Abstract
Exosomes, typically 30–150 nm in size, are lipid-bilayered small-membrane vesicles originating in endosomes. Exosome biogenesis is regulated by the coordination of various mechanisms whereby different cargoes (e.g. proteins, nucleic acids, and lipids) are sorted into exosomes. These components endow exosomes with bioregulatory functions related to signal transmission and intercellular communication. Exosomes exhibit substantial potential as drug-delivery nanoplatforms owing to their excellent biocompatibility and low immunogenicity. Proteins, miRNA, siRNA, mRNA, and drugs have been successfully loaded into exosomes, and these exosome-based delivery systems show satisfactory therapeutic effects in different disease models. To enable targeted drug delivery, genetic engineering and chemical modification of the lipid bilayer of exosomes are performed. Stimuli-responsive delivery nanoplatforms designed with appropriate modifications based on various stimuli allow precise control of on-demand drug delivery and can be utilized in clinical treatment. In this review, we summarize the general properties, isolation methods, characterization, biological functions, and the potential role of exosomes in therapeutic delivery systems. Moreover, the effective combination of the intrinsic advantages of exosomes and advanced bioengineering, materials science, and clinical translational technologies are required to accelerate the development of exosome-based delivery nanoplatforms.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Exosomes are a subset of extracellular vesicles (EVs) that are derived from various cells [1, 2]. Despite substantial advancements in the classification of EVs, they are generally divided into three major categories: microvesicles, apoptotic bodies, and exosomes [3–6]. These three categories are primarily distinguished by their morphology and mode of biogenesis. The particle size of microvesicles is generally 50–1000 nm, and they are generated via the budding of the plasma membrane [7]. Apoptotic bodies represent fragments formed during the process of programmed cell death and range from 50 to 5000 nm in diameter [8, 9]. In contrast, exosomes are small vesicles typically ranging from ∼30 to 150 nm in diameter, and exosome biogenesis involves in the endosomal pathway [10, 11].
Exosomes were originally hypothesized to be 'cellular dust' [12]. However, with advancements and more in-depth exploration, researchers have realized that exosomes serve as important mediators in intercellular communication [13, 14]. Exosomes participate in several physiological processes. Neuron-derived exosomes transfer miR-132 to endothelial cells to regulate the expression of vascular endothelial cadherin (VE-cadherin, also known as Cdh5), which is important for the maintenance of brain vascular integrity [15]. Oviduct- and uterus-derived exosomes contain PMCA4a, which contributes to the homeostasis of Ca2+ in sperm and prevents premature sperm capacitation [16]. Oligodendrocyte-derived exosomes can mediate signal transmission and are conducive to axonal health [17]. Exosomes also play important roles in immune responses [18, 19]. Based on the types of immune cells, exosomes may play a variety of regulatory roles in immune responses [20]. Mast cell-derived exosomes are rich in heat shock proteins 60 and A8, which contribute to the maturation of dendritic cells in mice [21]. Co-stimulatory molecules (such as CD40 and CD80) and major histocompatibility complex (MHC) class II from B cell-derived exosomes can activate CD4+ T cell clones [22]. Exosomes are also associated with pathological disorders [23, 24]. Glioblastoma cell-derived exosomes deliver specific proteins and mRNAs to recipient cells in lesions and stimulate the proliferation of glioma cells in vitro [25]. In an asthma model, epithelial cell-derived exosomes stimulate chemotaxis of undifferentiated macrophages. However, the inhibition of exosome proliferation leads to a reduction in monocytes and an improvement in asthmatic symptoms [26].
With an improved understanding of the functions of exosomes in intercellular communication, signal transmission, and immune homeostasis, studies on the application of exosome-based therapies in the various disorders have increased rapidly [27–29]. Most cells possess an exosome production mechanism, and exosomes may be taken up by surrounding cells or transported to other special sites and internalized by recipient cells [30, 31]. During this process, different cargoes (such as proteins and nucleic acids) in exosomes are transferred and regulate cellular function or gene expression in target cells [32, 33]. The structure of exosomes is similar to that of amphiphilic liposomes, and consists of an amphiphilic lipid bilayer and an aqueous core [34–36]. In drug-delivery systems, hydrophobic drugs are located in the bilayer, and hydrophilic drugs are scattered in the lumen [37–39]. Thus, exosome-based drug-delivery systems hold much promise.
Research has focused on the exosome properties responsible for physiological regulation and pathological modulation. However, a need for the exploration of the underlying principles of exosome biology has arisen because the underlying molecular mechanisms of exosome function remain unclear. Therefore, this review aims to summarize the biology, biochemical composition, uptake, isolation methods, characterization of exosome, and the potential of therapeutic delivery systems based on exosomes. Finally, we discuss targeted exosome-based delivery strategies, stimulus-responsive exosome delivery, and exosome-based liquid biopsy strategies.
2. The biogenesis of exosomes
Exosomes were first described in the 1980s, and research on exosomes has progressed rapidly, especially in the last 20 years [12, 40, 41]. Exosomes originate from the endosomal system (figure 1) [42]. The fusion of endocytic vesicles leads to the formation of early endosomes that contain cell surface proteins and soluble proteins from the extracellular environment [43]. Different cargoes, such as proteins, RNAs, DNAs or lipids, are sorted into multivesicular bodies (MVBs, later endosomes) by dynamically communicating with other organelles including the endoplasmic reticulum, mitochondrion, phagosome, trans-Golgi network, RNA granules, and micronuclei. After MVBs mature, they can fuse with lysosomes to be degraded or with the plasma membrane to secrete intraluminal vesicles (ILVs), also called exosomes, with a diameter ranging ∼30–150 nm in size. With an increased understanding of MVBs, we have gained new knowledge regarding their biological roles. Following the fusion of MVBs and autophagosomes, the resulting amphisomes move back to the plasma membrane to generate exosomes or are degraded by lysosomes [11, 44, 45].
Figure 1. The formation of MVBs is the central event in exosome biogenesis. Endocytic vesicle fusion forms early endosomes and further forms later endosomes. And then, different cargoes, such as proteins, RNAs, DNAs, or lipids, are sorted into later endosomes or MVBs by dynamically communicating with other organelles. After MVBs mature, they can fuse with lysosomes to be degraded or with the plasma membrane to secrete exosomes.
