Abstract
We explored appropriate technical setups for the detection of volatile organic compounds (VOCs) from exhaled cow breath by comparing six different polymer-based solid-phase extraction (SPE) cartridges currently on the market for gas chromatography/mass spectrometry (GC-MS) screening. Exhaled breath was sampled at a single timepoint from five lactating dairy cows using six different SPE cartridges (Bond Elut ENV (ENV); Chromabond HRX (HRX); Chromabond HRP (HRP); Chromabond HLB (HLB); Chromabond HR-XCW (XCW) and Chromabond HR-XAW (XAW)). The trapped VOCs were analyzed by dynamic headspace vacuum in-tube extraction GC-MS (DHS-V-ITEX-GC-MS). Depending on the SPE cartridge, we detected 1174–1312 VOCs per cartridge. Most VOCs were alkenes, alkanes, esters, ketones, alcohols, aldehydes, amines, nitriles, ethers, amides, carboxylic acids, alkynes, azoles, terpenes, pyridines, or sulfur-containing compounds. The six SPE cartridges differed in their specificity for the chemical compounds, with the XAW cartridge showing the best specificity for ketones. The greatest differences between the tested SPE cartridges appeared in the detection of specific VOCs. In total, 176 different VOCs were detected with a match factor >80%. The greatest number of specific VOCs was captured by XAW (149), followed by ENV (118), HLB (117), HRP (115), HRX (114), and XCW (114). We conclude that the tested SPE cartridges are suitable for VOC sampling from exhaled cow breath, but the SPE cartridge choice enormously affects the detected chemical groups and the number of detected VOCs. Therefore, an appropriate SPE adsorbent cartridge should be selected according to our proposed inclusion criteria. For targeted metabolomics approaches, the SPE cartridge choice depends on the VOCs or chemical compound groups of interest based on our provided VOC list. For untargeted approaches without information on the animals' metabolic condition, we suggest using multi-sorbent SPE cartridges or multiple cartridges per animal.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Exhaled breath sampling is a very new method in both human and animal research, but interest is growing in this low-invasive technique. Exhaled breath contains substantial information about the metabolism and health of an organism, as the volatile part of the metabolome (the volatilome) can be exhaled. The human volatilome consists of volatile organic compounds (VOCs) [1], a very heterogeneous group of organic substances, including carboxylic acids, alcohols, aldehydes, amides, esters, ketones, and terpenes, with molecular weights up to 400 Da and boiling points up to 250 °C [2]. To date, no standardized sampling procedure has been established for volatilome analysis, and the most appropriate technical setup has not been established [3–8]. Typically, three main sampling approaches are used: adsorbent cartridges, stainless steel canisters, and polymeric bags [9–11]. However, the compound detection may vary due to differences in portability, VOC background, sampling volumes, suitability for different compound groups and matrices, and VOC concentrations and stability during storage [9, 12]. VOCs are exhaled in breath at very low concentrations—in the ppt to ppb range [13], and due to their high volatility and the high humidity of exhaled breath from dairy cows [14], difficulties may arise during sampling and chemical analysis [9]. For example, stainless steel canisters and polymeric bags are limited in their storage volume and are susceptible to sample loss during storage until VOC analysis, leading to concentration problems [15]. Therefore, advantages can be gained through preconcentration methods that can prevent VOC losses during storage, separate the VOCs from the gaseous matrix of exhaled breath, and increase the VOC concentration to improve their detection [15]. One possibility is the use of Tenax TA thermal desorption tubes, which are already used for VOC sampling from human exhaled breath. The application of Tenax tubes for this purpose poses some problems, such as the very high cost per device and the limited storage time due to VOC losses [16]. In addition, sampling VOCs from humid gas, such as exhaled breath from dairy cows, leads to reduced recovery and reduces the ability of Tenax tubes to capture VOCs [14]. These problems can be circumvented by using polymer-based solid-phase extraction (SPE) cartridges, which are routinely used to preconcentrate VOC of interest from aqueous phases [17]. These allows a longer storage time due to the VOC extraction using solvents, are a cheaper and more efficient alternative for taking a large number of samples. In the case of VOCs, different cartridges may vary in their selectivity for different VOC chemical groups [18]; therefore, determining which SPE cartridge is appropriate for a particular VOC sampling application is important to ensure that the compound group or the specific VOC of interest will be detected with the highest specificity. To our knowledge, no performance evaluation has been published on polymer-based SPE cartridges for VOC sampling, especially from exhaled breath of ruminants. Therefore, the aim of the present study was to explore appropriate technical setups for the detection of VOCs from the exhaled breath of dairy cows by comparing six different polymer-based SPE adsorbent cartridges currently on the market for subsequent GC-MS screening. This provides the basis for selecting the optimal sample pre-treatment method for untargeted analysis of exhaled VOC.
2. Animals, materials, and methods
2.1. Animals
The experimental protocol complied with the Swiss animal welfare legislation and was approved by the Animal Care Committee of the Canton Fribourg, Fribourg, Switzerland (License No. 2021-38-FR). The experiment was conducted at the experimental farm of Agroscope (Posieux, Switzerland) and included five healthy, lactating (days in milk: 26–91 d; milk yield: 30.3–43.3 kg d−1), multiparous (2nd and 3rd lactation) Holstein Friesian dairy cows. The cows were housed together with 12 other cows not involved in this experiment in a free stall barn with a covered outdoor area. They were fed freshly cut herbage ad libitum and a concentrate mixture consisting of maize (45%), barley (16%), rapeseed cake (9%), oats (7%), and a mineral and vitamin premix. All cows had free access to fresh water.
2.2. SPE cartridges
Six different polymer-based SPE cartridges packed with different adsorbent materials (table 1) were tested. We used five SPE cartridges from Macherey-Nagel (HLB, HRP, HRX, XAW, XCW; Oensingen, Switzerland) and one from Agilent (ENV; Basel, Switzerland; ENV corresponds to the HLB SPE cartridge from Macherey-Nagel). The SPE cartridges were polymer-based, as polymer-based SPE cartridges are recommended for capturing both polar and nonpolar compounds [17, 19], and this seemed appropriate for the analysis of VOCs from exhaled breath. The comparability of the different SPE cartridges was ensured by confirming that all tested SPE cartridges had a column volume of 3 ml and an adsorbent weight of 200 mg. The air flow rate per minute through each SPE cartridge connected to the SPE sampling apparatus (figure 1(C)) was measured by connecting the vacuum pump used in this experiment (figure 1(D); Type HN 726.3FT.18, Neuberger, Balterswil, Switzerland) to the SPE sampling apparatus and a 5 l Tedlar bag (Sigma Aldrich, Buchs, Switzerland) at the end of each SPE cartridge to determine the air volume. Polymer-based SPE cartridges were chosen over silica-based SPE cartridges (i.e. C18 SPE) because silica gel cartridges are mainly recommended for capturing nonpolar and moderately polar compounds or for removing polar compounds, and this would have limited their applicability in studies on global volatile metabolomes [20]. In addition, silica gel cartridges are recommended for the extraction of liquid samples, where solvents can be used to counteract the drying action of the silica bed, which would negatively affect analyte retention [21]. These retention issues do not occur in a dry SPE bed [21], and because polymer-based SPE cartridges are recommended for capturing both polar and nonpolar compounds [17, 19], polymer-based SPE cartridges were considered more suitable for our purposes in the present study.
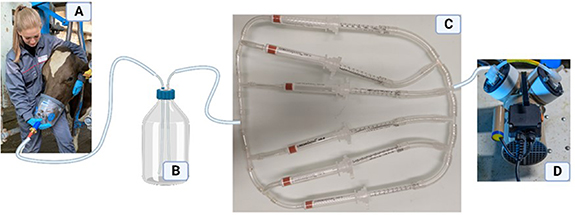
Figure 1. Technical setup for the sampling of exhaled breath from dairy cows. (A): tight-fitting face mask connected via silicone tubing to a vacuum pump; (B): bottle with internal standard solution; (C): adsorption cartridges for solid-phase extraction; (D): vacuum pump.
