Abstract
Each year wildland fires kill and injure trees on millions of forested hectares globally, affecting plant and animal biodiversity, carbon storage, hydrologic processes, and ecosystem services. The underlying mechanisms of fire-caused tree mortality remain poorly understood, however, limiting the ability to accurately predict mortality and develop robust modeling applications, especially under novel future climates. Virtually all post-fire tree mortality prediction systems are based on the same underlying empirical model described in Ryan and Reinhardt (1988 Can. J. For. Res. 18 1291–7), which was developed from a limited number of species, stretching model assumptions beyond intended limits. We review the current understanding of the mechanisms of fire-induced tree mortality, provide recommended standardized terminology, describe model applications and limitations, and conclude with key knowledge gaps and future directions for research. We suggest a two-pronged approach to future research: (1) continued improvements and evaluations of empirical models to quantify uncertainty and incorporate new regions and species and (2) acceleration of basic, physiological research on the proximate and ultimate causes of fire-induced tree mortality to incorporate processes of tree death into models. Advances in both empirical and process fire-induced tree modeling will allow creation of hybrid models that could advance understanding of how fire injures and kills trees, while improving prediction accuracy of fire-driven feedbacks on ecosystems and landscapes, particularly under novel future conditions.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Understanding and predicting fire-induced tree mortality
Millions of forested hectares burn annually, causing both positive and negative impacts on carbon storage, biodiversity conservation, hydrologic processes, and economic and social services (Bowman et al 2009). In fire-adapted and fire-dependent ecosystems, fire controls tree density and species dominance (Bond et al 2005), creating habitat that supports diverse plant and animal species that cannot persist in the absence of fire. However, fire-adapted ecosystems may be vulnerable to climate-driven alterations to fire regimes that are an emerging threat in recent decades, with observations of increasing fire size, frequency, and severity (Flannigan et al 2009, Pechony and Shindall 2010, Bowman et al 2014, Brando et al 2014, Fairman et al 2016, Seidl et al 2016, Liang et al 2017b). Across almost all biomes, fire season has lengthened for 25% of the Earth's vegetated surface and the burnable area has doubled since 1979 (Jolly et al 2015). Climate-mediated increases in fire severity and frequency are projected to cause large decreases in carbon stocks through loss in forested area (Liang et al 2017a) and are already causing forest declines in Australia where increased fire frequency is killing trees before maturity (Bowman et al 2014). Such changes in fire regimes can shift forests to non-forested states (Fairman et al 2016, Falk 2017, Walker et al 2018). Moreover, trees may be more sensitive to fire-caused injury following episodes of drought-stress (van Mantgem et al 2013), which may become more frequent in many regions with continued warming (Cook et al 2015). The global pervasiveness of fire highlights the importance of understanding how fire impairs and kills trees in order to accurately model those impacts for a wide range of applications and conditions.
Tree mortality is a critical mechanism through which fire limits ecosystem productivity, influences resource availability, and changes the structure and composition of vegetation (Bond et al 2005, Dantas et al 2016). In spite of the importance of fire-caused tree mortality, the underlying mechanisms remain poorly understood. This gap in understanding greatly limits our ability to accurately predict mortality from fire, estimate fire-driven feedbacks to the global carbon cycle, extrapolate to novel future conditions, and implement appropriate management actions to increase forest resilience to wildfire.
We review the mechanisms causing fire-induced tree mortality, suggest standardized terminology, describe applications and limitations to modeling approaches, and summarize key knowledge gaps. While the focus of this review describes tree mortality where the main stem dies (i.e. top-kill) and how that is modeled, we also address fire-induced tree injury and recovery via resprouting.
Mechanisms of fire-induced tree mortality
Plant and ecosystem responses to fire are categorized into either direct or indirect fire effects (Reinhardt et al 2001; see box 1 for glossary of key terms). Though most tree mortality is from direct effects, mortality from proximal, indirect effects often occurs in large trees and may account for a large proportion of forest biomass loss from fire (Van Mantgem et al 2011). Indirect mortality may be influenced by pre-fire stress from competition, drought, and disease, or by post-fire conditions such as elevated bark beetle populations. Because of these multiple interactions, predicting delayed tree mortality is less straightforward than predicting immediate, fire-caused tree mortality (Kane et al 2017b).
Box 1. A glossary of key terms. Main term is the recommended usage; previously used synonyms noted parenthetically.
