Abstract
Alveolar bone loss is widespread in all age groups and remains a severe hazard to periodontal health. Horizontal alveolar bone loss is the pattern of bone loss more commonly seen in periodontitis. Until now, limited regenerative procedures have been applied to treating horizontal alveolar bone loss in periodontal clinics, making it the least predictable periodontal defect type. This article reviews the literature on recent advances in horizontal alveolar bone regeneration. The biomaterials and clinical and preclinical approaches tested for the regeneration of the horizontal type of alveolar bone are first discussed. Furthermore, current obstacles for horizontal alveolar bone regeneration and future directions in regenerative therapy are presented to provide new ideas for developing an effective multidisciplinary strategy to address the challenge of horizontal alveolar bone loss.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
As a common type of periodontal lesion, alveolar bone loss is widespread among all ages [1–3]. Attempts to regenerate lost alveolar bone have been elusive, making it a severe hazard to periodontal health. Several factors, including periodontitis, tooth extraction, trauma, orthodontic treatment, and hormone levels, may contribute to alveolar bone loss, among which periodontitis is the most significant pathogenic factor [4–8]. Based on clinical observations, alveolar bone loss can be classified into two main types: vertical bone loss (also called intrabony defect) and horizontal bone loss (also called suprabony defect) [9] (figure 1). Vertical bone loss occurs in an oblique direction, leaving an intrabony type of periodontal pocket [10]. Horizontal bone loss renders the remaining bone edge almost perpendicular to the tooth surface, resulting in a suprabony defect.
Figure 1. Schematic representations of alveolar bone loss. (A) Healthy periodontal tissues. (B) Horizontal alveolar bone loss. (C) Vertical alveolar bone loss.
Download figure:
Standard image High-resolution imageThe 2009–2012 National Health and Nutrition Examination Survey investigated the clinical indications of alveolar bone loss in the United States adult population, indicating that 37.4% of all teeth had an attachment loss of ⩾3 mm and 10.6% of the teeth had a probing pocket depth of ⩾4 mm [11]. Specifically, horizontal alveolar bone loss is the type of bone defect commonly encountered in clinical practice [9, 12, 13]. A recent radiographic evaluation in patients with chronic periodontitis showed that 92.2% of the alveolar bone loss of affected teeth was the horizontal type of bone loss, and vertical defects represented only a small proportion of the teeth [14]. Common sites of horizontal bone loss are the anterior (aesthetic zone) and the posterior regions of the mandible [15]. Vertical bone loss usually occurs in the premolar or molar regions [12]. In addition, it has been shown that the extent of horizontal bone loss was correlated with the extent of vertical defects [16].
From a biological perspective, periodontal regeneration includes the repair of the defective alveolar ridge and root surface and the periodontal ligament (PDL) connecting them. The PDL plays a decisive role in the repair and regeneration process because it promotes the formation of new cementum by inserting collagen fibers on denuded root surfaces and providing continuity between alveolar bone and cementum [17]. Many classic studies have demonstrated that bone and gingival connective tissues lack periodontal regenerative capabilities since direct contact between osteoblasts/gingival fibroblasts and denuded root surfaces mainly results in root resorption and ankylosis during periodontal repair [18, 19]. New cementum and PDL formation occur only when PDL cells are preserved and proliferate in a coronal direction. Determining how to mobilize an appropriate number of stem cells to regenerate the new periodontal bone tissue, especially the vascularized bone, is a significant challenge during this phase. Advances have been made in cell culture and scaffold fabrication concerning periodontal regeneration based on stem cells [20]. However, they are still not sufficient to put into clinical scenarios. Of necessity are the optimization of cell culture methods and the accuracy of multiphasic scaffolds. In horizontal alveolar bone defect cases, the distribution of vascular and cellular resources is significantly altered and reduced due to a minimal existing healthy bony wall, making it particularly difficult for coordinated PDL proliferation on the root surface [21]. This poor biological structure leaves no space to accommodate blood clots or grafts and has higher requirements for cell colonization and scaffolds, not to mention achieving the ideal periodontal regeneration described above. In contrast, angular bone loss provides adequate mechanical and biological support for the cell–tissue structure and therefore responds well to conventional therapy, with open-flap debridement alone showing promising therapeutic effects [14]. This suggests that the anatomical type and biological structure of the periodontal defect can influence the outcomes of the regenerative procedure, with superior regeneration associated with deep, narrow defects and an increased number of residual bony walls [22].
From the clinical standpoint, it is well established that current periodontal regeneration is limited to treating vertical alveolar bone and Class II furcation defects [23]. However, treating horizontal alveolar bone defects, interdental craters, supracrestal parts of intrabony defects, or Class III furcations with a periodontal regeneration process is unpredictable. Indeed, a significant proportion of patients with horizontal alveolar bone defect respond inadequately to conventional treatment, making it a contraindication to periodontal regeneration by several clinicians. Therefore, more effective treatment modalities are urgently needed to solve this dilemma. Fortunately, many clinical and preclinical studies have achieved some positive results regarding horizontal alveolar bone regeneration, which will be the focus of the current paper and addressed in more detail in the later sections.
In order to achieve improved therapeutic effects, it is crucial to consider factors that may influence horizontal alveolar bone regeneration in the first place. These factors can be broadly classified into patient-related, periodontal, and treatment factors. Patient-related factors mainly include age and smoking. Older patients with smoking habits usually have lower regenerative potential [24, 25]. Oral hygiene control is an essential periodontal factor in preventing bacterial contamination from disrupting the healing process [26]. Moreover, teeth with gingival recession will result in limited new cementum formation and insertion of PDL fibers [27]. Regarding treatment factors, membrane exposure is a significant obstacle to periodontal regeneration. When membranes are exposed, an inflammatory infiltrate tends to occupy the space under the membrane, compromise the integrity of the membrane, and limit the regeneration process [28]. A successful guided bone regeneration (GBR) or guided tissue regeneration (GTR) surgery follows the so-called PASS principles, as follows: (a) primary wound closure, (b) angiogenesis, (c) space maintenance, and (d) stability of the wound [29]. Proper design of surgical procedures involved in this principle will allow colonization of wounds coronal to the alveolar crest by cells derived from the PDL and bone rather than by cells derived from lamina propria of the gingiva or bone alone for an excellent treatment outcome [17]. Innovations, improved materials, and new treatment modalities are required to address these challenges.