Download figure:
Standard image High-resolution imageTable 1. Different solid-phase extraction (SPE) cartridges used for the sampling of volatile organic compound (VOC) from dairy cows exhaled breath and their characteristics.
| SPE | Name | Surface chemistry | Mode | Particle size (μm) | Particle shape | Specific surface (m2 g−1) | Air flow rate | Recommended application for common use |
|---|---|---|---|---|---|---|---|---|
| ENV | Bond Elut ENV (ENV) | Polystyrene-divinylbenzene polymer | Reversed phase | 125 | Spherical | n.d. | 2.07 | Polar organic residues |
| HLB | Chromabond HLB (HLB) | Polystyrene-divinylbenzene polymer | Reversed phase | 60 | Spherical | 750 | 0.67 | Polar organic residues |
| HRP | Chromabond HRP (HRP) | Hydrophobic polystyrene-divinylbenzene copolymer | Reversed phase | 50–100 | Irregular | 1200 | 1.70 | Aromatic compounds, nitroaromatics from water, PAHs from oil, pesticides from water, and phenols from water |
| HRX | Chromabond HRX (HRX) | Hydrophobic spherical polystyrene-divinylbenzene copolymer | Reversed phase | 85 | Spherical | 1000 | 2.41 | Active ingredients from drugs from plasma/serum/urine, herbicides, PAHs from water, PCBs, pesticides, pharmaceuticals, and phenols |
| XAW | Chromabond HR-XAW (XAW) | Hydrophobic spherical polystyrene-divinylbenzene copolymer with secondary weak anion exchange activity | Mixed mode | 85 | Spherical | 850 | 0.96 | Acidic compounds, active ingredients from strongly matrix-contaminated materials, perfluorinated surfactants, and sulfonates |
| XCW | Chromabond HR-XCW (XCW) | Hydrophobic spherical polystyrene-divinylbenzene copolymer with weak cation exchange activity | Mixed mode | 85 | Spherical | 850 | 1.48 | Basic active ingredients from strongly matrix-contaminated samples, basic compounds, and quaternary amines |
SPE: solid-phase extraction; 'n.d.': not defined; reversed phase with non-polar surface; mixed mode (reversed phase and anion or cation exchange) with non-polar surface. a The air flow rate per minute through each SPE cartridge connected to the solid phase sampling apparatus (figure 1(C)) was measured by connecting the vacuum pump used in this experiment (figure 1(D); Type HN 726.3FT.18, Neuberger, Balterswil, Switzerland) to the SPE sampling apparatus and a 5 l Tedlar bag (Sigma Aldrich, Buchs, Switzerland) at the end of each SPE cartridge to determine the air volume.
2.3. VOC sampling from exhaled breath
Breath samples were collected from all cows between 0530 h and 0630 h on one day in October 2022 (temperature: 10.5 °C; relative air humidity: 99%; wind speed: 1.9 km h−1 [prevision meteo.ch]), immediately after morning milking and before morning feeding. For sampling, each cow was moved sequentially to a head gate located in the covered outdoor area of the barn. Exhaled breath was sampled using a tight-fitting face mask (Air One, Hippomed/Neu-Tec GmbH, Steinhagen, Germany; figure 1(A)), which was manually held over the nostrils and mouth of each cow for 4 min by experienced personnel, as described by Küntzel et al [3]. The face mask was connected by a silicone tube to a vacuum pump (figure 1(D); type HN 726.3 FT.18, Neuberger, Balterswil, Switzerland) to transport exhaled breath from the face mask (figure 1(A)) into the internal standard bottle (figure 1(B)), which contained 1 ml of an internal deuterated standard solution (100 ppb dimethylsulfide-d6, 10 ppb dimethylsulfoxide-d6 in acetonitrile) and then simultaneously through the six different SPE cartridges (table 1) connected by silicone tubes with identical tubing length (SPE sampling apparatus; figure 1(C)). To sample the next cow, the face mask was rinsed with water and dried with a paper towel before the SPE cartridges were replaced with six new cartridges that were rotated in their position within the SPE sampling apparatus.
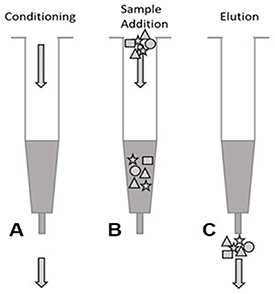
Prior to sampling, the 30 cartridges used (6 per cow) were prepared as follows. Residual components were removed, and reproducible interactions with the analytes were ensured by conditioning the SPE cartridges (figure 2(A)) by repeated rinsing with 3 × 3 ml of four solvents in the following order: NanopureTM water, methanol, acetone, and acetonitrile. The cartridges were then dried under nitrogen for 20 min. During exhaled breath sampling, the VOCs were captured on the highly porous surface of the SPE material (figure 2(B)). Within 2 h after sampling, the SPE cartridges were dried under nitrogen flow for 3 min, and 600 μl of acetonitrile was added to elute the captured VOCs from the SPE polymer (figure 2(C)). After 5 min, the VOCs dissolved in acetonitrile were flushed out with ambient laboratory air using a 20 ml air syringe and the eluents were stored in Eppendorf tubes at −40 °C until the VOC analyses.
Figure 2. Three-step procedure for solid-phase extraction (SPE) using SPE cartridges: (A): conditioning; (B): sample addition; (C): elution.
Download figure:
Standard image High-resolution image2.4. Sample preparation and VOC analysis
Samples for VOC analyses were thawed on ice for 1 h at room temperature. Quality control (QC) samples were prepared by mixing 10 μl of all samples and distributing the mixture in equivalent volumes into Eppendorf tubes [22]. Then, 100 μl of each sample (including the QC samples), were transferred to a 20 ml headspace vial and hermetically sealed with a silicone and Teflon septum (Macherey-Nagel AG, Switzerland). The vials were placed on a tray cooler at 4 °C and were analyzed immediately. The sample order was randomized using the RAND function in Excel to avoid systematic bias. Each batch started with two ambient laboratory air blanks, followed by three QC samples, and a QC sample was injected after every tenth sample. Untargeted analyses of VOCs were performed using dynamic headspace vacuum in-tube extraction (DHS-V-ITEX, CTC Analytics, Zwingen, Switzerland) gas chromatography-mass spectrometry (DHS-V-ITEX-GS-MS) based on the vacuum transfer in-tube extraction (DHS-VTT) protocol developed by Fuchsmann et al [23]. The DHS-V-ITEX-GC-MS instrument consisted of an MPS2 autosampler (Gerstel, Sursee, Switzerland) and an Agilent 7890B GC system coupled to an Agilent 5977B mass selective detector (Agilent Technology, Santa Clara, CA, USA). After 10 min incubation at 60 °C, the headspace of each sample was extracted for 10 min at 60 °C under vacuum (5 mbar) using a vacuum pump (Buchi V-300, Büchi, Flawil, Switzerland) and in-tube extraction materials equipped with a trap filled with Tenax TA (2/3 bottom)/Carbosieve S III (1/3 top) (ITEX2, Brechbühler, Switzerland) according to Fuchsmann et al [23, 24].
The VOCs were desorbed from the sorbent in the injector for 2 min at 300 °C under a nitrogen flow of 150–180 ml min−1. The injector was equipped with a glass liner filled with Tenax TA, conditioned at 250 °C for 60 min. After injection, the injector was heated to 250 °C at a rate of 12 °C s−1. The purge flow to the split vent was set at 100 ml min−1 after 2 min. The VOCs were separated on an Optima-5-MS fused silica capillary column (5% diphenyl - 95% dimethylpolysiloxane with low bleeding, 50 m × 0.20 mm × 0.5 μm film; Macherey-Nagel AG, Oensingen, Switzerland) with hydrogen as the carrier gas at a flow of 1 ml min−1 (corresponding to a velocity of 41 cm s−1). The oven temperature was programed as follows: 6 min at 40 °C, then heated to 280 °C at a rate of 10 °C min−1. The MS settings were as follows: transfer line at 230 °C, source temperature at 230 °C. The analytes were monitored in SCAN mode between 42.5 and 350 amu with a gain at 1 with a solvent delay of 5 min. The autosampler was controlled with the Cycle Composer V. 1.5.4 software (CTC Analytics, Zwingen, Switzerland) and the CIS 4 injector with Maestro1 software V.1.4.8.14/3.5 (Gerstel). The resulting analysis is semiquantitative; therefore, the VOC concentrations reported in the text refer to relative concentrations determined from the peak area of the VOCs (arbitrary unit).