Download figure:
Standard image High-resolution imageThe formation of MVBs is the central event in exosome biogenesis, and the generation of ILVs is an especially crucial event [46]. To date, researchers have proposed various mechanisms for this process. Generally, these mechanisms mainly include the endosomal sorting complex required for transport (ESCRT)-dependent pathway and ESCRT-independent pathways [47, 48]. The ESCRT includes four distinct complexes (such as ESCRT-0, ESCRT-I, ESCRT-II, ESCRT-III, and the associated AAA ATPase Vps4 complex), which could mediate the formation of ILVs [49, 50]. Of note, some studies have reported that the biogenesis of MVB could also occur without ESCRT. ILV formation in MVBs continues despite the simultaneous silencing of four ESCRT-associated protein complexes [48], indicating the presence of ESCRT-independent pathways. The apoptosis-linked gene 2 interacting protein X (Alix) could combine with syntenin and recruit ESCRT-III and VPS4 to realize ILVs formation, which is based on the Alix-dependent pathway [51, 52]. In a word, multiple mechanisms mediate the formation of ILVs, and the pathways may function synergistically. A thorough understanding of the biogenesis of exosomes is indispensable because it contributes to the further development of novel exosome-based therapies.
3. Biochemical composition of exosomes
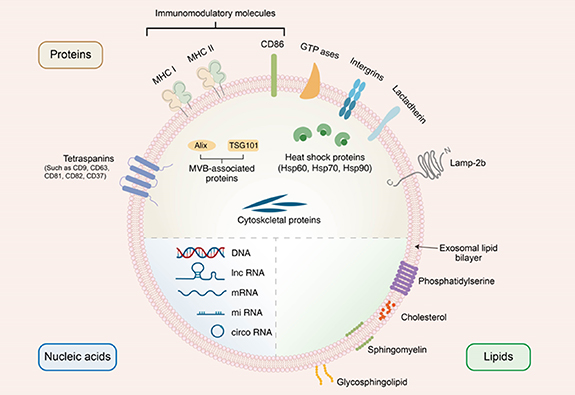
Insight regarding exosome content is actively pursued because the content determines exosome function and its role in intercellular communication to some extent. Exosomes contain different cargoes (figure 2). According to a report about the ExoCarta exosome database (www.exocarta.org), exosomes carry 9769 proteins, 2838 micro RNAs (miRNAs), and 1116 lipids. Their components vary from the cell type and are influenced by different cellular conditions or interventions.
Figure 2. Schematic diagram of the composition of exosomes. Exosomes are made up of a lipid bilayer, which allow them to interact and communicate with other cells in the body. Additionally, exosomes contain a variety of biomolecules, including proteins, nucleic acids, and lipids, which can be transferred between cells and participate a series of biological processes.
Download figure:
Standard image High-resolution image3.1. Proteins
Exosomes carry a large variety of proteins that have been identified in the exosome membrane or lumen. Exosomal proteins include (i) cell surface proteins, including tetraspanins (such as CD81, CD82, CD37, CD63, and CD9), CD86, MHC class II, MHC class I, lactadherin (LA), lysosome-associated membrane protein-2b (Lamp-2b), and integrins; (ii) membrane transport- and fusion-related proteins, including GTPases (such as Rabs, Ras, and Rho) and heat shock proteins (such as Hsp70 and Hsp90); (iii) MVB-associated proteins, including Alix and tumor susceptibility gene 101 (TSG101); and (iv) cytoskeletal proteins such as tubulin, actin, and cofilin [53–57]. Interestingly, integrins, LA, Lamp-2b and tetraspanins are involved in cell targeting and adhesion, while MHC class I, MHC class II, and CD86 proteins stimulate T cells [58]. Escola et al have confirmed that CD63 and CD81 are abundant in exosomes; these proteins are used as biomarkers of exosomes, along with other exosome tetraspanins such as CD9 [59]. Some proteins are selectively packed into exosomes via special way. For example, Hsp90, the major intercellular chaperone, can be sorted into exosomes by interacting with Rabs [60]. In addition, Hsp90 is responsible for proper protein folding and play a key role in the exosome biogenesis [61]. Alix acts as an exosomal scaffolding protein, binds to TSG101, and participates in ESCRT-independent pathways [62]. Notably, some exosome proteins exert therapeutic effects. For example, after preconditioning by tumor necrosis factor-α (TNF-α), adipose tissue-derived mesenchymal stem cell-derived exosomes contain high levels of WNT family member 3A, which promote bone tissue repair or regeneration [63]. Overall, proteins from exosomes play multiple roles and need more exploration.
3.2. Nucleic acids
Valadi et al have reported that mouse mast cell-derived and human mast cell-derived exosomes contain RNA, which can be delivered to other cells [64]. Subsequent studies have focused on nucleic acids contained in exosomes, such as miRNAs, long noncoding RNAs (lncRNAs), circular RNAs (circRNAs), and DNA [65, 66]. Typically, miRNAs, as a family of noncoding RNA molecules with length of 21–25 nucleotides, function in posttranscriptional gene regulation by binding to the 3'-untranslated section or open reading frames of mRNA and further influence signaling pathways or immune responses [67, 68]. For example, miR-222-3p from tumor-derived exosomes promotes signal transducer and activator of transcription 3-mediated M2 macrophage polarization by downregulating suppressor of cytokine signaling 3 [69]. lncRNAs are endogenous RNA molecules longer than 200 nucleotides that lack important open reading frames [70]. lncRNAs are selectively sorted into exosomes and can regulate tumor growth in multiple ways. One study has reported that exosome-derived lncRNA maternally expressed gene 3 inhibits tumor growth in osteosarcoma [71]. Another class of noncoding endogenous RNAs in exosomes are circRNAs, which are characterized by high evolutionary conservation and stability [72]. Many circRNAs play important roles in protein function or translation by acting as inhibitors of miRNAs or protein. For instance, circDLG1, as a miR-141-3p inhibitor, promotes gastric cancer progression and resistance to anti-PD-1-based therapy by increasing the expression of CXCL12 [73]. In comparison with exosomal RNA, exosomal DNA has been the topic of very few studies. However, exosomal DNA modulates tumor immunity by activating cytosolic DNA sensor pathways (such as STING and cGAS) and paracrine interactions. In addition, exosomal DNA reflects the mutational status, and may be utilized as a diagnostic 'liquid biopsy' material for personalized therapy in cancer [74].
3.3. Lipids
Lipids are one of the most essential components of exosomes and include cholesterol, sphingomyelin, glycosphingolipids, and phosphatidylserine [75, 76]. The relative abundance of exosome lipids may vary depending on the type or growth phase of the parental cell. Several research have shown that exosomes obtained from differently treated cells exhibit altered lipid compositions and modulated biological functions. For example, lipidomic analysis has shown increased levels of ether lipids in PC-3 cells upon the addition of the ether lipid precursor hexadecylglycerol. Furthermore, researchers have also observed an elevated level of ether lipids in exosomes released by ether lipid-rich PC-3 cells. Notably, ether lipid-rich PC-3 cells release more exosomes than untreated PC-3 cells, but these exosomes are similar in particle size [77]. Phosphatidylserine species are present in the inner leaflet of lipid bilayer of exosomes. However, phosphatidylserine is displayed on apoptotic and malignant cells and serves as an 'eat me' signal for phagocytes in the immune system [78]. Similarly, malignant cell-derived exosomes display phosphatidylserine, which can be considered as a promising exosome marker for the cancer diagnosis. Besides cancer, other diseases can also be detected using exosome lipid analysis. Compared with those of control volunteers, patients with hereditary α-tryptasemia show significantly lower levels of glycerophospholipids, glycerolipids, and sterols in urinary exosomes [79]. In neuropathological assessments, acid sphingomyelinase-enriched exosomes from the cerebrospinal fluid of patients with multiple sclerosis correlate with disease severity [80]. Exosome lipids have also shown potential as novel therapeutic targets. The amyloid-β peptide is central to Alzheimer's disease pathogenesis [81]. The intracerebral infusion of neuroblastoma N2a-derived exosomes decreases the levels of amyloid-β peptide and amyloid deposition in amyloid precursor protein transgenic mice, which has been attributed to the sequestration of glycosphingolipids on the exosome surface [82].
