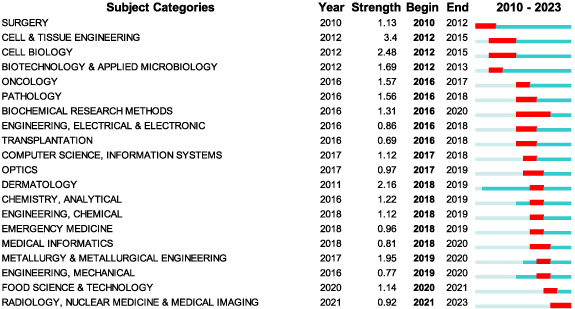
Between 2010 and 2023, citation bursts were observed in 22 out of the 78 associated academic disciplines. The timeline for this interval is represented by a blue line, while the periods of citation burst for each discipline are delineated by red line segments, annotated with their respective start and end years. Figure 5 highlights the top 20 disciplines that demonstrated considerable burst strength at various points within this timeframe. The field of Cell & Tissue Engineering exhibited a burst period from 2012 to 2015, reaching an apex burst strength of 3.40. More noteworthy, however, is the increasing diversity found in the temporal distribution of these citation bursts across different disciplines, signifying the evolving multidisciplinary nature of the field. This trend includes Cell Biology (2012–2015), Pathology (2016–2018), Dermatology (2018–2019), Metallurgy & Metallurgical Engineering (2019–2020), Food Science & Technology (2020–2021), and Radiology, Nuclear Medicine & Medical Imaging (2021–2023). A particular focus is placed on the most recent surge in the field of Radiology, Nuclear Medicine & Medical Imaging (2021–2023). This upward trend is attributed to the commencement of the 3D tissue production process, which typically begins with precise imaging of skin lesions, predominantly employing either computed tomography (CT) or magnetic resonance imaging. Despite offering high-quality images promptly, CT scans pose the risk of exposure to harmful ionizing radiation. On the other hand, the introduction of computer-aided design (CAD) has revolutionized the process by facilitating the creation of virtual models of desired tissues. This technology not only enables precise analysis but also allows for the assessment of the construct's behavior under various conditions. Furthermore, the integration of CAD/computer aided manufacturing (CAM) software has opened up avenues for the transformation of digital concepts into a language that is compatible with bioprinters [65].
Figure 5. The top 20 subject categories with the strongest citation bursts. Year: year of the first occurrence, strength: burst's strength, begin: burst's beginning year, end: burst's ending year.
Download figure:
Standard image High-resolution image3.2.2. Surge in keyword
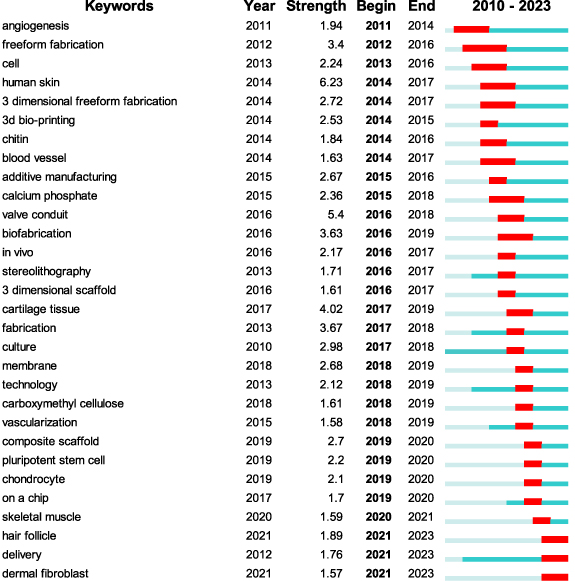
During the span of 2010–2023, keyword bursts were analyzed to discern active research areas within the domain of bio-printing skin. Among a total of 74 keywords exhibiting burst behavior at varying time junctures, figure 6 highlights the top 30 displaying the most potent burst strengths. For instance, the term 'freeform fabrication' underwent a burst from 2012 to 2016, reaching an apex burst strength of 3.4. Similarly, '3-dimensional scaffold' experienced a surge between 2016 and 2017, with a recorded burst strength of 1.61, while 'composite scaffold' showed a burst from 2019 to 2020, manifesting a burst strength of 2.7. Of particular interest among all bursting keywords are 14 terms that maintain their burst status until 2023, potentially signifying emerging areas of focus in this field. For example, the keyword 'hair follicle' demonstrated a burst with a strength of 1.89 from 2021 to 2023, concurrent with the term 'dermal fibroblast', which presented a burst strength of 1.57. Hair follicles fulfill various physiological roles such as offering protection against ultraviolet (UV) radiation, assisting in body temperature regulation, facilitating sweat drainage, and providing tactile perception [66]. On the other hand, dermal fibroblasts, located in the dermis, play a crucial role in synthesizing and secreting structural components of the skin, such as collagen and elastic fibers, along with other ECM molecules [67] and provide essential conditions for the survival and growth of 'hair follicle'. Therefore, the emergence of 'hair follicle' triggers the appearance of 'dermal fibroblast', and their simultaneous burst indicates that research in skin bio-printing goes beyond emphasizing the physical structure of the skin to also focus on its appendages and functions. This highlights the potential areas of interest in current and future investigations. (supplementary table 6).
Figure 6. The top 30 keywords with the strongest citation bursts.