| Bark char [bole char; bole scorch]: blackened residue of bark resulting from incomplete combustion and indicator of the duration the tree bole was exposed to flames and heat from the fire. Correlates to the heat pulse into tree and has been used as surrogate for cambium kill. |
| Bark char code [depth of char]: a classification system used as a proxy for the duration a tree bole was exposed to heating by fire (codes: unburned, light, moderate, deep). |
| Bark char height [bole char height; stem char height]: vertical height from ground of blackened bark on a tree bole. Typically measured as either maximum or average height. |
| Cambium kill: death of the vascular meristematic tissue (i.e. cambium tissue located between bark and secondary xylem/wood) during fire. Typically occurs on the lower portion of tree stems. |
| Cambium kill rating: method used to estimate the amount of cambium kill and stem injury from fire. Requires removing a small sample of bark at four locations at a tree's base. |
| Cavitation: process by which air in liquid water held under tension within plant xylem comes out of solution and expands to fill xylem elements, causing a break in the plant's water column and a decrease in hydraulic conductivity. |
| Crown kill [bud kill]: portion of a tree's buds (i.e. meristematic tissue that develops into branches, flowers, or foliage; usually at the end of stems), branches, and foliage that is killed during fire. |
| Crown scorch: portion of the tree's foliage that is killed during a fire. Foliage appears brownish red within days of fire. Crown scorch is usually expressed as a percent of either pre-fire crown volume or crown length. Mostly commonly, crown scorch estimates also include crown kill, but sometimes these injuries are measured separately. |
| Direct fire effect [first-order effect]: impacts from fire occurring during and immediately after a fire from heat-induced chemical processes; includes tree mortality solely from fire-caused injuries and not due to interactions with other stressors. |
| Duff: layer of moderately decomposed organic material, encompassing the fermentation (Oe) and humus (Oa) organic soil horizons. Duff occurs beneath the litter horizon (Oi) and above the underlying mineral soil. |
| Epicormic bud: dormant or adventitious bud on the stem or branch of a woody plant from which a shoot can arise after stimulation by stress or changes in light availability. |
| Fire severity: physical, biological, and ecological effects of a fire on ecosystem properties; in forests usually quantified by the level of tree mortality or the degree of soil heating. |
| Flame length: distance from the middle of the flaming zone at the base of the fire (usually the ground) and the average flame tip. |
| Heat flux: amount of heat released per unit area over time. |
| Hydraulic conductivity (Kp): ease with which water moves through the vascular xylem of a plant. |
| Indirect fire effect [second-order fire effect]: impacts from fire, occurring days to years after fire due to interactions with direct fire effects and other factors such as post-fire climate and insects. |
| Non structural carbohydrate (NSC): mobile, nonstructural carbon in plants not used in building structural biomass, but to buffer deficits in maintenance and growth demands. |
| Scorch height: the maximum vertical height at which lethal heating (i.e. reaches 60 °C) occurs during fire; used to estimate crown length scorched. It is mathematically related to fireline intensity and flame length. Many post-fire tree mortality models do not differentiate tissue types and assume all crown tissue (foliage, buds, branches) within the scorch height zone is dead. |
| Smoldering combustion: slow-moving, low-temperature, solid-phase burning of fuel without the presence of flames. |
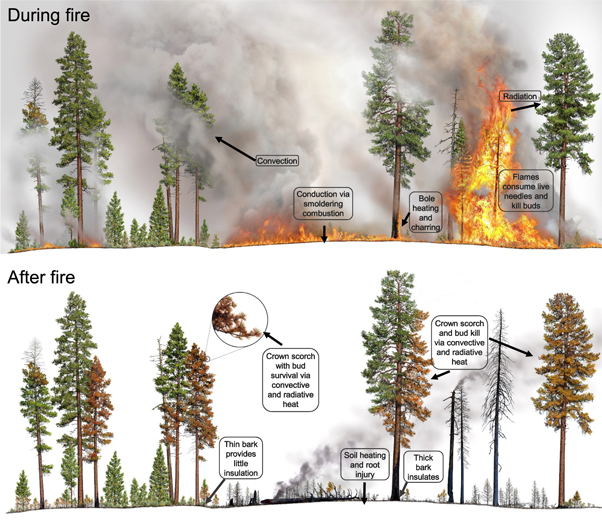
The assumed mechanism of direct tree death from fire is cambium necrosis via heat transfer to the crown, stem, and root tissue (figure 1) (Dickinson and Johnson 2001, Michaletz and Johnson 2007). Heat transfer occurs by convection, conduction, and radiation and all three processes can cause tree injury and mortality. Temperatures ≥60 °C cause immediate tissue death, although longer exposure at lower temperatures can also be lethal (Van Wagner 1973, Michaletz and Johnson 2007, Kelsey and Westlind 2017). Biophysical process models generally model heat transfer effects to a tree modularly, separating heat-caused injuries to the crown, stem, and root tissues (Michaletz and Johnson 2006, Michaletz and Johnson 2008, Kavanagh et al 2010, Chatziefstratiou et al 2013), as complete death to any of these tissues will result in tree death or top-kill if the tree is a resprouter. Although partial injuries to multiple parts of the tree can also lead to mortality, these interactions and indirect effects are not currently incorporated into any process models. We summarize the effects of heat transfer on a tree's crown, stem, and roots below, but refer readers to Dickinson and Johnson (2001) and Michaletz and Johnson (2007) for more detailed descriptions of combustion processes and direct fire-caused tree mortality.
Figure 1. Heat is transferred to living tissues of trees during fire (top panel), resulting in injuries to different parts of trees after fire (bottom panel). Fire causes injuries to different parts of trees—buds, foliage, cambium in the stem, and roots—through three different heat transfer processes. Combustion directly consumes live foliage and buds, small live branches, and small trees and causes tissue death. Convection, the movement of hot air—and radiation, heat traveling as energy waves, causes tissue death when temperatures are ≥60 °C for 1 s. Bole heating: Heat is conducted through the bark of trees, but because bark is a poor conductor it insulates the live cambium underneath from heat. Thick bark insulates larger trees of some species, while thin bark provides little insulation on smaller trees and thin-barked species. Soil and root heating primarily occurs through conduction during smoldering combustion of duff and large logs. Graphics by R Van Pelt.
Download figure:
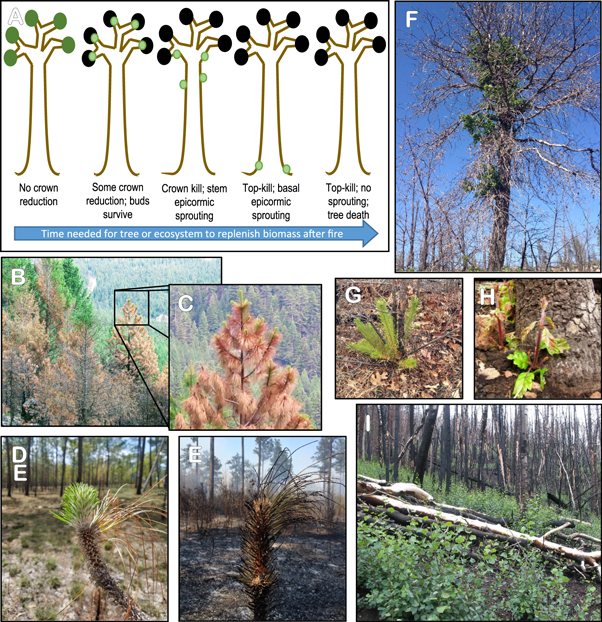
Standard image High-resolution imageTree crowns consist of foliage, buds, and branches. Convection is the dominant heat transfer process causing necrosis to crown tissue (Van Wagner 1973, Dickinson and Johnson 2001, Michaletz and Johnson 2006). Heated air in the fire plume can kill foliage and vascular tissue in buds and branches (figure 1). Fire-caused necrosis to the crown is often lumped into one estimate of injury called crown scorch (figures 2(A), (B)), but the difference between the amount of foliage versus buds killed can be large for some species (figures 1, 3) (Michaletz and Johnson 2007). Fires causing 100% bud necrosis, which also implies 100% foliage necrosis, result in immediate tree death in non-sprouting species. Partial bud necrosis reduces short-term photosynthetic capacity, necessitates mobilization of stored nonstructural carbohydrates (NSC) to rebuild foliage, and indicates heating to the surrounding branches and stem.