4. Exosome uptake
Exosomes are derived from donor cells and enable be taken up or internalized by recipient cells through various mechanisms, such as clathrin dependent/independent endocytosis, phagocytosis, and micropinocytosis [83]. Detailed studies on the mechanisms of viral uptake have provided meaningful paradigms for research on exosome uptake mechanisms. For example, the human immunodeficiency virus mediates infection via fusion with the plasma membrane [84]. However, various molecules from the exosome surface can interact with receptors or ligands on the membranes of recipient cells, implying exosomes may have complex cell-binding kinetics and transmit distinct signals [85].
The mechanisms of exosome uptake by recipient cells remain unclear. The first step of exosome uptake involves the targeting of the recipient cell. However, it remains unclear whether exosome uptake by different recipient cells is specific or nonspecific. Neuroblastoma cell-derived exosomes are indiscriminately taken up by neurons and glial cells [86]. In contrast, oligodendrocyte-derived exosomes are specifically taken up by microglia [87]. The second step of exosome uptake is the entry of exosomes into cells through internalization. This process is influenced by receptor proteins on the cell membrane. Proteoglycans and integrins participate in exosome uptake [88, 89], but evidence for the necessity of these proteins in the internalization of exosomes is lacking. The third step in exosome uptake is the release of exosome contents. Exosomes release their contents into the cytosol through membrane fusion. After internalization, the fate of exosomes follows a typical endosomal pathway. Eventually, exosomes fuse with lysosomes and are degraded.
5. Isolation of exosomes
Exosomes are utilized as drug-delivery carriers, and a variety of exosome-based therapeutics have been considered in clinical translation. An appropriate exosome isolation method is key to enabling exosome application in clinical translation. More specifically, the isolation method should exhibit the following key characteristics: high yield, high purity, repeatability, and scalability. We summarized five common exosome isolation methods: ultracentrifugation, size-exclusion chromatography, immunoaffinity capture, ultrafiltration, and polymer precipitation (table 1). A thorough understanding of the characteristics of these isolation methods is essential.
Table 1. Comparison of exosome isolation techniques.
| Isolation methods | Mechanism | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Ultracentrifugation | Particle density and size | Commonly used; cost-effective; relatively high purity | Time-consuming; exosome loss and damage; heavy dependence on equipment | [90–92] |
| Size-exclusion chromatography | Particle size | Preserves the integrity and biological activity of exosomes; time-saving | May not separate exosomes from similar particle sizes | [93, 94] |
| Immunoaffinity capture | Specific reaction | High specificity; relatively high purity | Low yield and limiting large-scale preparation | [92, 95–97] |
| Ultrafiltration | Particle size or molecular weight | Rapid; used on a large scale | Exosome loss and damage; blockage of membranes | [98, 99] |
| Polymer precipitation | Hydrophobicity | Cheap; simple; high yield | Low purity | [92, 100] |
5.1. Ultracentrifugation
Ultracentrifugation is regarded as the 'gold standard' for isolating exosomes and has become the most widely used isolation approach [101, 102]. This method exploits differences in particle density and size and involves the application of a gradually-increasing centrifugation speed to cell culture supernatants or biological fluids containing exosomes. For example, low-speed centrifugation (200 × g) is used to remove cells, followed by centrifugation at 2000 × g and 10 000 × g to remove cell fragments and debris, respectively. Finally, exosomes are obtained by high-speed centrifugation (110 000 × g). An increased centrifugation speed can accelerate separation but may destroy the integrity of exosomes [90]. Notably, ultracentrifugation requires researchers to spend more time removing supernatants and to set up the centrifugal conditions [91]. In addition, exosomes can be lost during the procedure of removing the supernatant and transferring the object to centrifuge tubes. Therefore, to increase the yield of exosomes, large volumes of cell culture supernatant or biological fluids are needed in the initial steps of ultracentrifugation.
5.2. Size-exclusion chromatography
Size-exclusion chromatography (also known as gel filtration chromatography) is a gentle and widely used chromatography technique based on differences in hydrodynamic radius [103]. As particles with a larger hydrodynamic radius are eluted rapidly and smaller particles can penetrate the pores, the elution time increases with decreasing hydrodynamic radius [104]. Exosomes are 30–150 nm in size while many proteins are below 10 nm; therefore, exosomes can be separated from proteins of different sizes. However, some larger proteins (such as chylomicrons and low-density lipoproteins) with particle sizes similar to those of exosomes are difficult to separate. To maintain a gradual elution process, the input volume should be far smaller than the column volume. Owing to size-exclusion chromatography is depended on the gravity flow, the integrity and biological activity of exosomes are well preserved [93].
5.3. Immunoaffinity capture
Immunoaffinity capture is also a gentle isolation technique based on the recognition of special surface biomarkers by immobilized antibodies on surfaces of magnetic beads, filters, or microfluidic devices [95]. The recognized fraction is collected by washing with a buffer and the unrecognized fraction is discarded. Exosome isolation is achieved by coating magnetic beads with antibodies that can bind to specific proteins on the exosome surface, such as TSG101, Alix, CD9, CD63, and CD81. In comparison with methods based on the physical properties of exosomes, immunoaffinity capture yields exosomes with greater purity and is suitable for proteomics, lipidomics, and RNAomics [96]. Because immunoaffinity capture depends on antibody recognition of exosome proteins, it may have the potential to capture a single type of exosome, leading to a low yield and limiting large-scale preparation [97]. In addition, this method also involves follow-up separation and purification steps.
5.4. Ultrafiltration
Ultrafiltration is a widely used technique that uses membrane filters and pressure to selectively retain or discard samples according to particle size or molecular weight. Exosomes can be obtained by applying pressure to drive the sample through membranes with 100 nm pores. This process is faster than ultracentrifugation and is amenable to large-scale use. However, shear stress produced by the application of inappropriate pressure can destroy the integrity of exosomes and may result in some larger contaminants being forced across the membrane [98]. In addition, larger contaminants may induce adhesion and blockage of membranes, which is unfavorable for the yield of exosomes and prolongs the processing time [99].