Download figure:
Standard image High-resolution image3.2.3. Reference burst
Upon an exhaustive analysis, an aggregate of 187 seminal articles were identified. Table 2 enumerates the top 30 publications that demonstrated the highest citation bursts from the period of 2010 through 2023. In the span of 2015–2019, a pivotal manuscript authored by Murphy and Atala [37] marked the first significant burst, with an exceptional burst strength of 31.16. This scholarly review provided a panoramic view of the field, underscoring the transformative influence of additive manufacturing, colloquially referred to as 3D printing, in spearheading advancements across multifaceted sectors including engineering, manufacturing, art, education, and health sciences. Within the sphere of regenerative medicine, 3D bioprinting facilitates the architectural design of intricate living tissues by incorporating biocompatible materials, cellular components, and ancillary support elements. This technological innovation surmounts technical hurdles and harbors promising applications in tissue regeneration, transplantation, pharmaceutical development, and toxicological research. The review article provides a comprehensive summary and introduction of the early technology of 3D biological printing of tissues, but does not elaborate on the limitations of this technology, including the most important issues of tissue survival and rejection. These are closely related to vascular generation, compatibility of biological inks, choice of printing methods, etc, which are also eternal topics in this field of study. With the development of technology, in addition to the successful construction of tissue structure, more emphasis is now placed on the retention of functions, such as hair follicles, sweat glands, stem cell culture and printing, etc. In addition, higher demands have been raised for the printing speed of tissues to avoid missing the optimal time for clinical transplantation, therefore, in-situ printing technology may become a better choice in the future. Another resonating article was penned by Lee et al [68], registering the second major burst, which captured substantial scholarly attention upon its unveiling. This publication exhibited a burst strength of 27.71 and maintained its influential status for half a dozen years, spanning from 2013 to 2019. It underscored the potentiality of 3D bioprinting in the realm of tissue engineering, particularly shedding light on the usage of human skin as an exemplary model. The study elucidated the unparalleled benefits of 3D bioprinting, such as shape fidelity, adaptability, reproducibility, and high throughput cultivation. A noteworthy submission was also made by Michael et al [40], recording a surge strength of 18.7 between 2015 and 2018. This groundbreaking investigation introduced a laser-assisted bioprinting modality to fabricate a fully cellularized skin substitute with meticulous 3D placement of fibroblasts and keratinocytes. This construct, when tested on athymic mice, exhibited successful integration with the surrounding tissue post 11 d, manifesting epidermal proliferation and collagen synthesis. The study thereby validates the feasibility of engineering complex tissues such as skin via 3D bioprinting.
Table 2. The references with citation bursts at different period.
| References | Year | Strength | Begin | End | 2010–2023 |
|---|---|---|---|---|---|
| Murphy and Atala [37] | 2014 | 31.16 | 2015 | 2019 | ▂▂▂▂▂▃▃▃▃▃▂▂▂▂ |
| Lee et al [68] | 2014 | 27.71 | 2014 | 2019 | ▂▂▂▂▃▃▃▃▃▃▂▂▂▂ |
| Michael et al [40] | 2013 | 18.7 | 2015 | 2018 | ▂▂▂▂▂▃▃▃▃▂▂▂▂▂ |
| Kolesky et al [69] | 2014 | 14.72 | 2015 | 2019 | ▂▂▂▂▂▃▃▃▃▃▂▂▂▂ |
| Skardal et al [70] | 2012 | 14.37 | 2015 | 2017 | ▂▂▂▂▂▃▃▃▂▂▂▂▂▂ |
| Koch et al [39] | 2012 | 14.37 | 2015 | 2017 | ▂▂▂▂▂▃▃▃▂▂▂▂▂▂ |
| Ozbolat and Yu [71] | 2013 | 11.49 | 2016 | 2018 | ▂▂▂▂▂▂▃▃▃▂▂▂▂▂ |
| Duan et al [72] | 2013 | 11.01 | 2015 | 2018 | ▂▂▂▂▂▃▃▃▃▂▂▂▂▂ |
| Malda et al [73] | 2013 | 10.6 | 2016 | 2018 | ▂▂▂▂▂▂▃▃▃▂▂▂▂▂ |
| Cui et al [74] | 2012 | 10.3 | 2013 | 2017 | ▂▂▂▃▃▃▃▃▂▂▂▂▂▂ |
| Pati et al [75] | 2014 | 9.78 | 2015 | 2019 | ▂▂▂▂▂▃▃▃▃▃▂▂▂▂ |
| Miller et al [76] | 2012 | 9.35 | 2014 | 2017 | ▂▂▂▂▃▃▃▃▂▂▂▂▂▂ |
| Ng et al [47] | 2016 | 8.81 | 2017 | 2019 | ▂▂▂▂▂▂▂▃▃▃▂▂▂▂ |
| Kang et al [77] | 2016 | 8.71 | 2018 | 2020 | ▂▂▂▂▂▂▂▂▃▃▃▂▂▂ |
| Murphy et al [78] | 2013 | 8.38 | 2016 | 2018 | ▂▂▂▂▂▂▃▃▃▂▂▂▂▂ |
| Xu et al [79] | 2013 | 8.13 | 2013 | 2018 | ▂▂▂▃▃▃▃▃▃▂▂▂▂▂ |
| Xu et al [80] | 2013 | 7.94 | 2016 | 2018 | ▂▂▂▂▂▂▃▃▃▂▂▂▂▂ |
| Hinton et al [81] | 2015 | 7.76 | 2016 | 2020 | ▂▂▂▂▂▂▃▃▃▃▃▂▂▂ |
| Markstedt et al [46] | 2015 | 7.76 | 2016 | 2020 | ▂▂▂▂▂▂▃▃▃▃▃▂▂▂ |
| Faulkner-Jones et al [82] | 2015 | 7.63 | 2018 | 2020 | ▂▂▂▂▂▂▂▂▃▃▃▂▂▂ |
| Derby [83] | 2012 | 7.43 | 2015 | 2017 | ▂▂▂▂▂▃▃▃▂▂▂▂▂▂ |
| Dababneh and Ozbolat [84] | 2014 | 7.34 | 2016 | 2019 | ▂▂▂▂▂▂▃▃▃▃▂▂▂▂ |
| Bertassoni et al [85] | 2014 | 7.33 | 2015 | 2019 | ▂▂▂▂▂▃▃▃▃▃▂▂▂▂ |
| Lee et al [52] | 2014 | 7.27 | 2017 | 2019 | ▂▂▂▂▂▂▂▃▃▃▂▂▂▂ |
| Owens et al [86] | 2013 | 7.05 | 2016 | 2018 | ▂▂▂▂▂▂▃▃▃▂▂▂▂▂ |
| Baltazar et al [87] | 2020 | 7 | 2021 | 2023 | ▂▂▂▂▂▂▂▂▂▂▂▃▃▃ |
| Zhu et al [88] | 2017 | 6.95 | 2019 | 2020 | ▂▂▂▂▂▂▂▂▂▃▃▂▂▂ |
| Vig et al [89] | 2017 | 6.74 | 2021 | 2023 | ▂▂▂▂▂▂▂▂▂▂▂▃▃▃ |
| Vig et al [89] | 2012 | 6.36 | 2015 | 2017 | ▂▂▂▂▂▃▃▃▂▂▂▂▂▂ |
| Bertassoni et al [90] | 2014 | 6.28 | 2015 | 2019 | ▂▂▂▂▂▃▃▃▃▃▂▂▂▂ |
| Merceron et al [91] | 2015 | 6.24 | 2016 | 2019 | ▂▂▂▂▂▂▃▃▃▃▂▂▂▂ |
Based on the analysis, a total of 45 articles demonstrated citation bursts until 2023. Table 3 presents the top 20 articles ranked by their strength index. Among these articles, 10 were categorized as 'original research' articles and 10 were classified as 'review' articles. Interestingly, most of these articles entered the period of citation bursts within one to two years after publication. Additionally, five of the articles experienced citation bursts four years after their initial publication. These findings indicate the rapid impact and recognition of these articles within the academic community.
