Abstract
There is a pressing need to develop a novel early-detection strategy for the precise evolution of small intestinal bacterial overgrowth (SIBO) in irritable bowel syndrome (IBS) patients. The current method based on a hydrogen breath test (HBT) for the detection of SIBO is highly controversial. HBT has many limitations and drawbacks. It often fails to indentify SIBO when IBS individuals have 'non-hydrogen-producing' colonic bacteria. Here, we show that hydrogen sulphide (H2S) in exhaled breath is distinctly altered for diarrhea-predominant IBS individuals with positive and negative SIBO by the activity of intestinal sulphate-reducing bacteria. Subsequently, by analyzing the excretion kinetics of breath H2S, we found a missing link between breath H2S and SIBO when HBT often fails to diagnose SIBO. Moreover, breath H2S can track the precise evolution of SIBO, even after the eradication of bacterial overgrowth. Our findings suggest that the changes in H2S in the bacterial environment may contribute to the pathogenesis of SIBO and the breath H2S as a potential biomarker for non-invasive, rapid and precise assessment of SIBO without the endoscopy-based microbial culture of jejunal aspirates, and thus may open new perspectives into the pathophysiology of SIBO in IBS subjects.
Export citation and abstract BibTeX RIS
Introduction
Irritable bowel syndrome (IBS), a common functional gastrointestinal disorder of unknown pathogenesis, is closely associated with abdominal discomfort, distension and bloating, diarrhea, constipation and even severe malabsorption as well as malnutrition [1–3]. Small intestinal bacterial overgrowth (SIBO) is another heterogeneous syndrome characterised by an increased number of bacteria (usually ⩾105 colony forming units ml−1) in the small bowel. Several pieces of evidence suggest a strong link between IBS and SIBO because the symptoms of both disorders overlap to a large extent [4, 5]. Moreover, it is still a debatable question whether subjects fulfilling the diagnostic criteria for IBS should be treated for SIBO, or individuals presenting with SIBO should be considered for the acute onset of IBS [6]. As a result, SIBO is often misdiagnosed and considerably under-diagnosed. A hydrogen breath test (HBT) by ingestion of glucose or lactulose has recently been proposed to be a viable non-invasive method for indirect evaluation of SIBO in contrast with the direct invasive endoscopy-based microbial culture of jejunal aspirates [7]. However, the protocols of the HBT in the diagnosis of SIBO have not yet been accurately standardised owing to its various limitations and drawbacks [8]. Moreover, the conclusions drawn from HBTs are highly controversial [9, 10], suggesting a pressing need to develop a new strategy which will make the detection of SIBO more sensitive, precise and specific.
Numerous studies [9–11] suggest that hydrogen sulphide (H2S) is produced in the human colon by naturally occurring H2-consuming microbes such as sulphate-reducing bacteria (SRB). The highly toxic substance H2S accumulates in the small intestine in presence of SIBO resulting in inflammation and damage of intestinal epithelial cells [12–15]. Some early evidence [16–18] suggested that the generation of H2S in the human colon may be involved in the onset and persistence of IBS. However, the precise role of H2S, particularly in the pathogenesis of SIBO, is not currently known. A new insight into the role of H2S is important to elucidate the pathophysiology of SIBO. Furthermore, to our knowledge, the potential links between H2S and SIBO have not yet been fully elucidated because of the unknown etiology and pathogenesis of IBS.
However, sulphate-reducing bacteria (SRB) use molecular H2 as a respiratory substrate during metabolism and reduce sulphate to H2S, which is then transported to the lungs through the bloodstream and finally excreted in exhaled breath. This activity suggests a tantalizing but unproven hypothesis about the possibility of exploiting the presence of breath H2S for non-invasive detection of SIBO as well as an indirect assessment of intestinal bacterial metabolism, and hence may initiate a novel early-detection strategy in the diagnosis of SIBO in patients with typical symptoms of IBS. Moreover, a number of pathways may exist for H2 disposal in the small intestine and there might be competition for the utilization of molecular H2 among a large number of bacterial and archaeal flora in the gut including methanogenic bacteria, SRB, acetogenic bacteria and other species [19, 20]. Therefore, unravelling the precise metabolic pathways involved in causing the changes of H2S in the small intestine and afterwards in the patient's breath remains a challenge, whenever an individual with IBS is at-risk of developing SIBO or during the preclinical phase of the bacterial overgrowth. It is noteworthy that a complete understanding of the role of molecular H2S in the pathogenesis of SIBO could be of importance in the development of novel therapies for SIBO along with better preclinical methodologies for treating SIBO.