5.5. Polymer precipitation
Polymer precipitation has often been applied in the isolation of macromolecules and viruses. The most widely used polymer for the exosome isolation is polyethylene glycol (PEG), which decreases exosome solubility by interacting with water molecules and results in the precipitation of exosomes [100, 105]. Commercial polymer precipitation-based kits for exosome isolation, such as ExoQuick (System Biosciences), have also been widely used. Specifically, exosomes are added to the PEG (with a molecular weight of 8 kDa) precipitation solution and incubated overnight at 4 °C. To isolate exosomes, above mixture requires to be centrifuged at a low speed. This method enables the obtention of high yields and allows high throughput of samples without requiring expensive equipment or complex procedures. However, some contaminating proteins, such as lipoproteins and immunoglobulins, are difficult to separate; thus, the main limitation of polymer precipitation is the low purity of exosomes [106].
6. Characterization of exosomes
Isolated exosomes should be comprehensively characterized by multiple techniques to obtain a holistic overview of their morphological and physicochemical properties and aid studies on the engineering of exosomes for delivery. Given the nanoscale size of exosomes, traditional imaging techniques lack adequate resolution and magnification to observe the morphology. To this end, transmission electron microscopy and scanning electron microscopy have been widely applied for the visualization of exosomes [107, 108]. Dynamic light scattering and nanoparticle tracking analysis are widely applied to determine the particle size of exosomes and the suitability of the dimensions of engineered exosomes for subsequent nanomedical applications [109, 110]. Exosome marker characterization can be performed to confirm the presence of exosomes in isolated samples. The International Society for Extracellular Vesicles has reported a statement on the characterization of exosome markers. Based on these guidelines, exosome markers should contain at least one transmembrane protein (such as CD63, CD81, or CD9), one cytosolic protein (such as TSG101 or Alix), and some proteins of interest [111].
7. Therapeutic modalities involving delivery of cargoes through exosomes
As natural carriers of cargo, exosomes exhibit great potential for therapeutic delivery. Exosomes show high biocompatibility and stability and low immunogenicity and toxicity [112–114]. Exosomes have inherent biochemical characteristics that contribute to their extensive application in drug-delivery systems (figure 3). Exosomes can be loaded with different cargoes, including proteins, genetic substances (miRNA and siRNA), and drug molecules (doxorubicin, paclitaxel, methotrexate, and curcumin), using physical, chemical, or biological methods [115–120]. After loading cargoes, exosomes can be administered in multiple ways, such as intravenous injection, subcutaneous injection, intraperitoneal injection, intranasal administration, and oral administration [121–124]. Thus, exosome-based drug-delivery systems serve as a promising approach to disease therapy.
Figure 3. Exosome-based delivery system for the delivery of different cargoes. The inherent characteristics of exosomes make them display great potential in delivering different cargoes, including proteins, genetic substances, and drug molecules.
Download figure:
Standard image High-resolution image7.1. Protein delivery
As a promising delivery system for therapeutics, multiple proteins are present on the surface and in the cytosol of exosomes. Some proteins are derived from parental cells and offer binding sites, which can combine with ligands of recipient cells for signal transduction and the delivery of exogenous specific proteins. For example, some proteins with strong immunogenicity exhibit antitumor effects by activating immune cells. Rivoltini et al have induced the transduction of TNF-related apoptosis-inducing ligand (TRAIL) in K562 cells and derived exosomes armed with TRAIL that control tumor progression in vivo by inducing TRAIL-mediated apoptosis in cancer cells [125]. CD47 is abundantly expressed on the membrane of various types of tumors and can impair the performance of macrophages to devour tumor cells by interacting with signal regulatory protein α (SIRPα) on phagocytic cells. To solve this problem, Koh et al have performed engineering modification with SIRPα variants on the surface to design a therapeutic exosome that antagonizes the interaction between CD47 and SIRPα, further promoting the phagocytosis of macrophages and inhibiting tumor progression in tumor-bearing mice [126]. Oxidative stress serves as a contributing cause in the pathological process of Parkinson's disease (PD), and the delivery of antioxidants (such as catalase and superoxide dismutase) to the lesion site is a promising therapeutic strategy [127]. Haney et al have constructed a novel exosome-based nanoplatform for catalase delivery, which effectively attenuates brain inflammatory response and increases the survival of neuron in a PD mouse model [128].
7.2. Nucleic acid delivery
Nucleic acids are inherent components of lumens of exosomes, affecting a variety of biological processes. However, these components may not be sufficient to regulate signaling pathways or perform special functions in pathological states. Therefore, engineering modification and material-processing methods endow exosomes with exogenous or endogenous nucleic acids and construct a functional exosome platform for therapy. Alvarez-Erviti et al have made a major contribution regarding siRNA delivery based on an exosome nanoplatform. In brief, dendritic cell-derived exosomes after loading with exogenous siRNA that silence the expression of BACE1 (an interference target for Alzheimer's disease). After intravenous injection of the exosomes into mice, mRNA levels and protein expression are downregulated [129]. The abovementioned study has demonstrated the therapeutic potential of the siRNA-exosome delivery system and, more importantly, promoted its application in other diseases. For example, exosomes loaded with siRNA targeting KRASG12D significantly downregulate KRASG12D mRNA levels and inhibit the growth of advanced tumors [130]. In a rat model of spinal cord injury, neuron-derived exosome-mediated siRNA delivery effectively blocks inflammasome activation [131].
Exosome-based miRNA delivery systems are also being extensively studied. As miRNA is easily degraded following intravenous injection, researchers have attempted to deliver miRNA using exosome nanoplatforms, which can overcome the abovementioned problem and improve its therapeutic effect. miR-145-5p is a tumor suppressive miRNA that is significantly downregulated in multiple types of cancers, such as hepatocellular carcinoma (HCC), colorectal cancer, and breast cancer [132–134]. Human umbilical cord mesenchymal stem cell-derived exosomes engineered to overexpress miR-145-5p can suppress the proliferation of pancreatic ductal adenocarcinoma cells and promote apoptosis [135]. Katakowski et al have demonstrated that marrow stromal cell-derived exosomes carrying miR-146b can significantly suppress glioma xenograft growth in a rat model [136]. In addition to miRNA, mRNA has also been identified as a potential therapeutic agent. However, the limitations of mRNA include large size and instability, which render crossing the membrane and entering the cytoplasm unfavorable. The use of exosomes can overcome these problems and expand the applications of mRNA. Kojima et al have designed a novel exosome production booster and specific mRNA packaging device that facilitates the delivery of therapeutic catalase mRNA to exosomes in target cells. This design effectively inhibits neurotoxicity and neuroinflammation in a PD model [137]. Notably, loading endogenous mRNA into exosomes is accompanied by protein translation, which may result in mRNA and the protein translated from RNA being packed together in exosomes and delivered to the target cells. Thus, future studies should aim to determine whether the therapeutic effect is conferred solely by mRNA delivered from exosomes and thereby facilitate the development of more precise therapeutic strategies.