Table 3. The references with citation bursts from beginning to 2023.
| Begin | End | Strength | Year | Type | Title |
|---|---|---|---|---|---|
| 2021 | 2023 | 7 | 2020 | Original research | 3D bioprinting of a vascularized and perfusable skin graft using human keratinocytes, fibroblasts, pericytes and endothelial cells [87] |
| 2021 | 2023 | 6.74 | 2017 | Review | Advances in Skin Regeneration Using Tissue Engineering [89] |
| 2021 | 2023 | 5.66 | 2019 | Original research | In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full Thickness Wound [8] |
| 2021 | 2023 | 5.57 | 2019 | Original research | Direct 3D bioprinted full-thickness skin constructs recapitulate regulatory signaling pathways and physiology of human skin [92] |
| 2021 | 2023 | 5.36 | 2020 | Original research | Rapid printing of bio-inspired 3D tissue constructs for skin regeneration [93] |
| 2021 | 2023 | 5.2 | 2020 | Original research | A biofabricated vascularized skin model of atopic dermatitis for preclinical studies [61] |
| 2021 | 2023 | 5.18 | 2019 | Review | Skin bioprinting: the future of burn wound reconstruction? [53] |
| 2021 | 2023 | 4.78 | 2020 | Review | In situ bioprinting—Bioprinting from benchside to bedside? [56] |
| 2021 | 2023 | 4.59 | 2017 | Review | 3D Bioprinting for Organ Regeneration [41] |
| 2021 | 2023 | 4.28 | 2018 | Review | Advances in the Biofabrication of 3D Skin In Vitro: Healthy and Pathological Models [94] |
| 2021 | 2023 | 4.03 | 2019 | Review | Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs [95] |
| 2021 | 2023 | 3.98 | 2018 | Review | Bioprinting of skin constructs for wound healing [19] |
| 2021 | 2023 | 3.97 | 2020 | Review | Overview of Current Advances in Extrusion Bioprinting for Skin Applications [96] |
| 2021 | 2023 | 3.87 | 2017 | Review | 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends [97] |
| 2021 | 2023 | 3.76 | 2019 | Original research | 3D bioprinting of collagen to rebuild components of the human heart [98] |
| 2021 | 2023 | 3.67 | 2017 | Original research | 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity [99] |
| 2021 | 2023 | 3.44 | 2020 | Original research | Polysaccharide-Based Bioink Formulation for 3D Bioprinting of an In Vitro Model of the Human Dermis [100] |
| 2020 | 2023 | 3.44 | 2018 | Original research | Tyrosinase doped bioink for 3D bioprinting of living skin constructs [59] |
| 2021 | 2023 | 3.42 | 2017 | Original research | A Gelatin-Sulfonated Silk Composite Scaffold based on 3D Printing Technology Enhances Skin Regeneration by Stimulating Epidermal Growth and Dermal Neovascularization [101] |
| 2021 | 2023 | 3.36 | 2019 | Review | Therapeutic strategies for skin regeneration based on biomedical substitutes [18] |
3.3. Emerging trends and novel advancements in the field
3.3.1. Temporal dynamics of keyword clusters
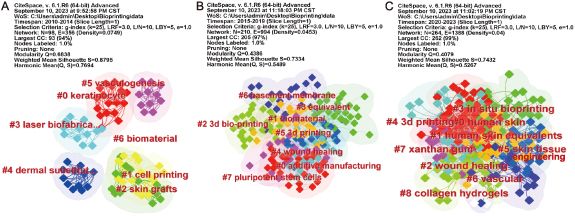
The keywords have close interconnectedness, and specific keywords can form distinct clusters based on their affinity. Identifying these clusters provides a more intuitive depiction of the different sub-fields within bioprinting for skin diseases or injuries research. The 14 year period was divided into three phases, and figure 7 illustrates snapshots of keyword clusters during each phase. In the first snapshot (2010–2014), 19 papers were analyzed, resulting in 7 clusters, including #0 keratinocyte, #1 cell printing, and #2 skin grafts (figure 7(A)). Moving to the second snapshot (2015–2019), 182 papers were examined, producing 8 clusters such as 0# additive manufacturing, #1 biomaterial, and #2 3D bio-printing (figure 7(B)). Finally, in the third snapshot (2020–2023), 413 papers were reviewed, leading to 9 clusters including 0# human skin, #1 human skin equivalents, and #2 wound healing (figure 7(C)).
Figure 7. The keyword clusters snapshots in four periods. (A) 2010–2014; (B) 2015–2019 and (C) 2020–2023.