Figure 2. Fire-caused tree mortality results from injuries to the crown, bole, and roots. Injuries to the crown: injuries to foliage and buds occur due to direct consumption during the fire, and convective and radiant heating during the fire which causes tissue death. The portion of the crown foliage killed (A), (B) is termed crown scorch and develops a characteristic red color soon after fire. Bud kill is typically assumed to equal crown scorch. Injuries to the bole and resistance to bole injuries: bark thickness, char depth, height, and the proportion of the circumference of the bole charred are indirect estimates of potential injury to the living cambium between bark and wood. Thick bark (C) protects vascular cambium and epicormic buds, increasing survival from fire. Even low-intensity fires kill the cambium of thin bark species (D). Direct measurement requires bark removal to determine if the cambium is dead (E; at arrow). Injuries to roots. Consumption of ground and surface fuels adjacent to the tree may be an important variable in ecosystems with deep accumulations of fuel (F). A surface fire burns near the bole of a tree (G). Thermal image of smoldering combustion near tree base after flaming has stopped (H). (Warmer colors = higher temperatures.)
Download figure:
Standard image High-resolution imageFigure 3. Fire-caused mortality models usually only predict top-kill, but resprouting has important consequences for rates of ecosystem recovery after fire. (A) The ability of trees to recover biomass after a given level of injury varies with species' traits. (B)–(D) Some species have protected or large buds that can survive temperatures that scorch and kill surrounding foliage, allowing branch survival and crown recovery. (E) Even large buds can be killed by high-intensity fire and direct flame contact. (F) Epicormic sprouting allows recovery along a tree's main stem, (G)–(H) stem base, and from (I) root suckering after top-kill. These adaptations to fire make it more likely that plant will photosynthesize, repair damage, replenish NSC reserves, and recover after fire-caused injuries.
Download figure:
Standard image High-resolution imageHeat transfer to the tree bole or stem occurs through conduction and radiation (figures 1, 2(D) and (E)) (Dickinson and Johnson 2001, Michaletz and Johnson 2007). Damage to the conductive tissues in the stem may be the most important primary cause of mortality in small trees and thin-barked species (Michaletz and Johnson 2008, Lawes et al 2011a, 2011b). Heating to the stem can damage phloem and xylem, and thus impair translocation of photosynthates to roots and water and nutrients to the crown (respectively), leading to eventual death (Midgley et al 2011). Bark thickness is an important determinant of tree resistance to fire (Brando et al 2012, Pausas 2015, Pellegrini et al 2017). Thick bark serves to protect the underlying vascular cambium and epicormic buds from fire and is the primary bark trait influencing heat transfer to the cambium (Bova and Dickinson 2005), though species have developed other adaptations that increase resistance to fire. For example, the relatively thin-barked eucalypts have higher than expected resistance to fire compared to other Australian species with thicker bark due to deeply buried buds that allow resprouting after fire (Lawes et al 2011a, 2011b).
Long-term smoldering combustion can conduct heat through soil, leading to lethal temperatures that kill roots and mycorrhizae. This impact decreases water transport and nutrient acquisition in the short-term and results in NSC drains to rebuild lost roots (Varner et al 2009, O'Brien et al 2010, Taudière et al 2017). Fire-caused tree mortality from root death alone is likely uncommon, as mineral soil is a poor conductor of heat and forest floor organic layers insulate soil and roots from flames (Hartford and Frandsen 1992, Hood 2010). The long-term heating required to kill roots would almost certainly also impact the tree stem, making any resulting tree death a combination of injuries to the roots and stem (figures 1, 2(F) and (G)). Although heat transfer to roots is less understood than to the crown or stem, there are models of soil heating from fire (Campbell et al 1994, Campbell et al 1995) that are used with the assumption that all roots located in the zone of lethal heating (i.e. ≥60 °C) are killed. This is likely an invalid assumption for larger roots (Michaletz and Johnson 2007).
Although it is widely recognized that fires can kill trees directly through heat injuries to tissues in the roots, stems, and crown, little physiological research has been conducted to identify cellular-level mechanisms causing mortality (Michaletz and Johnson 2007, Kavanagh et al 2010). This is perhaps unsurprising since even in the absence of fire, our current understanding of how trees die is limited (Hartmann et al 2015). Emerging physiological research on the mechanisms causing drought-induced tree mortality, described below, may also extend to fire-induced tree mortality.