Given the seriousness of the current situation, a high-level multidisciplinary strategy involving biologists, bioengineers, and dental clinicians should be developed to address the challenge of horizontal alveolar bone loss. This review summarizes recent advances, obstacles, and future directions in this field. We first discuss the biomaterials, growth factors, GTR membranes, and cells tested for horizontal alveolar bone loss regeneration. Next, we focus on clinical and preclinical approaches for horizontal alveolar bone regeneration. Furthermore, we highlight the obstacles of current treatment modalities and present future directions that will improve the field of horizontal periodontal regeneration research. This paper aims to provide a better perspective on horizontal alveolar bone loss and stimulate interest in developing innovative biomaterials, technologies, and multidisciplinary approaches for horizontal periodontal regeneration.
2. Biomaterials used for horizontal alveolar bone regeneration
Regenerative therapeutic approaches for horizontal bone loss usually involve using one or more elements: graft materials, GTR membranes, growth and differentiation factors, and cells.
2.1. Graft materials
Bone replacement grafts can be broadly classified into the human bone and bone substitutes. They can be further classified into autografts (obtained from the same person), allografts (from a different person of the same species), xenografts (from a different species), and alloplastic (from synthetic materials) [30]. While autografts and allografts are frequently used in periodontal therapy, xenografts and alloplastic are alternative materials for osseous grafts due to the limited number of human source materials.
2.1.1. Autografts
Harvested from patients' intraoral or extraoral sites [31, 32], autografts are non-immunogenic and contain osteoblasts and osteoprogenitor stem cells, offering great osteoinductive, osteoconductive, and osteogenic potential [33, 34]. The use of intraoral autogenous grafts for treating horizontal bone defects was reported only recently. One case report applied the composite bone graft mixed with autogenous bone and a new highly purified bovine bone to the horizontal bone defect and observed a complete 5 mm horizontal augmentation [35]. Another used a bovine bone mineral biomaterial and autogenous bone mixed with the bone morphogenetic protein-2/absorbable collagen sponge (BMP-2/ACS) and platelet-rich plasma (PRP) to fill a large alveolar ridge defect and found apparent supracrestal bone augmentation through cone-beam computed tomography (CBCT) [36]. In a prospective case series, particulate autogenous bone and an organic bovine bone-derived mineral mixed in equal proportions were applied to patients with knife-edge ridge surgical sites, and an average of >5 mm of lateral ridge augmentation was observed in the clinic [37]. Currently, autogenous bone is the gold standard for bone grafting [38].
2.1.2. Allografts
Allografts are a popular choice for treating periodontal defects, are available in large amounts, and do not need a second surgical site. However, allografts decelerate new bone formation compared to autografts [39]. Demineralized freeze-dried bone allografts (DFDBA) are the primary allograft used at present and have excellent biocompatibility, osteogenic potential, and osteoinductive molecules, such as BMPs [40]. Since DFDBA may lose some of its mechanical stability during the demineralization process, it should be applied with a space-maintaining material, especially when bone defects are not self-contained. When DFDBA was used as a graft material alone in horizontal bone defects, no significant differences were observed in either soft tissue parameters or the amount of new bone formation [41]. When DFDBA was used as a part of a combined approach, significant bone formation was observed in the interdental area compared to its application alone [41–43].
2.1.3. Xenografts
Xenografts are graft tissues obtained from non-human species and are almost nondegradable. The deproteinized bovine bone mineral (DBBM) is the most frequently used xenograft in periodontal regeneration due to its biocompatibility and osteoconductive ability [44]. A combination of DBBM and a collagen membrane was reported as an effective treatment modality for horizontal bone augmentation in 12 consecutive cases and a horizontal bone defects model of beagle dogs [45, 46]. In another case report, enamel matrix proteins (EMPs) and DBBM covered with a biodegradable membrane were applied jointly to reconstruct supracrestal alveolar bone loss. Radiographs showed that the original natural anatomy of the alveolar bone had been restored after bone grafting [47].
2.1.4. Alloplasts
Alloplastic grafts overcome the disadvantages of autografts in that they can be synthesized in different forms without disease transmission risk [48–50]. In general, hydroxyapatite (HA) and tricalcium phosphate (TCP) are two representative calcium phosphates, which demonstrate osteoconductive properties by releasing calcium and phosphate ions as 'raw materials' for new bone formation [51]. Moreover, TCP is degradable and can make space for the healing bone. In protected bone defects, TCP-based bone substitute materials exhibit faster bone healing than HA-based materials [39, 52, 53]. In studies of horizontal bone defects, TCP has been the most common alloplastic material [54]. Of its two crystallographic forms (α-TCP and β-TCP), β-TCP exhibits better biocompatibility and osteoconductivity [48]. When TCP was used as a carrier of growth factors like recombinant human growth factors-5 (rhGDF-5) or as a defect filler with another treatment mode in critical-sized supra-alveolar defects of dog models, the treated sites showed significant regeneration of newly formed bone and cementum [55, 56]. Lately, a new TCP, modified with monetite and zinc, was adopted in standardized defects of beagle dogs, showing a higher formation rate of newly mineralized tissues than xenograft or conventional GBR [57].
2.2. Barrier membranes
The concepts of GBR and GTR evolved in the late 20th century. Barrier membranes exclude epithelial and connective tissues from the wound area and create and maintain the space into which pluripotential and osteogenic cells freely migrate. Since then, it has become a standard of care for barrier membranes in treating periodontal bone and peri-implant defects and bone augmentation procedures [58, 59]. In addition, the GTR method has been confirmed to partially regenerate lost periodontal tissues with new orderly structures [60].