Several lines of evidence [10, 21, 22] also suggest that many IBS individuals with SIBO may have 'non-hydrogen-producing' bacteria and consequently the level of excreted hydrogen gas in their breath is not significant, but they can produce other gases, such as H2S prompted by the SRB organisms in the small intestine as a part of microbial fermentation. Under these circumstances, monitoring of only molecular H2 in the breath may often fail to correctly diagnose SIBO, suggesting that there might be a missing link between H2S and SIBO—an area of research that has never been explored before. Little is known so far about whether breath H2S can act as a potential biomarker to precisely track the evolution of SIBO in patients presenting with IBS.
Here, we investigated in the presence of SIBO whether H2S in exhaled breath is altered when IBS individuals are particularly recognized as diarrhea-predominant IBS (IBS-D). We focused particularly on IBS-D because subjects with IBS-D frequently show a higher frequency of SIBO than other subtypes of IBS (i.e. IBS with constipation or mixed IBS) [10]. We have shown that breath H2S can be used as a potential molecular marker for non-invasive evaluation of SIBO and even after in response to the standard eradication therapies of the bacterial overgrowth. We also explored the potential metabolic pathways underlying breath H2S alternation in the pathogenesis of SIBO and the molecular mechanisms linking breath H2S to SIBO.
Material and methods
Subjects
One hundred and fifty one individuals (n = 68 male and n = 83 female) with an average age of 46 ± 13 years (SD) with diarrhea-predominant IBS diagnosed according to Rome III criteria were enrolled for the study. HBTs and the 'gold-standard' culture of jejunal fluid aspirate were performed on the patients to confirm the positive and negative indication of SIBO. All subjects were requested to fill out a set of Rome III questionnaires before the breath tests that allowed the interpretation of IBS subtypes, as described and employed in several studies. Detailed information about the subjects' characteristics has been described in the supplementary material (supporting information (table S1) (stacks.iop.org/JBR/10/026010/mmedia)). Subjects taking any antibiotics or proton pump inhibitors, or being treated with drugs that interfere with gastrointestinal motility, were excluded from this study. Subjects with Crohn's diseases, ulcerative colitis, a previous history of gastric surgery, systematic sclerosis, liver cirrhosis, diabetes, COPD, halitosis or smoking, or taking any medication that hampers glucose metabolism, were also excluded from the present study. Moreover, subjects were not allowed to eat slowly adsorbed carbohydrate, fruits, vegetables, and legumes and fiber containing food 48 h prior to the study. We received approval from the Ethics Committee Review Board of AMRI Hospital, Salt Lake, Kolkata, India (study no.: AMRI/ETHICS/2013/2) and the current methods were carried out in accordance with the approved guidelines. Informed consent was obtained from all subjects. Administrative approval (ref. no.: SNB/PER-2-6001/13–14/1769) from the S N Bose National Centre for Basic Sciences, Kolkata, India was also obtained.
Breath sample collection and measurements
Before the breath test, all subjects were instructed to wash their mouth gently to avoid any kind of contamination of the ingested test meal with bacteria in the oral cavity. After overnight fasting (~10–12 h), an initial baseline breath sample was collected in a 750 ml breath sample collection bag (QT00892, QuinTron Instrument Co. USA). Then a test meal containing 50 gm of normal glucose dissolved in 250 ml of water was orally administered to the patient. From this point, breath samples were collected at 15 min intervals up to 120 min for the measurements of H2S and H2 in the breath. A standard residual gas analyzer (RGA)-based mass spectrometry (MS) technique was employed to measure the masses of H2S (i.e. 34 amu) in breath samples [23, 24]. The detailed measurement procedure by the RGA-MS system can be found in the supplementary material. However, the concentrations of H2 levels in breath samples were measured by utilizing an electro-chemical gas sensor-based equipment (Gastro + Gastrolyzer, Bedfont Scientific Ltd, model no.: CE0086). The procedure and steps followed in the present study are given in the supplementary material (figure S3).