All plants must maintain hydraulic conductivity and NSC pools to survive; therefore, focusing on hydraulic integrity and NSC provides a mechanistic framework to test the impacts of stress and disturbance on individual plant productivity and biomass (Anderegg et al 2015, Venturas et al 2017). Reduction of whole-plant hydraulic conductivity by more than 60% due to cavitation leads to hydraulic failure and death (Adams et al 2017b). Trees are most susceptible to cavitation at the ends of branches and roots, which act analogously to circuit breakers to relieve water tension and maintain hydraulic integrity of the main stem (Johnson et al 2016). The extreme temperatures trees experience during fire from the convective plume can cause vapor pressure deficits in foliage strong enough to cause cavitation, leading to immediate tissue death, or long-term reductions in hydraulic conductance (Kavanagh et al 2010, Midgley et al 2011), that can increase susceptibility to future stresses (e.g. drought, bark beetles). Experiments of tree stem heating show decreased sap flow, net photosynthesis, and stomatal conductance as early indicators of eventual tree death (Ducrey et al 1996, Smith et al 2017), which also suggest heat-induced cavitation occurs. In addition to causing cavitation, heating also may impair hydraulic conductivity by causing irreversible xylem deformation (Balfour and Midgley 2006, Michaletz et al 2012, Bär et al 2018). Experimental testing supports plume-induced hydraulic failure via cavitation, but evidence of xylem deformation is equivocal (West et al 2016, Bär et al 2018). Additional research with more species, under a wider range of heating scenarios, including actual fires are needed for an improved understanding of the cellular-level physiological damage caused by fire. For example, most studies to date have immersed cut stems into a water bath for the heat treatment, but it is unlikely that this method of heating accurately mimics conditions occurring during wildland fires. These initial experiments are important for testing of hypotheses, but our ability to measure physiological impacts remains rudimentary and basic methodologies need substantial improvement. Beyond these few experiments of heat effects to the stem and leaf/branch, there exists substantial uncertainty over the influence of root and mycorrhizal injuries on trees' ability to repair aboveground injuries from fire (Taudière et al 2017) or how fire affects long-term hydraulic vulnerability and sprouting ability in resprouting species (Pausas et al 2016).
Damage to photosynthetic tissues and the need to replace them constrain a tree's ability to maintain NSC pools by reducing acquisition of carbon through photosynthesis and increasing demand of carbon for repair. Plants must assimilate carbon and maintain NSC pools for myriad vital functions, including maintaining hydraulic integrity, defense, and respiration (Dietze et al 2014). In one of the only studies of fire effects on NSC, long-duration soil heating reduced root NSC concentration in coarse storage roots and led to short-term reductions in tree radial growth (Varner et al 2009). These effects persisted at least a decade post-fire, reducing defensive resin ducts and diminishing radial growth during subsequent droughts (Slack et al 2016). Death by carbon depletion seemingly can occur if NSC levels fall below critical thresholds (Martínez-Vilalta et al 2016), but evidence of these thresholds and the mechanisms involved are limited and seem inconsistent across tree species (Adams et al 2017b). Moreover, significant gaps in understanding remain regarding how to measure NSCs, how they are used for different plant functions, and their roles in buffering the impacts of abiotic and biotic stress and disturbance (Meir et al 2015).
Indirect causes of fire-induced tree mortality
The interactive nature of factors contributing to fire-induced tree mortality, particularly delayed mortality, suggests analogies to mortality patterns in unburned forests (figure 4). In unburned forests, tree mortality is often the result of multiple stressors, including competition, pest and pathogen activity, and short- and long-term climatic fluctuations (Das et al 2016). These interactions are likely also important in low- and mixed-severity fire regimes, in contrast to high-severity regimes where trees are typically immediately killed by fire or top-killed, in the case of resprouting species. Indicators of increased stress include decreased radial growth, reduced leaf area, low hydraulic conductance, and low NSC reserves (see above). For example, in a large study of unburned trees, trees that died had reduced tree growth in years preceding death compared to surviving trees (Cailleret et al 2016). These above lines of evidence, coupled with studies showing post-fire mortality may be worsened by pre-fire competition (van Mantgem et al 2018), suggest that low pre-fire growth may be an indicator of susceptibility to fire-caused injuries.
Figure 4. Pre-fire conditions such as drought and plant competition can increase vulnerability to fire through increased plant-stress and also by influencing the physical fire environment and increasing local fire intensity. (A) Pine tree showing signs of extreme drought stress. (B) Comparison of needles formed before acute drought stress in 2012 and in 2015 during drought. (C) A coast redwood/tanoak forest, impacted pre-fire by the invasive pathogen Phytophthora ramorum (sudden oak death) and then burned, contributing to increased tree mortality. Photo credit: Howard Kuljian. (D) Pitch tubes and resin streaming from bark beetle attacks after fire. (E) Bark removed from a dead white fir tree that burned 2 years prior, had bark beetle galleries from an attack pre-fire, and a white mycelia fan indicating Armillaria root rot infection—all three factors may have contributed to mortality. Photo credit: Tucker Furniss. (F) Dense, fire-excluded forests decrease water availability and can lead to extreme fire behavior and vulnerability to bark beetle-caused mortality. (G) High-intensity crown fire burning through a drought-impacted forest with recent tree mortality (i.e. red trees on left side of photo; photo credit: Carrie Vernon, NPS).
Download figure:
Standard image High-resolution imageDrought is a common stressor for conifers in the western US, and pre-fire drought has been shown to increase the likelihood of tree death following fire (van Mantgem et al 2013, van Mantgem et al 2018). This may become an important consideration in dry forests, where acute and chronic droughts are increasingly coupled with high temperatures and have been linked to forest die-backs even without fire (Allen et al 2015, Adams et al 2017a). In addition, fire-caused injuries can decrease subsequent growth (Slack et al 2016, Sparks et al 2017), reducing productivity and possibly increasing the likelihood of tree death in future fires.
Insects and pathogens can also increase stress before fire—decreasing growth, NSC reserves, and impairing hydraulic conductivity—as well as causing additional delayed mortality in trees that otherwise would have survived fire injuries (Parker et al 2006, Kane et al 2017b). Several post-fire mortality models include beetle attacks (Woolley et al 2012, Hood and Lutes 2017). Because bark beetles require living trees with healthy phloem to reproduce, trees killed immediately by fire are not suitable hosts. Host suitability and attraction after fire varies by tree and bark beetle species, but in general, bark beetles attack and kill trees with intermediate levels of both crown scorch and cambium injury, or higher levels of either crown scorch or cambium kill (Jenkins et al 2014). As fires can change both tree resistance to beetles after low-severity fire (Lombardero and Ayres 2011, Hood et al 2015, Kane et al 2017b) and local bark beetle population pressure (Jenkins et al 2014), post-fire mortality models may need to account for bark beetles to make accurate predictions. Additional basic research on the short and long-term impacts of fire on host tree defenses and attraction is needed. Likewise, research on the interaction of fire and pathogens is extremely limited (Parker et al 2006), though two studies have shown fire-pathogen interactions can cause additional mortality through synergistic feedbacks from infections both before and after fire (Metz et al 2013, Maringer et al 2016). These interactions are poised to increase with increasing rates of the introduction of non-native insects and diseases (Aukema et al 2010) and climate change-associated increases in bark beetle pressure (Kolb et al 2016).