2.2.1. Polytetrafluoroethylene (PTFE) membrane
Polytetrafluoroethylene (PTFE), a synthetic fluoropolymer, is nondegradable and has biologically inert properties [61]. A landmark animal study first demonstrated its excellent tissue compatibility [62]. Subsequently, the expanded PTFE (e-PTFE) and dense PTFE (d-PTFE) were modified to increase the rigidity and antibacterial properties of the material. Even in the early attempt, applying PTFE membrane in suprabony lesions could lead to new mineralized tissue formation in clinical practice [63, 64]. Later, the ePTFE membrane combined with other regenerative materials, including graft materials or growth factors, resulted in a significant clinical bone formation effect [43, 65, 66]. However, the nonabsorbable nature of the ePTFE membrane might lead to wound-healing complications [67–72], inflicting additional surgical trauma during its removal [73, 74]. In previous studies, the most frequently reported membrane exposure among PTFE membranes is the nondegradable e-PTFE membrane [75]. The subsequently developed d-PTFE barrier has better antibacterial properties and membrane integrity due to its smaller pore size [76]. Therefore, much effort has been directed toward developing a bioabsorbable barrier membrane.
2.2.2. Polyglycolide or polylactide (PGA or PLA) membranes
Synthetic aliphatic polyesters are used to fabricate degradable membranes, including polyglycolide (PGA) or polylactide (PLA) or their copolymers. They can be prepared reproducibly in almost unlimited quantities and are significantly superior to naturally occurring materials such as collagen. The molecular weight and structure of PGA or PLA can affect the rate and outcome of degradation [77]. Due to the presence of chiral carbon atoms, PLA polymers can be divided into three 3-dimensional configurations: dexo-PLA (PDLA), L-PLA (PLLA), and meso-PLA (PDLLA). PDLA and PLLA are optically active crystallizable materials with better mechanical and degradation properties than PDLLA [78]. Compared to PTFE membranes, the PGA or PLA membranes are both biodegradable and biocompatible, promote healing and closure of the tissues, and avoid extra exposure to the wound area [79]. Although there are fewer reports of membrane exposure after applying degradable membranes, we assume that this is associated with the development of the flap techniques, not the types of membranes used. Once exposed, however, it is more susceptible to infection and degrades more rapidly, adversely affecting the outcomes. The PGA or PLA membrane was introduced to the combined periodontal regeneration therapy as a comparatively newly developed material. In an animal experiment of critical-sized supra-alveolar periodontal defects, the PGA-trimethylene carbonate (TMC) membrane combined with recombinant human BMP-2 (rhBMP-2) showed significantly increased regeneration of alveolar bone height [80]. A clinical study reported enhanced bone regeneration when PLA membrane was combined to treat patients with edentulous ridge defects [81]. The PGA or PLA membranes appear to be a compatible biomaterial for horizontal bone regeneration.
2.2.3. Collagen membrane
Collagen membranes are predominantly made of type I collagen or a combination of type I and type III collagen. Their collagen composition is similar to that of periodontal connective tissues, which endows them with weak immunogenicity, cytotoxicity, and the ability to promote the chemotaxis of PDL and gingival fibroblasts [82, 83]. Collagen membranes are easy to manipulate, and their physiologic degradation enables them to calcify and ossify when placed close to the bone [58, 84]. These characteristics make collagen material superior to other biodegradable GTR or GBR barriers. However, collagen membranes have deficiencies such as unfavourable mechanical properties [85] and a fast biodegradation rate, resulting in inadequate barrier function [86–88]. In addition, the resorption rate of collagen membranes is unpredictable, with the half-life of non-cross-linked collagen membranes varying from 7 to 28 days. In contrast, the duration of cross-linked membranes is significantly prolonged for up to 4–6 months [58, 88–91].
For periodontal regeneration, collagen membranes were widely used in combination with various graft materials and growth factors in patients requiring vertical and horizontal ridge augmentation [35–37, 45]. These treatment modalities showed stable therapeutic effects, with significant bone augmentation adequate for implantation in most patients. In 2006, a bilayer collagen membrane combined with EMPs and DBBM was used in a patient with supracrestal alveolar bone loss caused by severe chronic periodontitis [47]. Periodontal conditions improved, and the alveolar bone was restored five years postoperatively. Until recently, collagen membranes were still considered a vital restoration component by various preclinical trials in attempts to restore horizontal bone defects [46, 56, 57].
2.3. Growth and differentiation factors
Growth and differentiation factors are essential for the development and growth of tissues and organs and have recently been shown to play a crucial role in wound healing/regeneration. Concerning periodontal regeneration, several growth and differentiation factors have also been evaluated as candidate technologies, including platelet-derived growth factor (PDGF), insulin-like growth factors I and II (IGF-I/-II), acidic and basic fibroblast growth factors (a/bFGF), enamel matrix protein derivative (EMD), and BMPs.
2.3.1. BMPs
A series of animal studies have confirmed the potential of BMP-2 to stimulate horizontal regeneration, with significant new bone formation found in defect areas [65, 66, 80, 92, 93]. One study reported significant new cementum formation with Sharpey's fibres [92]. However, one adverse effect of rhBMP-2 was that ankylosis was observed in almost all teeth near the sites receiving rhBMP-2. One study even reported a positive correlation between the extent of root resorption and rhBMP-2 concentration in a dog model of supra-alveolar periodontal defects [93]. Researchers used an extra membrane to separate the rhBMP-2 and the root surface in a similar animal experiment, successfully overcoming the side effects [94]. Given the limitations of rhBMP-2, RhBMP-12 was used as a new agent in the horizontal bone defect model [95]. Compared with rhBMP-2, the sites treated with rhBMP-12 showed less new bone formation, with smaller chances of ankyloses. More importantly, a functionally oriented PDL was observed between newly formed bone and cementum, suggesting that rhBMP-12 may positively affect periodontal regeneration without the side effects of rhBMP-2.