Statistical analysis
All the data were presented as mean ± SE (standard error). One-way analysis of variance (ANOVA) and non-parametric Mann–Whitney statistical analysis were performed to compare the normal and non-normal distribution of experimental data. A two-sided p value <0.05 was considered to indicate statistical significance. All the data were analysed using Origin Pro 8.0 (Origin Lab Corporation, USA).
Results
To investigate the possible role of H2S in the pathogenesis of SIBO, we first studied the time-dependent excretion kinetics of H2S in exhaled breath for numerous IBS-D patients (n = 151), diagnosed according to the symptom-based ROME-III criteria [25]. In the first set of experiments, we measured breath H2S alteration (i.e. % change in H2S, ΔH2S%), mass-spectrometrically following ingestion of an oral dose of natural glucose in patients with SIBO (n = 29) and without SIBO (n = 97) (supporting information (figure S4)). Jejunal fluid aspirate was cultured for these 126 subjects as the gold standard method for assessing SIBO. According to the usual convention of HBT, the patients were normally considered SIBO positive if H2 levels in their breath samples were ⩾10 ppm (positive HBT) within 60 min above the basal value (i.e. typically ⩽5 ppm) after oral glucose administration (n = 27). Numerous earlier studies [9, 26] have suggested that patients with this consideration are very likely to be indicative of a positive result of SIBO and these considerations are also established in the present study (figures 1(a) and (c)). However, in this investigation (figure 1(a)), individuals with SIBO positive (n = 27) exhibited significantly higher enrichments of ΔH2S% in breath samples as compared with SIBO negative (n = 92) individuals during the 2 h-excretion dynamics. A statistically significant difference (p < 0.001) in the ΔH2S% values in excretion dynamics established a marked distinction between IBS-D individuals with SIBO positive and SIBO negative (figure 1(b)).
Figure 1. Assessments of ΔH2S% and ΔH2 (ppm) values in exhaled breath associated with bacterial fermentation of normal glucose in presence (SIBO (+)) and absence (SIBO (−)) of bacterial overgrowth. (a) Excretion kinetics of ΔH2S% and ΔH2 (ppm) values for SIBO positive (SIBO (+)) and SIBO negative (SIBO (−)) individuals up to 120 min. Panels (b) and (c) indicate statistically significant differences of ΔH2S% (p < 0.001) and ΔH2 (ppm) (p < 0.001) values at 45 min between SIBO positive (SIBO (+)) and SIBO negative (SIBO (−)) individuals. Data are means ± SE.
Download figure:
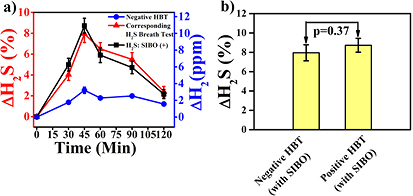
Standard image High-resolution imageWe next explored the excretion kinetics of breath ΔH2S% in a group of IBS-D patients (n = 25) where there was no significant increase of breath H2 levels above the basal value. Under these circumstances the test is not usually considered to be a positive indication of SIBO, despite the fact that we observed a significant increase of ΔH2S% in breath samples (figure 2(a)). The patients followed similar excretion profiles of ΔH2S% to those with positive SIBO, thus unveiling a missing link between breath H2S and SIBO when HBT fails to diagnose SIBO (figure 2(a)). The presence of SIBO in those IBS-D subjects was also confirmed by culture of jejunal aspirates on (n = 25) subjects. A statistically insignificant difference (p = 0.37) was observed in ΔH2S% in exhaled breath samples between negative and positive HBT subjects with SIBO infection at t = 45 min (figure 2(b)).