Download figure:
Standard image High-resolution imageThe first snapshot (2010–2014) represents the initial stage of development in the field of bioprinting for skin diseases or injuries. From keywords like #0 keratinocyte, #1 cell printing, and #4 dermal substitute, it can be inferred that the focus during this phase was on the single-layer printing technique of viable cells derived from keratinocytes or epidermal cells as an alternative to traditional skin grafts (#2 skin grafts). During this period, the regeneration of new blood vessels (#5 vasculogenesis) was acknowledged as an indispensable component in wound healing, considering its substantial influence on the overall success of skin regeneration. Moreover, a prominent cell printing technique during this phase was laser biofabrication (# laser biofabrication). This technique boasts several benefits, including a non-contact process that prevents nozzle clogging, and high resolution (50 μ), capable of printing single cells per droplet at high cell densities (108 cells ml−1) using low-viscosity cell suspensions (1–300 mPa s) [62, 102]. Nevertheless, it is crucial to underscore some disadvantages. Primarily, the potential risk of photonic cell damage due to laser exposure is noteworthy, particularly when metals are employed as a laser-energy-absorbing layer which could introduce cytotoxicity issues associated with metallic nanoparticles. Furthermore, scalability is constrained by the exorbitant cost of the laser system and the complexities inherent in controlling laser pulses [103]. The second snapshot (2015–2019) signals a progression in the field, marked by a shift towards 3D bioprinting as an emblematic form of additive manufacturing. This is suggested by key terms such as #0 additive manufacturing, #2 3D bio-printing, and #3D printing. Critical facets of 3D bioprinting involve selecting appropriate biomaterials (#1 biomaterial) that exhibit essential characteristics including good printability, mechanical stability, biocompatibility, biodegradability, non-toxicity, high availability, and high shape fidelity post the printing processes. Furthermore, this phase saw the exploration of induced pluripotent stem cells (#7 pluripotent stem cells), leveraging their wide differentiation potential and low immunogenicity for applications related to 3D printing of human skin and hair follicle regeneration. As observed in the most recent snapshot (2020–2023), #4 '3D printing' continues to be a prevailing research focal point. Concurrently, several emergent topics have arisen, including #3 'in situ bioprinting' with 42 articles, #6 'vascular' with 126 articles, #7 'xanthan gum' with 5 articles, and #8 'collagen hydrogels' with 381 articles. The traditional 'print-then-transplant' methodology in 3D printing presents multiple limitations such as delayed wound closure, escalated clinical protocol complexity, protracted hospital stays, and supplementary costs for patients. Manual manipulation of constructs during transfer to the wound site also confines material composition and amplifies the risk of contamination and infection transmission [104, 105]. Moreover, external bioreactors employed during the maturation process fall short of fully replicating the native physiological environment, potentially impeding tissue regeneration [106, 107]. These considerations have motivated the development of bedside bioprinting systems capable of depositing skin substitute structures in situ (#3 in situ bioprinting). In situ bioprinting is an important branch of bio-printing that involves directly printing bioinks into the defective site, considering the shape and characteristics of the damaged tissue or organ to achieve tissue or organ repair. This method enables accurate and rapid in situ bioprinting at the site of injury while repairing tissue based on its internal microenvironment. The vascular generation strategies (#6 vascula) in this phase differ from the primarily physicochemical stimulation-based vasculogenesis strategies (#5 vasculogenesis) observed in the first snapshot. Instead, the focus is on utilizing 3D bio-manufacturing technology to develop a complex angiogenesis system. In addition to viable cells, bioink materials consist of both natural biopolymers and synthetic biopolymers. Keywords like #7 xanthan gum and #8 collagen hydrogels represent the prevalent use of natural biopolymer protein gels as bioinks in current research. Supplementary table 7 provides detailed data for the third snapshot (2020–2023), offering insights into the core research areas of bioprinting for skin diseases or injuries during this recent stage. The 'representative keywords within clusters' help identify the key focus areas within the field.
3.3.2. The keyword alluvial flow visualization
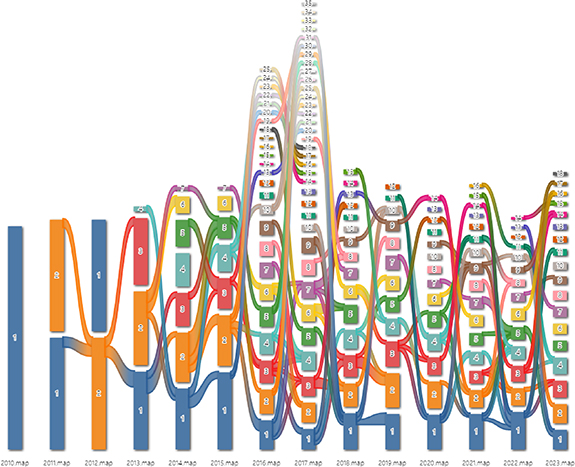
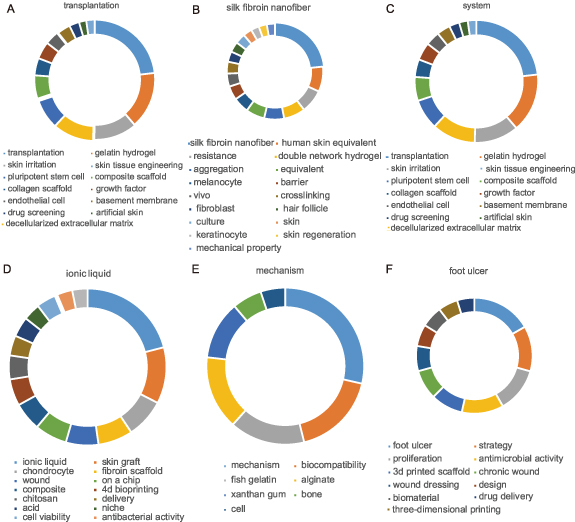
As depicted in figure 8, interconnected keywords coalesce to form distinct research modules. With the passage of time and consequent rearrangement of keywords, these modules may bifurcate or amalgamate, subsequently leading to the emergence of new modules. Certain keywords retain significant relevance throughout the span of 14 years, while others denote emergent research trends or gradually become obsolete. Supplementary table 8 within the supplementary materials divulges the most frequently encountered keywords for the top five modules each year. Interestingly, Module 1 consistently emerges as the most substantial research tributary (indicated by the color blue) over the past 14 years, barring 2011. This implies that Module 1 maintains enduring relevance. Furthermore, the keywords from the top six modules in 2023 were charted (figure 9). Module 1, titled 'transplantation', comprises 13 keywords such as transplantation, gelatin hydrogel, and skin irritation (figure 9(A)). Module 2, labeled 'silk fibroin nanofiber', encompasses 17 keywords including silk fibroin nanofiber, human skin equivalent, and resistance (figure 9(B)). Module 3, designated 'system', encapsulates 10 keywords like system, particle, and nanoparticle (figure 9(C)). Module 4, named 'ionic liquid', combines 15 keywords such as ionic liquid, chondrocyte, and wound (figure 9(D)). Module 5, known as 'mechanism', includes seven keywords such as mechanism, biocompatibility, and fish gelatin (figure 9(E)). Finally, Module 6, christened 'foot ulcer', contains 11 keywords including foot ulcer, strategy, and proliferation (figure 9(F)). These modules likely signify the prognostic trends in the realm of bioprinting for skin disorders or injuries in the subsequent five years or perhaps even longer.