2.5. Data processing and VOC identification
The MS signals were deconvoluted using the Masshunter Profinder software (version 10.0) in recursive mode (Agilent Technologies, Santa Clara, CA, USA). Missing values after automatic deconvolution due to signals below the detection limit were replaced by zero values, following Xia et al [25]. Manual peak integration was performed using MassHunter Quantitative Analysis software (version 12.1; Agilent Technologies, Santa Clara, CA, USA). The VOCs were identified according to the standard criteria for identification levels (1–4), as recommended by the metabolomics standards initiative: at level 1, metabolites are identified by comparing the resulting spectrum with the database (minimum match factor of 90%) and the calculated retention index (RI) with the reference RI (maximum relative difference of about ±10–15). Level 2 corresponds to spectra with a match factor >80% and a maximum relative difference in the calculated RI of ±15 of the reference RI. At level 3, metabolites are assigned to their compound classes based on their similar putative attributes with the compounds in a reference library. Level 4 corresponds to unknown compounds with a calculated RI > ±15 of the RI [26, 27]. The RI was calculated using the temperature-programmed Kovats index [28]. The following peak identification strategies were performed using the National Institute of Standards and Technology NIST/EPA/NIH mass spectral library (NIST17) (NIST, Gaithersburg, MD, USA):
- (i)To determine the number of VOCs captured by the SPE cartridges belonging to different chemical compound groups, VOCs were identified at least at level 3 (hereafter referred to as level 3 VOCs).
- (ii)For the determination of specific VOCs captured by the SPE cartridges used, both level 2 identified VOCs, tentatively identified VOCs (match factor >80%; reference RI not defined in reference databases), and level 4 VOCs were considered. The tentatively identified and unknown VOCs were further specified by their MS spectra.
3. Results and discussion
In this study, we compared the adsorption capabilities of six different SPE cartridges containing different polymer-based adsorbent materials for VOC contained in the exhaled breath of dairy cows. To the best of our knowledge, this is the first study of its kind.
3.1. Comparative analysis of the SPE cartridges
The average VOC numbers differed only slightly depending on the type of SPE cartridge used (table 2). The XAW SPE cartridges detected the highest average compound numbers, followed by HLB, XCW, HRX, ENV, and HRP. We used only polymer-based SPE cartridges, each with a column volume of 3 ml and an adsorbent weight of 200 mg to sample exhaled breath for 4 min. All SPE were used in parallel, resulting in a total air flow rate of 9.29 l min−1. In addition, we applied a uniform solvent for elution after VOC sampling. Therefore, the strongest effects on the specificity for VOC detection would arise from the adsorbent material, together with the SPE mode, chemical bond types, particle size, and shape. Table 1 shows that the differences in the number of VOCs detected are due to the SPE mode and their chemical bond type but not due to the difference in flow rate or specific surface area of the SPE.
Table 2. The total number of GC-MS peaks in exhaled breath from five dairy cows using six different solid-phase extraction (SPE) cartridges.
| Solid-phase extraction (SPE) cartridges | ||||||
|---|---|---|---|---|---|---|
| ENV | HRP | HLB | HRX | XAW | XCW | |
| Number of VOCs detected | 1216 ± 49.1 | 1174 ± 40.9 | 1242 ± 32.2 | 1227 ± 39.4 | 1312 ± 83.9 | 1229 ± 75.1 |
Total number of GC-MS peaks per cartridge observed after sampling exhaled breath from five dairy cows (shown as mean ± SD). ENV: Bond Elut ENV (polystyrene-divinylbenzene polymer); HRX: Chromabond HRX (hydrophobic spherical polystyrene-divinylbenzene copolymer); HRP: Chromabond HRP (hydrophobic polystyrene-divinylbenzene copolymer); HLB: Chromabond HLB (hydrophilic-lipophilic balanced N-vinylpyrrolidone-divinylbenzene); XCW: Chromabond HR-XCW (hydrophobic spherical polystyrene-divinylbenzene copolymer); XAW: Chromabond HR-XAW (hydrophobic spherical polystyrene-divinylbenzene copolymer with secondary weak anion exchange); XCW: Chromabond HR-XCW (hydrophobic spherical polystyrene-divinylbenzene copolymer with weak cation exchange).
For all six SPE cartridge types, the level 3 VOCs detected were those from the chemical classes (in decreasing order of VOC numbers detected) of alkenes, followed by alkanes, esters, ketones, alcohols, aldehydes, amines, nitriles, ethers, amides, carboxylic acids, alkynes, azoles, terpenes, pyridines, and sulfur-containing compounds (table 3). These chemical compound groups were detectable in the exhaled breath of all five cows and with each SPE cartridge used. The SPE cartridges can generally be classified into normal phase, reversed phase, ion exchange (anion and cation-exchange), and mixed-mode (combination of reversed phase and ion exchange) [29]. All SPE cartridges used in our study had reversed phase functionality. The wide range of detection of acidic, basic, and neutral compounds results from both the hydrophobic and hydrophilic parts of the SPE adsorbent materials used, which impart an amphiphilic character [19]. The hydrophilic character is induced by polar functional groups, such as polystyrene or polyvinylpyrrolidone, that contribute to the interaction with the polar functional groups of the VOCs, while the hydrophobic divinylbenzene part allows π–π interactions with the aromatic functional groups of VOCs [19, 30]. Therefore, all SPE cartridges were able to detect all chemical compound groups. In addition to the reversed phase character, the XAW and XCW cartridges had a weak anion and cation exchange, respectively, giving them a mixed-mode character.
Table 3. Volatile organic compounds (VOCs) in various chemical compound classes identified at level three after sampling from exhaled breath of five dairy cows using six different solid-phase extraction (SPE) cartridges (presented as mean ± standard deviation).