Figure 2. Time dynamics of ΔH2S% and ΔH2 (ppm) values in exhaled breath during fermentation of glucose for a group of IBS-D individuals with SIBO. (a) The excretion kinetics demonstrate the elevated ΔH2S% (SIBO(+)) and insignificant ΔH2 (ppm) (SIBO(−)) values over baseline for a group of IBS-D subjects infected with SIBO. Values are means ± SE. (b) Illustrates the statistically insignificant (p = 0.37) ΔH2S% level in breath between positive HBT and negative HBT individuals with SIBO infection.
Download figure:
Standard image High-resolution imageWe next determined an optimal diagnostic cut-off value of ΔH2S% in breath samples to distinctively track the evolution of SIBO using receiver operating curve (ROC) analysis (figure 3(a)). Individuals with ΔH2S% ⩾1.76% at t = 45 min were considered to be SIBO positive and this corresponded to 93.6% sensitivity and 89.4% specificity with 91.3% diagnostic accuracy (95% confidence interval). We subsequently explored the positive predictive value (PPV) and negative predictive value (NPV) of the present methodology to assess the probability of getting infected states when the actual test results are known. We found the diagnostic PPV and NPV of 98% and 91%, respectively between the IBS patients with SIBO and without SIBO.
Figure 3. Clinical validity of hydrogen sulphide breath test. (a) A receiver operating characteristic (ROC) curve with an optimal diagnostic cut-off point of ΔH2S% = 1.76% at t = 45 min, corresponds to 93.6% sensitivity, 89.4% specificity and 91.3% diagnostic accuracy. (b) Hydrogen sulphide breath test in response to the standard eradication therapies of the SIBO infection. A marked distinction (p < 0.001) for the ΔH2S% at 45 min was observed before and after the antibiotic therapy.
Download figure:
Standard image High-resolution imageWe further investigated the efficacy of the H2S breath test after 8 weeks of standard eradication therapy. A marked depletion of breath ΔH2S% values for individuals with SIBO (n = 20) was manifested (figure 3(b)) and all patients exhibited a significant improvement in symptoms after the eradication therapy, thus suggesting the clinical utility of the H2S breath test for eradication therapy in the area of biomedical science. As a result, we therefore posit that monitoring of H2S in breath samples is a valid and potentially robust new strategy for non-invasive assessment of SIBO, i.e. early detection and follow-up of patients after eradication of SIBO in IBS-D patients.
Discussion
Our findings demonstrate that there is a potential link between SIBO and breath H2S and this may open up the possibility of exploiting breath H2S for the non-invasive assessment of SIBO in IBS-D patients. In some earlier reports [27–29], it was suggested that some SRB residing in the small intestine have the ability to utilize hydrogen equivalents (such as acetate, formate etc), generated by the bacterial fermentation of ingested glucose to produce hydrogen sulphide. H2S produced by the intestinal bacteria is then transported to the lungs through the blood and eventually released in exhaled breath. Thus, the observation of the increase of H2S in breath samples, as demonstrated in figure 1(b), for IBS-D individuals positive for SIBO is possibly attributed to the bacterial activity of conversion of hydrogen equivalents to H2S.
The most striking finding of the study lies in the observation of H2S in breath when molecular H2 fails to track the precise evolution of SIBO. It has previously [8, 9, 15] been reported that many IBS individuals with SIBO may have H2-consuming SRB in the small intestine. These organisms use molecular H2 and consequently reduce sulphate in human body to sulphide, which is then rapidly hydrolyzed to H2S according to the following equation [30, 31]:

Therefore, the observation of the increase of H2S in breath samples as demonstrated in figure 2(a) for IBS-D individuals with SIBO positive is likely to be an effect of the activity of sulphate reduction by the SRB microorganisms. In view of these results, our findings suggest that H2S produced by the activity of SRB may contribute to the pathogenesis of SIBO and thus might be considered a potential biomarker for the detection of SIBO to provide a unique approach to treat the bacterial overgrowth in the human small intestine. An estimation of different diagnostic parameters to diagnose SIBO using the HBT and H2S breath test is shown in table 1. The present study confirms the clinical feasibility of a novel diagnostic methodology for the non-invasive assessment of SIBO in IBS patients without any endoscopy-based culture of jejunal fluid aspirate.