Figure 8. The keywords alluvial map 2010–2023. X axis: time slice. Y axis: counting of modules. Number: order of modules on each time slice sorted by the number of nodes.
Download figure:
Standard image High-resolution imageFigure 9. The keywords of top 6 modules in 2023. (A) Module 1; (B) Module 2; (C) Module 3; (D) Module 4; (E) Module 5; (F) Module 6.
Download figure:
Standard image High-resolution image3.3.3. The timeline visualization of references
Utilizing a timeline visualization predicated on citation duration enables the prediction of emerging, enduring, and potentially obsolete topics. Within the temporal map pertaining to bioprinting research centered on skin ailments or injuries, 14 clusters are discerned, ordered vertically in accordance with size (refer to figure 10(a)). Clusters #1 'regenerative medicine', #2 'multiple types of cells', #3 'soft hydrogel', and #7 'native skin tissue' constitute enduring themes. Despite not representing cutting-edge subjects, they maintain robust interconnections with other clusters. Conversely, clusters #6 'tissue development (skin substitutes)', #9 'nanofeature', #12 '4D bioprinting', and #13 'medical treatment' are perceived as less popular, potentially antiquated themes. These clusters exhibit fewer linkages to other conceptual groups and lack any demonstrable subsequent development within their respective timelines. Clusters #0 'wound healing', #4 'in-situ mineralization', and #5 '3D bioprinter' have been classified as burgeoning topics. Their activity from inception within the timeline signals potential future research hotspots. This evaluation is congruous with the snapshot analysis of keyword clusters illustrated in figure 7. For an intricate understanding of the emerging clusters, please refer to supplementary table 9.
Figure 10. The reference clusters map. (A) The citation timeline visualization; (B) the burst citation in #0, #4, and #5, (C) citation frequency distribution of the burst citation, X-axis: year, Y-axis: cited frequency.
Download figure:
Standard image High-resolution imageMoreover, several seminal works, marked by large nodes encircled in red, have been instrumental in advancing this subfield (figure 10(b)). Min D's 2018 publication [45], a highly cited piece from cluster #0, introduced a 3D bioprinting method that produces a full-thickness pigmented skin model, shedding light on its potential therapeutic and research applications. Groll J's 2019 work in BIOFABRICATION [108] from cluster #4, traced the evolution of 'bioinks' in biofabrication, outlining a clear distinction between bioinks and biomaterial inks. Li XD's 2020 review in CHEM REV [109] from cluster #5, detailed the history, principles, and applications of inkjet technology in biosciences, introducing the concept of 'bio-pixels' for enhanced comprehension of inkjet-based bioprinting. Vanaei S's 2021 paper in ENG REGEN [110], also part of cluster #5, explored commonly used biomaterials and techniques in 3D bioprinting, focusing on their applications in tissue regeneration and cancer research. Weng TT's contribution to J TISSUE ENG in 2021 [111], from cluster #0, reviewed 3D bioprinting techniques in skin tissue engineering, highlighting recent advances and future prospects. Fatimi A's 2022 article in GELS [112] from cluster #4, presented an in-depth review on natural polymer-based bio-inks, discussing progress, limitations, and future trends in large-scale tissue and organ fabrication using bioprinting. Finally, Ng WL's 2022 work in INT J BIOPRINTING [113] from cluster #5 demonstrated how thermal inkjet systems can enhance cell viability in sub-nanoliter bioprinting. The citation distribution of these key publications over recent years (figure 10(c)) indicates their continued relevance in upcoming years.
4. Summary and future perspectives
4.1. Nascent themes in bioprinting for skin diseases and injuries
This paper conducts a systematic investigation into the spatiotemporal dimensions of significant literature concerning bioprinting for skin impairments or injuries from 2009 to 2023, guided by scientometric analysis. Despite its nascent stage, the domain is experiencing rapid growth, marked by a remarkable increase in scholarly publications, extensive scientific collaborations, and a complex citation network. Shifts in focus within this arena occur over time, with recent surges in citations indicating an emphasis on terms such as 'hair follicle' and 'dermal fibroblast'. Similarly, the keyword cluster map incorporating '3D printing', 'in situ bioprinting', 'vascular', 'xanthan gum', and 'collagen hydrogels', coupled with clusters from the reference timeline map involving 'wound healing', 'in-situ mineralization', and 'bioprinter', elucidates the prevailing research frontiers and prospective course. Currently, 3D printing dominates the research theme, while in situ bioprinting shows considerable potential due to its dexterity and precision. The formation of new blood vessels represents a crucial phase in wound healing processes since a well-developed vascular network significantly influences the success of comprehensive skin regeneration. Thus, vascularization in engineered skin tissue stands as both a research hotspot and a challenge within the field of tissue engineering [114]. Moreover, the aspiration of tissue engineering extends beyond mere restoration of damaged tissues; it also strives towards mimicking skin functionality, making the development of fully functional dermal layers a key direction for future research [114]. This includes the fabrication and establishment of components like hair, sweat glands, and the ECM. The choice of an ideal bioink is paramount to the success of skin 3D printing, and the evolution of bioinks remains a consistent theme throughout the chronicles of biological printing. Bioinks are required to meet several conditions, including high biocompatibility, post-printing mechanical stability, and suitable printability. Factors such as bioink viscosity and hydrophilicity substantially influence the reliability of printing and cell encapsulation. Additional vital considerations include high printing resolution, accessibility, affordability, biomimicry, and immunological compatibility. Naturally sourced biomaterials like xanthan gum and collagen hydrogels provide a favorable environment for cell proliferation by mimicking the native ECM, demonstrating self-assembly, and exhibiting biodegradability and biocompatibility.
