Abstract
Three-dimensional (3D) cell-printing has been emerging as a promising technology with which to build up human skin models by enabling rapid and versatile design. Despite the technological advances, challenges remain in the development of fully functional models that recapitulate complexities in the native tissue. Moreover, although several approaches have been explored for the development of biomimetic human skin models, the present skin models based on multistep fabrication methods using polydimethylsiloxane chips and commercial transwell inserts could be tackled by leveraging 3D cell-printing technology. In this paper, we present a new 3D cell-printing strategy for engineering a 3D human skin model with a functional transwell system in a single-step process. A hybrid 3D cell-printing system was developed, allowing for the use of extrusion and inkjet modules at the same time. We began by revealing the significance of each module in engineering human skin models; by using the extrusion-dispensing module, we engineered a collagen-based construct with polycaprolactone (PCL) mesh that prevented the contraction of collagen during tissue maturation; the inkjet-based dispensing module was used to uniformly distribute keratinocytes. Taking these features together, we engineered a human skin model with a functional transwell system; the transwell system and fibroblast-populated dermis were consecutively fabricated by using the extrusion modules. Following this process, keratinocytes were uniformly distributed onto the engineered dermis by the inkjet module. Our transwell system indicates a supportive 3D construct composed of PCL, enabling the maturation of a skin model without the aid of commercial transwell inserts. This skin model revealed favorable biological characteristics that included a stabilized fibroblast-stretched dermis and stratified epidermis layers after 14 days. It was also observed that a 50 times reduction in cost was achieved and 10 times less medium was used than in a conventional culture. Collectively, because this single-step process opens up chances for versatile designs, we envision that our cell-printing strategy could provide an attractive platform in engineering various human skin models.
Export citation and abstract BibTeX RIS
1. Introduction
Human skin models have been used as skin substitutes [1, 2]. Beyond the use of such substitutes, the tissue models have recently received more attention as valuable alternative platforms for drug screening, and both fundamental and cosmetics research, in order to avoid animal testing [3–5]. It becomes more apparent that human skin models with high physiological relevance have to be developed because the importing and exporting of cosmetic products tested on animals has been completely forbidden since 2013 [6]. Although numerous studies have been made with a view to building in vitro skin models, such models rely on a manual seeding method [7]. The method makes it difficult to emulate complexities in human skin in a spatially controlled manner with multiple cell types, which would never accurately reflect complex physiological response [8]. In terms of engineering, moreover, current tissue models may have significant challenges, including fixed dimensions, high costs, low reproducibility, and limited applications.
The 3D cell-printing technique has emerged as a promising technology, allowing the realization of high shape fidelity and desirable functions through deposition of various cell types in a predefined spatial arrangement [9]. Based on this working principle, this technique can be divided into three main categories: extrusion-based, inkjet-based, and laser-assisted cell printing [10]. Because each process provides distinct features in terms of available material range, accuracy, resolution, and speed, all of these cell-printing technologies could serve as powerful platforms in the production of in vitro tissue models [11]. Most researchers have applied only one of these methods to recreate targeted tissues/organs in accordance with their cellular microenvironment [10, 12]. A few studies also demonstrated the potential of a droplet-based 3D cell-printing technique for the engineering of human skin models [13, 14]. It is probably due to the inherent cellular microenvironment of epidermis that it may be required to position keratinocytes with high spatial resolution onto the dermis region, which is hard to accomplish via the extrusion-based cell-printing method [15]. Despite such technical advancements, using only one of the three processes may have significant limitations in the engineering of multiscale tissue models having the complex morphological, biochemical, and biomechanical natures of native tissues/organs. In the field of skin tissue engineering, challenges remain in the development of fully functional models that recapitulate complexities in native skin [6, 8]. Moreover, although the 3D cell-printing technique allows for rapid, versatile, and customizable fabrication, the status of current in vitro skin models, which depend on multistep fabrication methods that use premade polydimethylsiloxane (PDMS)-based chips and commercial products (such as transwell inserts), may constrain the use of 3D cell-printing technology [16]. In this paper, we therefore suggest a novel cell-printing strategy to build up human skin tissue models with a functional transwell system, all in a single-step process using a hybrid 3D cell-printing system. The hybrid printing system—we name it the Integrated Composite tissue/organ Building System (ICBS)—was developed for the first time, allowing for the use of extrusion-based and inkjet-based dispensing modules at the same time. Considering the structural features of a native skin's microenvironment, we initially placed each module of ICBS in the right position. The 3D stabilized dermis was fabricated with favorable cellular morphology using the extrusion-dispensing module, significantly preventing collagen contraction. Using inkjet dispensing, keratinocytes were more uniformly distributed onto collagen matrices than by manual seeding. Collectively, we demonstrated the significant benefits of each module in the ICBS in the engineering of human skin models.