7.3. Drug delivery
With rapid advancements in biomedical technology, traditional drugs are an area of continuous innovation and development, and various dosage regimens are developed to meet clinical needs. As many drug targets are located in the cytoplasm of cells, drugs must cross plasma membranes to exert a therapeutic effect. However, poor solubility and premature degradation result in undesirable effects [138]. The lipid bilayer of exosomes can endow drugs with protection and facilitate endocytosis by recipient cells. In addition, the low immunogenicity of exosomes can reduce side effects. Tian et al have developed a feasible antitumor strategy by delivering the chemotherapeutic drug doxorubicin to tumor tissue in a mouse model using engineered exosomes, and this approach shows higher antitumor effects and lower side effects in tumor-bearing mice compared with those of free doxorubicin [139]. Sun et al have demonstrated that curcumin delivered by exosomes exhibits increased stability and a higher concentration in the blood compared with that of free curcumin. Additionally, curcumin-loaded exosomes protect against lipopolysaccharide-induced septic shock in mice [140]. Unlike traditional delivery systems (such as liposomes), the membranes of exosomes include transmembrane and membrane-anchored proteins, which contribute to endocytosis or uptake and facilitate the delivery of drugs. Exosomes are also equipped with the ability to penetrate biological barriers, including the blood–brain barrier. Therefore, exosomes show huge potential in the treatment of brain diseases [141]. Although exosomes have been regarded as robust drug delivery systems for several years and steady progress has been made, most research is still in the initial stage. The underlying interaction of drugs and exosome components is worthy of further exploration. On the other hand, the specific pharmacokinetics of the fusion and delivery process are still not well understood. Overall, substantial efforts are needed to develop exosome-based delivery for clinical use.
8. Targeted exosomes-based delivery strategies
The structure of the exosome membrane provides abundant sites for various surface modifications. To enhance the targeting performance and achieve targeted therapy, many researchers have investigated the reprogramming the exosomes based on the decoration of the lipid bilayer. We have summarized examples of the engineering exosomes as drug-delivery systems in recent years (table 2).
Table 2. Different exosomes modification strategies.
| Modification strategy | Method | Source | Targeting ligand | Therapeutic agent | Disease | Reference |
|---|---|---|---|---|---|---|
| Genetic modification | Lamp-2b | Dendritic cells | Chondrocyte-affinity peptide | miRNA-140 | Osteoarthritis | [142] |
| Lamp-2b | 293T cells | Her2 | miR-21 inhibitor | Colorectal cancer | [143] | |
| Lamp-2b | Mesenchymal stem cells | Ischemic myocardium-targeting peptide | / | Acute myocardial infarction | [144] | |
| Lamp-2b | 293T cells | tLyp-1 | siRNA | Lung cancer | [145] | |
| CD9 | 293T cells | HuR | miR-155 inhibitor | Liver injury | [146] | |
| CD63 | 293T cells | Apo-A1 | miR-26a | Liver cancer | [147] | |
| LA | Erythrocytes | Phosphatidylserine | / | Cancer | [148] | |
| Chemical modification | Click chemistry | Mesenchymal stem cells | cyclo(RGDyK) | Curcumin | Ischemic stroke | [149] |
| Electrostatic interactions | Mesenchymal stem cells | Cationized pullulan | / | Liver injury | [150] | |
| Hydrophobic interactions | Macrophages | AA–PEG | Paclitaxel | Pulmonary metastases | [138] | |
| Magnetic nanoparticle modification | Reticulocytes | SMCNCs | Doxorubicin | Liver cancer | [151] |
8.1. Genetic engineering
Genetic engineering is a feasible modification strategy for targeted exosome-based delivery. Traditionally, this method is used to effectively express peptides and proteins on the surfaces of exosomes by fusing the gene sequence of a polypeptide or protein to that of a specific exosome protein. Recently, researchers have successfully added glycans to the surface of exosomes to achieve targeting [152]. The construction of plasmid or viral vectors is required in the above process.
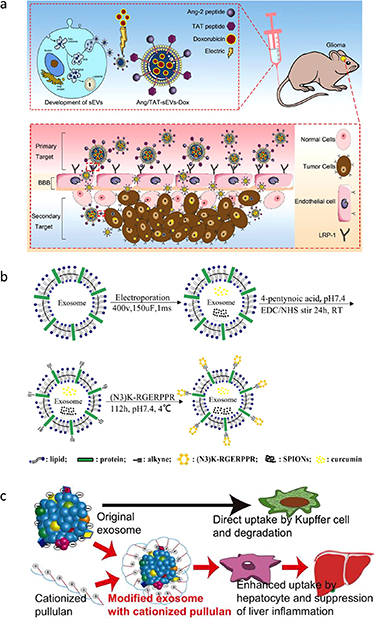
Lamp-1 and Lamp-2 are major protein components of the lysosomal membrane. Previous studies have confirmed that Lamp-1 and Lamp-2 can be used to favor the adhesion of cancer cells to basement membrane and endothelium and promote the migration of cancer cells [153, 154]. Furthermore, Lamp-2, which is highly expressed on several cancerous cells including cells of poorly differentiated gastric cancer, colon cancer and melanoma, can be used as a therapeutic target for site specific anticancer drug delivery [153, 155]. Lamp-2 undergoes alternative splicing of exon 9 to form the three isoforms Lamp-2a, b, and c, which have distinct transmembrane and cytosolic domains at the C termini [156, 157]. The N-terminal extramembrane domain of Lamp-2b can be fused to the targeting sequence. Lamp-2b is also abundant in dendritic cell-derived exosomes [58, 129, 158]. Plasmids, encoding the Lamp-2b-fused peptide, are used for the transfection of the donor cells to produce exosomes with effective targeting. Lamp-2b has been widely used as a surface protein to display a targeting motif. For example, exosomes expressing αγ integrin-specific iRGD peptide (CRGDKGPDC) fused to Lamp-2b show good targeting capabilities and efficiently deliver doxorubicin to integrin-positive breast cancer cells [139]. To improve ischemic stroke therapy, researchers have modified the surface of exosomes by fusing advanced glycation end-products (RAGE)-binding-peptide (RBP) to the N-terminal of Lamp-2b. RBP-linked exosomes efficiently deliver miR-181a to ischemic brain cells, which overexpress RAGE, to reduce RAGE signaling, inflammatory cytokines, apoptotic cells, and infarct volume after intranasal administration [159]. According to a recent report, due to the complexity of the blood–brain barrier, approximately 98% of small-molecule drugs and almost all large-molecule drugs are unable to cross the blood–brain barrier [160]. Zhu et al have simultaneously fused the Ang peptide (TFFYGGSRGKRNNFKTEEYC) and TAT peptide (YGRKKRRQRRRC) to the N-terminal of Lamp-2b, generating dual peptide‐modified exosomes that target glioma cells and the blood–brain barrier and significantly enhance blood–brain barrier permeation and glioma tumor penetration in the brain (figure 4(a)) [161]. These dual peptide‐modified exosomes are promising platforms for the treatment of brain disease. Despite the effectiveness of this genetic method, the peptide displayed on the exosome surface can be degraded in certain situations. Hung and Leonard have added a glycosylation motif to the N-terminal of Lamp-2b to suppress peptide loss and enhance stability [162].