4.2. Exploration of emerging topics
4.2.1. 3D printing
A 3D bioprinting is a fabrication technique that enables the creation of artificial scaffolds or tissue constructs through a sequential layer-by-layer deposition process. This technology offers individualized and adaptable solutions, outstripping traditional tissue engineering by allowing precise placement of bioactive molecules. Additional benefits encompass expediting the pace of skin construction, diminishing patient waiting periods, and aligning with transplantation needs for wounds with diverse areas and depths [37, 115, 116]. Additionally, recent advancements in 3D bioprinting have paved the way for replicating intricately organized biological constructs and directing living cells to accurately respond to these 3D-printed structures [41, 49, 117]. As a result, this technology has garnered escalating interest from researchers. For instance, the utilization of 3D-printed ECM facilitates concurrent and highly specific deposition of multiple types of skin cells and biomaterials, a feature that is absent in conventional skin tissue engineering approaches [51]. Furthermore, research has disclosed that a 3D-ECM crafted using 3D bioprinting can successfully stimulate specific cell differentiation in vitro and aid the regeneration of functional skin and its appendages in vivo [49]. Consequently, bioprinting yields potential advantages for skin regeneration and could revolutionize strategies employed in this discipline.
A 3D bioprinting methodologies are principally bifurcated into two categories: those that incorporate the printing of living cells into the structure, and those that do not. The latter comprises techniques such as fused deposition modeling, stereolithography (SLA), selective laser sintering, and low-temperature deposition manufacturing. Conversely, cellular bioprinting techniques utilize bioinks laden with viable cells to formulate the construct. These methods can be further delineated into four categories: laser-based, droplet-based, extrusion-based, and SLA-based bioprinting [37, 118]. Predominantly, the following four techniques are employed: inkjet-based printing—a variant of droplet-based technology [119], extrusion-based printing [119], laser-based printing [65], and SLA [17]. The operational mechanism of inkjet-based bioprinter parallels conventional 2D printers, replacing standard ink with biological materials and substituting paper for a specially prepared substrate. Droplets containing cell suspensions in an organic solvent are applied with stringent computer control [120]. With regards to the propelling forces required for cell deposition, three pressure generation methods exist: thermal, piezoelectric, and electrostatic [121, 122]. Inkjet-based bioprinting offers advantages including cost-effectiveness, fine resolution, and rapid cell dissemination. However, bioink viscosity poses a challenge, requiring high frequencies which might affect cell viability [37]. Extrusion-based bioprinting is capable of fabricating large structures in both horizontal and vertical orientations, with the aptitude to print highly viscous bioinks containing elevated cell densities [123]. The primary constraint lies in its low resolution, and the impact of shear stress on cell viability [124]. Laser-based printing engages a pulsed laser beam and leverages the absorption capacity of the ribbon structure post laser exposure [125]. It offers significant advantages such as transferring cells at high density onto the substrate, ensuring their survival [126]. The resolution of this technique can vary with alterations in parameters like viscosity, printing speed, pattern topology, and laser pulse energy. SLA executes a precursor hydrogel crosslinked through photoirradiation, facilitated by photoinitiators, a process known as photocuring. SLA printing provides high accuracy and precision fabrication, and the ability to print light-sensitive bioinks. Nevertheless, it is limited by a scarcity of biocompatible materials and a time-consuming UV crosslinking process, which could potentially harm biological components [127]. These printing strategies may be employed individually or synergistically to achieve optimal results [39, 128, 129].
4.2.2. In situ bioprinting
In-situ bioprinting represents a groundbreaking mobile skin bioprinting system that fuses imaging technology for wound topography mapping with in-situ cell delivery precision, tailoring the technology to each patient's unique requirements. In-situ skin bioprinting can be conducted either via a handheld device or a robotic printing platform. Handheld systems mandate surgeon-guided control of a portable instrument to deposit ink into the defect site. This method typically utilizes lower-capital-cost hardware and permits real-time modifications during fabrication to ensure precise accommodation within the wound bed [56]. Advantages entail affordability, provision for real-time adjustments, ensuring an exact correspondence between time-dependent wound bed geometries and printed constructs, high maneuverability, portability, and maintaining surgeon involvement in the delivery process. However, potential human error and user-dependence may influence printing accuracy and construct repeatability, presenting a challenge for regulatory approval, and larger wounds may impose physical strain on the surgeon. Alternatively, robotic bioprinting eliminates human error by using CAD software-directed hardware to deposit ink into a wound defect site. Despite necessitating additional preparatory steps to define wound bed geometry and develop a 3D skin substitute model, it offers higher print accuracy by reducing human error and user-dependence, making it ideal for treating larger wound areas. Drawbacks encompass higher capital costs, extra protocol steps prolonging wound closure and escalating cost, risk of confidentiality breaches of patient-specific digital data, and most systems do not account for body movement during printing, potentially harming healthy tissues.
4.2.3. Vascular
The formation of vascular networks is intrinsically tied to both natural tissue and stent development [130]. Typically, the healing process following a skin injury involves three stages: immediate hemostasis and inflammation, proliferation, and tissue remodeling [131]. During proliferation, a robust vessel network is rapidly formed at the injured sites. This transportation network plays a pivotal role in oxygen, nutrient, soluble signaling molecules transport and waste disposal [132]. Macrophage polarization is often considered integral to angiogenesis regulation and growth factor production [131]. In skin regeneration, increased angiogenic activity can expedite wound healing and effectively prevent necrosis. However, abnormal angiogenesis can lead to prolonged failed wound closure that could persist for several weeks or even months [133].
Two strategies exist in harnessing scaffolds to spur angiogenesis in skin repair. The first entails using a stent system to introduce complex physical or chemical cues stimulating vascular network formation. For instance, programming the release of growth factors can emulate bodily processes, ranging from initiating angiogenesis to inducing a mature vascular system. This strategy is prevalent in 1D and 2D scaffold applications [134]. The second approach involves using 3D bio-manufacturing technology to construct a sufficiently complex angiogenesis system, such as a dense, perfusible microvascular network, and a functional skin substitute. The primary objective here is to reproduce the host organ's structure and blood vessel network [134, 135].
4.2.4. Bioink
Creating intricate 3D matrices for wound healing and skin engineering through 3D bioprinting requires specialized, bioprintable materials termed as bioinks [22, 136]. A plethora of natural polymer hydrogels, including collagen, alginate, chitosan, gelatin, hyaluronic acid, and cellulose, have been employed as bioinks [38], alongside synthetic-based biopolymers like Poloxamer 407 [137] to enhance the mechanical properties of the 3D constructs [45, 138]. For bioprinting 3D ECM for skin substitutes, natural biopolymers are generally favored over synthetic biopolymers. Natural biopolymers provide superior biocompatibility and more closely mimic cells' natural microenvironment, advantageous for cell growth and tissue regeneration [134]. Regardless of their origins, these bioinks should possess certain essential properties such as good printability, mechanical stability, biocompatibility, biodegradability, non-toxicity, high availability, and high shape fidelity post-printing. For instance, polylactic acid with a relatively high processing (melting) temperature is unsuitable for bioprinting [51]. Also, the mechanical properties of biomaterials, such as stiffness, can impact cell differentiation to some extent [139, 140]. Therefore, an ideal bioink must meet various mechanical and biological requirements during and after printing.
