Abstract
The skin is the largest organ of the body, having a complex multi-layered structure and guards the underlying muscles, bones, ligaments, and internal organs. It serves as the first line of defence to any external stimuli, hence it is the most vulnerable to injury and warrants the need for rapid and reliable regeneration methods. Tissue engineered skin substitutes help overcome the limitations of traditional skin treatment methods, in terms of technology, time, and cost. While there is commendable progress in the treating of superficial wounds and injuries with skin substitutes, treatment of full-thickness injuries, especially with third or fourth degree burns, still looks murkier. Engineering multi-layer skin architecture, conforming to the native skin structure is a tougher goal to achieve with the current tissue engineering methods, if not impossible, restoring all the functions of the native skin. The testing of drugs and cosmetics is another area, where engineered skins are very much needed, with bans being imposed on product testing on animals. Given this greater need, 3D bioprinting is a promising technology that can achieve rapid and reliable production of biomimetic cellular skin substitutes, satisfying both clinical and industrial needs. This paper reviews all aspects related to the 3D bioprinting of skin, right from imaging the injury site, 3D model creation, biomaterials that are used and their suitability, types of cells and their functions, actual bioprinting technologies, along with the challenges and future prospects.
Export citation and abstract BibTeX RIS
1. Skin: anatomy and functions
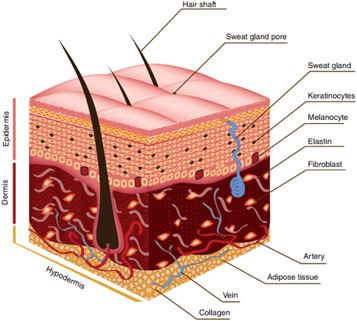
The skin, also called as integument is the largest organ of the body, accounting for about 15% of the total body weight in adults. Skin, along with its derivative structure constitutes the integumentary system [1]. The skin has a very complex multi-layered structure, which in turn consists of several other components such as cells, fibres, extra-cellular matrix (ECM), veins, capillaries, nerves, and the hair follicles. The skin predominantly consists of three layers namely epidermis, dermis, and hypodermis (also called as subcutaneous tissue). The epidermis is the outer most layer, consisting of Keratinocytes (KCs), the middle layer is the dermis, consisting of collagen and fibroblasts (FCs) and the inner most layer is hypodermis, consisting of lipocytes and collagen (figure 1). The constituents of three layers [2], in detail, are summarized in table 1. Malfunction of any one of these will cause undesirable skin conditions like rash, dermatitis, cellulitis, and even melanoma.
Figure 1. Anatomy of skin, showing the three layers (epidermis, dermis, hypodermis), the skin appendages (hair shaft, sweat gland) and the cellular constituents (keratinocytes and, melanocytes in epidermis; fibroblasts in dermis; adipose tissue at the base of hypodermis; collagen in all three layers). Reprinted by copyright permissions from [3].
Download figure:
Standard image High-resolution imageTable 1. Constituents and functions of the layers of skin.
| Layer | Constituents | Function |
|---|---|---|
| Epidermis | Keratinocytes | Guards from environmental damage by pathogens, heat, UV radiation and water loss |
| Melanocytes | Responsible for the production of the pigment melanin, protection from UV-B light exposure | |
| Merkel Cells | Associated with tactility (sense of touch) | |
| Langerhans Cells | Antigen-presenting immune cells | |
| Dermis | Collagen | Fibrous family of proteins responsible for stress-resistance and elasticity |
| Fibroblasts | Synthesizes ECM and Collagen, plays a critical role in wound healing | |
| Mast cells | Allergy response, anaphylaxis, wound healing and angiogenesis | |
| Hypodermis | Fibroblasts | Synthesizes ECM and Collagen, plays a critical role in wound healing |
| Lipocytes/Adipocytes | Energy storage in the form of fat | |
| Macrophages | Phagocytosis, adaptive immunity, wound healing |
Skin, mostly seen just as a fleshly covering, does a lot more vital functions than we could possibly imagine, including (i) Protective (barrier, UV light absorption, immune surveillance, mechanical), (ii) Perceptive (touch, temperature, pain), and (iii) Regulatory (thermal, hydration, excretory) functions. With all the above functions, skin thus aids in the maintenance of homeostasis [4].
Each and every component of the skin performs specialised functions, in addition to the general functions stated above. There are many reported works on the functions and mechanism of functions of keratinocytes [5–9], fibroblasts [10–13], melanocytes [14–19], merkel cells [20–24], Langerhans cells [25–30], mast cells [31–37], macrophages [28, 38–41], and other components of skin as well.
2. Compelling needs for artificial bio-mimic skin
Artificial skins are biological or synthetic substitutes for human skin, produced in laboratories. There are two compelling reasons as to why the world is in need of artificial skins: (i) wound healing and skin regeneration—especially for burn victims, and (ii) drug and skin care products (cosmetics) testing.
Injury or illness that causes loss of skin integrity will lead to substantial physiological imbalance and ultimately to significant disability, sometimes even fatality. The 2016 global wound management marke t is expected to hit $15 billion and forecasted to be worth over $22 billion in 2024 [42]. The global tissue engineered skin substitutes market was valued at USD 958.8 million in 2014 and is projected to reach USD 3873.5 million by 2023, expanding at a Compound Annual Growth Rate (CAGR) of 17.2% from 2015 to 2023 [43]. American Burn Association recorded that, in the US, the number of burn injuries receiving medical treatment per year are 486 000 and the number of hospitalizations per year for acute burn injuries are 40 000, per the 2016 report [44]. Fire, flames, electricity, scald and chemical products were the major causes of such acute burn injuries. The severity of the burn injury depends on the duration and intensity of exposure and can be classified accordingly [45, 46].
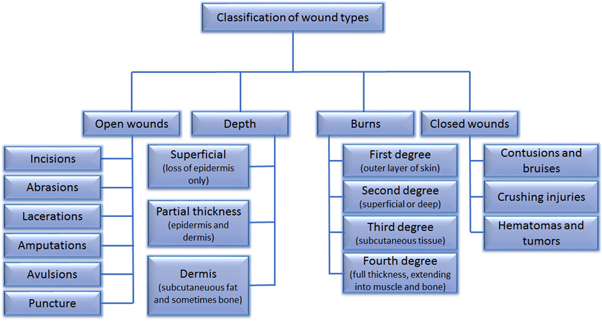
Of the classification shown in figure 2, third degree and fourth degree burns are considered as full-thickness injuries and are the most difficult to treat. This requires the regeneration of the whole skin structure, multi-layers with each having specialized cells, appendages, and other components. Not only is the thickness or volume of skin to be regenerated that matters, but restoring the functionality of the original skin is a necessity. Understanding the wound healing mechanisms [46–50] will help achieve the objective of making functional skins by emerging advanced technologies.
Figure 2. Classification of wounds based on four criteria namely open wounds, depth of the wound, burns, and closed wounds. Open wounds are those where the skin is broken and the underlying tissues are exposed to the external environment; depth refers to the depth of skin injury, where superficial wounds involves loss of epidermis only, partial thickness wounds involves loss of some part of dermis also along with epidermis and dermal wounds involves loss of both epidermis and dermis; burns, based on the extent of injury and severity can be classified into first up to fourth degree burns; closed wounds are those where the skin is intact and the underlying tissues are not exposed to the environment.
Download figure:
Standard image High-resolution imageThe second important reason that accelerates the need for artificial skins is the drug and skin-care products (cosmetics) testing. Not all drugs are mild enough to be tested directly on the humans. Researchers in pharmaceutical and skin care industries were and are using animals to test their products. However, testing the products on animals is not always predictive of responses in humans. In other words, the response to the same drug or product may be slightly or even drastically different from the response that is observed in animals, making the whole testing process unreliable, meaningless and absurd. Also, these testing procedures may cause discomfort and pain to the animals [51–53]. Many organisations and associations were formed since long, like Alternatives to Animal Testing (SCAAT) Steering Committee formed by The European Cosmetics Association (COLIPA) in 1992, the workshop on use of non-invasive methods in cosmetics testing organised by European Centre for the Validation of Alternative Methods (ECVAM) in 1994 [53–57], pressing the need to regulate the policies and procedures of industrial animal testing and if possible, to ban them and find other alternatives. The voice is louder now as ever before, after almost a decade. Due to increasing concerns on the animal welfare and constant pressure by newer and stronger organisations like PETA (People for the Ethical Treatment of Animals), urgency is created for alternative viable technologies. In summary, (i) People are concerned about using products which are tested on animals and many avoid such products; (ii) Many countries (including EU, Israel and India) had already banned industries from 'testing their products' on animals [58]; (iii) Other countries also are considering deploying the ban (like the USA 'The Humane Cosmetics Act (H.R.2858—114th Congress (2015–2016))') [59]; and (iv) Companies were classified as: Brands that DO NOT test on animals, Brands that DO test on Animals, Brands whose animal testing status is unknown. And people are encouraged not to buy the products of those companies that do product tests on animals [60, 61]. These are the most important reasons behind the need for artificial skins, not just an alternate, but bio-mimetic, fully-functional human skin, which is a big challenge but not impossible.
3. Current skin substitutes
Skin substitutes are defined as a heterogeneous group of substances that help in either temporary or permanent closure of many types of wounds, depending on wound coverage that vary based on wound and product characteristics [62]. Skin substitutes help when standard surgical therapies like flap coverage or surgical debridement is not desirable, which is decided on a case to case basis, depending on the type and extend of burn, shallow or deep wounds, the degree of burn, and the area of the body that is injured. There is no perfect or ideal substitute for the original skin per se but something is better than nothing always. Not to mention, researchers around the world, in interdisciplinary teams, comprising of scientists in mechanical, chemical, biomedical engineering, biological, and medical sciences are progressing towards the greater goal of bio-mimicking the original skin, a fully functional skin substitute, with all its intricacies in it. We are not there yet, but good news is we are moving closer. There are a few important characteristics that a perfect skin substitute is expected to have [62, 63] and they are summarized in table 2.