Figure 4. Different modification strategies for targeted delivery. (a) Ang peptide and TAT peptide were simultaneously fused to the N-terminal of Lamp-2b of exosomes, producing dual-targeting functional exosomes that can target glioma cells and the blood–brain barrier at the same time, significantly enhancing the permeability of the blood–brain barrier and the penetrability of tumor tissue. [161] John Wiley & Sons. © 2022 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles. (b) Schematic illustration of RGE-Exo-SPION/Cur synthesis: RGE (RGERPPR peptide) is modified on the surface of Raw264.7 cell-derived exosomes via a click chemistry reaction. Reprinted from [163], Copyright (2018), with permission from Elsevier. (c) The targeted accumulation of exosomes in the liver and the excellent anti-inflammatory effect via the modification on the surface of exosomes using pullulan. Reprinted from [150], Copyright (2018), with permission from Elsevier.
Download figure:
Standard image High-resolution imageIn addition to Lamp, the tetraspanin proteins (such as CD9, CD63, and CD81), which contain two extracellular loops and four transmembrane domains, can also be used to display targeting sequences [164]. For example, Apo-A1 can effectively bind to scavenger receptor class B type 1 receptor on the surface of HepG2 cells. The fusion of the small extracellular loop of CD63 with Apo-A1 facilitates the exosome delivery of miRNA-26a to SR-B1-expressing HepG2 cells, thus inhibiting hepatocarcinoma proliferation and migration [147]. According to another study, the fusion of the large extracellular loop of CD63 with a glycosylation domain facilitates the binding of exosomes to Sialyl Lewis-X (sLeX, a tetrasaccharide glycan), thus enhancing the ability of exosomes to target inflammatory cells [152]. In this study, researchers have developed a novel glycoengineering strategy to display sLeX on the surfaces of exosomes, which has prompted the use of different glycosyltransferases to load different kinds of glycosyl modifications to achieve the targeting of different cells. In a recent report, researchers have fused human antigen R (HuR) to the N -terminal of CD9. HuR is an RNA binding protein; thus, CD9–HuR functionalized exosomes deliver therapeutic miRNAs to the liver and spleen [146].
LA, transmembrane protein platelet-derived growth factor receptor (PDGFR), and glycosylphosphatidylinositol (GPI) can also be engineered to display a targeting motif. For example, anti-epidermal growth factor receptor (anti-EGFR) nanobodies can be displayed by the C1C2 domain of LA to increase the specific binding and uptake of exosomes by EGFR-overexpressing tumor cells, without affecting exosome characteristics [148]. To date, exosome-based drug delivery has shown broad application in disease treatment, but the potential of exosomes for immunotherapy has not been fully utilized. Recently, Cheng et al have genetically fused two genes encoding scFv fragments of αEGFR cetuximab and αCD3 UCHT1 to the transmembrane domain of PDGFR and genetically linked programmed death 1 and the OX40 ligand to the N- and C-terminal of CD9. Genetically engineered multifunctional immune-modulating exosomes (GEMINI-Exos) have been developed, which simultaneously target tumor-associated EGFR and immunomodulatory molecules [165]. This is the first report of the successful production of multifunctional exosomes via genetic engineering for targeted cancer immunotherapy and may offer a general strategy for developing exosome-based immunotherapeutics with desired pharmacological activities. In one study, through fusion with GPI-anchors, anti-EGFR nanobodies have been utilized to specifically target EGFR-positive (A431) cells [166]. This GPI-anchoring strategy offers a novel approach to the display of targeting ligands on the surface of exosomes.
8.2. Chemical modification
Chemical modification is largely based on covalent and non-covalent binding approaches. Click chemistry is a representative covalent binding reaction that utilize biocompatible compounds and efficient reactions to form irreversible chemical bonds under physiological conditions. The key and simple step in this reaction is the transformation of exosomal amine groups to alkyne groups. The copper (I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition reaction between organic azides and terminal alkynes, generally known as CuAAC, is an example of the click chemistry reactions that can be used to conjugate macromolecules and small molecules to the exosome surface [167]. For example, neuropilin-1, which is overexpressed in glioma, can bind to the peptide (RGERPPR). The display of RGERPPR on the exosome surface via a cycloaddition reaction with sulfonyl azide enables the targeted delivery of curcumin-loaded exosomes to glioma (figure 4(b)) [163]. Click chemistry has also shown potential in enhancing immune responses against tumor cells. For example, most tumor cells display a surface protein CD47, which interacts with SIRPα on phagocytes. This interaction limits the ability of macrophages to engulf and clear tumor cells. To inhibit tumor growth, Koh et al have utilized the click chemistry technique to connect dibenzocyclooctyne-derivatized SIRPα antibodies to exosomes modified with azide, and they have found that these modifying exosomes can be an effective strategy. These exosomes effectively block the CD47-SIRPα checkpoint on the surface of cancer cells [126]. This approach has shown promising results in preclinical studies and can potentially be developed into a new therapeutic strategy for cancer treatment. Notably, covalent binding reactions can provide stable decoration on the surface of exosomes, but may impair the inherent structure and function of exosomes. Furthermore, click chemistry offers several advantages over traditional conjugation methods, such as high efficiency, selectivity, and versatility, making it a promising tool for developing novel cancer immunotherapies.
In addition to these covalent modification techniques, non-covalent modification techniques are also being explored to produce targeted exosomes. The surface charge or lipophilicity of exosomes plays a key role in the distribution and targeting efficiency towards specific tissues or cells. Exosomes with a positive charge predominantly accumulate in the lungs, while anionic exosomes are mainly located in the liver or kidneys [168]. Modifying the surfaces of exosomes with a range of charges from biomaterials can control the accumulation of exosomes. This electrostatic interaction has potential therapeutic implications, as it could allow for targeted drug delivery to specific cells or tissues. For example, Tamura et al have modified exosomes with cationized pullulan, enhancing liver accumulation and therapeutic effects (figure 4(c)) [150]. A combination of biomaterials can enable exosomes to acquire new targeting abilities and improve their functions. In addition, another non-covalent modification strategy can be used, which involves inserting amphipathic molecules into exosomes [169–171]. Kim et al have inserted 1,2-dioleoyl glycero-3-phosphoethanolamine with PEG-conjugate (DSPE-PEG) into the exosome membrane. This experiment shows that aminoethyl anisamide (AA) binded to DSPE-PEG was able to conjugate to the exosome surface [138]. Similar to DSPE, cholesterol, which has a characteristic hydrophobic structure, can also be embedded in exosome membranes. Pi et al have modified exosomes by attaching cholesterol-connected RNA aptamers or folate to their surface via noncovalent binding reaction. The modified exosomes were then found to have an enhanced delivery capacity of miRNA and siRNA to the tumor region, resulting in a higher level of antitumor efficacy [172]. In addition to the lipid mentioned, there are other lipids that have been utilized to attach specific targeting molecules to the outer surface of exosomes. One such example is a diacyl lipid-DNA aptamer (sgc8) conjugate, which has been engineered for cancer cell-specific therapy [173]. These modified exosomes hold promising potential as drug carriers for tumor targeted drug delivery.