When designing bioinks, the selection and source of living cells become crucial considerations since they directly influence the immune response following the implantation of printed scaffolds [141]. Primary skin cells like fibroblasts, keratinocytes, and melanocytes are often preferred for co-culturing during the creation of skin bioprinting constructs [142]. The printed tissue/organ must consistently support normal cellular activities, including cell migration capacity and proliferation rate [42].
In addition to previously mentioned factors, tissue-engineered skin can also be enhanced by incorporating growth factors and other biomolecules (drugs, antibiotics, etc) [143, 144]. These participate in regulating several processes related to wound healing, with the ECM identified as a critical factor in growth factor regulation and delivery [145]. Key growth factors and cytokines that can be added to modulate wound healing include epidermal growth factor, basic fibroblast growth factor, transforming growth factor-b, platelet-derived growth factor, vascular endothelial growth factor, interleukin (IL)-1, IL-6, and tumor necrosis factor-a. These compounds influence reepithelialization, granulation tissue formation, matrix formation and remodeling, as well as inflammation levels [146, 147].
4.2.5. Functional skin
In the realm of skin regeneration, key challenges are centered on the absence of hair, sweat and sebaceous glands, as well as the inherent discoloration intrinsic to engineered skin [114]. Notably, specific stem cells—such as human bone marrow stem cells, embryonic stem cells, and adipose-derived stem cells—often function as 'bioink', given their potential for multilineage differentiation [148–152]. Consequently, melanocytes and stem cells can be integrated within bioprinted cells for the de novo synthesis of appendages, encompassing typical cyclic hair follicle growth and pigmentation of both hair and skin [114]. Beyond printing, a secondary step involving intracutaneous hair transplantation may also be necessary [95]. Sweat glands, crucial appendages that ensure skin hydration and body temperature regulation, present another challenge. Liu et al's 2016 study explored the bioprinting of a 3D matrix with a specific pore size and structural arrangement to induce the differentiation of epidermal progenitors into structures resembling sweat glands. Nevertheless, this structure disintegrated once removed from the supporting 3D architecture. Despite many researchers advocating for the potential of bioprinting in reconstructive surgery, it is clear more research is needed to overcome the aforementioned hurdles [153].
4.3. Major challenges and further directions
4.3.1. Major challenges
4.3.1.1. Bioink
One notable hurdle in skin bioprinting involves bioink, with seeding cells functioning as the basic units of natural skin. While modern cell culture techniques have improved cell generation for bioprinting, there remain concerns about the adequacy of readily producible cells for clinical application of bioprinted skin constructs [141]. Currently, cell viability can be maintained within biological materials [154], yet these materials do not fully emulate the bioelasticity of native skin. Ideally, an appropriate material would facilitate 3D scaffold printing for seeding cells while also replicating the electrophysiology of natural skin. Consideration should be given to developing novel materials for optimal bioink. For instance, gelatin hydrogels are frequently used as bioink due to high biocompatibility [22, 38, 47, 68, 155, 156]. However, the limitations of the printed construct, including severe contraction, rapid degradation, and limited lifespan, must not be disregarded [70, 157]. Whilst chemical cross-linkers can mitigate such weaknesses, they also introduce potential toxicities and cause damage [158–161]. Therefore, optimizing materials for scaffold printing remains a primary challenge that warrants future research.
4.3.1.2. Inadequate blood supply
Whilst some researchers have employed 3D printing technology to fabricate multi-scale vascular networks, such as straight pipelines [52, 162] and dendritic channels [163], these constructed vessels still fail to meet the specifications of native skin's blood vessels. This discrepancy stems from the fact that natural vessels incorporate cells and other essential components for functional blood vessels, and hence differ from bioprinted blood vessels composed solely of biological materials. Moreover, the complexity of human skin's vascular network necessitates further advancements in micro-vessel fabrication via bioprinting technology. Recently, Ruixing et al studied the use of a GelMA-HAMA-fibrin scaffold in 3D bioprinting for enhanced vascularization and wound healing. They found this scaffold, when loaded with cells and confined forces, promotes neovascularisation and wound repair, suggesting hydrogel composites' potential as mechanically-loaded tissue-engineered scaffolds to optimize vascularization and introduce novel wound healing strategies [164]. Additionally, their another research revealed the role of yes associated protein in vascular branch formation and emphasized the importance of precise mechanical control for effective vascular network generation [165]. Likewise, Mirabella et al [166] proposed an approach where implantation of 3D printed grafts containing endothelial-cell-lined lumens instigates spontaneous, geometrically guided generation of collateral circulation in ischemic settings. They highlighted how these vascular patches can salvage perfusion of distal tissues, thereby preventing capillary loss, muscle atrophy, and loss of function. These findings suggest the promising application of 3D bioprinting technology in bioprinting skin constructs, notwithstanding the lack of studies wherein printed blood vessels were directly applied in skin repair.
4.3.1.3. Skin appendages
The current bioprinted skin constructs lack essential components such as hair follicles, sweat glands, sebaceous glands, and other skin appendages, constituting another major obstacle for 3D skin bioprinting [141]. The implementation of stem cell biological printing [167, 168] could potentially address this issue. Yet, in terms of the bioprinting of stem cells, epidermal stem cells, and other biological entities closely related to skin hair follicles, substantial work remains crucial for future explorations.