Table 2. Characteristics of an ideal skin substitute.
| Able to resist infection |
| Able to prevent water loss or dehydration |
| Able to withstand the shear forces and wound hypoxia |
| Cost-efficient and affordable |
| Easy to prepare, store and use |
| Wide availability and Long Shelf life |
| Lack of antigenicity |
| Flexible in thickness |
| Durable with long-term wound stability |
| Can be conformed to irregular wound surfaces |
| Provides permanent wound coverage |
| Recreates dermal and epidermal components |
Quantification of each of the properties listed in table 2 is not straight forward and there is no catalogue readily available to compare which is acceptable, which is not and what is best. It is up to the clinicians to decide how these tenets affect the product selection and availability, depending on the type of cases they are dealing with and the practice environment. There are many commercially available products, each having its own advantages and disadvantages, strengths and limitations, giving choices for the practitioners to choose. Comparison of some of these products is dealt with, in the later part of this section.
3.1. Classification of skin substitutes
Skin substitutes can be classified based on their durability, single or multi-layer, the type of layer, and composite substitutes. Skin substitutes may be broadly classified as temporary or permanent and biological or synthetic, which is more practical. In response to the classification proposed by Balasubramani et al [64], which is based on the plasticity and composition, Kumar et al [65] had added upon to give a comprehensive framework for classification of skin substitutes, as given in table 3.
Table 3. Classification of Skin Substitutes.
| Class | Sub-classes | Examples |
|---|---|---|
| Class I: Temporary impervious dressing materials | (i) Single layer Materials | - Biological dressing substitute (potato peel, amoniotic membrane) |
| (ii) Bi-layered Tissue Engineered Materials | - Synthetic dressing substitute (synthetic polymer sheet (Tegaderm®, Opsite®), polymer foam or spray | |
| TransCyte® | ||
| Class II: Single layer durable skin substitutes | (i) Epidermal Substitutes | cultured epithelial autograft (CEA), Apligraft® |
| (ii) Dermal Substitutes | bovine collagen sheet, e.g. Kollagen®, | |
| porcine collagen sheet, bovine dermal matrix, e.g. Matriderm®, human dermal matrix, e.g. Alloderm® | ||
| Class III: Composite skin substitutes | (i) Skin graft | Allograft, Xenograft |
| (ii) Tissue Engineered Skin | Dermal regeneration template, e.g. Integra®, Biobrane® |
There are other ways of classification too. Dorothy et al [66] classifies the skin substitutes in to three classes namely Acellular, Cellular-Allogenic, and Cellular-Autologous; Auger et al [67] reports three classes namely Epidermal substitutes, Dermal substitutes, and Bi-layer substitutes. Some of the current skin substitutes available commercially are tabulated in table 4. Many papers have reported the detailed information, application, clinical trial results of the listed skin substitutes [63, 66–69].
Table 4. Engineered Skin substitutes (Data taken from [66–68]).
| Product | Company | Description | Application |
|---|---|---|---|
| Alloderm | LifeCell Corporation, Branchburg, NJ | Acellular (freeze-dried) allogeneic dermis | Treat full- and partial-thickness wounds |
| Apligraf | Organogenesis Inc., Canton, MA | Allogeneic keratinocytes seeded over dermal scaffold (bovine collagen sponge) containing allogeneic fibroblasts | Treat venous and diabetic foot ulcers |
| Biobrane | UDL Laboratories Inc., Rockford, IL | Porcine collagen chemically bound to silicone/nylon membrane | Temporary covering of partial-thickness burns and wounds |
| CellSpray | Avita Medical. Perth, Australia | Preconfluent autologous keratinocytes delivered into a suspension for spray | Treat partial-thickness burns and chronic ulcers |
| Cymetra | LifeCell Corporation, Branchburg, NJ | Micronized particulate acellular cadaveric dermal matrix | Wound filler in plastic surgery |
| Dermagraft | Advanced Biohealing Inc., La Jolla, CA | Cryopreserved (viable cells) allogeneic fibroblast-derived dermal matrix | Treat full-thickness diabetic foot ulcers |
| Epicel | Genzyme Tissue Repair Corporation, Cambridge, MA | Confluent autologous keratinocytes from skin on petrolatum gauze backing | Treat full- and partial-thickness burns and chronic ulcers |
| Epidex | PharmaTec | Confluent autologous keratinocytes from hair follicles outer root sheath on silicone membrane | Treat full- and partial-thickness burns and chronic ulcers |
| EZ-Derm | Molnlycke Health Care, Sweden | Aldehyde-crosslinked porcine dermal collagen | Treat full- and partial-thickness wounds |
| FortaFlex | Organogenesis Inc., Canton, MA | Acellular porcine small intestine submucosa | Treat full- and partial-thickness burns, venous and diabetic ulcers |
| Hyalograft 3D | Fidia Advanced Biopolymers, Italy | Esterified hyaluronic acid matrix seeded with autologous fibroblasts | Treat full- and partial-thickness wounds |
| Hyalomatrix | Medline Industries Inc., Mundelien, IL | bi-layered, sterile and flexible layer made of a derivative of hyaluronic acid (HA) in fibrous form with an outer layer comprised of a semipermeable silicone membrane | Treat pressure ulcers, diabetic foot ulcers and deep second-degree burns |
| ICX-RHY | Intercytex, Ltd, Manchester, UK | suspension of human dermal fibroblasts (HDFs) in cell storage medium | treat a variety of skin related problems including Epidermolysis Bullosa and scar contractures |
| Integra | Integra Life Science Corporation, Plainsboro, NJ | Acellular. Temporary silicone epidermal substitute over dermal scaffold made of collagen and chondroitin-6 sulfate | Treat partial- or full-thickness burns |
| LaserSkin (Vivoderm) | Fidia Advanced Biopolymers, Italy | Subconfluent autologous keratinocytes seeded on esterified laser-perforatedhyaluronic acid matrix | Treat full- and partial-thickness burns and chronic ulcers |
| MatriDerm | MedSkin Solutions Dr Suwelack AG, Germany | Acellular scaffold made with bovine collagen types I, III, V and elastin | Treat partial- or full-thickness burns |
| Myskin | CellTran Ltd, Sheffield, UK | Subconfluent autologous keratinocytes seeded on specially treated silicone sheet | Treat partial-thickness burns and chronic ulcers |
| OASIS | Smith&nephew plc, London, UK | Acellular porcine small intestine submucosa | Treat full- and partial-thickness burns, venous and diabetic ulcers |
| OrCel | Ortec International, Inc., New York, NY | Allogeneic keratinocytes seeded over dermal scaffold (bovine collagen sponge) containing allogeneic fibroblasts | Treat skin graft donor sites and mitten-hand surgery for epidermolysis bullosa |
| Permacol | Tissue ScienceLaboratories, Andover, MA | Processed dermal xenograft | Temporary burn coverage & clean partial thickness wounds |
| Permaderm | Regenicin Inc., Little Falls, NJ | Autologous keratinocytes seeded onto dermal substitute made with autologous fibroblasts in bovine collagen matrix | Treat chronic wounds |
| Repliform | Acellular human dermal allograft | Urological plastic surgery applications | |
| Suprathel | Institute of Textile and Process Engg, Denkendorf, Germany | Synthetic Epidermal substitute made of DL-Lactatide monolayer | Partial-thickness burns and skin graft donor sites |
| TissueTech | Fidia Advanced Biopolymers, Italy | Combination of Hyalograft 3D and Laserskin | Treat partial- or full-thickness burns and chronic ulcers |
| VCT-01 | Organogenesis Inc., Canton, MA | Keratinocytes seeded on top of dermal substitute made with fibroblasts secreting their own ECM | Treat partial thickness wounds |
3.2. Limitations of current skin substitutes
Skin substitutes help in the restoration and regeneration of skin to a greater extent but they are not without limitations. Mimicking the original skin in all aspects including functionality has not been done successfully to date, to the best of our knowledge. There are many reasons as to why we did not succeed, such as the technological limitations to have multi-layered bio-mimicking structure, with each layer made of different cells and appendages. However, the most important challenges are vascularization and innervation. Boyce et al [70] give an insightful discussion on the limitations of the skin grafts based on the wound healing physiology [71]. Lack of organogenesis is stated as one big short-coming of the current models. Other disadvantages include mechanical fragility, susceptibility to microbial contamination, lesser rates of engraftment, time delay in healing, and of course the cost.
Most of the skin substitutes are predominantly acellular and even if they are cellular, they only use two types of cells, namely fibroblasts and keratinocytes, most of the cases, only either of them. This prevents the substitute from being fully functional like the native skin [66]. Another limitation was pigmentation [66], which can also be solved if the pigment secreting cells are made available in the substitutes. Vascularization and innervation is a challenge, especially for drug and cosmetics testing, where fully functional skin is a requirement and not a choice. In wound healing applications, fully functional skin is not needed because the healthy skin can provide nourishment to the injured area where artificial skin is grafted.
4. Major approaches in tissue engineering of skin
Having said that about the limitations of the current skin substitutes, it is important to understand the approaches in using advanced skin regeneration strategies such as 3D bioprinting. There are two fundamental strategies for tissue engineering in general [72–77], namely (i) bottom-up approach and (ii) top-down approach.
The bottom-up approaches aim to fabricate a larger complex tissue by biological sintering of smaller building blocks, at micrometric scale, mimicking the natural tissue architecture. The building blocks may be cell aggregates, cell-laden hydrogel, polymer microbeads with a homogenous or heterogeneous composition. These smaller blocks are spatially arranged in 3D to form a biomimetic complex architecture. Cell sheet technology, which aims at building up functional thick tissues without the use of biodegradable scaffolds and bioreactor by layering individual cell sheets of few microns thick and bottom-up assembly of cell-laden microgels are most common examples [78, 79]. Some of the other technologies used in this approach are laser-assisted techniques like direct writing [80, 81], laser-induced forward transfer [82, 83], inkjet printers or Drop-On-Demand systems [84, 85], and microdispensing techniques [86].
The top-down approaches aims at processing larger bulk materials into smaller complex structures. Top-down approaches generally involves the use of biodegradable scaffolds. The cells are seeded on to the scaffolds; growth factors are added to aid cell growth and proliferation, usually preserved in a bioreactor that provide appropriate mechanical and hydrodynamic stimuli for maturation in to a 3D tissue construct. Gel casting [87, 88], sol-gel process, replication of polymer sponge [89, 90], solvent casting and particulate leaching [91], phase separation technique [92], freeze drying, electro-spinning [93–95], selective laser sintering (SLS) and stereolithography apparatus (SLA) [96–104] are some of the processes used in the fabrication of biodegradable scaffolds.