Most previous studies of fire-caused tree mortality have ignored density-dependence (Wooley et al 2012, Grayson et al 2017), modeling tree mortality as if individual trees were alone in space. Density can influence fire-induced tree mortality through two main ways: by affecting local fire behavior and through competition with neighboring trees. Forest structure influences fuel arrangement, local moisture, and fire-atmosphere interactions, thereby also influencing fire behavior that causes direct injury to trees (Agee and Skinner 2005, Stephens et al 2012). Indirectly, competition can influence fire-induced tree mortality (Das et al 2011), since it limits access to aboveground and belowground resources, thereby increasing stress. Increased fire-induced mortality from competition has been documented (Platt et al 1998, Yu et al 2009, Hammond et al 2016, van Mantgem et al 2018), with slower growing trees more likely to die given the same level of fire-caused injury than faster growing trees (van Mantgem et al 2003, Nesmith et al 2015). Alternatively, decreases in tree density and competition after fire can increase resource availability, potentially compensating for the short-term impacts of injury to release surviving trees (Alfaro-Sánchez et al 2016). Additional research is needed using density-dependent models to determine how pre- and post-fire competitive interactions influence mortality.
Empirical modeling approaches
Most research into fire-induced tree mortality is empirical and uses logistic distribution models where the binary outcome is tree status, either alive or dead. These empirical logistic regression models are used in fine-scale software tools for fire management planning (Reinhardt et al 1997, Reinhardt and Crookston 2003, Reinhardt and Dickinson 2010, Andrews 2014), process-based landscape succession models (Sturtevant et al 2009, Keane et al 2011), and dynamic global vegetation models (DGVMs) of the terrestrial carbon cycle (Thonicke et al 2010, Kelley et al 2014) (table 1). Empirical models are applied to predict mortality at one of two scales: the probability of individual tree mortality or the proportion of tree mortality by size class and species (or functional type) (Hood et al 2007).
Table 1. Commonly used post-fire tree mortality modeling applications by scale. See suggested primary references for detail on models and assumptions used in each software system.
| Spatial scale | Temporal scale | Application and primary user | Software systems and primary references | |
|---|---|---|---|---|

|
Tissue | Seconds—minutes | Predicting lethal crown kill height and stem (i.e. cambium) kill | FOFEM submodel (Van Wagner 1973); FireStem2D (Chatziefstratiou et al 2013) |
| Researcher | ||||

|
Individual tree | 0–3 years | Predicting mortality for post-fire salvage logging | FOFEM (Reinhardt et al 1997, Hood and Lutes 2017) |
| Manager; researcher | ||||

|
Stand-Forest | Years—decades | Prescribed fire planning; estimating different treatment and expected wildfire effects related to tree-mortality objectives | FOFEM (Reinhardt et al 1997); FFE-FVS (Reinhardt and Crookston 2003) |
| Manager; researcher | ||||
|
Landscape | Decades—centuries | Spatially explicit predicted changes in tree populations, cover type, and carbon pools under different management, climate, and disturbance regime scenarios | Fire-BGC (Keane et al 2011); |
| Researcher | LANDIS-II (Sturtevant et al 2009) | |||
|
Biome-Global | Years—decades | Estimating carbon pools, NPP, and vegetation dominance; examining controls driving patterns in cover type and productivity | LPX-Mv1 (Kelley et al 2014); SPITFIRE (Thonicke et al 2010) |
| Researcher | ||||
Post-fire tree mortality (i.e. top-kill) has been traditionally modeled as a function of fire injury (e.g. crown scorch, bark char) and tree characteristics (e.g. species, bark thickness, height, and diameter) (figure 5(A)) (Ryan and Reinhardt 1988, Woolley et al 2012). Some models also incorporate fuel consumption and fire residence time (Peterson and Ryan 1986, Thonicke et al 2010). Crown scorch has been shown to be most important predictor of fire-caused conifer mortality (Sieg et al 2006), but is less influential for resprouting species (Lawes et al 2011b). The simplest scenario to predict is when a surface or crown fire completely consumes the tree crown (i.e. terminal branches and foliage) via direct flame contact, killing all crown meristematic tissue and causing immediate tree death. In other words, when flame length exceeds tree height, tree death can be predicted relatively accurately. Mortality from convective and radiative heating from surface fires is more challenging to predict when portions of the tree crown remain alive, especially for conifer species with large buds that can survive even when needles are scorched and killed. Predictive computer systems (Reinhardt and Crookston 2003, Andrews 2009, Thonicke et al 2010) typically estimate crown kill height (also called scorch height as crown scorch and kill are undifferentiated) from fire intensity based on flame length using the equation developed by Van Wagner (1973); some systems also allow direct input of anticipated crown kill height (Reinhardt et al 1997). Crown kill height is then used to calculate crown scorch as either a percentage of the crown length or volume (Reinhardt et al 1997). Less commonly, the percent of crown length or crown volume scorched is estimated directly via post-fire field observations and used as a model input (Lutes 2012). Some models use bole or stem char height in addition to or in replacement of crown scorch (Hély et al 2003, Woolley et al 2012), especially for deciduous trees (Brando et al 2012).
Figure 5. (A) Conceptual model representing current modeling approaches to predicting post-fire tree mortality. Common inputs are shown in ovals. Additional, less-common inputs are shown in boxes. Black arrows represent causal pathways that are usually included in the models, while gray arrows include pathways that are included less often. No physiological processes are explicitly represented. (B) Conceptual model of the physiological processes that contribute to post-fire tree growth and mortality. Species are first grouped and assigned response functions to heat flux of living tissues (tan box), susceptibility to insects and disease, and competition tolerance (blue boxes). Short-term damage (red boxes) leads to long-term impairment of physiological status (green boxes), specifically the depletion of carbohydrate reserves or decreases in hydraulic function. Either of those factors, or their combined influence, can cause changes to tree growth and immediate or delayed tree mortality. Exogenous factors (blue boxes) can directly cause damage, or alter the physical environment (purple boxes) leading to damage, impaired functioning, or improved functioning. Positive correlation (+), negative correlation (−), Species dependent (+/−).