Growth/differentiation factor-5 (GDF-5), also known as cartilage-derived morphogenetic protein-1, is another member of the BMP family of proteins [96, 97]. An in vitro study demonstrated that GDF-5 might provide an environment conducive to periodontal wound healing/regeneration by affecting PDL cell proliferation in a dose-dependent manner [98]. Concerning supra-alveolar periodontal defects, several animal studies evaluated the sites treated with recombinant human GDF-5 (rhGDF-5), which showed increased bone formation and greater PDL and cementum regeneration [55, 99, 100]. Only one study observed limited ankylosis, suggesting that rhGDF-5 may be another potential candidate for periodontal wound healing/regeneration [55].
2.3.2. bFGF
The bFGF appears to be the most recognized form of the FGF polypeptide family. It stimulates wound healing and tissue repair by promoting angiogenesis, cell proliferation, and non-collagenous protein synthesis. It is widely used in periodontal-related studies and can promote new periodontal tissue formation [101–104]. Concerning horizontal alveolar bone defects, regeneration therapy consisting of collagen-binding bFGF (CB-bFGF) showed significantly increased new bone formation and considerably suppressed epithelial downgrowth in a rat model [105]. Although limited research has been conducted, the potential of sustained-release CB-bFGF composite material for improved periodontal regeneration in the vertical axis is worth intensive studies.
2.3.3. EMD
As an extract of porcine foetal amelogenin, EMD is a potent factor in regenerating the periodontal attachment apparatus on teeth with advanced periodontal defects [106]. Moreover, the highly conserved structure and function of amelogenins allow EMD to be applied in other species without triggering allergic or immunologic reactions [107]. In some clinical studies, the therapeutic effects of EMD were evaluated in patients with supra-alveolar bone defects, and a significant clinical benefit was suggested [47, 108–110]. The EMD treatment showed consistently better clinical improvements compared to conventional flap debridement, especially at sites with deep pockets. In animal studies, the slight influence of EMD on bone regeneration was verified again. In addition, histological examinations revealed the ability of EMD to reduce junctional epithelium along the root surface and enhance the formation of new cementum [111, 112].
2.3.4. Platelet concentrates
Platelets are indispensable for hemostasis and are a rich source of growth factors, including PDGF, vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), and transforming growth factor-β (TGF-β) [113]. Different platelet concentrates, such as PRP, pure PRP (P-PRP), leukocyte-PRP (L-PRP), and platelet-rich fibrin (PRF) have been widely used in periodontology, including horizontal bone loss.
In a clinical study, the application of autologous PRF resulted in significant improvements in probing depth and clinical attachment gain of 15 patients with horizontal bone loss [114]. In another study, PRF matrix (PRFM) assisted by intramarrow penetration (IMP) significantly improved clinical indications and alveolar bone measurements of patients with horizontal bone defects [115]. Concerning ridge augmentation in edentulous areas, platelet concentrates enhanced bone regeneration and increased horizontal bone gain and percentage of vital bone in clinical trials of combined procedures [36, 81, 116]. In addition, both the graft handling and biological abilities of PRP and PRF were improved through mixing with graft materials. Although it is difficult to judge the therapeutic effects of platelet concentrates due to their component complexity and individual variations, combined application of various growth factors may represent a general trend in periodontal regeneration. Table 1 presents the features of the main biomaterials and growth and differentiation factors.
Table 1. Features of main biomaterials, and growth and differentiation factors.
| Main type of materials | Advantages | Limitations | ||
|---|---|---|---|---|
| Graft materials | ||||
| Human bone | Autografts |
|
|
|
| Allografts |
|
|
| |
| Bone substitutes | Xenografts |
|
|
|
| Alloplasts |
|
|
| |
| Barrier membranes | ||||
| Nondegradable | ePTFE membrane |
|
|
|
| Biodegradable | PGA or PLA membranes |
|
|
|
| Collagen membrane |
|
|
| |
| Growth and differentiation factors | ||||
| BMPs |
|
|
|
|
| bFGF |
|
|
| |
| EMD |
|
|
| |
| Platelet concentrates |
|
|
|
|
Abbreviations: FDBA, freeze-dried bone allografts; DFDBA, demineralized freeze-dried bone allografts; DBBM, deproteinized bovine bone mineral; TCP, tricalcium phosphate; ePTFE, expanded polytetrafluoroethylene; PGA/PLA, polyglycolide/polylactide; BMPs, bone morphogenetic proteins; bFGF, basic Fibroblast growth factors; EMD, enamel matrix protein derivative; GDF-5, growth/differentiation factor-5; PRP, platelet-rich plasma; P-PRP, pure platelet-rich plasma; L-PRP, leukocyte- and platelet-rich plasma; PRF, platelet-rich fibrin; TGF-β, transforming growth factor β; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor; IGF, insulin-like growth factor; PDL, periodontal ligament.
2.4. Cell-based therapy
Advances in stem cell biology and regenerative medicine have driven the development of cell-based therapeutic approaches in periodontal regeneration to improve the efficacy and predictability of current therapies [117, 118]. Stem cells can be used to replenish destroyed cells under certain conditions due to their proliferative and multipotent properties, offering a new concept for periodontal regeneration [119, 120]. Several studies have applied them successfully in horizontal bone defects.
The bone marrow stromal cells (BMSCs) have useful clinical applications in oral and maxillofacial surgery [121]. BMSCs were first applied for repairing experimental horizontal alveolar bone defects in a dog model [122]. Canine BMSCs were induced to differentiate into osteoblasts in vitro and seeded on calcium alginate to prepare a cellular scaffold. Twelve weeks after the scaffold was implanted, the height of the repaired alveolar bone increased to 48.59% of the height of the normal alveolus. Two case reports have shown the clinical application of a novel cellular allograft (containing native mesenchymal stem cells and osteoprogenitor cells) [123] and autologous bone marrow mononuclear cells in patients with horizontal bone loss [124]. At postoperative follow-ups, clinical and radiographic improvements in bone regeneration were observed.