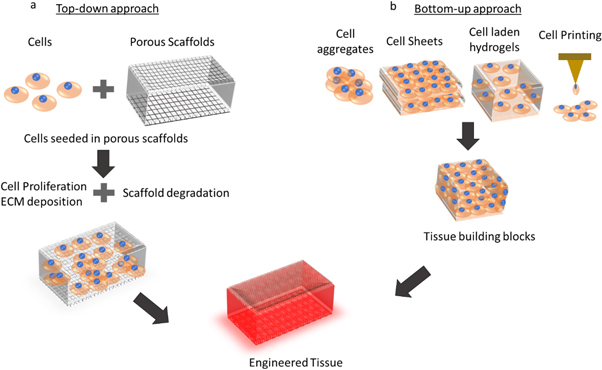
Both of the approaches have their own pros and cons. Bottom-up approach is said to have the potential to improvise the vascularization in 3D constructs, by perfusing them with intraorgan vascular trees [3] and the feasibility of depositing multiple-cell types with desired spatial orientation in 3D. However, one major drawback in this approach is the inability of certain cell types to secrete sufficient amounts of the extra-cellular matrix (ECM), migration of cells and inability to form cell-cell junctions [75]. Top-down approach has many advantages such as the structural stability, ease of cell migration on the scaffold structure thus forming cell-cell junctions and better cell-cell interactions, and availability of a range of biodegradable, biocompatible materials for fabrication of scaffolding structure. The main disadvantage is the lack of understanding on the cell-scaffold interaction in detail. The mechanical and surface properties of the scaffold will affect the cell viability, growth and proliferation of different types of cells. Also, if stem cells are seeded on to the scaffolds, it may differentiate in to different types of cells based on the scaffold properties [105–109]. Though bottom-up approach is credited with the advantage of being scaffold-free, some of the approaches like extrusion bioprinting involves fabrication of 3D structures with cell laden hydrogels. For reasons like increasing the cell density, cells can be further added to the bioprinted constructs (printed from cell-laden hydrogels by extrusion bioprinting), where the properties of the bioprinted construct influences the behaviour of cells that are being added. Hence, understanding the cell-material interaction becomes critical in both the approaches. Figure 3 diagrammatically explains both the approaches.
Figure 3. Top-down versus Bottom-up approach in Tissue Engineering. (a) Top-down approach involves culturing of cells in a porous scaffold, cells grow, proliferate, migrate on the scaffold structure and creates ECM while the scaffolds undergo biodegradation and finally a matured tissue is obtained. (b) Bottom-up approach use cell aggregates, cell sheets or cell-laden hydrogels to produce modular blocks or tissue building blocks, which are then assembled together to form engineered tissues.
Download figure:
Standard image High-resolution imageIn order to successfully fabricate a bio-mimic, fully-functional skin substitute, it is important to have the advantages of both the approaches; thus, overcoming the limitations in using either of them separately. Integration of both the bottom-up and top-down approaches [110] is an excellent idea, yet to be experimented for skin tissue engineering. In bone tissue engineering, Ouyang et al [111] used an integrated approach for engineering ligament analogues. They used bottom-up approach to fabricate a bone marrow stromal cells (bMSCs) sheet in the presence of ascorbic acid and top-down approach to fabricate a scaffold made of poly (L-lactide) (PLLA) and assembled them together using wrapping technique and it is reported to be a promising method for fabricating tissue-like and functional ligament analogues. Sargeant et al [112] also reported an integrated approach for hybrid bone implants, where self-assembly of peptide amphiphile (PA) nanofibers (bottom-up approach) takes place within the pores of metallic Ti–6Al–4 V foams (top-down approach). Though the integrated approach possesses many advantages, technical advancements are necessary to make the approach feasible for tissue engineering [110]. With 3D bioprinting, this integration is possible. As explained earlier, both bottom-up (inkjet printers/Drop-On-Demand systems) and top-down approaches (SLS, SLA) are possible with 3D bioprinting technologies and hence, the integration is also not impossible. A hybrid system, consisting of various tenets of 3D bioprinting can achieve this integration. Especially for 3D bioprinting of skin, bottom-up approach may be used for constructing each layer of the skin, the epidermis or dermis and then these layers can be put together using top-down approaches.
5. 3D Bioprinting of skin
3D Bioprinting of skin is a popular research area now and big players in the market are investing to further research in this area. L'Oreal USA, the largest subsidiary of the world's leading cosmetics company, recently (May 2015) signed a Research Collaboration Agreement with a 3D Bioprinting company, Organovo Holdings, Inc. to 3D bioprint skin models using the NoveGen Bioprinting platform of the latter, for testing their cosmetic products [113]. Procter & Gamble (P&G), another big player in consumer goods, launched a research project in Singapore (in May 2015), seeking research collaborations from academia on 3D bioprinting applications, especially for workable skin models [114]. Rokit, a market leader in 3D printing technology in Korea, announced recently (July 2015) that it is participating in a government funded project for the development of bioprinting technology and the first focus will be on bioprinting of human skin tissue [115]. Therefore, there is a huge demand for this technology and potential for immediate commercialization, if successfully developed.
Broadly speaking, there are three important steps in 3D Bioprinting process, namely, Pre-processing (3D Model Generation, Bio-ink preparation, etc), 3D bioprinting, and Post-processing (using a bioreactor for tissue maturation). In general, the process flow given by Murphy et al [116] holds good for most of the bioprinting processes (as given in figure 4).
Figure 4. A typical process for bioprinting 3D tissues involving six steps namely Imaging (imaging the injured site/tissue), design approach (image converted to 3D models; approach can be either printing the biomimetic structure along with cells, self-assembly of cells or constructing mini-tissues to be assembled later), material selection (based on the intended application, can be natural or synthetic polymers, ECM or combinations of them), cell selection (based on the intended application, can be stem cells or differentiated cells), bioprinting (using commercial or custom-made bioprinters) and application (post-processing in a bioreactor for maturation, grafting or implantation at the injury site). Reprinted with permission from [116].
Download figure:
Standard image High-resolution image5.1. Imaging, 3D modelling and design approach
The first and most important step in 3D Bioprinting is the process of imaging. This involves scanning of the injured tissue or part of the body. Three scanning or medical imaging technologies, x-ray, Computed Tomography, commonly called as CT scan, and Magnetic Resonance Imaging or MRI are widely available and used in hospitals. Though the images from these scanning methods can be then converted to a 3D model using available software, in order to apply it for tissue or organ bioprinting, specialized techniques are required. For instance, a clinician requires to replace part of injured skin in the visible areas of a burn victim, may be on the face or hands. If the imaging system does not have the capability to scan and differentiate the colour of the skin, then exact or at least a closer representation of the victim's skin colour cannot be replicated in the printed skin and hence will not be aesthetically appealing. There are some technologies that are being developed to solve this problem.
Most clinicians, especially plastic surgeons depend on either free-hand 2D sketches on picture printouts or computerized picture morphing for defining the goals of facial aesthetic procedures [117, 118]. These sketches and morphs are then used for discussion with the patients to know their needs and wishes on the facial modification. Though creation of 3D facial shapes is possible with CT-scans [119, 120], the radiation effect and the costs are impractical for aesthetic procedures. Other hardware dependent techniques like laser or stereophotogrammetric scanners [121–123] are too expensive and much complex to handle. To overcome the complexity of hardware dependent methods, many hardware independent software techniques were proposed such as shape from shading [124], structure from motion [125, 126], shape from silhouette [127, 128], and statistical facial models [129], but all have limitations [130]. Oliveira-Santos et al [130] proposed a hardware independent web-based application, which used 2D-digital pictures to create 3D representation of a patient's face for planning the aesthetic procedures. The three important steps in this application are (i) 2D feature and contour points detection (from the 2D-digital image); (ii) 2D to 3D face reconstruction; and (iii) Texture mapping. The greatest advantage of this application is that it is completely internet-based and no skilled manpower is required to use, unlike the CT or MRI. The same method can be used for scanning the injured skin, to analyse the requirements for skin graft. Though the 3D reconstruction is not as precise as other advanced methods, its simplicity and ease of use gives this technique an edge over others and can be used in places where affordability is the main issue. Hani et al [131] used various algorithms for useful wound data acquisition from laser scanned images. They used two methods for solid construction and volume computation namely mid-point projection and convex hull approximation, also called as Delaunay tetrahedralization. The main aim of this procedure is to determine critical wound data namely wound top area, true surface area, depth, and volume of the ulcer wounds in patients, in order to continuously monitor the healing progress throughout the treatment period. AutoCAD software is used to reconstruct the model, subject to the two methods earlier, from the input laser scan images; these reconstructed 3D models were then used to 3D print wax models of ulcer wounds; wound parameters were measured and compared against the surface scan measurements. This method can also be used to other wound causes like the burn injury, to assess the wound parameters, thus aiding the clinicians to get a customized skin graft for each injury site. Active dynamic thermography (ADT) or non-destructive thermography (NDT) is a non-contact imaging technique used widely in quality inspection of material defects in many industries including aerospace and automobile [132]. Recently, this technology has gained much attention in medical applications, for characterizing cutaneous lesions [133, 134] and burns [135–138]. Non-homogeneity of burn wounds, which refers to demarcation of different zones based on the severity of the injury, scaling from first to fourth degree can be done using ADT [135]. Many parameters including the healing time within three weeks are also assessed previously using this technique [136, 138]. Pirindeze et al [139] examined the optical resolution of ADT, to define the resolution limits of this system and to establish a set of parameters for thermal simulation using this system. In fact, they used human skin of varying thicknesses for this resolution study and have successfully demonstrated the ability of ADT's resolution to differentiate details of human skin as thin as 0.025''. ADT can be another potential technology for 3D imaging of burns or wounds in the pre-processing stage of 3D bioprinting.
The imaging and 3D modelling systems are summarized in table 5.