Download figure:
Standard image High-resolution imageBark thickness or tree diameter at breast height, which is strongly correlated with bark thickness, is used in many models as a surrogate for resistance to basal heating (Ryan and Reinhardt 1988, Hood et al 2008, Kelley et al 2014) because bark thickness has the largest influence on heat transfer to the underlying cambium due to bark's poor conductivity (Bova and Dickinson 2005). DGVMs and some landscape models that predict fire-caused mortality values by large grid cells and cohorts use a simplified approach with constant parameters for scorch height and bark thickness based on broad plant functional type or species grouping (Thonicke et al 2010).
Advantages and limitations to empirical models
The current structure of empirical models relies on simple, external measures of observable tree injuries that are proxies for the fire's actual heat flux (figure 5(A)). This structure makes it possible to predict fire-caused tree death for a range of flame lengths as long as species, tree diameter, and height are known. For non-resprouting species and those where crown scorch and bud kill are equal, the current framework seems to work reasonably well if the bark thickness coefficient (i.e. predicted relationship of bark thickness based on species and diameter) is correct and delayed mortality due to insects is not a factor (Hood et al 2007, Grayson et al 2017, Kane et al 2017a). When the above conditions are not met, model performance is reduced due to over-simplification of species responses, extrapolation beyond the models' underlying data, the inability to quantify long-term effects on tree-to-ecosystem productivity (see below), and difficulty correctly incorporating indirect effects on mortality.
Many fire behavior and effects software use fire-induced mortality algorithms developed from a limited number of conifers and use a simplified crown-injury response to predict mortality for numerous additional deciduous and conifer species (Reinhardt et al 1997, Reinhardt and Crookston 2003, Thonicke et al 2010, Andrews 2014). These software systems use a sub-model estimating crown scorch height from flame length based on one study of four species (Van Wagner 1973). Further error is introduced if crown length scorched must be converted to crown volume scorched to meet model requirements (Peterson 1985, Hood et al 2007). The scorch height model cannot differentiate between foliage kill and bud kill, an important distinction that can modify tree survival through post-fire crown recovery (Hood et al 2010). As a result, the most commonly used empirical models predict mortality of several western conifers with reasonable certainty, but perform poorly for others, especially when simplified models with fewer inputs are used over more complex ones (Hood et al 2007, Kane et al 2017a). Model accuracies have not been evaluated across size ranges or for the majority of tree species, especially the diverse angiosperms. The focus on western US conifer species explains why crown scorch and bark thickness are the primary model inputs. The former predictor is problematic for deciduous species, as crown scorch cannot be assessed during the leafless dormant season. Therefore, some models use bole char in lieu of crown scorch (Brando et al 2012, Keyser et al 2018), but unlike crown scorch, bole char cannot be predicted by standard wildland fire behavior models. In addition, surface fuel consumption at the base of trees is not typically included in models, but may be of particular importance for shallow-rooted species, seedlings, and in long-unburned areas (see below).
Empirical mortality models predicting top-kill of the main stem account for stem resistance to fire by using either a species-specific model or a multi-species model with species-specific bark-thickness scaling functions based on tree diameter. The relative investment of bark thickness to stem diameter varies by species (Pausas 2015, Pellegrini et al 2017). Bark thickness-tree size relationships used in US fire effects software systems are linear, which is supported by research showing bark thickness scales linearly with diameter in some common western US conifers from small-to-large trees (10–50 cm basal stem diameter) (van Mantgem and Schwartz 2003) and angiosperms (Rosell 2016). However, bark thickness-tree size relationships generally have not been validated for many species and across diameter ranges. Jackson et al (1999) illustrated several relationships between size (or age) and bark thickness allocation in North American Pinus and Quercus species, with some species allocating preferentially to bark early, some linearly, and others only as adults (a sigmoidal pattern). Additionally, the thickness of the outer versus inner bark is likely more important for protection from wildfire and the ratio of these bark components varies by species (Pausas 2015, Rosell 2016). These unaccounted differences can under- or over-predict bark thickness, which can distort tree mortality estimates (Zeibig-Kichas et al 2016). These shortcomings are further exacerbated by the diversity in bark morphology and moisture that can influence heat transfer to the underlying tissues (Chatziefstratiou et al 2013).
Well-known exceptions to coniferous tree responses to fire include bud survival when foliage is scorched and sprouting from epicormic buds and below-ground bud banks (Meier et al 2012, Burrows 2013, Pausas et al 2018) (figure 3). For example, burning during dormant seasons or periods when active growth has ceased can reduce bud kill and subsequent tree mortality (Harrington 1987, Valor et al 2017) and indeterminate-growth species can sustain higher levels of crown loss if burning occurs at the beginning of the growing season compared to later in the season (Weise et al 1987). Likewise, species that can resprout from epicormic buds can survive higher levels of crown injury (Bond and Midgley 2001). Moreover, resprouting following top-kill (i.e. mortality of the aboveground ramet but not the genetic individual) confuses the very concept of fire-caused mortality (Midgley et al 2010). In ecosystems where resprouting following top-kill is common (e.g. savannas, temperate deciduous forests), ramets may still follow standard patterns of mortality (e.g. bark thickness confers fire resistance), but successional patterns and recovery times are faster than ecosystems dominated by non-sprouting species (Kelley et al 2014, Pausas and Keeley 2017).