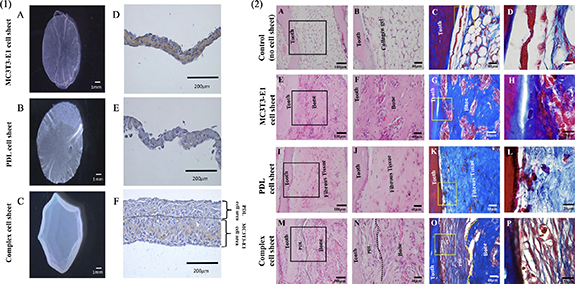
Another cell type used frequently to achieve periodontal regeneration is PDL stem cells (PDLSCs) [125]. PDLSCs possess stem cell characteristics and have a unique potential to fabricate three-dimensional PDL tissue [126]. A combination scaffold of PDLSC sheets, mixed bone grafts, and an absorbable GTR membrane was applied in a dog model of canine horizontal periodontal defects, with favorable clinical results [56]. Similarly, when critical-sized supra-alveolar periodontal defects were treated with PDLSCs implanted in a demineralized bovine bone mineral with 10% collagen, histological findings at three months showed periodontal regeneration with the formation of new cementum, new bone, and connective tissue attachment on the previously exposed root surface [127]. The PDLSC sheets transplanted around the denuded root surface might help with the newly formed cementum. Recent research has evaluated the effectiveness of autologous, allogeneic, and heterogenic sources of stem cells used for periodontal bone defects [128]. Both autologous and allogeneic PDLSC treatments showed significantly improved periodontal tissue regeneration, indicating that allogeneic cells derived from the PDL may have a potential similar to autologous cells. One complex cell sheet was recently reported by layering PDL cells on top of the osteoblast-like cells (MC3T3-E1 cells) during fabrication and turning the PDL cell side towards the root surface and the MC3T3-E1 cell side towards the bone tissue side [129]. After eight weeks, complete recovery of periodontal tissue injury was observed. The complex cell sheet might have the potential to reproduce the bone–ligament structure similar to natural periodontal tissues (figure 2).
Figure 2. (1.A–C) Fabrication of cell sheets by temperature responsive culture dishes. (1.D–F) Immunohistochemical staining. MC3T3-E1 cell sheet showed positive staining for anti-mouse- osteocalcin (OCN) antibody, PDL cell sheet did not show any positive staining for anti-mouse-OCN antibody, Complex cell sheet showed positive expression for anti-mouse-OCN antibody in MC3T3-E1 cells space and negative staining in the PDL cells area, PDL cells and MC3T3-E1 cells area is demarcated using black dotted lines. (2.H&E) and azan staining of ectopic transplants. (2.A–D) Control, without any cell sheet transplantation shows only collagen gel structure around the tooth, (2.E–H) MC3T3-E1 cell sheet transplants showed only bone like tissue formation, (2.I–L) PDL cell sheet transplants with only fibrous tissue generation, (2.M–P) Complex cell sheet transplants showed both PDL and bone-like tissue (PDL-like and bone-like tissue is demarcated using black and yellow dotted lines). (A)–(P) Reproduced from [129]. CC BY 4.0.
Download figure:
Standard image High-resolution imageDespite recent advances, some difficulties in applying cell therapy to horizontal periodontal regeneration still exist. First, periodontal cell delivery strategies are hampered by inherent difficulties in isolating and purifying true patient autologous stem cells and identifying the appropriate ex vivo culture before reimplantation. Additionally, the selection of the scaffold or carrier is also worth consideration. Under ideal conditions, an appropriate carrier may enhance the survival and local retention of certain cells, and cell therapy may, in turn, promote the regenerative capacity of these carriers, especially for bone grafts. However, sometimes, the differences between the scaffold-alone and cell scaffold groups were not significant [127]. Finally, biosecurity is crucial in cell therapy, as immune rejection and transmission of bacterial, viral, or other pathogens may lead to life-threatening reactions [130]. Therefore, further investigations are necessary on controlling cell transformation and more considerations about balancing the effectiveness, the availability, and the potential risks of all these different choices. Table 2 summarizes the features of primary cell-based therapy.
Table 2. Features of main cell-based therapy.
| Cell types | Study approach | Models/Subjects | References | Advantages | Limitations | |
|---|---|---|---|---|---|---|
| BMSCs |
| Animal study | Mongrel dog experimental horizontal alveolar bone defects model | [122] |
|
|
| Clinical Study | A 48-year-old woman with interproximal horizontal bone loss | [123] | |||
| Clinical Study | A 23 year female with severe horizontal bone loss | [124] | |||
| PDLSCs |
| Animal study | Beagle dog horizontal periodontal defects model | [56] |
| |
| Animal study | Beagle dog with critical-size-6 mm supra-alveolar periodontal defects model | [127] | |||
| Animal study | Mouse periodontal tissue injury model | [129] | |||
Abbreviations: BMSCs, Bone marrow stromal cells; PDLSCs, periodontal ligament stem cells; GTR, guided tissue regeneration; PDL, periodontal ligament; MC3T3-E1, osteoblast like cells.