| Solid-phase extraction cartridges | ||||||
|---|---|---|---|---|---|---|
| Chemical compound group | ENV | HRP | HLB | HRX | XAW | XCW |
| Aldehydes | 10.40 ± 4.34 | 10.20 ± 2.77 | 12.60 ± 2.88 | 13.00 ± 3.39 | 9.80 ± 3.56 | 9.60 ± 2.30 |
| Alcohols | 23.20 ± 4.32 | 14.40 ± 2.70 | 18.60 ± 4.88 | 17.40 ± 4.16 | 9.80 ± 3.05 | 21.20 ± 3.83 |
| Alkanes | 63.80 ± 7.66 | 68.60 ± 6.35 | 64.40 ± 4.10 | 64.20 ± 5.63 | 65.20 ± 5.40 | 61.80 ± 7.33 |
| Alkenes | 85.20 ± 10.85 | 86.80 ± 5.40 | 77.40 ± 2.61 | 82.80 ± 4.15 | 83.40 ± 8.73 | 82.80 ± 4.97 |
| Alkynes | 3.40 ± 1.34 | 2.20 ± 1.10 | 4.00 ± 1.22 | 4.20 ± 1.30 | 4.00 ± 1.00 | 4.00 ± 1.22 |
| Azoles | 2.40 ± 1.67 | 4.20 ± 1.79 | 3.20 ± 1.30 | 2.60 ± 1.82 | 2.80 ± 0.84 | 2.20 ± 0.84 |
| Amides | 3.20 ± 2.28 | 4.40 ± 1.82 | 5.60 ± 1.52 | 3.60 ± 1.14 | 5.60 ± 2.70 | 3.60 ± 1.95 |
| Amines | 8.20 ± 3.90 | 5.60 ± 2.70 | 7.20 ± 2.39 | 9.20 ± 4.71 | 8.80 ± 4.38 | 7.40 ± 2.70 |
| Carboxylic acids | 4.60 ± 0.55 | 3.60 ± 1.14 | 4.80 ± 2.17 | 4.60 ± 1.14 | 3.20 ± 1.30 | 3.60 ± 1.52 |
| Esters | 27.60 ± 4.39 | 28.40 ± 5.50 | 36.40 ± 5.73 | 34.40 ± 3.71 | 32.20 ± 8.17 | 29.20 ± 2.68 |
| Ethers | 6.20 ± 1.79 | 4.80 ± 0.84 | 5.00 ± 1.22 | 5.60 ± 1.67 | 4.60 ± 1.82 | 5.60 ± 1.52 |
| Ketones | 21.20 ± 3.63 | 19.20 ± 3.27 | 23.60 ± 3.29 | 23.80 ± 2.77 | 31.40 ± 11.26 | 24.20 ± 4.21 |
| Nitriles | 7.20 ± 2.68 | 5.40 ± 2.19 | 6.80 ± 1.92 | 8.00 ± 2.35 | 6.40 ± 3.78 | 7.60 ± 1.82 |
| Pyridines | 1.00 ± 1.00 | 1.40 ± 1.14 | 2.80 ± 1.48 | 1.00 ± 1.41 | 1.00 ± 1.22 | 1.40 ± 1.34 |
| Sulfur compounds | 0.80 ± 0.84 | 0.80 ± 0.45 | 1.80 ± 0.84 | 2.60 ± 1.14 | 0.60 ± 0.89 | 2.00 ± 1.22 |
| Terpenes | 1.60 ± 0.55 | 2.20 ± 1.10 | 1.00 ± 0.71 | 2.60 ± 1.67 | 2.20 ± 0.45 | 2.00 ± 0.71 |
| Others | 19.40 ± 4.22 | 16.20 ± 3.11 | 16.60 ± 3.44 | 18.00 ± 5.10 | 19.40 ± 5.18 | 20.20 ± 2.17 |
Compound identification (match factor >50%) using the National Institute of Standards and Technology NIST/EPA/NIH mass spectral library (NIST17). Number of volatile organic compounds (VOCs) within chemical groups shown as mean ± standard deviation (SD) from sampling of five cows per SPE cartridge used. ENV: Bond Elut ENV (polystyrene-divinylbenzene polymer); HRX: Chromabond HRX (hydrophobic spherical polystyrene-divinylbenzene copolymer); HRP: Chromabond HRP (hydrophobic polystyrene-divinylbenzene-copolymer); HLB: Chromabond HLB (hydrophilic-lipophilic balanced N-vinylpyrrolidone-divinylbenzene); XCW: Chromabond HR-XCW (hydrophobic spherical polystyrene-divinylbenzene copolymer); XAW: Chromabond HR-XAW (hydrophobic spherical polystyrene-divinylbenzene copolymer with secondary weak anion exchange); XCW: Chromabond HR-XCW (hydrophobic spherical polystyrene-divinylbenzene copolymer with weak cation exchange).
The number of level 3 VOCs within a particular chemical compound group differed between the SPE cartridges used (table 3). The greatest difference between the SPE cartridges was noted for the association with ketones, where the highest numbers of VOCs were detected using XAW. The XAW adsorbent cartridge has a mixed-mode character, which imparts a higher efficiency for binding VOCs from complex matrices compared with cartridges with only a reversed phase character [29]. Mixed-mode cartridges can also form ionic interactions (anion or cation exchange). In particular, the weak anion exchanging capacity of the XAW adsorbent cartridge allows interactions between the positively charged groups on the SPE adsorbent cartridge and the negatively ionized or ionizable groups of the VOCs (anionic parts) [19]. The higher sensitivity of XAW for capturing ketones may be due to the enhanced formation of anionic enols within the keto-enol tautomerism, reinforced by the alkalinity of bovine saliva (pH 8.55–8.90 [31]) within the humid matrix of the exhaled breath [32] (>95% relative humidity) [33]. Due to their lower charge compared to carboxylic acids, ketones can better elute from the surface of the SPE adsorbent material before reverting to the amplified keto form, which is enhanced by the neutral pH of the acetonitrile elution solvent. With the XAW cartridge, a greater number of carboxylic acids, which form stronger ionic interactions, could be detectable by lowering the pH during the elution process.
The use of the XCW cartridge, which has an additional weak cation exchange section, did not improve the detection of basic chemical compounds. Further investigation of the effect of elution pH on the detection of different compound groups is required, as strongly acidic or basic compounds might only be eluted by changing the pH. Other moderate differences between the detection of VOCs from specific chemical compound groups were observed using the ENV polymer, which showed the best specificity for alcoholic compounds, while HRP was best for alkanes, alkenes, and azoles; HLB was best for esters; and HRX was best for aldehydes. Alkanes and alkenes are not typically produced by physiological processes involving the organism's metabolism, but are environmental pollutants originating from vehicle exhaust, gasoline evaporation, biomass burning, the use of volatile chemical products (solvents, paints, pesticides, detergents, personal care products, etc) and vegetation emissions, and can also be contaminants in plants used as animal feed [34]. Very small differences were found between the different SPE cartridges for the detection of amides, amines, alkynes, carboxylic acids, ethers, nitriles, pyridines, sulfur-containing compounds, and terpenes.
In total, 176 specific VOCs were detected from the physiologically relevant compound groups of aldehydes, alcohols, azoles, amides, amines, carboxylic acids, esters, ethers, ketones, nitriles, pyridines, sulfur-containing compounds and terpenes (table 4). Differences in the detection of these VOCs were observed between the five cows and between the SPE cartridges used. This could reflect animal-specific metabolism. The largest number of specific VOCs was captured with the XAW cartridge (149), followed by ENV (118), HLB (117), HRP (115), HRX (114), and XCW (114). The different numbers indicate some differences in the detected VOCs among the six SPE cartridges used, indicating their different specificity for particular VOCs. More insight into the differences is provided by the chromatograms, which differed according to the SPE adsorbent cartridge in terms of the peaks detected (figure 3). The main differences, with more peaks detected, occurred at a retention time (RT) between 8 and 9 min with the XAW cartridge; RT 10–12 min with XAW and XCW; RT 18–19 min with HLB and XAW; RT 23–24 min and 26–27 min with HRP and XAW.
Figure 3. Comparison of chromatograms of exhaled breath from one dairy cow obtained using six different solid-phase extraction (SPE) cartridges. Differences in the peaks detected between the different chromatograms highlighted with black circles. ENV: Bond Elut ENV (polystyrene-divinylbenzene polymer); HRX: Chromabond HRX (hydrophobic spherical polystyrene-divinylbenzene copolymer); HRP: Chromabond HRP (hydrophobic polystyrene-divinylbenzene-copolymer); HLB: Chromabond HLB (hydrophilic-lipophilic balanced N-vinylpyrrolidone-divinylbenzene); XCW: Chromabond HR-XCW (hydrophobic spherical polystyrene-divinylbenzene copolymer); XAW: Chromabond HR-XAW (hydrophobic spherical polystyrene-divinylbenzene copolymer with secondary weak anion exchange); XCW: Chromabond HR-XCW (hydrophobic spherical polystyrene-divinylbenzene copolymer with weak cation exchange).