Empirical models are inherently limited to the underlying data distributions, creating uncertainty in accuracy when extrapolating beyond initial data ranges and for novel conditions. The data used to develop current empirical models have limited scope in terms of species, sizes, and life history strategies. Furthermore, the data were collected primarily from fires occurring in the 1980s to the early 2000s, and therefore performance has not been evaluated under the hotter climate anticipated in many areas. Because increased temperatures exacerbate plant moisture stress via increased vapor pressure deficits (Breshears et al 2013), it is critical that we further our understanding of fire-drought interactions on tree death. The overwhelming focus of tree mortality research has been on moderate-sized trees, with very few studies including small trees (i.e. ≤10 cm DBH), but fuels treatments and prescribed burning objectives often involve killing small trees. It would be useful to know how effective such prescribed burns are for killing small trees and if models need re-parameterization for predicting small tree mortality. Limited evidence suggests that higher levels of damage may be needed to cause mortality in smaller trees (Engber and Varner 2012). While crown injuries are still influential for small trees, basal scorch and ground char can be more important because of thin juvenile bark (van Mantgem and Schwartz 2004, Battaglia et al 2009). Likewise, large, old conifers often experience elevated mortality after fire, through a combination of factors: damage to roots from smoldering combustion in fuel accumulations near the tree base, fire burning in existing fire scars, low leaf area relative to carbon demands, and decreased hydraulic conductance (Kolb et al 2007, Hood 2010). In addition, some bark beetle species preferentially attack larger-diameter trees, thereby increasing post-fire mortality of these trees that likely would have survived based solely on fire-injuries (Hood and Bentz 2007, Kolb et al 2016). To accurately predict mortality of small or very large trees, different or additional predictor variables may need to be incorporated into models.
Perhaps the most limiting aspect of current empirical models is that predictions are binary–either the tree survives or dies from fire. This approach is appropriate for predicting individual tree mortality, but constrains modeling how sub-lethal fire-caused injuries affect tree growth. Fire-driven changes in stand structure through loss of photosynthetic biomass and reductions in hydraulic conductivity due to injury that further constrains photosynthesis can alter stand and ecosystem-scale gas exchange and productivity patterns for years (Nolan et al 2014, Smith et al 2016). Although spatially explicit ecosystem process models already include algorithms of fire-induced tree mortality (table 1) and factor changes in the competitive environment on subsequent projections of tree growth, additional research could allow inclusion of fire injury on post-fire growth and vulnerability of surviving trees, as shown in figure 5(B). In summary, empirical models can effectively predict binary mortality outcomes, but due to the lack of widespread model evaluation and uses that often extrapolate far beyond models' scopes, we do not know how well empirical models work for numerous species, tree sizes, and geographic regions, nor can we predict fire-caused changes in productivity.
Fire-induced tree mortality is governed by a complex suite of direct and indirect factors that simple linear models cannot easily accommodate. Past disturbance, stress, bark beetles, fungi, competition, season, and soil type may all impact fire-induced tree mortality (Hood and Bentz 2007, Youngblood et al 2009, Fettig et al 2010, Das et al 2011), making delayed tree mortality difficult to predict (Eidenshink et al 2007, Kane et al 2017b). The challenges presented by multiple interactions mirror difficulties in describing instances of tree mortality in unburned stands, which also may be caused by the effects of multiple stressors (Das et al 2016). While attempts have been made to improve post-fire tree mortality model performance by adding additional variables, such as species identity, pre-fire climate, season of fire, tree vigor, insects and pathogens, or other local conditions (Varner et al 2007, Woolley et al 2012, van Mantgem et al 2013, Nesmith et al 2015), these alternative models were developed from smaller, regional datasets and vary widely in inputs, making comparisons difficult. Moreover, attempts at model evaluation and tests of transferability to different regions have been restricted to a few species and geographic locations (Hood et al 2007, Woolley et al 2012, Ganio and Progar 2017, Grayson et al 2017, Kane et al 2017a), limiting the applicability of these models outside the original range of data and creating challenges to incorporating them into widely used fire effects software programs.
A roadmap for future research and model implementation
Despite the limitations of empirical modeling approaches, they are useful and many of the limitations can be resolved or improved. The following research priorities should be explored simultaneously to advance our understanding of and ability to predict fire-induced tree mortality (box 2). First, improvement to existing empirical models and development of new empirical models should continue, so that managers who rely on these models to make decisions can do so with higher accuracy–given an understanding of model limitations and uncertainty in their predictions. Software systems have embedded post-fire tree mortality models that predict mortality far beyond the data used to parameterize the models. Therefore, benchmark datasets are needed to allow model evaluation and quantify uncertainty across species, sizes, and geographic regions. Second, research is needed to make the connections from fire behavior, to energy release, to tissue damage of specific tissues, to the effects of the fire on whole plants—i.e. mortality, survival with reduced fitness, or survival with full recovery. The dose-dependent response approach developed for quantifying reductions in productivity associated with fire-related tree injuries rather than a binary outcome (Smith et al 2016, Sparks et al 2016) offer great promise. Fire behavior models based on fluid dynamics are beginning to model heat flux at scales relevant for plant tissues, but the connection between heating and physiological damage in different tissues, and how that varies with ontogeny, phenology, and morphology is not understood. Detailed knowledge of individual species responses will be limiting. We suggest that grouping species based on similar traits (e.g. bark thickness, sprouting ability, morphological architecture, and hydraulic strategies) and developing functional responses to heat flux, insect and disease, and competition could offer an immediate improvement to the existing empirical modeling framework. Third, we need a better understanding of the basic physiological impacts of fire on hydraulic failure and NSC maintenance and how these impacts on individual tissue scale to affect whole tree functioning and death (Venturas et al 2017, Michaletz 2018). Also, biophysical models only account for direct fire effects, but incorporating indirect effects such as insects and competition would improve understanding of delayed tree mortality. Focusing on these lines of research will help answer some of the remaining outstanding questions about fire-induced tree mortality (box 2), and improve our ability to predict fire-induced tree mortally both at immediate time scales and under novel future climates.