3. Current procedures of horizontal alveolar bone regeneration
3.1. Single procedure
3.1.1. Animal studies
Preclinical studies with a single procedure have commonly been used to determine the therapeutic effects of growth factors. One advantage of animal studies is that histological changes in periodontal tissues can be clearly observed during regeneration. In 1997, rhBMP-2 with a sponge-type carrier was placed over horizontal circumferential defects created in beagle dogs [92]. Twelve weeks after the surgery, considerable new bone formation and new cementum with Sharpey's fibres were observed in the rhBMP-2-treated sites. This effect was verified again in an animal model of critical-sized, supra-alveolar, and periodontal defects created around the premolars of beagle dogs [93]. Compared with the control group, supra-alveolar periodontal defects receiving different concentrations of rhBMP-2 exhibited extensive alveolar regeneration. Cementum regeneration was also observed, with a new fibrous attachment formed between bone and cementum. In another animal study with the same defect model, rhBMP-12 carried by an ACS was used as a new agent [95]. Eight weeks after surgery, rhBMP-12/ACS achieved comparable cementum regeneration and more chances of functionally oriented PDL formation. Although bone regeneration was less than rhBMP-2/ACS, sites receiving rhBMP-12/ACS showed lower chances of ankylosis. Periodontal regeneration potential was demonstrated by a novel injectable rhGDF-5/poly (lactic-co-glycolic acid) (PLGA) construct [100]. The rhGDF-5/PLGA construct was injected into the surgically created pockets over the buccal roots of the second and fourth mandibular premolars. At four weeks, PDL and cementum regeneration was two folds greater in the control group, while increased horizontal bone formation was observed at sites receiving rhGDF-5/PLGA. At six weeks, the rhGDF-5/PLGA group showed increased maturity of the newly formed bone. Recently, the efficacy of CB-bFGF was tested in a rat horizontal alveolar bone defect model [105]. This construct and collagen powder effectively promoted bone regeneration in horizontal bone defects. Unfortunately, the defect area was confined to only alveolar bone; therefore, the effect of CB-bFGF on cementum regeneration was not evaluated. However, not all studies applying growth factors alone have reported positive results for bone and cementum regeneration, and conclusions may differ for a certain kind of growth factor. EMD was used in bony defects created on the mesial aspect of the mesial root of the first maxillary molars in Wistar rats [111]. Compared with the control group, only the EMD carrier propylene glycol alginate was applied; the EMD group showed a similar amount of new bone formation 12 weeks after surgery. In a more recent study of a similar model, EMD did not affect bone or cementum regeneration compared with untreated controls [112].
3.1.2. Clinical studies
Histological evaluation is unpractical in clinical studies; therefore, current clinical evaluations and diagnoses are mainly based on periodontal probing and x-ray examinations, which can partly reflect the degree of new bone formation. Clinical studies have adopted graft materials, barrier membranes, and growth factors separately. TCP and DFDBA grafts are the two graft materials adopted separately in patients with horizontal bone loss. Two studies [41, 131] reported a certain degree of clinical symptom improvements, such as probing depth reductions and clinical attachment level gains, but little evidence of new bone formation was observed through radiographic examinations. As a representative nonabsorbable membrane, the ePTFE membrane was once popular and singly tried in horizontal bone defects. Although studies have reported that applying PTFE membrane in suprabony lesions could lead to new mineralized tissue formation, measuring methods in these early attempts have not been accurate, mainly based on probing diagnosis without visual inspection by radiography [63, 64]. For the application of growth factors, some clinical studies evaluated the therapeutic effects of EMD and platelet concentrates separately in patients with supra-alveolar bone defects. EMD treatment showed better clinical improvements in probing depth, attachment level gain, and gingival recession, which were more pronounced at periodontal sites with high probing depths [47, 108–110]. However, the results showed no significant increase in radiographic bone levels, consistent with preclinical studies. Concerning the evaluation of platelet concentrates, clinical studies have found inconsistent effects on bone regeneration. In a clinical trial of 15 patients, 45 sites with horizontal bone loss were treated with autologous PRF gel, PRF gel and PRF membrane or open flap debridement (OFD) [114]. Re-evaluation at nine months revealed that the PRF gel alone significantly decreased probing depths and clinical attachment gain compared to baseline and control. However, there was no significant difference in gingival recession and radiographic bone levels nine months after surgery between the three groups, indicating that PRF alone may have little effect on horizontal bone regeneration.
3.2. Combined procedure
With the advances in material science, treatment planning, and clinical skills, researchers could integrate various regeneration elements into one combined structure, using more than one regeneration factor, which has become the mainstream of today's regeneration therapy.
3.2.1. Graft material and barrier membrane
Early in 1999, a combination of graft material and barrier membrane was first adopted in a clinical study [43]. DFDBA was used with an e-PTFE membrane in patients with advanced horizontal bone loss. The results indicated successful supracrestal regeneration of horizontal defects, with the mean attachment gain for the seven patients studied ranging from 2.6 to 3.0 mm 2 years postoperatively. Radiographically, coronal apposition of bone was clearly seen between 18 and 24 months in certain sites of the treated areas. Another clinical study used a combination of DFDBA and calcium phosphate cement (CPC) with a membrane to augment bone in the interdental area with horizontal bone loss [42]. At six months, there was a significant bone fill and trabecular formation in the interdental area and a reduction in tooth mobility. The reconstructive technique using bone grafts and barrier membranes showed apparent therapeutic effects for new bone formation in patients with horizontal bone loss. However, regeneration of cementum and functional PDLs were rarely reported using this treatment design.
3.2.2. Growth factors and barrier membranes
The efficacy of a combined procedure using growth factors and barrier membranes was explored in a series of preclinical animal studies using BMP-2 [65, 66, 80]. The PGA-TMC membrane with rhBMP-2 in biodegradable hyaluronan (Hy) carrier was adopted in Hound Labrador mongrel dogs with artificially created supra-alveolar, periodontal defects [80]. The study showed that rhBMP-2 significantly enhanced alveolar bone augmentation and soft tissue healing when combined with the PGA-TMC membrane; however, ankylosis compromised regeneration in sites receiving PGA-TMC/rhBMP-2. Similarly, the effects of BMP-2 and ePTFE membrane were evaluated in the same animal model [65]. Bone regeneration and ankylosis increased significantly in jaw quadrants receiving rhBMP-2/ACS with ePTFE membrane compared to ePTFE membrane alone [65]. In addition, compared to rhBMP-2/ACS alone, the regenerated bone area was significantly enhanced in jaw quadrants receiving rhBMP-2/ACS with the ePTFE membrane [66]. These findings suggested that the barrier membrane appears suitable as a protector to enhance rhBMP-2/ACS-induced alveolar bone formation. To avoid ankylosis caused by BMP-2, one study placed an extra polymer-coated gelatin sponge (PGS) spacer membrane between PGS containing rhBMP-2 and the root surface in horizontal circumferential periodontal defects of beagle dogs [94]. Twelve weeks after surgery, sites treated with rhBMP-2/PGS and spacer membrane exhibited enhanced new bone formation and connective tissue attachment with cementum regeneration compared to the physiological saline/PGS group. Although sites treated with rhBMP-2/PGS alone showed more bone formation than sites treated with rhBMP-2/PGS and spacer membrane, the latter sites showed no ankylosis [94].