Download figure:
Standard image High-resolution imageTable 4. Volatile organic compounds (VOCs) from the physiologically relevant chemical groups (aldehydes, alcohols, azoles, amides, amines, carboxylic acids, esters, ethers, ketones, nitriles, pyridines, sulfur containing compounds and terpenes) detected using six different solid phase extraction (SPE) cartridges (highlighted in brackets with the number of cows, where these VOCs were detected) after sampling from exhaled breath of five dairy cows.
| SPE cartridge | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volatile organic compounds | BM | RT (min) | CAS number | m/z | Level | Match factor | RI calc | RI ref | ENV | HRX | HRP | HLB | XAW | XCW |
| 2-Butenal | BM-001 | 5.853 | 4170-30-3 | 70 | 1 | 90.2 | 665 | 657 | (5) | |||||
| 2-Pentyn-4-one | BM-002 | 6.009 | 7299-55-0 | 82 | 2 | 81.5 | 669 | 672 | (5) | (5) | (5) | (5) | (5) | |
| Pyridine, 2-nitro-t | BM-003 | 6.302 | 15009-91-3 | 78 | 3 | 83.8 | 675 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| 4,6-Heptadiyn-3-onet | BM-004 | 6.314 | 29743-27-9 | 78 | 3 | 80.8 | 676 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-005 | 6.669 | — | 57 | 4 | — | 683 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-006 | 6.688 | — | 78 | 4 | — | 684 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| 2-Propanol, 1-methoxy- | BM-007 | 6.705 | 107-98-2 | 75 | 2 | 89 | 684 | 672 | (5) | (5) | (5) | (5) | (5) | (5) |
| 4-Penten-2-ol | BM-008 | 6.949 | 625-31-0 | 71 | 2 | 83.3 | 690 | 688 | (5) | (5) | (5) | (5) | (5) | (5) |
| Acetoin | BM-009 | 6.978 | 513-86-0 | 88 | 2 | 85.2 | 690 | 700 | (1) | (1) | ||||
| Unknown | BM-010 | 7.482 | — | 78 | 4 | — | 702 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-011 | 7.869 | 498-60-2 | 90 | 4 | — | 715 | — | (1) | |||||
| 2,4-Dimethylfuran | BM-012 | 7.883 | 3710-43-8 | 96 | 2 | 89 | 716 | 703 | (5) | (5) | (5) | (5) | (5) | (5) |
| 3,4-Dimethylfuran | BM-013 | 7.889 | 20843-07-6 | 96 | 2 | 86.5 | 716 | 724 | (5) | (5) | (5) | (5) | (5) | (5) |
| Pyrazine | BM-014 | 8.488 | 290-37-9 | 80 | 1 | 99.4 | 736 | 740 | (5) | |||||
| 3-Penten-1-ol, (Z)- | BM-015 | 8.508 | 764-38-5 | 68 | 2 | 86.9 | 737 | 725 | (5) | (5) | (5) | (5) | (5) | (5) |
| 3-Buten-1-ol, 3-methyl- | BM-016 | 8.511 | 763-32-6 | 86 | 2 | 85.8 | 737 | 734 | (5) | (5) | ||||
| 3-Penten-2-one | BM-017 | 8.580 | 625-33-2 | 84 | 2 | 85.1 | 739 | 739 | (5) | |||||
| Unknown | BM-018 | 8.605 | — | 84 | 4 | — | 740 | — | (5) | |||||
| Unknown | BM-019 | 8.686 | — | 70 | 4 | — | 743 | — | (5) | (5) | ||||
| Unknown | BM-020 | 8.723 | — | 86 | 4 | — | 744 | — | (5) | |||||
| Unknown | BM-021 | 8.972 | — | 98 | 4 | — | 752 | — | (5) | |||||
| 1,3,5-Cycloheptatriene | BM-022 | 9.743 | n.d. | 61 | 2 | 87.6 | 772 | 765 | (5) | (5) | (5) | (5) | (5) | (5) |
| 2-Butenal, 3-methyl- | BM-023 | 9.946 | 107-86-8 | 84 | 1 | 90 | 785 | 783 | (5) | |||||
| Unknown | BM-024 | 9.958 | — | 91 | 4 | — | 786 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-025 | 10.279 | — | 100 | 4 | — | 797 | — | (5) | |||||
| Hexanal | BM-026 | 10.321 | 66-25-1 | 82 | 1 | 90.1 | 798 | 802 | (5) | |||||
| 4-Pentenal,2,2-dimethyl-t | BM-027 | 10.339 | 5497-67-6 | 83 | 3 | 83.3 | 799 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| 1-Hexyn-3-olt | BM-028 | 10.346 | 105-31-7 | 83 | 3 | 83.7 | 799 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-029 | 10.348 | — | 84 | 4 | — | 799 | — | (5) | |||||
| 3.5-Dimethyl-1.6-heptadien-4-olt | BM-030 | 10.425 | 19549-66-7 | 85 | 3 | 84 | 802 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| Hydroperoxide, hexylt | BM-031 | 10.438 | 4312-76-9 | 85 | 3 | 90.2 | 802 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| Acetic acid, butyl ester | BM-032 | 10.753 | 123-86-4 | 73 | 2 | 87.8 | 815 | 812 | (5) | (5) | (5) | (5) | (5) | |
| Unknown | BM-033 | 10.919 | — | 104 | 4 | — | 822 | — | (1) | |||||
| Furfural | BM-034 | 11.235 | 98-01-1 | 95 | 2 | 81.4 | 834 | 835 | (5) | (5) | ||||
| Unknown | BM-035 | 11.359 | — | 129 | 4 | — | 839 | — | (1) | |||||
| 3-Hexen-2-one | BM-036 | 11.413 | 763-93-9 | 83 | 1 | 90.3 | 842 | 845 | (5) | (5) | ||||
| 2-Pentanone, 4-hydroxy-4-methyl- | BM-037 | 11.427 | 123-42-2 | 83 | 1 | 90 | 842 | 841 | (5) | (5) | ||||
| 2-Pentanone, 3-methylenet | BM-038 | 11.436 | 4359-77-7 | 98 | 3 | 90.1 | 842 | n.d. | (5) | (5) | ||||
| Unknown | BM-039 | 11.863 | — | 63 | 4 | — | 860 | — | (5) | (5) | ||||
| Unknown | BM-040 | 11.897 | — | 106 | 4 | — | 861 | — | (1) | |||||
| Unknown | BM-041 | 11.997 | — | 91 | 4 | — | 865 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-042 | 12.034 | — | 106 | 4 | — | 867 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| 1-Methoxy-2-propyl acetate | BM-043 | 12.103 | 108-65-6 | 72 | 1 | 91.6 | 869 | 870 | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-044 | 12.116 | — | 93 | 4 | — | 870 | — | (2) | |||||
| Unknown | BM-045 | 12.217 | — | 106 | 4 | — | 874 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| 3-Heptanone | BM-046 | 12.520 | 106-35-4 | 85 | 2 | 87.2 | 886 | 887 | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-047 | 12.623 | — | 103 | 4 | — | 890 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Butanedioic acid, phenyl-t | BM-048 | 12.710 | 635-51-8 | 104 | 3 | 89.2 | 894 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| Fumaronitrile | BM-049 | 12.717 | 764-42-1 | 78 | 2 | 81.3 | 894 | 917 | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-050 | 12.794 | — | 103 | 4 | — | 897 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Heptanal | BM-051 | 12.846 | 111-71-7 | 81 | 2 | 86.6 | 899 | 901 | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-052 | 12.847 | — | 106 | 4 | — | 899 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-053 | 12.848 | — | 106 | 4 | — | 899 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-054 | 12.851 | — | 105 | 4 | — | 899 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-055 | 12.852 | — | 106 | 4 | — | 899 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-056 | 12.885 | — | 91 | 4 | — | 901 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Ethanol, 2-butoxy- | BM-057 | 13.032 | 111-76-2 | 87 | 2 | 88.7 | 908 | 909 | (5) | |||||
| Unknown | BM-058 | 13.036 | — | 79 | 4 | — | 908 | — | (1) | |||||
| 2,4-Hexadienal, (E,E)- | BM-059 | 13.047 | 142-83-6 | 81 | 2 | 83.1 | 909 | 911 | (5) | |||||
| Unknown | BM-060 | 13.103 | — | 118 | 3 | — | 911 | — | (1) | |||||
| Dimethyl sulfone | BM-061 | 13.132 | 67-71-0 | 79 | 1 | 92.3 | 913 | 919 | (5) | (5) | (5) | (5) | (5) | |
| Unknown | BM-062 | 13.716 | — | 110 | 4 | — | 941 | — | (5) | |||||