We finally considered a cell-printing strategy to produce a 3D human skin model with a functional transwell system in a single-step process. Our transwell system indicates a supportive porous 3D construct made using an extrusion module, enabling the maturation of our skin model without the aid of commercial transwell inserts. As a consequence, our skin model revealed a morphology similar to that of native skin in terms of histological appearance. With a 98 percent reduction in cost and a 90 percent reduction in the amount of medium used compared with conventional transwell culture, it is also a cost-effective technique. We believe that this strategy could present an opportunity for a rapid, simple, and versatile design in the field of skin tissue engineering, facilitating the development of various functional human skin models.
2. Materials and method
2.1. Materials preparation
Polycaprolactone (PCL) (FDA-approved, molecular weight = 70 000–90 000 g mol−1) was supplied by Polysciences (Warrington, PA, USA). Gelatin powder from porcine skin (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in distilled water (DW) at 60 °C to make solutions with various concentrations of gelatin hydrogel. For the extracellular matrix (ECM)-based dermal compartment, various concentrations (w/v %) of collagen type 1 hydrogels were prepared by dissolving lyophilized collagen sponge derived from porcine skin (Dalim Tissen, Seoul, Korea) in 0.02 M of acetic acid solution and then neutralizing with cold 0.5 M NaOH solution, while avoiding gelation and bubble formation.
2.2. System development
We developed the ICBS as a combinative platform with positional accuracy and repeatability of ±2.5 μm and ±1 μm in the x and y motion directions and ±10 μm in the z motion direction. This platform accommodated two dispensing modules: an extrusion-based module and an inkjet-based module. The system provides six individually controllable heads through which nine different biomaterials can be used. Collectively, the ICBS can use five biomaterials with the extrusion-based dispensing module and four biomaterials with the inkjet-based dispensing module. With the extrusion module, biocompatible synthetic polymers could be melted with a heating system (TCD-200EX, Iwashita Engineering, Tokyo, Japan) and extruded. Pneumatic pressure was adjusted with a controller having a range of 30–500 kPa (SuperΣCMII-V5, Musashi Engineering, Tokyo, Japan). Hydrogels were also extruded, using another type of pneumatic controller system with a pressure range of 5–200 kPa (SuperΣCMII-V2, Musashi Engineering, Tokyo, Japan) that enables us to print the hydrogel at a higher resolution than with the pneumatic controller with the range of 30–500 kPa. The thermo-sensitive hydrogels could also be regulated, using a home-made cooling system. Meanwhile, four drop-on-demand PH-46 inkjet heads (MicroFab, Texas, USA) and fluid reservoirs were combined into our cell-printing system, together with fittings and tubing. The four-channel pressure controller was then connected to the components to maintain a slight adverse pressure for optical jetting. Unlike the pneumatic controllers triggered by static pulses, the JetDrive III controller (MicroFab, Texas, USA) is operated under dynamic pulses capable of producing droplets continuously. Therefore, to trigger the JetDrive III controller in the ICBS, a pulse generator was added to it, to convert static pulses into dynamic pulses.
2.3. Cell culture and preparation for printing
Human primary dermal fibroblasts (HDFs) and epidermal keratinocytes (HEKs) were purchased from ATCC (Manassas, VA, USA). The HDF samples were cultured in Dulbecco's Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S) solutions. The fibroblast growth medium was changed every other day. The HEK samples were cultured in an M154CF (Gibco, UK) medium containing 1% human keratinocyte growth supplement (HKGS) (Gibco, UK), 0.07 mM CaCl2, and 1% P/S. The keratinocyte growth medium was also changed every other day. The cells were split before they reached 80% confluency. In this experiment, 2.5 × 105 cells ml−1 of HDF at passage 8–10 and 1 × 106 cells ml−1 of HEK at passage 3–4 were prepared for cell-printing. The HDFs were suspended in 1/10 volume of 10× reconstitution buffer with the total amount of pregel and resuspended in collagen hydrogel. Distilled water was then added to the prepared collagen bioink, so that the final collagen concentration reached 2% (w/v). The HEK samples were suspended in the keratinocyte growth medium for printing due to their preference for exposure to air over submersion in an ECM [15]. All components—including syringes, tubing, nozzles, and reservoirs—were sterilized with a 70% alcohol and distilled water mixture and dried under the culture hood with a UV lamp. The collagen bioinks were loaded in a chilled plastic syringe, avoiding any bubbles. The HEK-populated medium was loaded into a 2 ml plastic reservoir for printing.