3.2.3. Growth factors and IMP
In a clinical study of nine patients with horizontal bone defects, PRFM was used with OFD and IMP (OFD + IMP + PRFM) [115]. After nine months, probing pocket depth and clinical attachment level showed a significant difference between the OFD + IMP + PRFM and control groups. At this time, the radiographic assessment also showed significantly better new alveolar bone deposition at the PRFM-treated sites.
3.2.4. Graft materials, growth factors, and barrier membranes
Only one case report combined graft material, growth factor, and barrier membrane to treat horizontal bone defects. This combined procedure was used in a patient with supracrestal alveolar bone loss caused by severe chronic periodontitis [47]. The probing depths and clinical attachment levels in the maxilla averaged 4.46 mm and 5.48 mm, respectively, at baseline. After debridement, the root surfaces were conditioned, and EMPs were applied to stimulate cementum and PDL formation. The bovine bone mineral was placed in the supracrestal defects to help regenerate the lost alveolar bone. In addition, the graft materials around maxillary anterior teeth were covered with a biodegradable polytetrafluoroethylene membrane. Probing depths in the maxilla decreased by 1.91 mm, and clinical attachment levels increased by 2.28 mm in five years. Radiographs showed that the original natural anatomy of the alveolar bone had been restored following the grafting procedure.
3.2.5. Cell therapy, graft materials, and barrier membranes
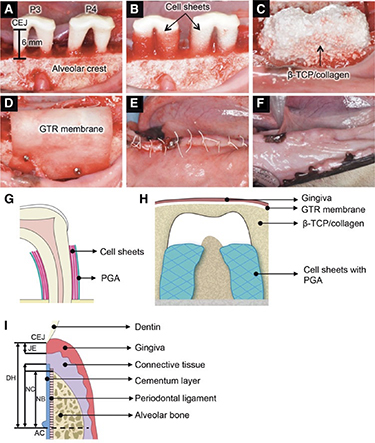
The combined application of cells and other materials was also a common design reported by several studies in horizontal bone defects (mentioned in section 2.4) [123, 124, 127]. One animal study combined cell therapy, graft materials, and barrier membranes in a canine horizontal periodontal defect model [56]. Three-layered PDLSC sheets were transplanted around denuded root surfaces in autologous and allogeneic groups [56]. A mixture of β-TCP and collagen gel was placed on bone defects and covered with an absorbable GTR membrane wrapped over the component (figure 3). Eight weeks after surgery, alveolar bone regeneration was observed in both the autologous and allogeneic transplantation groups. Inflammatory markers from peripheral blood sera showed a slight difference between the two groups. The allogeneic transplantation group showed significant regeneration of dense collagen fibres in the middle portion of the defects, which attached perpendicularly to the cementum-like tissue layer.
Figure 3. Allogeneic transplantation of PDL-MSC sheets in a canine horizontal defect model. The photographs (A)–(E) show the surgical procedures used for dogs. (F) At 8 weeks after transplantation, no visible infection, suppuration, or adverse reactions were observed. (G) Schematic illustration of three-layered cell sheets and polyglycolic acid (PGA) sheet. (H) Schematic illustration of transplanted site. (I) The illustration shows the histometric parameters. B-TCP, b-tricalcium phosphate; CEJ, cementum–enamel junction; DH, defect height; JE, junctional epithelium; NC, newly formed cementum; NB, newly formed bone; AC, alveolar crest (bottom of bone defects). (A)–(H) Reproduced from [56]. CC BY 4.0.
Download figure:
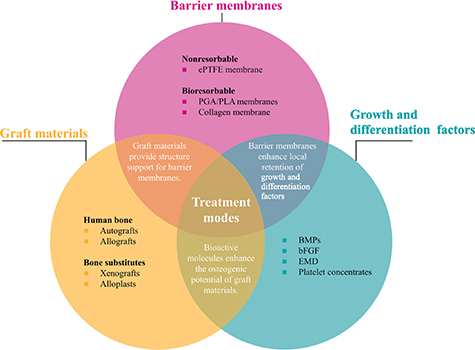
Standard image High-resolution imageTable 3 summarizes the current procedures of horizontal alveolar bone regeneration. Both animal and clinical studies using a combined procedure have reported significant regeneration of new alveolar bone in horizontal bone defects. Therefore, the combined procedure will remain the major treatment modality for periodontal horizontal regeneration (figure 4). However, regeneration of cementum and functional PDLs have rarely been reported, which is the key to achieving complete periodontal regeneration. Considering the complexity of periodontal tissues, the current use of regeneration elements is still oversimplified without managing coordinated spatiotemporal regeneration of different tissues within the defect area.
Figure 4. Different treatment modes of combined procedures used in horizontal periodontal regeneration. Graft materials, barrier membranes, and growth and differentiation factors are three key elements commonly used in combined procedures. Generally, graft materials provide structure support for barrier membranes; barrier membranes enhance local retention of growth and differentiation factors; growth and differentiation factors enhance the osteogenic potential of graft materials.