Table 5. Summary of imaging and 3D modelling systems.
| Imaging/3D modelling methods | Pros | Cons | Reference |
|---|---|---|---|
| CT-scan | Accurate measurement of tissue depths | Radiation exposure, High cost | [120, 121] |
| Stereophotogrammetric scanners | Fast capture speeds | Complex to handle Very expensive | [121–123] |
| Hardware independent methods (Shape from shading, Structure from motion, Shape from silhouette, Statistical facial models) | Sophisticated hardware not required, relatively inexpensive | Used in research environments and not optimized for clinical use, limitations on speed and accuracy | [124–129] |
| Web-based application | Completely web-based, no hardware required | Less reproduction accuracy, sub-optimal 3D reconstruction | [130] |
| Active dynamic thermography (ADT) | Accurate measurement of tissue/burn depths, High resolution | Necessity of using proper thermal models of living tissues, controlled experimental conditions required | [135–139] |
Colour recognition is another important aspect, aiding to replicate the exact skin colour of the patient in engineered skin grafts. Xiao et al [140] developed a colour reproduction system for processing of soft tissue prostheses accurately and automatically using advanced technologies like 3D Bioprinting. A Minolta CM-2600d spectrophotometer was used for colour measurement, along with SpectraMagic NX Color Data Software to decipher values in CIELAB values [141]. The study used Zcorp Z510 3D colour printer. A protocol was developed using many mathematical models based on 240 training colours. Of all the mathematical models, the third-order polynomial regression based on least-square fitting provided the best model performance for colour profile. Based on the colour profile, the colour reproduction system was established and parameters such as accuracy of colour reproduction, performance of colour repeatability and colour gamut were evaluated using 14 known human skin shades. The printed prostheses demonstrated successful colour reproduction of all these 14 skin shades. Simulation of colour gamut for the proposed bioprinting system was done and except the extreme skin shades of dark and light, majority of skin colours are reproduced in the 3D bioprinted prostheses. The colour gamut is shown in figure 5.
Figure 5. Colour gamut of 3D printing system for 14 human skin colours: (a) top view. and (b) side view. Reprinted with permissions from [140].
Download figure:
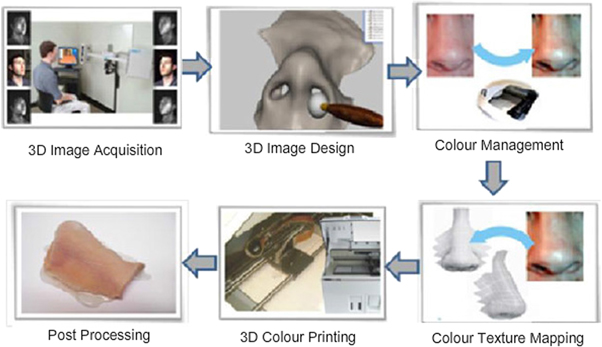
Standard image High-resolution imageThe 3D colour image reproduction system developed by Xiao et al [142] is represented in figure 6.
Figure 6. 3D colour image reproduction system for facial prostheses involving six steps namely 3D Image acquisition (facial photos taken in different angles), 3D image design (processing of the acquired image and converting them to 3D models), colour management (chosen based on the human skin shade), colour texture mapping (texture matched to the original facial skin), 3D colour printing and post processing. Reprinted with permission from [142].
Download figure:
Standard image High-resolution imageJang et al [143] proposed a spectrum-based colour reproduction algorithm for make-up simulation of 3D facial avatar. All the colour processing is based on RGB data as of now and its inability to represent a real make-up simulation on a 3D facial avatar encouraged them to work on a spectrum-based colour reproduction process, which takes into account the intrinsic characteristics of the objects and also the illuminant. The performance evaluation of the system was found acceptable. This method can be coupled along with other colour reproduction techniques to recognize the skin colour of the patient and also replicate the same colour in 3D bioprinted skin grafts or prostheses. Wang et al [144] developed a gradient-based multi-spectral method for face liveliness detection, which explored the reflectance of all the distinctive regions in a face and their spatial relationship, unlike the available methods, a complement to the previous pixel-based methods. Application of this technology in 3D imaging, especially for medical applications like skin wound imaging, is yet to be explored.
The design approach should be based on the severity of the injury. If the wound or burn is a partial thickness injury, then epidermal layer skin graft should be fine. When the severity of the injury is of higher degree, multi-layered structure is required. If the wound area to be treated is smaller, scaffold-free methods may be acceptable as against the larger wound area, where scaffold based approach is necessary to facilitate easy cell migration, forming cell clusters and cell-cell junctions and finally tissue formation.
5.2. Materials
Material selection is one of the key steps in 3D Bioprinting. Biomaterials are a group of materials which are biocompatible and biodegradable. Since the most common type of materials used is polymer, they are also called as biopolymers. Based on their source of production, they can be broadly divided in to two, namely natural polymers, and synthetic polymers.
Natural biopolymers are those that are naturally occurring, including (i) proteins (such as collagen, gelatin, albumin, thrombin, fibrinogen, etc), and (ii) polysaccharides (such as chitosan, chitin, cellulose, etc) [145]. Biocompatibility, biodegradability, hydrophilicity, biological characteristics, resemblance to the native ECM are strong reasons why natural polymers are preferred in tissue engineering applications.
Synthetic biopolymers are man-made polymers which includes polylactic acid (PLA), polyglycolic acid (PGA), poly(ε-caprolactone) (PCL), poly(lactic-co-glycolic acid) (PLGA), poly(propylene fumarate), polyanhydrides, polycarbonates, polyorthoesters, polyurethanes, and polyphosphazenes. Synthetic biopolymers are known for their excellent mechanical properties, which are important for a scaffold material. Key advantage is the ability to tailor the mechanical properties and degradation kinetics, by altering the polymer structure, to suit various biomedical applications, for example, bone graft or skin graft [146].
Both of the above types suffer from certain limitations. Natural biopolymers, in spite of having the greatest advantage of mimicking the native tissue ECM, suffer from poor mechanical properties. On the other hand, synthetic biopolymers have the advantage of better mechanical properties but very different from the natural ECM. And hence, researchers use composite polymers, which has both natural and synthetic biopolymer constituents, thus combining the advantages of both and overcoming the limitations. This is extremely important with 3D bioprinting because, in addition to the basic requirements of a biopolymer, there is another important factor to be considered while preparing the bio-ink, which is 'printability'. The requirements of an optimal biomaterial [145] are biocompatibility with tissues, biodegradability at the ideal rate corresponding to the rate of new tissue formation, nontoxicity, nonimmunogenicity, optimal mechanical properties, adequate porosity and morphology for transporting of cells, gases, metabolites, nutrients, and signal molecules both within the biomaterial and between the biomaterial and the local environment, and printability.
The most common natural biopolymers used in skin tissue engineering are collagen, gelatin, chitosan, and silk fibroin; the synthetic biopolymers being PCL, PLA, PLGA, and PLLA. The composite biopolymers are formed by combining one from each group. Electrospinning is one of the most successful technologies to produce nanofibers of the above biopolymers, which serve as the scaffold material for skin tissue regeneration. A list of such electro-spun nanofibrous scaffolds is tabulated in table 6 (data taken from [147]). This information is included in this review as most of the biopolymers or composites that are electro-spun can also be 3D bioprinted using inkjet and Drop-on-Demand systems. They are grouped into four main types, viz. natural, synthetic, natural composite (two or more natural biopolymers), and composites (at least one natural and one synthetic biopolymer).
Table 6. Electro-spun nanofibrous scaffolds for skin tissue engineering (data from [147]).
| Polymer | Type | Special feature | Reference |
|---|---|---|---|
| Collagen | Natural | Reduced wound contraction compared to freeze-dried scaffolds | [148] |
| Higher cell proliferation in aligned fibres compared to random scaffold | [149] | ||
| Gelatin | Natural | Well-stratified dermal and epidermal layers including a continuous basal keratinocyte layer | [150] |
| Cross-linking gelatin nanofibers with genipin improves cell proliferation but cell viability is reduced | [151] | ||
| Promotes cell adhesion and spreading of type I collagen, suitable for wound dressing [152, 153] | [152, 153] | ||
| Silk fibroin | Natural | Oxygen plasma-treated SF nanofibers showed higher cellular activities than the untreated ones | [154, 155] |
| Chitosan | Natural | Sonication of chitosan nanofibers resulted in higher porosity and increased cell proliferation | [156] |
| Fibrinogen | Natural | Suitable for wound repair or hemostatic products | [157] |
| Good mechanical properties, suitable for wound dressing | [158] | ||
| Significantly increased peak stress and modulus values for the fibrinogen cross-linked with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and fibrinogen-genipin cross-linked scaffolds | [159] | ||
| Collagen/Silk fibroin | Natural composite | Better cellular responses in hybrid nanofibrous scaffolds than that of blend nanofibrous scaffolds | [160] |
| Chitosan/Silk fibroin | Natural composite | Addition of silk fibroin enhanced the mechanical properties of nanofibers | [161] |
| Chitosan/Collagen | Natural composite | Showed keratinocyte migration and wound re-epithelization-a prerequisite for healing and regeneration | [162] |
| Chitosan/Chitin nanocrystals | Natural composite | Addition of chitin nanocrystals as well as crosslinking had a positive impact on the mechanical properties | [163] |
| PCL | Synthetic | Enhanced cell proliferation and wound healing due to the surface degradation of the polymer under physiological conditions and the formation of functional groups like hydroxyl and carboxyl groups that promoted cell proliferation | [164] |
| A micro-machined human skin pattern mould was used as a collector in an electrospinning setup to replicate the pattern onto the surface of the electrospun mat; exhibits guidance of cells along the skin pattern without significant deterioration of pattern geometry | [165] | ||
| PLLA | Synthetic | Nanofiber scaffolds enhanced epidermal skin cell migration across the wound when compared to a control group without scaffold. Aligned nanofibers promoted the infiltration of endothelial cells into the scaffolds | [166] |
| PDLLA-LL | Synthetic | Hybrid microfibers possessed a unique porous high surface area mimicking native extracellular matrix (ECM) | [167] |
| PLGA | Synthetic | Cells acquired a well spread morphology and collagen type III gene expression was significantly up-regulated | [168] |
| Good cellular penetration, with no adverse inflammatory response outside the scaffold margin and with no capsule formation around the periphery | [169] | ||
| The cell attachment and spreading were several times higher in the nano-/microfiber composite scaffolds than in the microfibrous scaffolds without nanofibers | [170] | ||
| Highly porous scaffold provided mechanical support for cells to maintain uniform distribution; better cell migration and collagen secretion | [171] | ||
| PCL/Chitosan | Composite | The presence of chitosan aided a significant improvement in the hydrophilicity of the scaffold, bioactivity and protein adsorption | [172] |
| Amino-remained chitosan-graft-poly (ε-caprolactone) (2/8) mats with a moderate surface zeta-potential (ζ=3 mV) were the best in promoting the cell attachment and proliferation | [173] | ||
| Novel polysaccharide-coated PCL fibre mats remarkably combine the mechanical resistance typical of PCL with the surface properties of chitosan | [174] | ||