2.4. Performance of each module for production of human skin models
Considering the inherent microenvironment of native skin cells [15, 17], we applied the extrusion-based dispensing module for the dermis and the inkjet-based dispensing module for the epidermis. In this way, the benefits of each module in the engineering of the dermis and epidermis were verified. Granular PCL was loaded in a stainless steel syringe to fabricate a supportive mesh. The engineered dermis was fabricated as follows. First, the loaded PCL was heated to 80 °C and extruded with compressed air; the PCL mesh of 22 mm diameter, 400 μm height, and 100 μm pore size was fabricated; and then a 2% solution of collagen bioink was sequentially dispensed onto the mesh. Finally, the entirety of the construct was achieved, of at least 22 mm diameter and 2.5 mm height, including the height of the PCL mesh. We additionally fabricated two dermal constructs as groups for comparison; one was made of PCL anchor and fibroblast-populated collagen, and the other of fibroblast-populated collagen only. The experimental groups are described later in more detail, in figure 3(A). The contraction was next observed for 7 days because it is known to occur within a week [18]. For qualitative analysis, the constructs were imaged inside their respective wells in 12 well plates at 1, 3, 5, and 7 days of culture. For quantitative analysis, the top surface areas of imaged samples were determined through the border using ImageJ software [19].
To verify the superiority of the inkjet-based dispensing module in engineering epidermis layers, two approaches were tested (printed versus manual). A quantity of 1 × 106 cells ml−1 of HEK was loaded in the cell culture medium and uniformly onto the premade collagen matrices. For manual seeding, 10 μl of 1 × 106 cell suspension was added onto the collagen matrices as well [7, 18]; that is, the same number of cells that was used and located onto the collagen matrices, in order to compare our method with the existing manual method. Bright field images were captured every 12 h with a microscope (CX41, Olympus, Japan) until 100% confluency was reached. In addition, the cell proliferation rate was evaluated for 60 h using Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan). The CCK-8 stock solution was diluted (1:10) in cell culture medium to constitute a working solution, in which the samples were submerged for 150 min. Then, 100 μl of the submerged solution was extracted from the cell culture plates and moved into a plate of 96 wells. The extracted solution was analyzed through measurement of the absorbance at 450 nm, using a microplate reader (Asys UVM 340, Biochrom, Cambridge, UK). The samples were washed in phosphate-buffered saline (PBS) solution until the working solution was completely removed from the samples, followed by incubation with fresh medium.
2.5. Tight junction analysis of printed keratinocytes compared with manually-seeded keratinocytes
We also examined the morphology of the keratinocytes tested in the two approaches (printed versus manual) using immunofluorescence staining. It was assumed that printed keratinocytes could express a tight junction (E-cadherin) with a more uniform distribution than the manually-seeded keratinocytes because of the precise nature of the inkjet module. After fixation, permeabilization, and blocking, the samples were stained with rabbit antihuman E-Cadherin antibody (Abcam, Cambridge, MA, USA) diluted 1:100 in a 1× PBS solution containing 1% bovine serum albumin (BSA) for 1 h at room temperature. A secondary antibody of Alexa Fluor 488 goat antirabbit antibody (1:100; Invitrogen, CA, USA) was subsequently submerged in the samples for 1 h at room temperature and then counterstained with DAPI solution diluted 1:1000 for 5 min. Finally, the stained samples were imaged using confocal laser scanning microscopy (Olympus FV 1000, NY, USA).
2.6. Live and dead assay
A live/dead cell assay kit (Invitrogen, CA, USA) was used to assess cell viability and morphology. The samples were submerged in dye solution composed of ethidium homodimer (EthD-4 μM) and calcein AM (2 μM) in a 1× PBS solution, followed by incubation at 37 °C for 30 min. A fluorescent microscope (Axiovert 200, Zeiss, Jena, Germany) was utilized to capture the stained samples. The live cells appeared in green and the dead cells in red.