Through the application of external forces, such as magnetism, targeted delivery can also be realized [151, 163, 174]. Exosomes can be secreted from a variety of cells, of which reticulocytes (RTCs) are the main source of exosomes in the blood. Exosomes secreted by RTCs contain a variety of membrane proteins, including transferrin (Tf) receptors. Researchers have conjugated holo-transferrins to Fe3O4 superparamagnetic nanoparticles and anchored them to exosomes through Tf–Tf receptor interaction. This technology can not only separate exosomes from blood, but also endow exosomes with a tumor-targeting ability [151]. The combination of simplicity and versatility has made this strategy a powerful tool, but the poor biocompatibility and low specificity of magnetic materials still need to be addressed [174, 175].
9. Stimuli-responsive exosome delivery
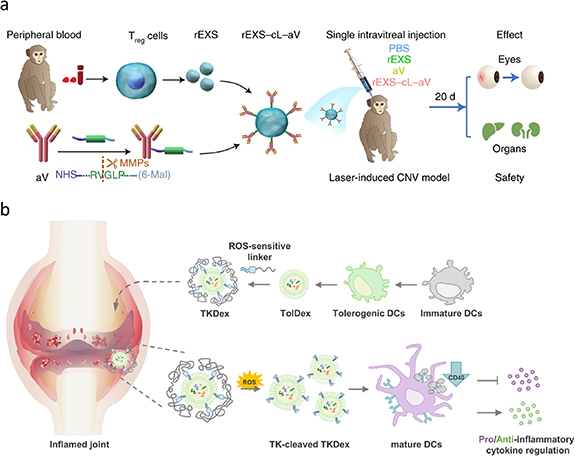
With a deep understanding of mechanisms of different diseases and the rapid advancements in materials science and biomedicine, research on stimuli-responsive drug-delivery systems have reached new heights. Stimuli-responsive delivery systems realize the precise control of on-demand drug delivery in response to endogenous stimuli (e.g. pH, temperature, enzymes, and reactive oxygen species) or exogenous stimuli (e.g. ultrasound, magnetic field, electrical stimulus, and light) [176–179]. To further improve the precision of drug delivery and reduce off-target-induced toxicity, exosome-based stimuli-responsive delivery systems have attracted considerable attention and have been widely applied to different models of disease. For example, Tian et al have developed an exosome-based combination therapeutic modality by engineering regulatory T cell-derived exosomes and modified anti-vascular endothelial growth factor antibodies on the surface using a specific peptide linker, which is able to be cleaved by highly expressed matrix metalloproteinases in lesion sites. This rational design significantly suppresses inflammation and ocular neovascularization in nonhuman primate models of choroidal neovascularization (figure 5(a)). More importantly, this work opened up a new paradigm in exosome-based stimuli-responsive delivery systems [180]. In addition, Lee et al have developed a reactive oxygen species-responsive therapeutic strategy that introduces thioketal linker-embedded PEG on the membrane of dendritic cell-derived exosomes. The overexpression of reactive oxygen species in the lesion cut off the linker and facilitated cellular uptake and immunoregulation (figure 5(b)) [181].
Figure 5. Stimuli-responsive exosome delivery systems. (a) Schematic illustration for the matrix metalloproteinase-responsive therapeutic strategy in the choroidal neovascularization cynomolgus monkey model. Reproduced from [180], with permission from Springer Nature. (b) The illustration of ROS-sensitive tolerogenic dendritic cell-derived exosomes in the inflamed joint of rheumatoid arthritis. Reprinted from [181], Copyright (2021), with permission from Elsevier.
Download figure:
Standard image High-resolution image10. Exosome-based liquid biopsy strategies
In addition to their multiple therapeutic effects, exosomes also have great diagnostic value. For example, tRNA-derived small RNA is significantly increased in plasma exosomes from patients with liver cancer [182], and exosomes from patients with early pancreatic cancer express a special protein glypican-1 [183]. These findings suggest that exosomes could serve as a promising biomarker for disease diagnosis. Liquid biopsy has been widely used in fields such as oncology, cardiovascular, and immune diseases by sampling cerebrospinal fluid, saliva, pleural fluid, blood, ascites, and urine to detect potential biomarkers. Currently, common biomarkers for liquid biopsy include circulating tumor DNA, cell-free DNA, and circulating tumor cells [184]. Exosomes are expected to become new biomarkers for non-invasive liquid biopsy. In 2016, Exosome Diagnostics (ExoDx) proposed the first approved exosome-based liquid biopsy product ExoDx ™ Lung. In the diagnosis of non-small cell lung cancer patients, ExoDxTM Lung has a specificity of 100% and sensitivity of 88% for detecting EML4-ALK mutations, which is more sensitive than cfDNA. In addition, the emergence of liquid biopsy of exosomes is also expected to reduce the unnecessary number of tissue biopsies in prostate cancer patients [185]. ExoDx Prostate IntelliScore (EPI) is a noninvasive liquid biopsy that can detect three RNAs (ERG, PCA3, and SPDEF) in the urine exosomes of prostate cancer patients with prostate specific antigen levels ranging from 2 to 10 ng ml−1, and further to predict the likelihood of developing high-grade prostate cancer [185, 186]. According to prospective studies published by ExoDx, setting the validated cut-point at 15.6 can avoid 26% of unnecessary prostate biopsy, with sensitivity and negative predictive values of 93% and 89%, respectively [187]. In 2019, EPI became the first fluid biopsy of exosomes certified by FAD, which not only demonstrated the clinical importance of EPI detection, but also an important milestone in the clinical application of non-invasive fluid biopsy of exosomes.