| PCL/Collagen | Composite | Anisotropic nanofibers significantly triggered integrin β1 signalling pathway | [175] |
| Collagen was immobilized on aminolysed PCL nanofibers using glutaraldehyde as cross linker, enhancing cell proliferation | [176] | ||
| PCL and collagen nanofiber matrices support the attachment and proliferation of human dermal fibroblasts; suitable for dermal substitutes | [177] | ||
| Coaxial electrospinning of collagen-PCL showed superior cell proliferation than roughly collagen-coated PCL | [178] | ||
| Acellular scaffold strength increased with increasing PCL content but the mechanical strength of the Engineered Skin was not enhanced by the use of collagen–PCL blended scaffolds, after a minimum amount of PCL (10%) | [179] | ||
| Collagen gel coated PCL nanofibrous scaffolds showed better cell responses than PCL/collagen nanofibrous matrices | [180] | ||
| PCL/Gelatin | Composite | Coaxially electrospun PCL/gelatin scaffolds exhibited increased cellular adhesion and metabolism versus PCL alone or gelatin-PCL blended fibre scaffold | [181] |
| Acetic acid-doped TFE solvent system was used to prevent phase separation during electrospinning | [182] | ||
| PCL/Gelatin/Collagen | Composite | Collagen type I-modified PCL/gelatin scaffold was successful in maintaining characteristic shape of fibroblasts, besides good cell proliferation than the PCL/gelatin scaffold | [183] |
| PLA/Chitosan | Composite | Core–shell structure nanofibers of PLA/chitosan easier to fabricate than double-needle electrospinning | [184] |
| Aligned PLA/chitosan fibrous scaffold fabricated using a novel collector made of parallel blades; PLA/chitosan nanofiber has enhanced degradation than the pure PLA fibres | [185] | ||
| PLA/Gelatin | Composite | Modified PLA/gelatin (7/3) scaffold is more suitable for fibroblasts attachment and viability than the PLA or gelatin nanofiber alone | [186] |
| PDLLA/Chitosan | Composite | Dual-layer chitosan/PDLLA structure result in aligned migration and directional penetration of skin fibroblasts into 3D domain | [187] |
| PLLACL/Collagen | Composite | PLLACL/collagen scaffolds showed better cell differentiation of MSCs than PLLACL scaffolds | [188] |
| PLLACL/Gelatin | Composite | Fibroblasts proliferation, morphology, CMFDA dye expression and secretion of collagen were significantly increased in plasma-treated PLACL/gelatin scaffolds compared to PLACL nanofibrous scaffolds | [189] |
| PLGA/Chitosan | Composite | Hybrid PLGA/chitosan and core/shell structured PLGA/chitosan membranes showed better cytocompatibility than the PLGA membrane in adhesion, viability assays as well as morphology observation | [190] |
| PLGA/Collagen | Composite | Highly porous fibrous mats of PLGA and Type I collagen were produced by blend electrospinning; inoculation of collagen enhanced the cell attachment, proliferation and extracellular matrix secretion, which were found to be dependent on the amount of collagen in the composite scaffold | [191] |
| PLGA/collagen nanofibrous membranes were functionally active in responses in human fibroblasts, and were very effective as wound-healing accelerators in early-stage wound healing | [192] | ||
| PCL/PLGA/Chitosan/Gelatin | Composite | The upper layer of PCL/PLGA nanofibers provided mechanical support and reduced degradation rate of the hydrogel layer. The lower layer of porous chitosan/gelatin hydrogel could retain moisture. | [193] |
As mentioned earlier, in addition to the satisfaction of basic requirements of a biomaterial, the bio-ink should be printable. Ideal properties of bioprinting hydrogels are tabulated in table 7 [197]. Malda et al [194] summarizes the properties of bio-ink or hydrogels, under two headings: (i) rheology, which includes viscosity, shear thinning, and yield stress and (ii) crosslinking mechanisms of hydrogels namely physical, ionic, chemical, stereocomplex or thermal crosslinking. Nakamura et al [195] discussed the roles of hydrogels for biofabrication, especially with inkjet and 3D bioprinting technologies. Murphy et al [196] evaluated the properties of twelve different hydrogels, which are commercially available and commonly used in research. These evaluated properties are relevant to bioprinting of skin such as gelation time, swelling or contraction, stability, biocompatibility, and printability. The twelve hydrogels that are evaluated are Collagen Type I, Collagen/Fibrin, Fibrin, ExtracelTM Hydrogel, ExtracelTM UV, Tyramine substituted hyaluronic acid (TS-NaHy)-CorgelTM, Methylcellulose-Hyaluronan (MC-HA), Chitosan, Chitosan/Collagen, Alginate, Alginate/Gelatin, and Polyethylene Glycol Diacrylate (PEGDA). The results revealed that Extracel-UV, the photocrosslinkable HA-based hydrogel was the best candidate for bioprinting, possessing the desirable properties for bioprinting application. The regulatory, commercial and financial aspects of each of the hydrogels were also discussed briefly. Wang et al [197] recently reported a state-of-the-art review of smart hydrogels for 3D bioprinting; 'smart' referring to the responsiveness of the hydrogel to various external stimuli such as pH, temperature, light, electric, and magnetic field. The potential of combining smart hydrogels with 3D bioprinting, their challenges and future prospects were also reviewed.
Table 7. Ideal bioprinting hydrogel properties [197].
| Printability | Viscosity |
| Shear-thinning property | |
| Response and transition time | |
| Sol-gel transition stimulus | |
| Biocompatibility | Degradability |
| Cell binding motifs | |
| Non-toxic | |
| Non-immunogenic | |
| Mechanical properties | Stiffness |
| Elasticity | |
| Strength | |
| Shape and structure | Pore size |
| Micro/Nano structure |
Material selection is a key for any bioprinting process. For 3D bioprinting of skin, there is wide range of natural, synthetic, and composite biopolymers available, which can be selected based on the type of printing process used and bio-ink formulation can be done with or without cells, based on the tissue engineering approach that is aimed at. With the development of smart hydrogels, 3D bioprinting of functional skin will no more be just a dream, but a realistic goal that is achievable in the near future.
5.3. Cell selection
One of the most important factors that decide the success of tissue engineering is the choice of suitable cells, assuring persistence and function of the regenerated tissue for the patient's life time [198]. The most common type of cells used extensively in current skin substitutes is keratinocytes, which forms the outer epidermal layer. There are many advantages in using keratinocytes in skin tissue engineering; they can be cultured, maintained and propagated in the laboratory with ease [199, 200] and their resistance to senescence is so great that they can be passaged for many hundreds of generations [201]. However, there are overwhelming limitations that prevent the sole use of keratinocytes to engineer a fully functional skin substitute. The first major drawback is the time required to culture these cells. It takes about 3 weeks for a 2 sq.cm biopsy to expand on a surface, sufficient enough to cover the whole body (73 sq.m), which may take even longer for elderly patients. Scarring is another problem, referred to as hypertrophic scarring. The presence and degree of scarring is directly proportional to the healing time, which is obviously longer when keratinocytes are used [202]. Fibroblasts come next to keratinocytes, present in the dermal layer of natural skin and obviously used in the dermal skin substitutes. They represent a diverse population of cells, with the ability to differentiate along a lineage from highly proliferative progenitor fibroblasts. They are ubiquitous, static in nature and provide support and maintenance to the tissues. The inherent problem of long-term fragility of keratinocytes can be overcome by having a layer of dermal fibroblasts underneath it. It is reported that there are specialized subsets of fibroblasts and each respond to the different phases of wound healing and skin regeneration. And also, the fact that fibroblasts from different sites of the body possesses different functional properties, should be considered before selection for tissue engineering, as it may affect their suitability for dermal substitutes [203]. The bilayer or composite skin substitutes, normally referred to as full-thickness skin grafts, consist of both keratinocytes and fibroblasts, representing the epidermal and dermal layers respectively. The production process of such a composite is complex, involving many phases [204, 205] and there is no contention on the optimal product on long-term [206, 207]. Though the epidermal keratinocyte and dermal fibroblast composite is better than being individually used, there are so many other cells and appendages that are required to be present in order to make it fully functional per se. Irregular pigmentation, for example, is a limitation, which can be overcome by co-culturing melanocytes along with keratinocytes, which ensures normal pigmentation by secreting melanin [208]. Vascularization is another problem, which can be managed to some extent by incorporating endothelial cells to facilitate angiogenesis. Genetically modified keratinocytes, which overexpresses vascular endothelial growth factor (VEGF) were reported to promote vascularization [209]. Still, integration of pilosebaceous units, with hair follicles and sebaceous glands to the skin substitutes, remains an unsolved challenge [202].
Most of the current tissue engineering strategies depend on a sample of autologous cells from the damaged host tissue or organ. It becomes increasingly difficult to obtain the cells from the damaged host, especially when the injury or damage is extensive. Alternative cell sources become a necessity in such cases, which is debated in the field of regenerative medicine. In order for the tissues and organs to maintain a balance between cell loss and replacement, the tissues must possess cells that are capable of self-renewal and differentiation and hence, stem cells are looked upon as a promising source for future skin regeneration and skin tissue engineering [210]. Many types of stem cells that inhabit the skin have been identified and located which includes epidermal stem cells (interfollicular stem cells and bulge stem cells), that are widely used recently, dermal stem cells [211], sebaceous stem cells, melanocyte stem cells, hair follicle stem cells [212, 213], sweat gland stem cells, endothelial stem cells, mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), and neural stem cells [214, 215]. When using stem cells for bioprinting or tissue engineering in general, the relationship between regeneration and development should be understood well, when undifferentiated stem cells are used as the source from which differentiated cell types arise, thereby creating or regenerating functional tissues [210]. Also, there are many ethical and technical problems, controversies surrounding stem cells to be overcome before we can attempt bioprinting stem cells, utilising their full potential [216–218].
Having reviewed the types of cells that can be used for skin substitutes, 3D bioprinting has the luxury of using any kind of cells, or combination of cells, suspended in the same solution or bio-ink or different cells in different bio-inks, that can be fed through different nozzles in a multi-nozzle bioprinting system. Based on the type of cells chosen, the bio-ink composition varies in order to satisfy the basic requirements of a printable bio-ink.
5.4. 3D bioprinting processes
Bioprinting can be defined as a 'computer-aided transfer processes for patterning and assembling living and non-living materials with a prescribed layer-by-layer stacking organization in order to produce bio-engineered structures serving in regenerative medicine and other biological studies' [219]. The bioprinting techniques can be classified under three main topics namely, Laser-based bioprinting or laser-induced forward transfer [80, 220–225], Inkjet bioprinting/droplet-based bioprinting [226–231], and robotic dispensing/extrusion/deposition bioprinting [232–239] (refer to figure 7).