2.7. Fabrication of human skin models with functional transwell system and histological verification
In the ICBS, the extrusion-based dispensing module was applied to the fabrication of the transwell system and engineered dermis. The inkjet-based dispensing module was used to uniformly place keratinocytes onto the engineered dermis. The transwell system indicates a 3D PCL construct that enables us to maturate our skin model without additional commercial products. Our strategy was performed as follows. Molten PCL in a 10 ml steel syringe was dispensed through a steel nozzle of diameter 250 μm through pneumatic pressure of 700 kPa; then, 25% gelatin hydrogel loaded in a 10 ml plastic syringe was filled within the internal pores of the fabricated PCL framework, using an extrusion module. This process was repeated until it reached the predesigned dimensions; in sequence, PCL mesh was fabricated onto the hybrid 3D PCL construct. Lastly, HDF-populated 2% collagen bioink was extruded onto the mesh while fabricating a PCL wall that could block medium penetration during air-liquid interface conditions. This process was repeated until the wall height reached at least 3.5 mm. The optimum concentration of gelatin hydrogel was determined from the gel with its intended functional performance being that it could support the pregel collagen for at least 30 min, which is the minimum time needed for crosslinking collagen at 37 °C (figure S1 is available at stacks.iop.org/BF/9/025034/mmedia for supplementary information). While incubating the collagen, the HEK-containing medium was suspended in a plastic reservoir and uniformly distributed onto the cross-linked dermis using the inkjet-based dispensing module. The final construct was submerged in the coculture medium composed of DMEM/F12 (3:1) supplemented with 0.4 μg ml−1 hydrocortisone, 10 ng ml−1 cholera toxin, 180 μm adenine, 5 μg ml−1 human recombinant insulin, 5 μg ml−1 human apo-transferrin, and 5 μg ml−1 triiodothyronine, followed by incubation in a humidified atmosphere of 5% CO2 at 37 °C. During this incubation period, the sacrificial hydrogel was dissolved and completely removed by washing with PBS solution. The cell culture media was changed every other day for 3–5 days under submerged conditions. The construct was next grown at the air-liquid interface by adjusting medium levels. The dermis and epidermis compartments were also bioprinted without any supportive PCL mesh or transwell system. The compartments were then cultured in commercial transwell inserts (poly (ethylene terephthalate) membranes, 3 μm pore size, Corning, USA) inserted in a 6-deep well plate (Corning). This is a process that may have common ground with existing methods for cell-printing in producing human skin models [13, 20]. We therefore defined this as 'existing fabrication'.
For histological analysis, the matured skin tissue models were fixed in 4% paraformaldehyde solution for 1 h and were then embedded in a paraffin block after dehydration and washing. The paraffin blocks were sectioned (10 μm) and used for an H&E staining process. The staining was performed based on a standard protocol [20]. Specifically, the 10 μm sections were stained with hematoxylin solution at room temperature for 8 min and washed several times with tap water. The samples were sequentially counterstained with eosin Y solution for 5 min. Dehydration was next performed through immersion in 95–100% ethanol. Slides were then mounted with a xylene-based mounting medium. The stained samples were finally examined using an optical microscope (CX41, Olympus, Japan).
3. Results
3.1. Development of integrated composite tissues/organs building system (ICBS)
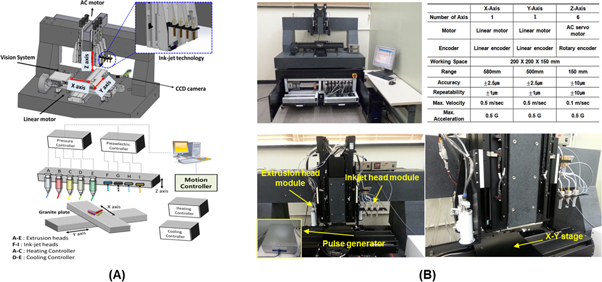
The schematic diagrams in figure 1 show the entire procedure for fabricating 3D-printed human skin models with a functional transwell system in a single-step process. First, we developed a 3D cell-printing system that we named the Integrated Composite tissue/organ Building System (ICBS). It is a combinatory fabrication platform that can simultaneously run extrusion and inkjet-based dispensing modules. The 3D CAD images were drawn using a 3D modeling software package (Solidworks, Concord, MA, USA) (figure 2(A)). The hardware of ICBS was initially designed according to the working principle of an extrusion-based dispensing module. A home-made pulse generator was connected to the system to manipulate an inkjet-based dispensing module at the same time. The detailed descriptions regarding the system are shown in figure 2(B). Fundamental experiments on each dispensing module were carried out under various fabrication conditions for leveraging our cell-printing system with optimal printing conditions (supplementary information figures S2 and S3).
Figure 1. Schematics showing the entire procedure to realize our cell-printing strategy. (A) Hybrid system development allowing for simultaneous use of extrusion-based dispensing modules and inkjet-based dispensing modules. (B) Fabrication of stabilized collagen-based 3D dermal compartment using extrusion modules. (C) Inkjet printing of keratinocytes with uniform distribution using inkjet modules. (D) Fabrication and maturation of 3D-printed human skin model with functional transwell system, all in a single-step process.