Table 1. Different diagnostic parameters to diagnose SIBO using HBT and H2S breath test.
| Test | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| H2 breath test | 46.0% | 90.4% | 69.0% | 78.2% | 76.0% |
| H2S breath test | 93.6% | 89.4% | 98.0% | 91.0% | 91.3% |
We finally elucidated the potential metabolic pathways (figure 4) underlying the mechanisms responsible for the alteration of H2S in breath samples whenever an individual is at-risk of bacterial overgrowth in the small intestine. When a dose of glucose is orally administered to the IBS-D patients with SIBO, the ingested glucose disposal takes place in the small intestine where it is transformed into pyruvate through the glycolysis pathway [32], and subsequently hydrogen is produced as a part of the bacterial fermentation from other host-residing bacteria in the small intestine. Hydrogen equivalents (e.g. lactate, formate) are also generated by the oxidation of organic compounds [26, 30]. SRB present in the small intestine use the hydrogen equivalents to produce energy for their growth through the hydrogen-cycling pathway [33, 34]. Part of the hydrogen equivalents is converted to hydrogen by cytoplasmic hydrogenase complexes in the cytoplasm of SRB. Hydrogen is then diffused to the periplasm and subsequently metabolized to protons (H+) and electrons (e−) by the activities of periplasmic hydrogenase enzyme. However, the remaining hydrogen equivalents in the cytoplasm lead to the formation of pyruvate, which is transformed into carboxylic acid salt and this is supposed to be cycled to the periplasm via the activities of cytoplasmic hydrogenases. In the periplasm, carboxylic acid salt is subsequently metabolized by dehydrogenase (dh) enzymes to form protons (H+) and electrons (e−). The protons essentially provide the proton-motive force for the generation of ATP and electrons are cycled back to the cytoplasm through the cytochrome c3 network [35] to accomplish the reduction of sulphate ( ).
).
Figure 4. Potential metabolic pathways for variation of hydrogen sulphide (H2S) in breath associated with small intestinal bacterial overgrowth (SIBO). Orally administered glucose (G) disposal takes place in the small intestine and subsequently hydrogen and hydrogen equivalents are formed as a part of the bacterial fermentation by a primary fermenter (PF). The hydrogen equivalents are utilized by sulfur reducing bacteria (SRB) following the hydrogen cycling pathway to produce protons (H+) and electrons (e−). The electrons are cycled to the cytoplasm through the cytochrome c3 network to accomplish the reduction of sulphate ( ) to H2S. Part of the total H2S, along with H2 formed in the fermentation process, is then transported to lungs through the bloodstream and eventually excreted in exhaled breath. Conversely, owing to the inactivity of hydrogen cycling pathway in some SRB, H2 formed in bacterial fermentation acts as an electron donor. The electrons are cycled to the cytoplasm via the cytochrome c3 network and form hydrogen sulphide in the dissimilatory sulphate reduction process. Part of the total H2S formed in this process is finally excreted in exhaled breath.
) to H2S. Part of the total H2S, along with H2 formed in the fermentation process, is then transported to lungs through the bloodstream and eventually excreted in exhaled breath. Conversely, owing to the inactivity of hydrogen cycling pathway in some SRB, H2 formed in bacterial fermentation acts as an electron donor. The electrons are cycled to the cytoplasm via the cytochrome c3 network and form hydrogen sulphide in the dissimilatory sulphate reduction process. Part of the total H2S formed in this process is finally excreted in exhaled breath.