Box 2. Outstanding questions about fire-induced tree mortality.
| What is the uncertainty and predictive accuracy in existing empirical models predicting tree death, and how does it vary across species, geographic regions, and tree sizes? |
| An active information archiving network is essential to provide benchmark datasets for model evaluation and to promote collaboration and discourse about research findings. |
| How does ontogeny, phenology, and morphology modulate tissue sensitivity to lethal heating? |
| Morphological and chemical characteristics influence heat transfer, water loss, vulnerability to cavitation, etc, but limited work has been conducted at temperatures experienced during wildland fires. Such basic work is needed across a range of species, sizes, and growing periods to allow improvement in predicting tree mortality and productivity for dormant versus growing season burning, differential species sensitivities to fire, and fire-drought interactions. Tissue lethal exposure time is unresolved for the elevated temperatures that occur in fires. Instantaneous tissue death is assumed at 60 °C, but what causes death at lower temperatures over longer time periods ? Models should account for heat flux to tissues from flaming and smoldering combustion fire phases. |
| Do injuries to different tree tissues (crown, stem, and roots) cause mortality in additive or synergistic ways? |
| Currently, separate process models exist for crown and stem components, but not for roots. Coupled physical-physiological model integration is needed to predict whole-tree mortality and differential tissue death to allow top-kill with and without epicormics and root sprouting. Conduction of heat to underlying mineral soils and roots is poorly characterized. |
| How does pre-fire tree state due to drought stress (prolonged and acute), disease, competition, etc influence NSC levels, vulnerability to cavitation during fire, repair, and tree mortality? |
| Knowledge of pre-fire state and post-fire resource allocation is needed to develop tree-level process-based models and improve predictions of empirical models. These models should incorporate post-fire stress or release from competition, indirect fire effects, and climate. |
| Are there logical functional groupings based on species traits and vulnerabilities? |
| Grouping species with similar plant architecture, physiological strategies, or functional responses could allow development of more general response functions to heat flux, drought stress, insects and disease, and competition without needing exhaustive research for individual species. |
Although current logistic models can accurately predict mortality for some species (figure 5(a)), they are far removed from the actual physiological and ecological processes that cause immediate and delayed post-fire mortality (figure 5(b)). Other empirical analysis techniques that can detect nonlinearities and contingent relationships (e.g. classification and regression trees, path analysis) could help identify interactions and provide insight into the mechanisms of fire-induced tree mortality, laying a foundation for future advances in process-based models of fire-induced mortality. Some attempts to model fire-induced mortality with path analysis have been made (Menges and Deyrup 2001, Youngblood et al 2009, van Mantgem et al 2018). These models allow better accounting of the strength and direction of direct and indirect influences on post-fire tree mortality, but also require a priori hypotheses of effects and interactions. Applying different modeling techniques does not necessarily mean dauntingly complicated models. For example, the likelihood of death increases sharply around 70% crown scorch in some conifers, which has led to the use of piecewise regression to identify simple thresholds of mortality in predictor variables (Fowler et al 2010, Grayson et al 2017).
Existing research and data already provide a foundation upon which existing models and planning tools could be improved to make more accurate predictions and explicitly quantify uncertainty in predictions. Planning tools could report expected ranges of mortality (i.e. 95% C.I.) and allow for the inclusion of additional observations (e.g. bark beetle attacks, cambium kill) where a higher degree of model accuracy is desired. Given the development of easy to acquire gridded climatic data, such as PRISM (Daly et al 2002) or TerraClimate (Abatzoglou et al 2018), incorporating climatic variables, such as water stress, into widely used fire effects software could provide expected mortality levels given a range of pre-fire climates. Also, older models deserve to be re-evaluated: the empirical model developed by Peterson and Ryan (1986) allows for different lethal heating thresholds in the crown due to seasonal effects and crown morphology. Though the provided temperatures are unsubstantiated, this model provides a way forward, linking fuel consumption and fire behavior to predict resulting tissue injury and tree death.
Ultimately, a whole-tree coupled physical-physiological model is necessary to predict physical heat transfer and resulting fire effects based on living plant physiological traits, and thus the prediction of fire-induced tree mortality and growth. However, such a model would still have a host of limitations and uncertainties (Adams et al 2013). While a whole-plant process model is not yet available, independent, tissue-specific models exist to predict circumference and height of cambium kill (Chatziefstratiou et al 2013) and differences in crown scorch and bud kill heights (Michaletz and Johnson 2006).
The wide-ranging applications associated with fire-induced tree mortality (table 1) do not lend itself to a one-size-fits-all approach, and it seems unlikely that empirical models will be replaced due to the need to balance model complexity with model application. Instead, empirical models should be refined for use in land management applications in the near-term, while heating and physiological process models should be developed and linked to create a hybrid-based approach to improve mechanistic understanding to predict mortality under novel scenarios.
Accurate predictions of fire-induced tree mortality with quantified uncertainty are needed for models used in planning, post-fire management, predicting future landscape dynamics, and feedbacks to the global carbon cycle. Fire is expected to become increasingly prevalent in many ecosystems due to climate change (Flannigan et al 2009, Jolly et al 2015). Direct fire effects may be exacerbated during periods of climatic stress, such as drought, where xylem function may be further compromised or more easily disrupted by heat effects of fire in stems and crowns (Kavanagh et al 2010, Michaletz et al 2012), as well as potentially increased indirect fire-induced mortality due to bark beetles (Kolb et al 2016). Many critical questions remain about fire-induced tree mortality (box 2). Taken together, these reasons underscore the need for increased research on the fundamental processes of post-fire tree mortality coupled with the development of better management tools.
Acknowledgments
We acknowledge funding from the Joint Fire Science Program under Project JFSP # 16-01-04-8. Discussions with Robert Mitchell, Matthew Dickinson, Joseph O'Brien, Robert Keane, Kathleen Kavanagh, Kevin Hiers, Tara Keyser, Adam West and others during the 'Fire-induced tree mortality: Empirical modeling, physiology, and integrative approaches' special session of the 2018 Fire Continuum Conference provided insight into knowledge gaps and improved understanding of fire-induced tree mortality. The comments of Jon Keeley and anonymous reviewers improved previous versions of this manuscript. All uncredited photos were taken by a co-author. Any use of trade names is for descriptive purposes only and does not imply endorsement by the US government.