Download figure:
Standard image High-resolution imageFigure 2. Integrated composite tissues/organs building system (ICBS) enabling simultaneous use of extrusion-dispensing and inkjet-dispensing modules. (A) A schematic diagram and CAD modeling of the ICBS. (B) Front and side views of the ICBS, displaying each tool with which the ICBS is equipped, and a detailed specification.
Download figure:
Standard image High-resolution image3.2. Engineered dermal compartment using extrusion-based dispensing technique
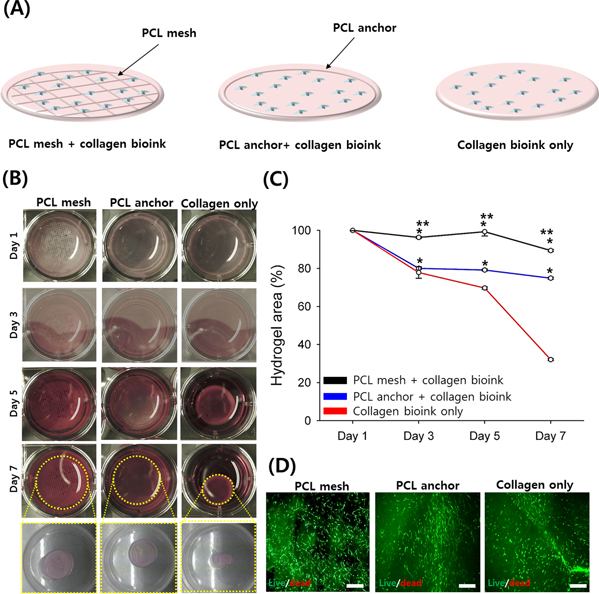
We fabricated three different collagen-based constructs using the extrusion-based dispensing technique (figure 3(A)). To examine the effects of the supportive PCL mesh on construct shrinkage, the samples were cultured in a fibroblast culture medium for 7 days and imaged at days 1, 3, 5, and 7 (figure 3(B)). A graph was plotted for quantification analysis (figure 3(C)). Collectively, these results revealed only a contraction of 32.1% ± 0.5% for the fibroblast-populated collagen in the initial area after 7 days, whereas the fibroblast-populated collagen with the PCL anchor and mesh showed much smaller contraction values of 77.4% ± 0.3% and 89.4% ± 0.6%, respectively. More importantly, the collagen with PCL mesh displayed not only the minimum contraction rate but also a more stabilized cellular morphology than the other groups (figure 3(D)). These results indicate that the collagen with PCL mesh could stabilize our skin tissue model during tissue maturation, potentially leading to improved morphology and long-term maintenance [21].
Figure 3. Engineering of dermal compartments using extrusion-based dispensing module equipped in ICBS. (A) Schematic diagram of bioprinted collagen matrices according to the experimental groups. (B) Contraction measurement at day 7. (C) Quantification analysis of the contraction using ImageJ software (* p < 0.05 compared with collagen bioink only; ** p < 0.05 compared with PCL anchor + collagen bioink). (D) Image and dead images of the contracted compartments at day 7 to reveal their morphology and viability. (Green: live cells. Red: dead cells. Scale bar: 200 μm.)
Download figure:
Standard image High-resolution image3.3. Uniform distribution of HEKs using inkjet-based dispensing module
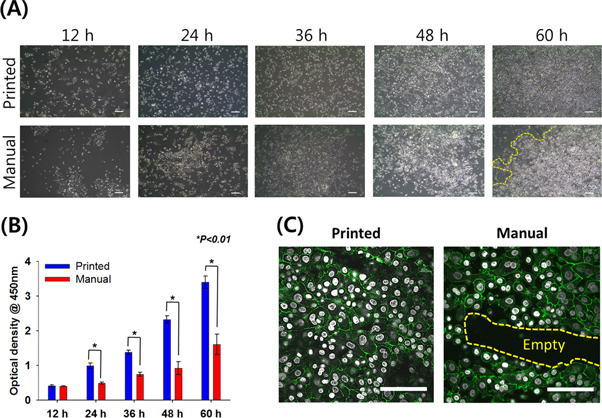
The inkjet-based dispensing module was used to enhance the epidermis formation process through positioning keratinocytes with high spatial resolution. To verify its potential feasibility, two approaches were examined (printed versus manual). On a microscopic level (figure 4(A)), we observed that the printed keratinocytes provided a further uniformly distributed area after 12 h compared with the manually-seeded keratinocytes arranged in clusters. The printed keratinocytes showed a uniformly distributed proliferation rate throughout the collagen matrices, leading to nearly 100% confluency within 60 h. Our quantitative analysis also revealed that the printed keratinocytes provided a much higher proliferation rate, probably because the uniform distribution could enhance cell-to-cell communication (figure 4(B)). Furthermore, we investigated morphology characters of HEKs before the formation of epidermis layers, which is the presence of a tight junction that plays a central role in the process of epithelial morphogenesis [15]. The samples were stained for the adhesion protein E-cadherin antibody at day 3. We observed that the junction protein was highly visible in both approaches (figure 4(C)). More importantly, the protein of printed cells was evenly expressed throughout the collagen matrices, whereas the empty region was observed in the samples prepared by the manual method. This observation indicates that the inkjet-based dispensing module may be capable of positioning cells with higher spatial resolution, leading to enhancement of keratinocyte differentiation.