| Unknown | BM-063 | 14.207 | — | 106 | 4 | — | 965 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Benzaldehyde | BM-064 | 14.209 | 100-52-7 | 77 | 1 | 96.7 | 965 | 970 | (5) | (5) | (5) | (5) | (5) | (5) |
| Benzenamine, 4,4-(1,2-ethanediyl)bis-t | BM-065 | 14.216 | 621-95-4 | 106 | 3 | 80.3 | 965 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| 1-((2-Pyridinylcarbonyl)oxy)-2,5-pyrrolidinedionet | BM-066 | 14.220 | n.d. | 78 | 3 | 81.3 | 965 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-067 | 14.574 | — | 75 | 4 | — | 982 | — | (1) | |||||
| 1,1-Carbonyldiimidazolet | BM-068 | 14.625 | 530-62-1 | 68 | 3 | 81.2 | 985 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| 5-Hepten-2-one, 6-methyl- | BM-069 | 14.644 | 110-93-0 | 108 | 2 | 82.8 | 986 | 988 | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-070 | 14.705 | — | 86 | 4 | — | 989 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| 1,2-Ethanediol, diacetate | BM-071 | 14.706 | 111-55-7 | 86 | 2 | 81.7 | 989 | 993 | (5) | (5) | (5) | (5) | (5) | (5) |
| Sorbic acid vinyl ester | BM-072 | 14.771 | 42739-26-4 | 95 | 2 | 83.2 | 992 | 990 | (5) | |||||
| Unknown | BM-073 | 14.779 | — | 95 | 4 | — | 992 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-074 | 14.878 | — | 118 | 4 | — | 997 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| 2-Cyclopenten-1-one, 3,4-dimethyl- | BM-075 | 14.905 | 30434-64-1 | 95 | 2 | 81 | 998 | 986 | (2) | |||||
| Octanal | BM-076 | 14.964 | 124-13-0 | 81 | 1 | 91.5 | 1001 | 1000 | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-077 | 14.992 | — | 119 | 4 | — | 1003 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| β-Methoxyethoxymethyl chloridet | BM-078 | 15.138 | 3970-21-6 | 89 | 3 | 80.4 | 1011 | n.d. | (1) | |||||
| 2(5H)-Furanone, 3,5,5-trimethyl-t | BM-079 | 15.134 | 50598-50-0 | 111 | 3 | 88.1 | 1011 | n.d. | (5) | (5) | (5) | (5) | ||
| 3-Carene | BM-080 | 15.289 | 13466-78-9 | 93 | 2 | 85.3 | 1019 | 1013 | (5) | (5) | (5) | (5) | (5) | (5) |
| o-Cymene | BM-081 | 15.440 | 527-84-4 | 119 | 2 | 85.5 | 1028 | 1028 | (5) | (5) | (5) | (5) | (5) | (5) |
| p-Cymene | BM-082 | 15.440 | 99-87-6 | 119 | 1 | 91.1 | 1028 | 1027 | (5) | (5) | (5) | (5) | (5) | (5) |
| 2-Cyclohexen-1-one, 4,5-dimethyl- | BM-083 | 15.457 | 5715-25-3 | 124 | 2 | 85.5 | 1029 | 1016 | (5) | |||||
| 1-Hexanol, 2-ethyl- | BM-084 | 15.491 | 104-76-7 | 112 | 2 | 89.4 | 1031 | 1019 | (5) | (5) | (5) | (5) | (5) | (5) |
| 2-Ethyl-1-hexanol, trifluoroacetate | BM-085 | 15.492 | 53 800-08-1 | 112 | 2 | 85.1 | 1031 | 1019 | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-086 | 15.496 | — | 120 | 4 | — | 1031 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-087 | 15.522 | — | 120 | 4 | — | 1031 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-088 | 15.517 | — | 120 | 4 | — | 1032 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| 4-Pentenal, 2-ethyl- | BM-089 | 15.522 | 5204-80-8 | 57 | 2 | 85.1 | 1032 | 1034 | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-090 | 15.536 | — | 105 | 4 | — | 1033 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| D-Limonene | BM-091 | 15.615 | 5989-27-5 | 68 | 1 | 91.9 | 1038 | 1031 | (5) | (5) | (5) | (5) | (5) | (5) |
| Limonene | BM-092 | 15.619 | 138-86-3 | 68 | 2 | 89.9 | 1038 | 1036 | (5) | (5) | (5) | (5) | (5) | (5) |
| Benzyl alcohol | BM-093 | 15.658 | 100-51-6 | 108 | 2 | 84.1 | 1040 | 1036 | (5) | (5) | (5) | (5) | (5) | (5) |
| cis-3-Hydroxy-dl-prolinet | BM-094 | 15.674 | 4298-05-9 | 86 | 3 | 80.5 | 1041 | n.d. | (3) | (3) | (3) | |||
| Unknown | BM-095 | 15.712 | — | 111 | 4 | — | 1043 | — | (1) | |||||
| 2-Cyclohexen-1-one, 3-methyl- | BM-096 | 15.454 | 1193-18-6 | 82 | 1 | 90.7 | 1028 | 1039 | (5) | |||||
| Unknown | BM-097 | 16.068 | — | 105 | 4 | — | 1063 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-098 | 16.088 | — | 91 | 4 | — | 1063 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| 1-Octyn-3-olt | BM-099 | 16.133 | 818-72-4 | 97 | 3 | 80.5 | 1066 | n.d. | (5) | |||||
| Unknown | BM-100 | 16.134 | — | 109 | 4 | — | 1066 | — | (4) | |||||
| 2-Pentadecyn-1-olt | BM-101 | 16.135 | 2834-00-6 | 93 | 3 | 85.6 | 1066 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| Acetophenone | BM-102 | 16.248 | 98-86-2 | 105 | 2 | 86.6 | 1073 | 1065 | (5) | (5) | (5) | (5) | (5) | (5) |
| 1,2-Propanedione, 1-phenyl- | BM-103 | 16.249 | 579-07-7 | 105 | 2 | 87.7 | 1073 | 1166 | (5) | (5) | (5) | (5) | (5) | (5) |
| Acetophenone | BM-104 | 16.250 | 98-86-2 | 105 | 1 | 92.1 | 1073 | 1078 | (5) | (5) | (5) | (5) | (5) | (5) |
| 6-Octen-1-ol, 3,7-dimethyl-, formate | BM-105 | 16.270 | 105-85-1 | 82 | 2 | 81.3 | 1074 | 1275 | (1) | |||||
| Octanenitrile | BM-106 | 16.375 | 124-12-9 | 96 | 2 | 81.4 | 1080 | 1085 | (5) | (5) | ||||
| 1-Octanol, 3,7-dimethyl- | BM-107 | 16.439 | 106-21-8 | 83 | 2 | 86.4 | 1083 | 1196 | (5) | (5) | ||||
| Benzaldehyde, 3-methyl- | BM-108 | 16.544 | 620-23-5 | 119 | 1 | 95.1 | 1089 | 1086 | (5) | (5) | ||||
| Unknown | BM-109 | 16.544 | — | 91 | 4 | — | 1090 | — | (5) | (5) | ||||
| Unknown | BM-110 | 16.547 | — | 120 | 4 | — | 1089 | — | (5) | (5) | ||||
| Benzaldehyde, 2-methyl- | BM-111 | 16.551 | 529-20-4 | 119 | 2 | 87.2 | 1090 | 1085 | (5) | (5) | ||||
| Indan, 1-methyl- | BM-112 | 16.702 | 767-58-8 | 117 | 2 | 89.7 | 1098 | 1087 | (5) | (5) | (5) | (5) | (5) | (5) |
| Nonanal | BM-113 | 16.794 | 124-19-6 | 82 | 1 | 92.2 | 1104 | 1102 | (5) | (5) | (5) | (5) | (5) | (5) |
| Aziridine, 1,2,3-trimethyl-, trans-t | BM-114 | 16.805 | n.d. | 85 | 3 | 80.1 | 1104 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| 4-Isoxazolecarbonitrile, 5-aminot | BM-115 | 16.843 | n.d. | 109 | 3 | 80.2 | 1106 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-116 | 16.872 | — | 94 | 4 | — | 1108 | — | (1) | |||||
| 2-Cyclohexen-1-one, 3,5-dimethyl-t | BM-117 | 16.909 | 1123-09-7 | 82 | 3 | 90.3 | 1111 | n.d. | (5) | |||||
| Heptanoic acid, 4-methoxyphenyl estert | BM-118 | 16.935 | 56052-15-4 | 124 | 3 | 80.3 | 1112 | n.d. | (5) | |||||
| Unknown | BM-119 | 16.945 | — | 84 | 4 | — | 1113 | — | (5) | |||||
| 6-Methyl-3,5-heptadiene-2-one | BM-120 | 17.128 | 1604-28-0 | 109 | 2 | 85.6 | 1124 | 1110 | (1) | |||||
| Isophorone | BM-121 | 17.249 | 78-59-1 | 82 | 2 | 85 | 1132 | 1121 | (1) | |||||
| Unknown | BM-122 | 17.667 | — | 95 | 4 | — | 1158 | — | (4) | (4) | (4) | (4) | (4) | (4) |
| 2-Octanol, 2-methyl-6-methylene- | BM-123 | 17.668 | 18479-59-9 | 81 | 2 | 81.6 | 1158 | 1160 | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-124 | 17.737 | — | 79 | 4 | — | 1162 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1 S)- | BM-125 | 17.738 | 464-48-2 | 95 | 2 | 80.6 | 1162 | 1147 | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-126 | 17.819 | — | 119 | 4 | — | 1167 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| cis-7-Decen-1-al | BM-127 | 17.845 | 21 661-97-2 | 97 | 2 | 85.6 | 1169 | 1179 | (5) | |||||