Figure 7. Three main bioprinting technologies (a) Laser-assisted bioprinting focuses laser pulses on to the donor slide, thus creating high pressure to propel droplets of cell-laden hydrogel on to the collector slide (b) Inkjet printing ejects droplets of biopolymer or cell-laden hydrogels through a nozzle by either thermal energy application (electrically heating to produce vapour bubbles that forces droplets to come out through the nozzle) or piezoelectric actuator (actuation of piezoelectric crystals by applying electrical energy at high frequencies). (c) Extrusion or robotic dispensing bioprinters extrude biopolymers or cell-laden hydrogels through the nozzle by applying air pressure (pneumatic) or mechanical systems (piston or screw).
Download figure:
Standard image High-resolution imageLaser-induced forward transfer utilizes laser energy to deposit cell-encapsulated droplets of required volume, in any defined 3D spatial arrangement. The main components of such a system are the donor slide, the collector slide and laser generation system. The donor slide has a conductive coating for laser energy absorption and a layer of the cell suspension or bioink that is to be bioprinted. The collector slide acts as the substrate, which can be biopaper or scaffolds or culture plates. When laser pulses are focussed on the donor slide, the conducting layer absorbs the laser energy, creates a high gas pressure on absorption of laser energy and as a result, droplets of cell suspension are propelled towards the collector slide. Required spatial arrangement can be obtained by controlling the path of the laser beam. Inkjet printing is the second bioprinting technique. They are also called Drop-on-Demand systems as it propels drops of cell suspension or bioink on to the substrate. Based on the mechanism of how droplets are generated, they are classified as thermal and piezoelectric based inkjet printing. In thermal inkjet printing, a heater or a heating filament produces a vapour bubble in the nozzle, which propels the droplet out of the nozzle onto the substrate. In piezoelectric based systems, the changes in the volume of piezoelectric crystal on application of electric pulses, expels the droplet through the nozzle. Third comes the robotic dispensing systems. In this type, the bioink is extruded out of the nozzle by application of mechanical force. The mechanical force can be pneumatic, piston-based or screw type. An extensive review of these technologies has been done by Murphy et al [116], Dababneh et al [219], Malda et al [194] and Ozbolat et al [240]. Arslan-Yildiz et al [241] had reviewed the recent advances in bioprinting technologies, current markets, approaches, and biomedical applications of bioprinting.
5.4.1. Laser assisted Bioprinting (LaBP) of skin tissue
Current 2D cell culture and 2D tissue engineering do not well mimic the native tissue as the original natural tissue is in a 3D environment. Studies have already proved that cells behave differently in 2D and 3D environment and it is better with in 3D space as it mimics the complex 3D-microenvironment architecture in vivo [242–244]. Laser cell printing or Laser assisted Bioprinting (LaBP) or Laser induced forward transfer, is one technology which can precisely place cells in 3D spatial arrangement. Advantages of LaBP includes capability of positioning small volumes of cell suspensions or bioink, down to a few hundred femtolitres, with high resolution [220, 245] and ability to print high density cell suspensions [246] or hydrogel precursors or bioink with any desired viscosity, which is the main limitation with ink-jet bioprinters, which can only handle lower viscosity ranges. Schiele et al [247] comprehensively reviewed the laser-based direct write cell printing technologies, giving an overview of different realisations and denominations of this technique. Research group from Hannover Medical School, Germany along with Laser Zentrum Hannover, had used this technique to print skin tissue [248, 249].
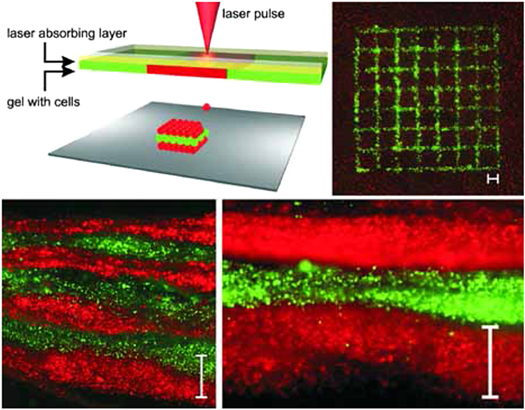
Koch et al [248] used LaBP to arrange the vital cells of skin tissue, namely the keratinocytes and fibroblasts embedded in collagen, in a 3D arrangement, representing multicellular skin grafts that are analogous to the native archetype. The bio-ink used in this study is made of (a) murine cell line NIH-3T3 fibroblasts, representing the dermal layer, (b) keratinocytes cell line HaCaT from adult human skin, representing the epidermal layer, (c) suspended in a hydrogel, collagen, which is the main component of ECM in native skin, and (d) a sheet of MatridermTM, which is a commercial dermal skin substitute, to serve as a scaffold or substrate. The main focus of this study was the arrangement of these skin cells under a set of different conditions or parameters, namely 3D cell pattern, cell density and matrix material, in a way that the microenvironment is comparable to the native skin tissue and to study the tissue formation process. The experimental set-up [250] is shown in figure 8. It consists of two co-planar glass slides. The upper one is coated underneath with an energy absorbing layer or laser absorbing layer, to be specific; a 60 nm thin gold layer coating is used in this study and a layer of the cell suspension that is to be transferred or bioprinted, which is keratinocytes or fibroblasts suspended in collagen hydrogel. The lower glass slide acts as the substrate; scaffold or hydrogel layer can be positioned on this plate on which the cells will be printed. For better understanding, the upper glass slide is the 'donor-slide' and the lower glass slide is the 'collector-slide'. The cell suspension is printed on to the collector-slide from the donor-slide using laser induced forward transfer. Laser pulses are focussed on the donor slide, which causes localized evaporation in the gold layer beneath, the absorption of laser energy generates a high gas pressure, thereby propelling the subjacent cell suspension towards the lower glass slide, which is the collector-slide. The droplet size of the cell suspension transferred depends on a number of parameters namely the laser pulse energy, the gap between the upper and lower glass slide, the laser spot size, thickness of the laser absorbing layer and the thickness of the cell suspension layer.
Figure 8. Top left: sketch of the laser printing setup. The cell–hydrogel compound is propelled forward as a jet by the pressure of a laser‐induced vapour bubble. Layer‐by‐layer, a 3D cell pattern is generated. Top right: A printed grid structure (top view) of fibroblasts (green) and keratinocytes (red) demonstrates micropatterning capabilities of the laser printing technique. Bottom: Seven alternating colour‐layers of red and green keratinocytes (left; detail right). Each colour‐layer consists of four printed sublayers. Scale bars are 500 μm. Reprinted with permission from [248].
Download figure:
Standard image High-resolution imageA bilayer skin construct, consisting of keratinocytes dominated outer epidermal layer and fibroblasts dominated the inner dermal layer was printed; 20 cell-collagen sublayers of NIH-3T3 Fibroblasts being printed first on a sheet of MatridermTM, followed by 20 cell-collagen sublayers of HaCaT keratinocytes over it.
Important factors that indicate a successful skin tissue construct were studied on the printed bilayer construct including immunohistochemistry, analysis of gap junction coupling, post-printing cell proliferation, development of a basement membrane between epidermis and dermis like the natural skin, analysis of adherens and gap junctions (which is important for mechanical connection between cell-cell and cell-ECM). The cells remained in the printed pattern, i.e. as bilayer, and did not intermix even after 10 days in culture; proliferation post-printing was good; development of the basal lamina serves as a sign of skin tissue formation; better adherence of the cells to neighbouring cells and the ECM shown by the extensive formation of adherens junctions all go to say that this was a unique successful first demonstration of 3D arrangement of vital cells using LaBP method as multicellular grafts analogous to the native skin.
Michael et al [249], from the same group, used the same technique to print the bilayer skin construct and tested it in vivo in nude mice. In this work, same kind of cells used in the previous study namely NIH3T3 Fibroblasts and HaCaT keratinocytes suspended in collagen were used. A bilayer skin construct, having 20 layers of fibroblast-containing collagen and 20 layers of keratinocyte-containing collagen were printed onto a sheet of MatridermTM (2.3 cm × 2.3 cm, followed by incubation under submerged conditions overnight in an incubator. The next day (defined as Day 0), nine round pieces (diameter 6.0 mm) were removed from the large construct with a biopsy punch, three of which were implanted into the skin fold chambers in vivo (one per mouse, four independent printing processes, tested in 12 animals in total). The remaining six pieces of each printing process served as in vitro controls. Two of them were directly fixed on Day 0 to depict the situation at the beginning of the experiments, whereas the remaining four pieces were raised to the air-liquid-interface. In vitro controls were then fixed on Days 5 and 11 (duplicates per time point) and in vivo specimen on Day 11.
The results were promising. The printed skin constructs placed in to the full-thickness wounds in the dorsal skin chamber of nude mice, in vivo, were fully connected to the surrounding tissue when explanted after 11 days. Multi-layered epidermis was formed by the printed keratinocytes, with initial differentiation and stratum corneum. Adherens junctions, which are the main indicators of tissue formation, detected by the E-cadherin, could be found in the epidermis, both in vivo and in vitro. The printed fibroblasts produced collagen, which is their main function, in both in vivo and in vitro conditions, staying partly on the top of the underlying matrix and partly migrated in to the matrix. Above all, some blood vessels were found growing from the wound bed and the wound edges towards the printed cells.