Figure 4. Superiority of inkjet-based dispensing module in cell-printing human primary keratinocytes with homogenous distribution. (A) Representative photomicrographs of printed keratinocytes and manually seeded keratinocytes over 60 h in culture for qualitative analysis (scale bar: 200 μm). (B) Cell proliferation rate of printed and manually seeded keratinocytes over 60 h for quantitative analysis. (C) Comparison of manually seeded and printed keratinocyte through immunofluorescence labelling examination of E-cadherin. White: nuclei. Green: E-cadherin. Yellow border line: loosely packed cells (empty region). Scale bar: 200 μm.
Download figure:
Standard image High-resolution imageFigure 5. Schematic diagram elucidating our cell-printing strategy to deposit 3D human skin models with a functional transwell system in a single-step process. A: sacrificial material (gelatin) in extrusion head. B: supporting material (PCL) in extrusion head. C: cell-containing collagen bioink in extrusion head. D: cell containing medium in inkjet head. These cell-printing processes are shown from side views.
Download figure:
Standard image High-resolution image3.4. Fabrication of 3D-bioprinted human skin tissue with transwell system in a single-step process
Taking these features of each module together, we established a cell-printing strategy to produce 3D human skin tissue models with a functional transwell system in a single-step process (figure 5). The characterization of our transwell system and commercial transwell insert was first compared in air-liquid interface conditions to verify its feasibility (figure S4 of supplementary information). The observation indicated that our transwell system could also maintain air-liquid interface conditions. In addition, we revealed that human skin models of varying shapes could also be fabricated in accordance with our potential purpose through designing our transwell system (figure S5 of supplementary information). This strategy was also highly cost-effective, with a 98 percent reduction in costs compared with the use of commercial transwell inserts and a 90 per cent reduction in the amount of medium used.
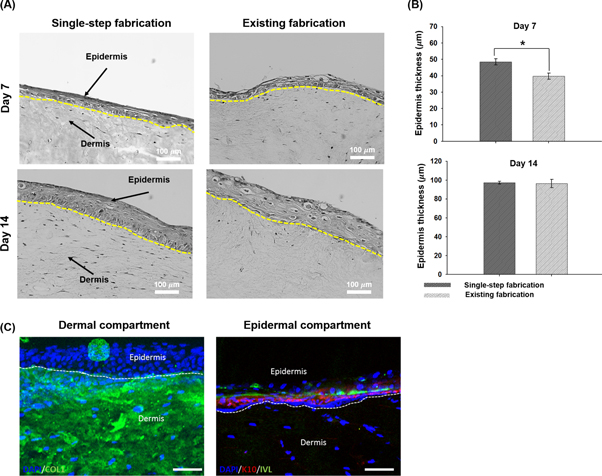
After culturing our tissue model in the air-liquid interface conditions for 14 days, the H&E-stained sections at days 7 and 14 were examined (figure 6(A)). The histological appearance showed that fibroblasts in the matured skin were stretched over day 14, comparable to what is observed in a native dermis [17]. This appearance also revealed that keratinocytes were stratified at day 14. The thickness of stratified epidermis (97 ± 3 μm) also remained within the range of native human epidermis (75–150 μm) [22]. The results indicate that our transwell system could successfully make matured skin tissue models. More importantly, our bioprinted skin model included significantly improved structural features of the dermis and epidermis when compared with the skin model fabricated based on the existing method, although both were comparable in epidermis thickness (figure 6(B)). Moreover, we observed expressions of proteins of 3D cell-printed dermis and epidermis by examining representative respective markers; collagen type I was produced in matured dermis and early/late differentiation markers were expressed in stratified epidermis in our system (figure 6(C)). Collectively, it should be noted that our bioprinted skin model could include structural morphology similar to that of native skin.