Download figure:
Standard image High-resolution imageMethionine and cysteine are two essential amino acids which play a central role as the source of  ion in the human body. SRB uptake the
ion in the human body. SRB uptake the  ion and convert it to adenosine phosphosulphate (APS) to initiate their energy metabolism. The successive reductions of sulphate to sulphite (
ion and convert it to adenosine phosphosulphate (APS) to initiate their energy metabolism. The successive reductions of sulphate to sulphite ( ), trithionate (
), trithionate ( ) and thiosulphate (
) and thiosulphate ( ) are mediated by the cytochrome c3 electron transport network along with a series of reductases. Concurrently, electrons are passed to the dissimilatory sulphite reductase (Dsr) transmembrane complex, which then leads to the reduction of
) are mediated by the cytochrome c3 electron transport network along with a series of reductases. Concurrently, electrons are passed to the dissimilatory sulphite reductase (Dsr) transmembrane complex, which then leads to the reduction of  to H2S and
to H2S and  . This
. This  ion is again recycled to produce H2S, subject to the availability of electrons from the cytochrome c3 network [36]. Thus H2S produced via the hydrogen cycling pathways would then follow different escape routes. A major part of H2S is oxidized in different physiological processes for detoxification of liver and muscle tissue. The remaining part of the total H2S along with H2 formed in the fermentation process of anaerobic bacteria is then transported to the lungs through the bloodstream and eventually excreted in breath. Thus elevated levels of both H2 and H2S are observed in exhaled breath, as exhibited in figure 1(a).
ion is again recycled to produce H2S, subject to the availability of electrons from the cytochrome c3 network [36]. Thus H2S produced via the hydrogen cycling pathways would then follow different escape routes. A major part of H2S is oxidized in different physiological processes for detoxification of liver and muscle tissue. The remaining part of the total H2S along with H2 formed in the fermentation process of anaerobic bacteria is then transported to the lungs through the bloodstream and eventually excreted in breath. Thus elevated levels of both H2 and H2S are observed in exhaled breath, as exhibited in figure 1(a).
Conversely, the cytoplasmic hydrogenases and transmembrane complexes that are supposed to be involved in hydrogen cycling have a highly variable distribution [30, 31]. Hydrogen cycling is not necessary when intestinal H2, produced in anaerobic fermentation of glucose, plays a pivotal role in serving as the electron donor [30]. The periplasmic metabolism of H2 by Fe-only hydrogenase directly establishes the electrochemical gradient in the periplasm that is necessary for ATP synthesis and the growth of microorganisms [30]. The electrons are cycled to the cytoplasm via the cytochrome c3 network, which leads to the generation of H2S in the dissimilatory sulphite reduction process. Thus, only elevated level of H2S was observed in exhaled breath, as demonstrated in figure 2(a).
Conclusions
We have demonstrated that H2S is altered in exhaled breath in response to the glucose-stimulated bacterial environment whenever a diarrhea-predominant irritable bowel syndrome patient has bacterial overgrowth in the small intestine. To investigate how breath H2S alters, we have taken a step towards elucidating the potential metabolic pathways linking the alteration of H2S in exhaled breath and SIBO in IBS-D patients. Our data support that breath H2S could be used as a potential non-invasive biomarker to accurately identify the presence of SIBO either at the onset of the syndrome or after the eradication of the disorder. Although several important gaps remain in our understanding, our findings may open new perspectives into the pathophysiology of SIBO and hence may pave the way for a novel strategy in the diagnosis of SIBO using H2S breath analysis, even when the present hydrogen breath test fails to diagnose SIBO. Moreover, a new insight into the role of H2S in the pathogenesis of SIBO is raising the possibility of exploration of new pharmacological targets to prevent or treat the deleterious effects of SIBO.
Acknowledgments
This project was funded by the 'Rapid Grant for Young Investigators (No. BT/PR6683/GBD/27/477/2012)' from the Department of Biotechnology (DBT, India) to MP; GDB and AD were funded by the DST Inspire Fellowship, whereas SS was supported by fellowship from S N Bose Centre. The authors declare that they have no competing interests. We thank all volunteers who participated in this study.