| Unknown | BM-128 | 17.881 | — | 119 | 4 | — | 1171 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Benzaldehyde, 3-ethyl- | BM-129 | 17.926 | 34246-54-3 | 134 | 2 | 86.9 | 1174 | 1168 | (3) | (4) | (3) | |||
| Benzaldehyde, 4-ethyl- | BM-130 | 18.195 | 4748-78-1 | 91 | 2 | 86.3 | 1190 | 1180 | (5) | |||||
| Unknown | BM-131 | 18.220 | — | 131 | 4 | — | 1191 | — | (5) | |||||
| Ethanone, 1-(4-methylphenyl)- | BM-132 | 18.283 | 122-00-9 | 91 | 2 | 89.4 | 1196 | 1183 | (5) | |||||
| Ethanone, 1-(3-methylphenyl)- | BM-133 | 18.285 | 585-74-0 | 133 | 2 | 83 | 1196 | 1182 | (1) | |||||
| Benzaldehyde, 2,4-dimethyl- | BM-134 | 18.334 | 15764-16-6 | 105 | 2 | 88.3 | 1198 | 1190 | (5) | |||||
| Unknown | BM-135 | 18.423 | — | 128 | 4 | — | 1205 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-136 | 18.444 | — | 128 | 4 | — | 1206 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Decanal | BM-137 | 18.445 | 112-31-2 | 81 | 2 | 89.4 | 1206 | 1200 | (5) | (5) | (5) | (5) | (5) | (5) |
| 7-Octen-1-ol, 3,7-dimethyl-, (S)- | BM-138 | 18.447 | 6812-78-8 | 81 | 2 | 89.4 | 1206 | 1211 | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-139 | 18.510 | — | 131 | 4 | — | 1211 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| cis-7-Decen-1-al | BM-127 | 17.845 | 21661-97-2 | 97 | 2 | 85.6 | 1169 | 1179 | (5) | |||||
| Unknown | BM-128 | 17.881 | — | 119 | 4 | — | 1171 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Benzaldehyde, 3-ethyl- | BM-129 | 17.926 | 34246-54-3 | 134 | 2 | 86.9 | 1174 | 1168 | (3) | (4) | (3) | |||
| Benzaldehyde, 4-ethyl- | BM-130 | 18.195 | 4748-78-1 | 91 | 2 | 86.3 | 1190 | 1180 | (5) | |||||
| Unknown | BM-131 | 18.220 | — | 131 | 4 | — | 1191 | — | (5) | |||||
| Ethanone, 1-(4-methylphenyl)- | BM-132 | 18.283 | 122-00-9 | 91 | 2 | 89.4 | 1196 | 1183 | (5) | |||||
| Ethanone, 1-(3-methylphenyl)- | BM-133 | 18.285 | 585-74-0 | 133 | 2 | 83 | 1196 | 1182 | (1) | |||||
| Benzaldehyde, 2,4-dimethyl- | BM-134 | 18.334 | 15764-16-6 | 105 | 2 | 88.3 | 1198 | 1190 | (5) | |||||
| Unknown | BM-135 | 18.423 | — | 128 | 4 | — | 1205 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-136 | 18.444 | — | 128 | 4 | — | 1206 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Decanal | BM-137 | 18.445 | 112-31-2 | 81 | 2 | 89.4 | 1206 | 1200 | (5) | (5) | (5) | (5) | (5) | (5) |
| 7-Octen-1-ol, 3,7-dimethyl-, (S)- | BM-138 | 18.447 | 6812-78-8 | 81 | 2 | 89.4 | 1206 | 1211 | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-139 | 18.510 | — | 131 | 4 | — | 1211 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-153 | 20.969 | — | 95 | 4 | — | 1380 | — | (5) | |||||
| Unknown | BM-154 | 21.376 | — | 104 | 4 | — | 1393 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Dodecanal | BM-155 | 21.377 | 112-54-9 | 95 | 2 | 85.7 | 1411 | 1409 | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown-063 | BM-156 | 21.462 | 1653-30-1 | 97 | 4 | 83.2 | 1417 | 1307 | (5) | (5) | (5) | (5) | (5) | (5) |
| 1,1'-Biphenyl, 2-methyl- | BM-157 | 21.525 | 643-58-3 | 167 | 2 | 83.6 | 1422 | 1438 | (5) | (5) | ||||
| Unknown | BM-158 | 21.987 | — | 109 | 4 | — | 1458 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| 2-Hexyl-1-octanolt | BM-159 | 22.010 | 19780-79-1 | 111 | 3 | 84.3 | 1460 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-160 | 22.013 | — | 99 | 4 | — | 1460 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-161 | 22.236 | — | 91 | 4 | — | 1477 | — | (5) | (5) | ||||
| Hexyl octyl ethert | BM-162 | 22.561 | 17071-54-4 | 85 | 3 | 81.4 | 1502 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-163 | 22.604 | — | 117 | 4 | — | 1505 | — | (1) | |||||
| 2,4-Di-tert-butylphenol | BM-164 | 22.800 | 96-76-4 | 191 | 2 | 81.9 | 1521 | 1513 | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-165 | 22.938 | — | 111 | 4 | — | 1533 | — | (5) | (5) | ||||
| 1-Dodecanol,3,7,11-trimethyl | BM-166 | 23.405 | 6750-34-1 | 83 | 2 | 84.9 | 1571 | 1571 | (5) | |||||
| Unknown | BM-167 | 23.686 | — | 83 | 4 | — | 1593 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-168 | 23.689 | — | 97 | 4 | — | 1594 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-169 | 23.692 | — | 85 | 4 | — | 1594 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-170 | 23.872 | — | 111 | 4 | — | 1609 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| 2,4,4-Trimethyl-1-hexenet | BM-171 | 23.881 | 51174-12-0 | 71 | 3 | 82.3 | 1610 | n.d. | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-172 | 25.150 | — | 85 | 4 | — | 1720 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-173 | 25.959 | — | 110 | 4 | — | 1793 | — | (2) | (2) | (2) | (2) | ||
| Unknown | BM-174 | 26.257 | — | 97 | 4 | — | 1821 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| Unknown | BM-175 | 26.389 | — | 98 | 4 | — | 1834 | — | (5) | (5) | (5) | (5) | (5) | (5) |
| cis-11-Hexadecenal | BM-176 | 26.389 | 53939-28-9 | 81 | 2 | 82.1 | 1834 | 1809 | (5) | (5) | (5) | (5) | (5) | (5) |
Identified using National Institute of Standards and Technology NIST/EPA/NIH mass spectral library (NIST17) (match factor >80%) after manual peak integration using MassHunter Quantitative Analysis software; RT: retention time (min); CAS: chemical abstracts service registry number; Level: identification level; BM exhaled breath metabolite number; RI: Retention-Index; RI ref: reference RI after comparison from the NIST chemistry web book; RI calc: calculated RI; RI n.d.: no retention index available in the literature with respect to a comparable analytical method (non-polar column 5 ms, ramp temperature); t: Tentatively identified (match factor >80%; reference RI not defined or > ±15 from calculated RI; Unknown: unknown VOCs (match factor >80%; reference retention index (RI) greater than ±15 of the calculated RI); ENV: Bond Elut ENV (polystyrene-divinylbenzene polymer); HRX: Chromabond HRX (hydrophobic spherical polystyrene-divinylbenzene copolymer); HRP: Chromabond HRP (hydrophobic polystyrol-divenylbenzol-copolymer); HLB: Chromabond HLB (hydrophilic-lipophilic balanced N-vinylpyrrolidone-divinylbenzene); XCW: Chromabond HR-XCW (hydrophobic spherical polystyrene-divinylbenzene copolymer); XAW: Chromabond HR-XAW (hydrophobic spherical polystyrene-divinylbenzene copolymer with secondary weak anion exchange); XCW: Chromabond HR-XCW (hydrophobic spherical polystyrene-divinylbenzene copolymer with weak cation exchange).