Figure 6. Histological appearance of mature skin models prepared through single-step and existing fabrication processes for 14 days. (A) Samples of H&E-stained sections at days 7 and 14 (yellow dashed line indicates the border between epidermis and dermis). (B) Quantification of the thickness of epidermis at different times of culture at air-liquid interface (* indicates p < 0.05). (C) Immunofluorescence analysis of the human skin model fabricated in the single process (DAPI: nucleus (blue), collagen type I (COL): representative ECM of dermis (green), K10: early differentiation marker, and involucrin (IVL): late differentiation marker).
Download figure:
Standard image High-resolution image4. Discussion
The 3D cell-printing techniques (inkjet-based, extrusion-based, and laser-assisted) have become indispensable in the development of artificial tissue models by allowing simultaneous and highly specific deposition of multiple types of cells and biomaterials [11]. The techniques have been recently used in conjunction with polydimethylsiloxane (PDMS)-based microfluidic devices to create more biomimetic 3D human tissue chips [23–26]. Despite such technological advances, the fact that current in vitro models depend on multistep fabrication methods, which use premade PDMS-based chips and commercial products, may be tackled in leveraging 3D cell-printing technology. Moreover, challenges still exist in the development of fully functional tissue models that recapitulate complexities in the native tissue [24, 25, 27]. Several recent approaches have focused on simpler fabrication techniques as a promising alternative to the current fabrication techniques [24, 28, 29]. Our previous research also confirmed such a possibility by adopting one-step fabrication of an organ-on-a-chip with spatial heterogeneity that relies on extrusion-based technology [30]. This study exhibited the development of varying chip platforms, using printable PCL as a housing material instead of PDMS. Although the extrusion-dispensing module facilitates the production of structurally correct 3D models, one of the major shortcomings is the lack of high-resolution systems due to the nature of the extrusion process itself. It indicates the necessity for a more versatile 3D cell-printing technology for tissue engineering advancement [12]. In this respect, our 3D cell-printing system may serve as a promising fabrication platform for the development of further biomimetic tissue models.
Over the past decade, collagen type I hydrogel has been widely used for engineering human skin models because of its high biocompatibility and representing of ECM components in native skin [7, 31]. However, one of the significant drawbacks in using the collagen is its poor mechanical properties, leading to contraction, fast degradation, and limited lifespan [7, 21]. This flaw also necessitates waiting for 5–7 days before planting keratinocytes because it is difficult to form a well-stratified epidermis onto the collagen matrices suffering from the contraction. Although several studies have displayed reinforced dermal compartments, such as Hyalograft-3D™ and photocrosslinkable gelatin hydrogel, they still rely on a manual seeding method, and some of their ECM components were less suitable than the collagen type I [21, 32]. These limitations indicate that a stabilized collagen-based dermis should be engineered. However, it may be difficult to reinforce an engineered dermis using the existing cell-printing strategies because they should use droplet-based dispensing modules requiring low-viscosity hydrogels [13, 14]. To overcome this challenge, Koch et al [13] printed 20 layers of fibroblast-encapsulated collagen onto commercial Materiderm™. However, this strategy might limit us from positioning multiple cells in the dermis. More recently, Lee et al demonstrated that the dimension of bioprinted collagen constructs remained unchanged [20]. Yet, more supporting explanations regarding the detailed mechanism remain for study. We hypothesized that the presence of 3D-printed supportive PCL mesh could stabilize collagen-based dermis and prevent the severe contraction during tissue maturation. As a result, the shrinkage of the engineered dermis was significantly reduced when compared to the collagen without PCL. As major drawbacks of engineering human skin models, application of collagen hydrogels as dermal compartments exhibited weak stability and restricted lifespan due to their severe shrinkage [21]. These weaknesses limit their use in elucidating the mechanisms regulating balanced growth and differentiation in a stabilized tissue. In this respect, our results indicate that the stabilized dermis incorporating the PCL mesh facilitates the fabrication of long-term human skin models and provides a favorable microenvironment in simulating the physiological response of tissues.