Download figure:
Standard image High-resolution imageTable 3. Current procedures of horizontal alveolar bone regeneration.
| Study approach | Materials | Models/Subjects | Conclusions | References | |
|---|---|---|---|---|---|
| Single procedure | |||||
| Animal study (histological evaluation) | Growth and differentiation factors | rhBMP-2 | Beagle dog experimental horizontal circumferential defects model |
| [92, 93] |
| rhBMP-12/ACS | Hound Labrador mongrel dog supraalveolar periodontal defects model |
| [95] | ||
| rhGDF-5/PLGA | Hound Labrador mongrel dogs with periodontal pockets (3 × 6 mm, width × depth) model |
| [100] | ||
| CB-bFGF | Rat horizontal alveolar bone defect model |
| [105] | ||
| EMD | Wistar rat mesial root bone defect of first maxillary molar model |
| [111, 112] | ||
| Clinical study (periodontal probing diagnosis and x-ray examination) | Growth and differentiation factors | EMD | Patients with supra-alveolar bone defects |
| [47, 108–110] |
| Autologous PRF gel, PRF gel and PRF membrane | Patients with horizontal bone loss |
| [114] | ||
| Graft materials | TCP, DFDBA | Patients with horizontal bone loss |
| [41, 131] | |
| Barrier membranes | ePTFE membrane | Patients with horizontal bone loss |
| [63, 64] | |
| Combined procedure | |||||
| Clinical study | Graft materials + Barrier membranes | DFDBA + ePTFE membrane | Patients with advanced horizontal bone loss |
| [43] |
| DFDBA + CPC + degradable membrane | A 38-year-old female with the interdental area and horizontal bone loss |
| [42] | ||
| Animal study | Growth and differentiation factors + Barrier membranes | rhBMP-2 + PGA-TMC membrane + hyaluronan carrier | Hound Labrador mongrel dog with critical size, 5–6 mm, supra-alveolar, periodontal defects model |
| [80] |
| rhBMP-2/ACS + ePTFE membrane | Hound Labrador mongrel dog critical-size, 5–6 mm, supra-alveolar, periodontal defects model |
| [65] | ||
| rhBMP-2 + PGS spacer membrane | Beagle dog horizontal circumferential periodontal defects |
| [94] | ||
| Clinical study | Growth and differentiation factors + intramarrow penetration | OFD + IMP + PRFM | Patients with horizontal bone defects |
| [115] |
| Clinical study | Graft molecules + Barrier membrane | DBBM + EMP + ePTFE membrane | One patient with supracrestal alveolar bone loss caused by severe chronic periodontitis |
| [47] |
| Animal study | Cell therapy+ Graft material+ Barrier membrane | PDLSC sheet + mixture of β-TCP collagen gel+ GTR membrane | Beagle dog horizontal periodontal defect model |
| [56] |
Abbreviations: rhBMP-2, recombinant human bone morphogenetic protein-2; rhBMP-12, recombinant human bone morphogenetic protein-12; ACS, absorbable collagen sponge; PLGA, poly (lactic-co-glycolic acid); rhGDF-5, recombinant human growth/differentiation factor-5; CB-bFGF, collagen-binding- basic fibroblast growth factor; EMD, enamel matrix protein derivative; PRF, platelet-rich fibrin; TCP, tricalcium phosphate; DFDBA, demineralized freeze-dried bone allografts; ePTFE, expanded polytetrafluoroethylene; CPC, calcium phosphate cement; PGA-TMC, polyglycolic acid-trimethylene carbonate; ACS, absorbable collagen sponge; PGS, polymer-coated gelatin sponge; OFD, open flap debridement; IMP, intramarrow penetration; PRFM, platelet rich fibrin matrix; EMP, enamel matrix proteins; GTR, guided tissue regeneration; PDLSC, periodontal ligament stem cells; PDL, periodontal ligament.
4. Conclusion and future direction
Currently, shortcomings still exist in biomaterials, and growth and differentiation factors adopted in regeneration therapy, impeding stable and efficient treatment results. Autografts are still the best graft materials with exclusive osteogenesis abilities, while synthetic graft materials offer only a partial solution to managing localized bone loss. Combined application of current graft materials is an excellent way to overcome their limitations and retain their advantages simultaneously. Although bioabsorbable membranes have clear advantages over nondegradable barriers, defects, including unfavourable mechanical properties and fast biodegradation rate, are unsolved. Various growth factors targeted on specific periodontal tissues, such as BMP-12 or GDF-5, should be tested with different or multi-phased carriers to achieve directed differentiation locally. In addition, more attention should be paid to the anti-infection capacity of all the materials.
Under current treatment modalities, alveolar bone tissue regeneration is achievable for horizontal periodontal defects during clinical practice. In fact, related techniques have already been widely adopted in implant dentistry for combined implant–periodontal therapy. In a recent study, orthodontic treatment was first carried out on elongated maxillary incisors with horizontal bone defects. Surprisingly, the shape of the bone defects was changed to a vertical type on some teeth after orthodontic intrusion. Periodontal regenerative therapies were then performed on these angular bone defects to improve bone morphology [132]. This combined orthodontic–periodontal treatment improved the horizontal alveolar bone loss of the defective sites, which suggests the importance of multidisciplinary therapy.
Finally, our ultimate goal is complete periodontal regeneration, which requires highly coordinated spatiotemporal regeneration of all the tissues during the narrow window of the restoration period. The tissue engineering approach, utilizing an advanced 3D scaffold as a retentive architecture to maintain the spatial position of various regeneration materials, may have the potential to overcome the limitations of the current clinical treatment modality and may even have the ability to guide and coordinate the healing process of various tissues at the same time. Currently, it is possible to use computer-aided design software to develop patient-specific scaffolds based on specific defect parameters extracted from computed tomography scans. In a recent case report, CBCT scans of peri-osseous defects were used to design a customized polycaprolactone scaffold fabricated via selective laser sintering [133]. Future studies should focus on improving the design and biological properties of the current scaffolds, enabling the utilization of a greater variety of materials with appropriate spatiotemporal arrangement while maintaining high manufacture resolution at the same time. Future studies should achieve further clinical benefits with advances in the properties of new biomaterials, the increased structure design of material complex, and more specific bioagents targeted on each periodontal tissue.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC, Nos. 81701005 and 82071150) and the Sichuan Science and Technology Program (No. 2021YFS0193).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Conflict of interest
The authors declare no conflicts of interest.