Since the major differences between the SPE cartridges tested were observed for the detection of specific VOCs, a list of detectable VOCs according to the SPE adsorbent cartridge used is provided in table 4. Many studies remain challenging because many VOCs have not yet been identified [13]; therefore, we have provided MS spectra for both tentatively identified and unknown VOCs, in addition to their RTs and calculated RIs, to aid in their future identification (figures S1 and 2). This list of 176 specific VOCs allows the selection of an appropriate SPE adsorbent cartridge based on specific compounds of interest and in terms of possible markers to assess the specific metabolic state and health of the animals.
One such compound may be 2-ethyl-1-hexanol. Its increase in exhaled breath has been reported as a marker of infection with Mycobacterium avium subsp. paratuberculosis in cattle [35], and this compound was detected at low levels using all six SPE cartridges. Due to the large differences in SPE adsorbent material-specific VOC detection, the use of several different cartridges per animal, as in the present study, allows improved recovery of a wider range of VOCs and provides more flexibility, especially in untargeted approaches [36]. After sampling and elution, the eluents from the different cartridges used per animal can be mixed to allow a single analysis step for the multiple cartridges used simultaneously. Self-packed multi-bed SPE cartridges may also be suitable. For this purpose, the SPE material with the greatest coverage of the metabolite spectrum should be used. Based on our study, XAW seems to be the most suitable SPE cartridge, as it gave the highest number of detected peaks and specific VOCs.
3.2. Technical strengths and limitations of using SPE cartridges and future improvement possibilities
In our experiment, the aldehydes butenal, decanal, cis-7-decen-1-al, heptanal, hexanal, cis-11-hexadecenal, nonanal, octanal, and tetradecenal and the carboxylic acid esters acetic acid butyl ester and propanoic acid, 2-methyl-, 2-propenyl ester were detected. These and other VOCs, especially from the chemical groups of aldehydes and carboxylic acid esters, differed in their detectability from a previous study by Polvara et al [9]. They compared the performance of two methods for VOC sampling from a biomass storage plant using polymeric sampling bags and different sorbent tubes for automatic desorption (ATD) of the sampled emission from the polymeric bags. In particular, the above-mentioned aldehydes and esters of carboxylic acids, were not detectable using the ATD but were detected in the polymeric sampling bags [9]. This observation illustrates that large differences in results may arise due to differences in the choice of sampling and storage devices, as well as the analytical technique used.
3.2.1. Sampling
The sampling of VOCs from exhaled breath, as described in our study, poses a risk of contamination with ambient VOCs from the barn, regardless of the VOC storage device used. This problem could be overcome in future studies by sealing the face mask from the ambient air and connecting it to a container of VOC-free air as the inhalation source for the cow. This modulated face mask could then be connected to multiple VOC storage devices. Furthermore, Teflon instead of silicone tubes can be used for further exhaled breath sampling to eliminate the step of removing silicone derived contaminants such as siloxanes from the data set after VOC analysis. Another VOC sampling method could be cryogenic condensation using liquid nitrogen or dry ice, as this would stabilize VOCs at low temperatures and allow the storage of a relatively high VOC concentration within a small container [37]. However, this would require special equipment, such as liquid nitrogen and glassware, and the complexity of the method would limit the sampling throughput. By contrast, transport and handling of polymeric bags, thermal desorption tubes, and SPE cartridges does not require extensive infrastructure, and these materials can be easily used in the field. While SPE cartridges and polymeric bags are also very cost-effective, thermal desorption tubes are expensive and are therefore not as suitable for a high sample throughput. Another option is to combine VOC sampling with in-field VOC analysis. This method is possible for targeted approaches, such as those using proton-transfer-reaction time-of-flight MS.
3.2.2. Storage
During sample storage, contaminants from the sampling device, such as plasticizers, can contaminate the sampled exhaled VOCs [8]. We conducted an additional exhaled breath sampling experiment using glass bulbs (Supelco, Bellefonte, PA, United States) that were washed three times with NanopureTM water and methanol and dried with nitrogen for 20 min. Using this sampling device, no animal-derived VOCs could be identified because their peaks were too strongly overlaid by contaminant-derived peaks. Similarly, Fido et al [8] described high levels of plasticizer contamination in exhaled breath samples using polymeric bags as sampling devices. This contamination can be avoided by sampling and storing VOCs directly on the SPE cartridges or thermal desorption tubes. While thermal desorption tubes are not suitable for medium-term storage, the use of SPE cartridges provides high VOC stability and easy storage without the gaseous carrier matrix in a refrigerator for sample analysis at a later time.
3.2.3. Analysis
For VOCs stored in sampling bags, analysis by secondary electrospray ionization-MS is possible without any previous steps. For the SPE cartridges, chemical solvents are required to elute the VOCs from the SPE adsorbent surface, as described above. In the future, the SPE adsorbent material could be removed from the cartridge and the VOCs thermally desorbed in a glass vial. Our DHS-V-ITEX-GC/MS analysis further enhances VOC detection even at low concentrations by providing a second concentration step through the polymer-filled ITEX needle and further focusing the VOCs within the Tenax-filled liner prior to separation in the GC. This method of exhaled breath sampling, which we have used in dairy cows, could also be used for human exhaled breath sampling for non-invasive exhalome studies.
Further research is needed to determine the extent to which other sampling and environmental conditions (humidity, temperature, wind speed, etc) affect VOC detection by different SPE cartridges, under what conditions the negative effects occur, and how these conditions may affect VOC recovery.
4. Conclusion
In general, we have demonstrated the suitability of polymer-based SPE cartridges for the sampling of VOCs from exhaled dairy cow breath. Our findings indicate that the choice of an appropriate SPE adsorbent cartridge has an enormous influence on what VOCs will be detected and identified, thereby affecting the quality and validity of the results.
For this reason, polymer-based SPE adsorbent cartridges should be selected based on the specific study purpose and objective:
- (i)Targeted approaches according to the known metabolic conditions of experimental animals for the detection of potential markers or specific chemical compound groups of interest: Selection of the appropriate SPE adsorbent cartridge depending on the detection of the VOCs or chemical compound groups of interest based on our provided list of VOCs.
- (ii)Untargeted approaches without information on the metabolic status of the animals: use of multi-sorbent SPE cartridges or use of multiple cartridges per animal, including the adsorbent cartridges with the greatest coverage of the metabolite spectrum (e.g. the XAW cartridge).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical statement
The experimental protocol complied with the Swiss animal welfare legislation and was approved by the Animal Care Committee of the Canton Fribourg, Fribourg, Switzerland (License No. 2021-38-FR).
Funding
This research received no external funding.
Supplementary data (3.3 MB PDF)