4.3.2. Further directions
4.3.2.1. Multifunctional biological scaffold
Multifunctional bioscaffolds denote a 3D structure that amalgamate various bioactive agents, bioinert components, and molecules to augment cell-biomaterial interactions, inhibit infections during regulated biodegradation, and overall stimulate skin regeneration [169]. These bioscaffolds, crafted from a myriad of natural and synthetic biomaterials, can perform simultaneous functions such as the delivery of bioactive agents and pharmaceutical molecules, control of stem cell behavior, and guidance of cellular growth and differentiation [170, 171]. The design of multifunctional bioscaffolds, actively involved in providing biological cues to guide and stimulate cellular activities like attachment, proliferation, migration, growth, and differentiation, is extensively researched using organic, inorganic, and hybrid (organic–inorganic) materials [172, 173]. Application of such multifunctional bioscaffolds has demonstrated varied degrees of success in skin regeneration across both in vitro and in vivo models [174–179], necessitating controlled attributes such as homogeneous porous 3D structure, interconnected porosity, and mechanical properties to facilitate carriage of cells and bioactive molecules for wound healing and skin regeneration [180]. For example, in our latest research achievement [181], we have innovatively developed a living Chinese herbal scaffold that provides a sustainable oxygen and nutrient supply for long-term healing processes. By successfully encapsulating traditional Chinese herbal medicine (Panax Notoginseng Saponins [PNS]), and a living autotrophic microorganism (microalgae Chlorella pyrenoidosa [MA]) into the scaffolds, we achieved a gradual release of PNS from the scaffolds which facilitated cell adhesion, proliferation, migration, and tube formation in vitro. In addition, these scaffolds could produce sustainable oxygen under light illumination due to the photosynthetic activity of the live MA, offering a protective effect against hypoxia-induced cell death. Leveraging these distinctive attributes, our in vivo experiments demonstrated that these living Chinese herbal scaffolds could effectively alleviate local hypoxia, enhance angiogenesis, and thus expedite wound closure in diabetic mice. This showcases their significant potential for use in wound healing and other tissue repair applications.
4.3.2.2. Four-dimensional (4D)
In an effort to boost wound healing, recent studies have investigated emerging technologies such as microfluidic technology, which regulates the discharge of bioactive molecules or cells via electric field manipulation [182, 183], and 4D printing technology, which preconditions polymer materials to assume a specific form upon exposure to certain stimuli [184]. The concept of 4D printing was introduced by Skylar Tibbits in 2014 as a technique capable of temporal adaptation or transition between structures [185]. Its application in tissue engineering introduces the dimension of 'time' into 3D bioprinting, leading to the inception of 4D bioprinting [186], enabling printed constructs to evolve over time—a key factor in 4D bioprinting design. Both pre- and post-printing stimulations in the 4D process should ensure stability of the printed constructs [187]. As an emerging technology, 4D bioprinting integrates time as the fourth dimension with 3D bioprinting [188], triggering predetermined conformational changes in bioprinted architectures using live cells and stimuli-responsive materials [189]. As such, dynamic 4D bioprinted constructs can morphologically adapt to various stimuli including temperature, humidity, pH, ions, light, and magnetic or electric fields [190].
4.3.2.3. Artificial intelligence and big data in bioprinting
Given bioprinting's inherent complexity, future prospects may involve leveraging machine learning (ML) alongside an array of computational methods. Here, ML has the potential to overcome complexities involved in transposing biological tissue models derived from imaging into 3D tissue models with cellular precision and tissue properties [191, 192]. Moreover, integrating ML with Big Data—predominantly from contemporary clinical imagery—could address complexities arising from multiscale and multiparametric aspects when the number of mutable parameters exceeds during processing and post-processing phases. Diagnostic images, experimental data, and scientific literature could serve as substantial sources of Big Data for 3D bioprinting [193]. In conclusion, 3D bioprinting, a potentially transformative tool in tissue engineering, could be significantly enhanced through the incorporation of ML.
4.3.2.4. Integration of various techniques
We propose that laboratory fabrication of a 3D skin construct will likely depend on the synergy of diverse technologies. For instance, while skin generated via self-assembly lacks appendages, these can be synthesized via bioprinting. To foster their maturation and functionality, bioprinting appendages within a construct destined for self-assembly into skin could provide critical support. Incorporating human umbilical vein endothelial cells during the bioprinting process could potentially enhance vascularization. The resultant construct could then serve as a graft, affixed using fibrin glue. Thus, comprehensive studies exploring various combinations remain necessary to yield the most effective skin model for both laboratory testing and grafting purposes, rendering it a promising regenerative strategy for skin loss.
In sum, despite existing challenges, the future of 3D bioprinting technology in skin regeneration holds significant promise. Numerous teams are currently employing this technology to fabricate viable skin tissues for transplantation, fostering advancements in the development of skin equivalents for wound-healing therapies [70, 194, 195]. Insights gleaned from diverse fields including bioengineering, chemistry, materials science, and cell biology have laid a robust foundation, propelling progression towards optimal 3D bioprinted skin substitutes and their clinical translation into the future. Moreover, it is important to acknowledge some unavoidable limitations in our study: we strived to adhere to the best practices for systematic literature searches and broadly covered literature across various disciplines. However, due to specific constraints (like language restrictions, publisher access limitations, etc), we might not have been able to access all related literature. Additionally, selective reporting and publication bias could exist in the literature review. These factors could potentially influence the accuracy and comprehensiveness of our analysis. Our work provides researchers with a comprehensive historical overview and understanding of evolving trends in bioprinting for skin diseases or injuries, potentially illuminating new directions for further exploration.
Acknowledgments
I would like to express my gratitude to my friend, Wang Dan, for her efforts in organizing the charts and figures in this paper.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Authors' contributions
The research conception and design were orchestrated by Gui-Xue Wang, Fei Teng, and Zhi-Qiang Wang. The responsibility of data acquisition fell upon Fei Teng and Wei Wang. Data interpretation and analysis were conducted by Fei Teng and Wei Wang. Zhi-Qiang Wang secured funding for the study. Manuscript composition was handled by Fei Teng and Wei Wang, while critical revisions for intellectual content were made by Gui-Xue Wang and Zhi-Qiang Wang. All authors have read and given their approval for the final manuscript draft. Each author has made substantial contributions to the manuscript and has approved the version submitted.
Ethics approval and consent to participate
Data for this study originates from the WoSCC, thereby negating the requirement for Institutional Review Board approval.
Funding
This research received funding from the Natural Science Foundation of Chongqing, China (CSTB2022NSCQ-MSX1143), with the Rapid Service Fee financed by the authors themselves.
Conflict of interest
The authors declare no conflicts of interest.
Supplementary data (<0.1 MB CSV)
Supplementary data (<0.1 MB CSV)
Supplementary data (<0.1 MB CSV)
Supplementary data (<0.1 MB CSV)
Supplementary data (<0.1 MB CSV)
Supplementary data (<0.1 MB DOCX)