Epidermis layers are stratified through keratinocyte differentiation, which are crucial for physiological response in an in vitro system [33]. In a manual seeding method [7, 18], largely, tens of microliters of medium with hundreds of thousands of keratinocytes were added to the center of the engineered dermis, where a concave area was formed due to contraction, potentially resulting in discrete cell clusters that could yield poor epidermis formation. Physiologically, keratinocytes have to establish a confluent monolayer before developing stratified epidermal layers, indicating that the keratinocytes have to be placed onto a dermis region with high spatial resolution [15]. In this regard, it is obvious that an inkjet-based dispensing module would provide opportunities to overcome the limitations in the manual method. Although a few attempts revealed the possibility in printing keratinocytes using droplet-based dispensing modules, the cells were embedded in collagen and stacked up to over 2 layers [13, 20]. This might hinder the keratinocyte differentiation because keratinocytes should be in an air-liquid interface with cell-to-cell junctions [15]. In addition, none of the other studies exhibited keratinocyte behaviors on two approaches (manual versus printed). We therefore aimed to explore the capabilities of cell printing in engineering epidermis layers by comparing the inkjet-printing with the manual seeding. Our results revealed that the uniformly-printed keratinocytes reach nearly 100% confluency within 60 h, whereas the manually-seeded keratinocytes were overlapped at 60 h with discrete cell clusters. To confirm this phenomenon, cell proliferation rate and immunoreactivity were also examined. The printed cells showed the higher cell proliferation rate, probably because of enhanced cell-to-cell interaction. Although manually-seeded and printed cells expressed E-cadherin, the printed cells expressed more uniform junction markers throughout the collagen matrices. Overall, these results suggest that the inkjet-based dispensing module could provide a powerful tool in forming epidermis layers including not only keratinocytes but also melanocytes in the future.
We developed a new 3D cell-printing strategy to produce a 3D skin tissue model with functional transwell system in a single-step process. It was hypothesized that a 3D-printed PCL porous construct enables us to maturate the engineered skin tissue without the aid of commercial transwell inserts. After 14 days at the air-liquid interface, fibroblasts were stretched in the matured dermis and the thickness of stratified epidermis remained in the range of native human epidermis [22]. This observation suggests that our PCL construct, termed as a transwell system, enables the maturation of our skin model. Several researchers revealed the potentials of 3D cell-printed human skin models incorporating fibroblast and keratinocyte cell lines [13, 20]. Using inkjet-based or laser-based techniques, they demonstrated that not only could the fibroblasts exist in the dermal compartment after the printing process, but also the thickness of the epidermis increased under the air-liquid interface. Despite such outcomes, the specific markers did not verify the maturation of the human skin model. For example, the organized keratinization and stratification, which is the key characteristic of the epidermis, should be investigated. In the dermal compartment, moreover, representative ECM production by the printed fibroblasts should be verified. It may also be essential to use human primary skin cells rather than using such cell lines for proper tissue maturation in vitro [20]. In our 3D-printed skin model incorporating human primary skin cells, the specific makers for each compartment were properly expressed, as shown in figure 6(C). In this respect, our 3D printed skin model might provide a morphology more like that of native skin compared with the others developed from a cell-printing technique. In addition, our integrated 3D cell printing strategy could facilitate more structural biomimicry reflected in the native skin through placement of each module in the right positions.
At present, most of the studies, including conventional and 3D cell printing approaches, have to rely on commercial transwell inserts to establish matured human skin tissue models. Several skin models were also made in conjunction with premade PDMS-based biochips for dynamic culture, termed as skin-on-a-chip [34–36]. However, these additional devices are often complicated, time consuming, and expensive [37]. Additionally, these fabrication techniques could be tackled in leveraging 3D cell-printing technology. On the other hand, our strategy would lead to opportunities for a simpler, more versatile, and more cost-effective design in the engineering of various human skin tissue models. Based on this strategy, we could demonstrate the proof-of-concept possibility through fabrication of a novel human skin model with a perfusable transwell system including inlet and outlet tubes by newly designing our transwell system (figure S6 of supplementary information). We envision that our cell-printing system and strategy could be used as an enabling platform for the development of fully functional human skin models.
5. Conclusions
In this article, we presented a new strategy for direct cell-printing of a 3D human skin model with functional transwell system via our versatile 3D cell-printing system. To this end, we first developed an Integrated Composite tissues/organs Building System (ICBS) allowing for the use of extrusion-based and inkjet-based dispensing modules at the same time. A 3D human skin model with functional transwell system was fabricated with the ICBS in a single-step process. The transwell system indicates a supportive porous 3D construct comprising PCL, enabling the maturation of bioprinted skin tissue models without the aid of expensive and confined commercial transwell inserts. Although additional morphology examinations were not conducted in this work, we demonstrated that our skin model could provide a morphology similar to that of the native skin. In this model, fibroblasts were stretched in the matured dermis and a thickness of the stratified epidermis was also obtained within the range of native human epidermis. This strategy was also cost-effective, with a 50 times reduction in cost and 10 times less medium being used compared with the conventional transwell culture. Because this novel strategy could open up chances for a simpler and more versatile design, our cell-printing system and strategy holds significant potential as a novel platform in engineering various human skin models as well as other tissue models.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF, grant No. 2010-0018294), funded by the Government of Korea (MSIP).