11. Conclusions and further perspectives
Exosomes have attracted considerable attention in many research areas. Although multiple studies have focused on the basic properties of exosomes for physiological or pathological regulation, most underlying molecular mechanisms remain unclear, highlighting the need for more efforts to explore the underlying principles of exosome biology. Ongoing advances in technology have promoted the development of therapeutic or diagnostic exosome applications. Ongoing technological advances have promoted the development of therapeutic exosome applications. Notably, exosome coating technology showed great potential in drug delivery systems. For example, dacarbazine-loaded carbon quantum dots upon coating exosome could enhance the antitumor efficacy. Exosomes not only improved the integrity of nanomedicine, but also facilitated the targeted delivery to cancer cells via heparan sulfate proteoglycan receptors [188]. In another study, exosome coating endowed nanogel (loaded with pituitary adenylate cyclase-activating polypeptide and estrogen) with good stability and the ability to cross the blood–brain barrier, ameliorating perimenopausal depression by reducing proinflammatory cytokine and oxidant stress, and promoting neural synaptic plasticity [189]. Therefore, exosome coating technology provides new insight for treatment of different diseases.
Recently, microfluidic chip with low detection limit and high accuracy has shown great potential in drug screening. The integration of exosome and microfluidic chip may exhibit broad perspective. By mixing exosome with drugs on a microfluidic chip and detecting signal changes after the interaction between exosome and drugs, the impact of drugs on exosome could be evaluated, and further screen out potential therapeutic drugs. Additionally, exosome-based machine learning for biomarker discovery have rapidly emerged. Researchers conducted machine learning on 114 602 exosomal RNAs from 118 patients with HCC and 112 healthy individuals. Nine exosomal RNAs (MTRNR2L8, FTL, PPBP, TMSB4X, S100A11, S100A9, ACTB, exo_circ_22106, and exo_circ_79050) were identified as markers for HCC prediction and the accuracy reach to ∼85% [190]. Based on machine learning and combine with multi-omics analyses, we may explore the prediction role of exosome in different tumor types or even subtypes, and further account for their heterogeneity. Given that machine learning fully utilizes large-scale molecular databases for profiling and predicting, researchers could explore more precise exosome-based drug delivery systems. Biomimetics strategies also provide a new direction for biologic therapies [191]. Biomimetic exosomes produced by cell membranes self-assembly, synthetic biology approach, and biohybrid may have advantages in quality control and reduce the heterogeneity of exosomes. Owing to low price, broad sources, and stable properties, biomimetic exosomes show a great potential as novel delivery system.
There are various factors affecting exosome-based drug delivery systems. Firstly, exosomes from different sources have different protein to lipid ratios, which can affect delivery [192]. Secondly, the different isolation methods mentioned above can lead to different yields and qualities of exosomes, which is also the key to developing exosome-based drug delivery systems [193]. In addition, surface modification of exosomes can increase the content of targeted delivery to the target area [194]. The route of administration is also a crucial factor. Different types of diseases require different routes of administration to achieve optimal delivery effects. For example, intratumoral injection is suitable for tumor treatment [195], intravenous administration is more suitable for liver diseases [196], and intranasal administration is meaningful for delivering drugs to the brain [197].
It is worth noting that drug loading is a crucial factor for exosome-based drug delivery systems [198]. The traditional methods of loading therapeutic agents into exosomes, such as electroporation, ultrasound, and chemical transfection, have low loading efficiency and are prone to damage the integrity and functionality of exosomes, which hinders the progress of exosomes in biomedical applications. Recent studies have shown some advanced drug loading technologies to improve the loading efficiency. Among them, the use of microfluidic devices have achieved high-throughput loading of therapeutic agents into exosomes. The 'exosome nanoporator (ENP)' proposed by Hao et al is an advanced microfluidic device that utilizes mechanical compression and fluid shear to create transient pores of different sizes in the exosome membrane, which promotes the diffusion of therapeutic substances from the surrounding solution into the exosomes while maintaining exosome integrity. The ENP device consists of 30 000 nanochannels with a channel width of 2 μm and an optimal depth of 130 nm. These characteristics give it high sample processing capability and avoid channel blockage. Future researchers can adjust the size of transient pores to match the expected size of cargos by adjusting the dimensions and flow rate of nanochannels [199]. Chiang et al invented asymmetric cell electroporation technology, which allows exosomes to load high mRNA and siRNA. Compared with traditional electroporation, asymmetric cell electroporation can achieve large-scale preparation of drug loaded exosomes [200].
Over the past 20 years, a number of preclinical studies have evaluated the value of exosome-based delivery systems in various diseases. Notably, some exosome-based strategies have been approved for application in several clinical trials. In a phase I clinical trial, ascites-derived exosomes combined with granulocyte–macrophage colony-stimulating factor have been used as immunotherapy for patients with advanced colorectal cancer. This form of immunotherapy is safe, well-tolerated, and induces a beneficial tumor-specific antitumor cytotoxic T lymphocyte response [201].
Compared to cell membrane coating and lipid-nanoparticles/proteo-lipid vehicles, exosomes have lower immunogenicity and higher biocompatibility. In addition, exosomes show an advantage in targeted delivery due to the specific surface proteins of exosomes, which facilitate interaction between exosomes and their target cells. However, there are several limitations that we have to face. Although various isolation methods have been developed, the purity of exosomes after isolation is not sufficient for clinical applications. Furthermore, physical or chemical modifications and the incorporation of drugs may affect the integrity and composition of exosomes, and the optimum storage conditions (including temperature and humidity) of exosomes require elucidation. Another main limitation is the lower production of exosomes, which has extremely hampered the translational perspective. Therefore, it is important to scale-up exosome production. Large-scale cell culture is a key step for harvesting sufficient exosomes. Researchers have developed several 3D dynamic cell culture bioreactors that provide more spreading space and are more suitable for cell viability. For example, Watson et al reported that exosome production increased 5–10 folds when human embryonic kidney cells were cultured in a hollow-fiber bioreactor [202]. Stirred tank bioreactors are also widely applied in the exosome large-scale production, which could realize a robust and high-yield exosome production process [203].
Based on the multiple functions of exosomes in different diseases, exosomes show a promising translational perspective, which relies on a good manufacturing practice compliant production platform and more rigorous standards of purity and quality. In addition, shifting from experimental isolation to commercial production at a minimal cost will also promote the translation of exosomes and further exploration of exosome-based biomedical therapies. In a word, the development of exosomes requires advancements in technology as well as close cooperation among basic scientists, materials scientists, bioengineering scientists, and clinicians.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (Grant No. 81971105), the Science and Technology Department of Jilin Province (YDZJ202201ZYTS677), and Norman Bethune Program of Jilin University (2022B02) to ZNG, and the Norman Bethune Health Science Center of Jilin University (2022JBGS03), Science and Technology Department of Jilin Province (YDZJ202302CXJD061, 20220303002SF), and Jilin Provincial Key Laboratory (YDZJ202302CXJD017) to YY, the National Natural Science Foundation of China (Grant No. 82301464) to H M.
Data availability statement
No new data were created or analyzed in this study.
Conflict of interest
The authors declare no conflict of interest.