5.4.2. Robotic dispensing based 3D-bioprinter
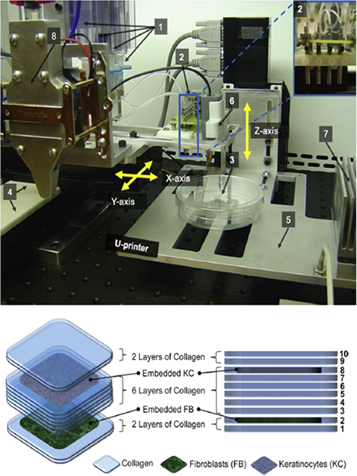
Lee et al [251] reported a novel method to construct stratified skin construct in 3D, layer by layer, using robotic cell dispensing technology. The schematic of the device is given in figure 9. The printer mainly consists of an array of four micro valves as dispensers and a three-axis Cartesian robotic stage. The dispensers, each with a pneumatically driven control mechanism, were mounted to the horizontal (x–y) robotic stage, which controlled the timing and location of dispensing of printable materials including hydrogel precursors and cell suspensions. The entire device was housed in a laminar flow hood. The MATLAB computing environment was used to generate the robot control codes dictating the dispensing spatial coordinates. Primary adult human dermal FBs and primary adult human epidermal KCs were used along with Type I collagen as hydrogel precursor for a scaffold material. A total of 10 collagen layers were sequentially printed on a planar square in a 60 mm tissue culture dish. FB and KC layers were located in second and eighth layer of the collagen layers, counted from bottom, respectively. The five collagen layers sandwiched between the FB and KC layers demonstrate the ability to print spatially distinctive cell layers. Post-printing, the printed tissue construct was cultured in 37 °C, 5% CO2 in KC media. There are two important and unique advantages of this device. The first is the new crosslinking mechanism introduced and the second is printing on non-planar surfaces. Instead of the traditional method of dipping the printed construct into the crosslinking solution bath, here, the nebulized mists of crosslinking agents were coated on the surface before and after printing each layer. This new mechanism engenders the second advantage of printing on non-planar surfaces, which was successfully demonstrated by printing on 3D non-planar Polydimethylsiloxane (PDMS) substrates. This characteristic of this method makes it possible to print the tissues directly on to the wound surfaces in vivo, even on the most complex, non-planar, irregular wound area. One limitation of this system was reported to be the cap for thickness of printed tissues at 100 um, as the resolution achieved was in the order of 700 to 1000 um. Improvements in the dispensing system and printing mechanism should be made for increasing the resolution. With thicker tissues comes the problem of providing adequate perfusion, which can be overcome by introducing fluidic channels inside the hydrogel.
Figure 9. (Top) Picture of the modular tissue printing platform shown with: [1] 4 syringes as 'cartridges' to load cell suspensions and hydrogel precursors; [2] an array of 4-channel dispensers; [3] target substrate; [4] horizontal stage; [5] vertical stage; [6] range finder; [7] vertical stage heater/cooler; [8] optional independent heating/cooling unit for the dispenser. Inset: close-up view of the 4-channel micro-dispensers. (Bottom) Schematic of layer-by-layer printing of the multi-layered skin cells and collagen. Reprinted with permission from [251].
Download figure:
Standard image High-resolution imageLee et al [252] utilised a similar system but with eight independently controlled cell-dispensing channels, instead of four, which can be utilised to place cells, ECM, scaffold materials, and other growth factors in any user-defined 3D arrangement. Each dispenser is independently operated by electromechanical valves, as in the previous system [251] and mounted on a three-axis, high precision, xyz robotic stage, with the option to precisely control the volume of dispensed droplets, with range as low as 15 nL, with high precision. FBs (HFF-1) and KCs (HaCaT) were used to construct the 3D skin tissue in this study. The schematic is shown in figure 10. A total of six collagen layers for the dermis and two collagen layers for the epidermis were printed to obtain the desired dermal and epidermal thicknesses. FBs proliferated within the dermal layer and maintained a sparse distribution. KCs proliferated more rapidly on the top of the collagen matrix and fully covered the surface in 4–7 days. No cell invasion across the dermal and epidermal layers was observed, which is a notable advantage. A comparison of skin tissues fabricated via 3D bioprinting and manual deposition was made, which showed that printed skin samples retain their form, dimensions and shape, whereas manually deposited structures shrink, buckle and form concave shapes under submerged culture condition after 7 days. In vitro tests namely the viability tests and immunohistochemistry tests indicated the development of the printed construct in to a skin tissue, mimicking the epidermal and dermal layers of native skin.
Figure 10. Construction of three-dimensional (3D) skin tissue. (a) Layer-by-layer printing of collagen matrix, KCs, and FBs to construct the dermal and epidermal compartments in a single structure. (b) Schematic of the 3D printed skin tissue showing the cross-section (left) and top view (right). Reprinted with permission from [252].
Download figure:
Standard image High-resolution image5.4.3. Cryogenic extrusion based direct-plotting system
Kim et al [253, 254] studied an extrusion based cryogenic direct-plotting system for skin tissue engineering. The device set-up is shown in figure 11 [253]. The cryogenic 3D plotting system composed of a 3D robot system, a dispensing system, and a cryogenic manufacturing stage. The cryogenic refrigeration system consisted of two compressors, circulating silicone oil to cool the stage, and a circulation pump. A collagen scaffold of required thickness and pore size were processed by computer-driven direct deposition, layer by layer with a plotting needle under controlled pneumatic pressure on a cryogenic stage at −40 °C and the ambient temperature being 10 °C, to enable the printed collagen strands to freeze almost instantly as it touches the substrate. The nozzle was coated with silicone rubber to prevent clogging. The plotted scaffold was immediately placed in a freeze-dryer at −76 °C for 3 days, followed by immersion in 50 mM 1-ethyl-(3-3-dimethylaminopropyl) hydrochloride (EDC) solution in 95% ethanol for 24 h at room temperature for cross-linking. Keratinocytes and fibroblasts are co-cultured on the scaffolds then and in vitro studies performed. The well designed pore structure of the scaffold enabled the cells to proliferate, readily migrate in to and differentiate in the scaffold. However, the structure suffered low shape stability due to highly porous structure and poor mechanical properties of collagen.
Figure 11. Fabrication procedure for 3D collagen scaffolds. (a), Schematic diagram of the desired scaffold (20X20X1.5 mm3); (b) schematic diagram of the cryogenic plotting and processing system at −40 °C (left) and images of the fabricated scaffold (right); (c) complete freeze-drying of the fabricated 3D scaffold at −76 °C over 3 days. (d) Electron micrograph of scaffold curing in 50 mM EDC in 95% ethanol for 24 h at room temperature. Scale bar = 1 mm. Reproduced from [253] with permission of The Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageIn order to overcome the limitation of poor mechanical properties, Kim et al [254] plotted a core/shell structure scaffold using the same device set-up. Alginate and collagen were printed coaxially with alginate providing required mechanical strength to the scaffold. In vitro tests proved that the core/shell scaffold had completely similar cellular behaviour to that of the pure collagen scaffold, with the added advantage of enhanced mechanical stability. In addition to in vitro, in vivo studies had been conducted on adult male BALB/c/Bkl-nu/nu mice. The plotted scaffold with co-cultured keratinocytes/fibroblasts, implanted as a dermal substitute on the wound area, provided good granulation tissue formation and rapid vascularization in the dermis and the scaffold. This technique can be used to co-extrude other natural or synthetic biopolymers along with collagen or even cell-laden bioink and expanded further with other growth factors and peptides in the core area to make functional skin substitutes. However, one disadvantage of this system is that the whole process takes place in a cryogenic environment unlike the other systems where the processes take place at room temperature.
5.4.4. Microfluidic approach based skin printer
Leng et al [255] from University of Toronto, developed a skin printer based on a microfluidic approach, which is widely accepted as a technique that will aid in vascularization of tissue engineered constructs. The experimental schematic is shown in figure 12. The device [256] consists of an annular gear pump, a microfluidic flow focussing device or a micro-fabricated cartridge and a collecting drum. The ECM used in this study is a mixture of alginate and collagen. The base layer, which is indicated in blue colour shown in figure 12, carried to the device exit via a set of parallel microchannels, a time-varying content of the bioink. Additional layers located above can contain a different cell suspension, like keratinocytes (though not used in this study), or focussing streams containing the cross linker can be printed, indicated by red colour streams in figure 12. There was a rotating drum downstream that collected the printed sheets, which in addition to the shear stress exerted by the focusing fluid, aids in continuous formation of hydrogel or bioink sheets, image based inspection and better collection. Human fibroblast cells were incorporated within a continuously extruded biopolymer sheet with precise spatio-temporal control over the cell location and cell seeding density (which is 1.94 million cells/mL in this study). Various shapes of biopolymer sheets like spots and parallel strips were printed, with fibroblasts seeded in to the structure. The patterns can also be hollow to promote vascularization while providing relevant thickness. In vivo testing was carried out on immunodeficient mice by performing excisional skin biopsy and replacing the excised skin with printed biopolymer sheets patterned with fibroblasts. Trichrome and keratin 14 staining on the wounded site revealed that the printed skin construct led to keratinization and better wound healing. The greatest advantage of this device is the shortest lead time for printing skin, which is reported to be 10 mm s−1, i.e. it will take only 48 min to print a 1 m2 skin construct.
Figure 12. Illustration of proposed skin printer for formation of cell-populated skin substitute in a microfabricated printer cartridge and application in vivo. The base layer, which is indicated in blue colour is carried to the device exit via a set of parallel microchannels. Additional layers located above can contain a different cell suspension or focussing streams containing the crosslinker can be printed, indicated by red colour streams [255]. Reproduced with permission from Chemical and Biological Microsystems Society.
Download figure:
Standard image High-resolution image5.4.5. In situ bioprinting of skin
Binder et al [257] from Wake Forest University developed a novel device for in situ bioprinting of skin. The criteria for developing this system includes: (i) portability; the system must be capable of being quickly transported to patients with extensive burn wounds, (ii) must accommodate a wide range of body types, (iii) capable of tailoring cell therapy to individual patient's specific needs, (iv) ease of sterilization, (v) allows for repeated treatments, and (vi) inexpensive and easy maintenance. The first two criteria were accomplished using a movable lightweight frame on which an XYZ plotting system was mounted. The dimensions of the system are large enough to cover the torso of an average patient but small enough to easily pass through most doorframes. During the treatment, the system is positioned over the patient's wound area and locked at the optimal position. The powerful locks prevent the frame movement while bioprinting, thus preserving both the mobility of the system and its delivery precision. A belt-driven plotting system with 100 μm precise movements aids the XYZ axes in precision delivery of cell suspension on to the patient's body. The movement of Z axis can be independently controlled, thereby allowing the delivery system to track with the curvature of patient's body and thus capable of accommodating many different body types. A laser based wound scanning system was coupled with the cartridge-based delivery system which helps in tailoring the cell therapy according to individual patient's need. The laser scanning system gathers length, width, and depth data about the wound; gathered data is processed to obtain a functional wound map and based on the wound map the software determines the exact cell composition and thickness of different areas of skin. The most important components of the device are cartridge-based delivery system, consisting of a number of nozzles, laser scanner for wound scanning, the mobile frame, and XYZ axes. In vivo experiments were performed on the porcine wound model, as porcine skin is similar to human skin. The bioprinting procedure was divided in to two stages. In the first stage, skin from the shoulder area of the pig was removed using a dermatome for preparation of fibroblasts and keratinocytes for bioprinting. These cells isolated were then cultured and mixed with biopolymers namely fibrin and type I collagen to make the bioink. After expanding these cells in culture, four full-thickness 10 cm × 10 cm wounds were excised through the panniculus carnosus layer of the thoracic dorsa on the animals. Prior to surgery, the print head, cartridges, and tubing were sterilized. No part of the printer was in direct contact with the animal at any point during the printing procedure. The wound area was placed under a portable bioprinter and dermal cells and matrices were directly bioprinted onto the wound bed. One layer of 10 million fibroblasts (1.0 × 105 cells cm−2) was bioprinted at 2 mm intervals between drops with thrombin sprayed from the atomizing nozzles simultaneously to form a fibrin/type I collagen hydrogel, followed by a 15 min break to allow complete conversion of fibrinogen to fibrin and finally another layer of 10 million keratinocytes was bioprinted above the fibroblast layer. Results were very much promising. Complete re-epithelialization could be witnessed at 8 weeks. Keratinocytes proliferated over the course of 8 weeks to completely cover the wound and their epithelium was found connected to the advancing wound edge. Pre-labelled autologous fibroblasts and keratinocytes as well as allogeneic fibroblasts were visible in the wound at 8 weeks, demonstrating that bioprinted skin cells can survive, proliferate, and form skin tissue in an immuno-competent large wound model. One limiting factor reported is that the performance of the laser imaging system is not up to the mark in vivo because of the breathing motion of the patient. This could be overcome by replacing the laser scanner with imaging system that can take instantaneous snapshots of the wound and create the wound map. Certain optimizations are also required to be made before using it for treating human wounds.
Sofokleous et al [258] worked on developing a portable handheld electrohydrodynamic (EHD) multi-needle spray gun for biomedical applications. The main component of the EHD spray gun is a multi-needle device assembly with 1–2 brass needles, a dremel glue gun used as the spray gun case heat-shrinkable tubing with adhesive inner liner, high current wire, and silicone tubes. The number of brass needles can range from one up to four, based on the application. The working principle of an EHD spraying/spinning of liquids is application of an electric force to the surface of a conducting liquid to produce fibers and such a system consists of a high voltage supply, which creates the required electric field between a charged capillary filled with the material to be processed in liquid state and a ground collector. When the electrostatic force overcomes the surface tension of the liquid at the capillary tip, a fine jet emanates from the nozzle tip. PLGA and Polymethysilsesquioxane (PMSQ) solutions were tested using the device. Simple, multicomponent and multifunctional products such as encapsulated fibres and particles having sub-micrometre to micrometre scale dimensions were successfully generated with the spray gun at different angles relative to a defined reference angle and deposited in a marked area of interest, proving its flexibility and portability. Though no cell laden bioink have been printed and tested, the spray gun technology can evolve in to a portable handheld in situ bioprinter in the future, facilitating ease of use by the clinicians.
The summary of all the skin bioprinting technologies are given in table 8.
Table 8. Summary of skin bioprinting technologies.
| Uniqueness | Cellular components | Size of the tissue construct | Cell viability | Resolution | Speed | In vivo animal model | In vivo results | Scalability | References | |
|---|---|---|---|---|---|---|---|---|---|---|
| Laser based bioprinting | First demonstration of 3D arrangement of vital cells by LaBP as multicellular grafts analogous to native archetype; the individual layers of the skin construct do not intermix with each other | FBs (Murine cell line NIH-3T3) and KCs (cell line HaCaT from adult human skin) embedded in collagen | 10 mm × 10 mm × 2 mm | Proved vitality of the printed cells 10 days after printing | Droplet volumes of 0.1–1 nL | — | — | — | Low | [248] |
| Possibility to position different cell types in an exact three-dimensional (3D) spatial pattern | NIH3T3 FBs and HaCaT KCs suspended in collagen | — | Normal cell viability | — | — | Male BALB/c-Nude mice | After 11 days, the borders of the skin constructs and the surrounding mouse skin were tightly grown together; neither inflammatory/necrotic processes nor contraction of the wounds could be detected; small blood vessels could be found. | Low | [249] | |
| FBs and KCs, along with hMSCs were printed using laser bioprinting due to their high potential in regeneration of human skin and new application possibilities of stem cell therapy | NIH3T3 FBs and HaCaT KCs, Bone marrow–derived hMSC (Bm-hMSCs), Adipose-derived hMSC | 9.6 mm × 9.6 mm | FBs and KCs showed exponential growth starting on day 1 up to day 6; continuously increasing proliferation of Bm-hMSC until day 4 after printing | Droplet sizes of 80 to 140 μm diameter | 1200 printed cell droplets per minute | — | — | Low | [250] | |
| Robotic dispensing | A method to create multi-layered engineered tissue composites; direct, on-demand fabrication of the 3D tissue composites on non-planar surfaces [250]; First study of printing both FB and KC in a single experiment with the formation of dermal/epidermal-like distinctive layers in a 3D hydrogel scaffold [251] | Primary adult human dermal FBs and primary adult human epidermal KCs, Type I collagen | — | There was no significant difference in cell viabilities between printed cells and manually plated cells as demonstrated in the viability assay | Droplet volumes of 7.6–10 nL | — | — | Medium | [251, 252] | |
| Cryogenic plotting | A novel system for the cryogenic plotting of 3D scaffolds developed to overcome the problems in fabricating highly hydrophilic natural polymers into a scaffold. | Normal human KCs and FBs; Type I collagen for fabricating porous scaffolds | 18.4 mm × 18.3 mm × 1.4 mm | Good cell migration and differentiation; stratum corneum was well expressed similar to the normal skin tissue | — | — | — | — | Low—Medium | [253] |
| Cryogenic plotting | The hybrid core/shell (collagen/alginate) scaffolds fabricated by cryogenic plotting, exhibited good structural stability, increased Young's modulus about seven times compared to pure collagen scaffold, and good cell viability. | Normal human KCs and FBs; Type I collagen and alginate for fabricating porous scaffolds | 10 mm × 10 mm × 2 mm | Cell proliferation of core/shell (collagen/alginate) scaffold was completely similar to that of the pure collagen scaffold | — | — | Adult male BALB/c/Bkl-nu/nu mice | Provided good granulation dermal tissue formation and rapid vascularisation; the epidermis was completely covered after 14 days. | Low—Medium | [254] |
| Microfluidic Skin printer | A scalable approach and uniquely addresses the need to provide skin substitutes at affordable prices that are easy to handle and apply, reduced wound recovery times; enables the continuous formation of cell-populated single-layer, bilayer, and vascularized sheets | Human FBs | up to 35 mm wide sheets | Normal cell viability | — | 10 mm s−1 equivalent to 1 sq.m in 48 min | Immunodeficient mice | Better wound healing and keratinization, suggesting an improved skin regeneration | High | [255, 256] |
| In situ skin printer | Fist demonstration of in situ skin printing on porcine wound model | FBs and KCs harvested from Porcine model suspended in fibrin and Type I collagen | 10 cm × 10 cm | — | — | — | Porcine wound model | Complete re-epithelialization could be witnessed at 8 weeks; KCs proliferated over the course of 8 weeks to completely cover the wound and their epithelium was found connected to the advancing wound edge. | Medium | [257] |
6. Challenges and future prospects
Having reviewed in detail the various processes of 3D bioprinting skin and its advantages, it is important to look at the realistic deployment potential of these technologies clinically. No technology is perfect and all of them suffers from certain limitations and pose challenges. 3D bioprinting is not an exception too. Current skin grafts suffer from the greatest limitations, in terms of long healing time and cost, which is reported to be over $50 000 to cover 40% burn area on an average male [66, 69, 259, 260]. Though 3D bioprinting has the potential to reduce the healing time considerably, the cost factor should be taken in to account. Affordability is the first challenge that is to be overcome. The next biggest challenge is to bioprint a fully functional skin substitute. Though most of the functions of the native skin can be mimicked in the bioprinted skin by using more types and number of cells in addition to keratinocytes and fibroblasts, like the melanocytes or Langerhans cells or even hair follicle cells, path to vascularization and innervation in engineered tissues still remains murky. Use of stem cells is one option but the ethical issues, cost and skill requirement hinders from utilising them to their full potential. Genetically modified keratinocytes, which overexpresses vascular endothelial growth factor (VEGF) were reported to promote vascularization [209] and it is worth trying to use them to see how well it can help the vascularization in bioprinted tissues, provided with all the other growth factors and tissue environment in a bioreactor. The neural crest stem cells are a multipotent cell population that can give rise to a diversity of neural and non-neural cell types in the adult, such as melanocytes, neurons, and glial cells of the sensory and autonomic ganglia of the peripheral nervous system, and some endocrine cells [215, 261]. All these enhancements will benefit the wound healing application, where the bioprinted skin substitute will be implanted on a patient's body or printed in situ, either way, the healthy part of the skin can provide nourishment to the grafted part. But, the drug testing and cosmetics testing remains a challenge as the skin substitutes will not be grafted on to humans but it requires a biomimetic skin substitute ex vivo, with all functionality of the native skin so that the drugs or cosmetics can be tested accurately. Application in personalized or precision medicine is another prospect which can be benefitted much with 3D bioprinting of skin. As a matter of fact, all the steps in bioprinting skin explained in this paper, right from imaging of wound site to the application, makes it a natural choice for personalized or precision medicine care. Another potential application is the study of melanoma and other types of skin cancer. In vitro 3D tumour models with engineered tumour microenvironment (TME) [262] are very helpful for studying the modus operandi of cancer cell proliferation, migration and metastasis. Different melanoma cell types or numbers could be printed in designated skin areas or layers and monitored for proliferation, motility, survival, and drug response [263]. Even before initiating the treatment process for a cancer patient, clinicians can bioprint cancer models to see how each patient's actual tumour responds to the drug and is there a possibility of metastasis. Such sophisticated 3D skin cancer models can be engineered with 3D bioprinting technology, for example, the recent 'metastasis-on-a-chip' platform [264, 265]. From a policy standpoint, standardization and government support for clinical implementation is the need of the hour. 3D bioprinting is thus far a promising technology but lacks coordinated efforts from multi-disciplinary teams. A collaborative approach, including experts from various domains including engineering, medicine, biomedical, and biochemistry will further strengthen its prospects and bolster its potential to be available quickly in serving the people and saving their lives.