Abstract
Low in macro-porosity electro-spun scaffolds are often associated with foreign body response, whilst macro-porous electro-spun scaffolds have low mechanical integrity. Herein, compressed, macro-porous and collagen (bovine Achilles tendon and human recombinant) coated electro-spun poly-ε-caprolactone scaffolds were developed and their biomechanical, in vitro and in vivo properties were assessed. Collagen coating, independently of the source, did not significantly affect the biomechanical properties of the scaffolds. Although no significant difference in cell viability was observed between the groups, collagen coated scaffolds induced significantly higher DNA concentration. In vivo, no signs of adverse tissue effect were observed in any of the groups and all groups appeared to equally integrate into the subcutaneous tissue. It is evidenced that macro-porous poly-ε-caprolactone electro-spun meshes with adequate mechanical properties and acceptable host response can be developed for biomedical applications.
Export citation and abstract BibTeX RIS
1. Introduction
Electro-spun meshes are extensively used in biomedicine, as subject to the polymer used and its concentration and the fibre dimensionality and orientation two- and three- dimensional scaffolds can be produced with controllable mechanical properties and immune response; and with ability to promote cell adhesion and directional migration/growth; to either maintain cell phenotype or to control cell differentiation; and to direct neotissue formation [1–4]. Additionally, numerous methods have been developed to functionalise electro-spun meshes (e.g. blending, multi-layering, dual electro-spinning, co-axial electro-spinning, electro-spinning-co-electro-spraying) enabling them that way to deliver bioactive/therapeutic cargos in a controlled and localised manner [5–9]. Yet again, the relatively low mechanical properties of electro-spun meshes in combination with their dense structure have jeopardised their wide acceptance and use in clinical practice. Considering that the dimensionality and the shape of pores of hernia meshes have been shown to modulate tissue integration in vivo [10, 11], recent efforts are directed towards the development of micro-porous electro-spun meshes with sufficient mechanical resilience to be able to withheld local forces.
Although controlled porosity electro-spun meshes have been realised [12–15], the introduction of porosity affects the overall structural integrity of the mesh [15, 16]. Using compression and thermal ablation, we have developed electro-spun meshes with circular pores and 30% porosity with clinically relevant mechanical properties [15, 17], using poly-ε-caprolactone (PCL). PCL is an FDA approved, slow in degradation and hydrophobic semi-crystalline polyester that is commonly used in biomedicine, as it can easily and relatively economically be processed into various scaffold conformations, with clinical indication specific mechanical properties [18, 19]. In vitro data have revealed that PCL degradation initiates with the non-enzymatic hydrolytic cleavage of the ester groups and subsequently the polymer becomes more crystalline (as the amorphous regions are primarily degraded) and of low (≤3000 kDa) molecular weight, primarily due to intracellular degradation by macrophages and giant cells and to a lesser extent by fibroblasts [20, 21]. However, due to the hydrophobicity [22] and surface chemistry [23] of PCL, cell adhesion and the subsequent in vitro and in vivo response can be impeded [23–26]. Therefore, functionalisation with collagen (COL) is commonly used, considering that COL is the most abundant extracellular matrix (ECM) protein and it is favoured for biomedical applications [27]. One should note though that electro-spun COL alone scaffolds are not suitable for biomedical applications due to their poor mechanical properties [28]. Electro-spun co-extruded COL/PCL scaffolds are also not suitable, as the addition of COL has been shown to significantly weaken the overall tensile properties in comparison to PCL alone scaffolds [28, 29]. Further, the fluoroalcohols that are customarily used in the electro-spinning process have been shown to irreversibly denature COL [30, 31]. Post-fabrication COL coating not only does not compromise mechanical integrity and does not denature collagen, but it has also been shown to promote cell attachment and growth (e.g. endometrial stem cells [32], kidney cells [33], dermal fibroblasts [34]).
Considering the above, herein we developed electro-spun macro-porous PCL meshes, which were subsequently coated with human recombinant collagen type I (hR COL) or bovine Achilles tendon collagen type I (BAT COL). The produced scaffolds were assessed in vitro (morphological, mechanical and cytocompatibility analyses) and in vivo (rat subcutaneous implantation).
2. Material and methods
2.1. Scaffolds fabrication
Electro-spinning was performed as has been described previously [15, 17, 35–37] with slight modifications. Briefly, 200 mg ml−1 of PCL (PURASORB® PC-12, Corbion, Netherlands) were dissolved in 2,2,2-trifluoroethanol (TFE; 99.8%, Acros Organics, Ireland) and were extruded at 100 μl min−1 from three syringes simultaneously (to reduce manufacturing time) through an 18 G stainless steel blunt needle (EFD Nordson, Dublin, Ireland). Upon application of high voltage (20 kV) between the needle and the collector (20 cm distance), the solvent evaporated, and the fibres were collected on a rotating mandrel (60 revolutions per min). The electro-spun scaffolds were compressed at Proxy Biomedical (Spiddal, Ireland) at 282 N cm−1 for 20 min at a temperature of 25 °C. Electro-spun scaffolds were then laser cut to introduce porosity with 2 mm diameter circular pores in a simple 0/90° array pattern until 30% surface porosity was introduced. Some compressed electro-spun scaffolds were coated with 5 mg ml−1 hR COL (CollPlant, Israel) or BAT COL (Vornia Biomaterials, Ireland). After coating, the scaffolds were allowed to air dry in a laminar flow hood at room temperature (RT) overnight and then were crosslinked (to increase stability) with 1-ethyl-3-[3-dimethy- laminopropyl]carbodiimide (EDC): N-hydroxy- sulfosuccinimide (NHS) in 0.05M 2-(N-morpholino) ethanesulfonic acid (MES) buffer at pH 5.5 in a 3:1:5 ratio respectively, as has been described before [38–40]. The following day, three phosphate buffered saline (PBS) washes were carried out and then the meshes were allowed to dry at RT.
2.2. Morphology assessment
Gross visual observations were performed with a stereomicroscope (Olympus, Japan). For finer details, the electro-spun scaffolds were mounted onto a carbon disk, gold sputter coated and imaged with a Hitachi S-4700 scanning electron microscope (Hitachi High-Technologies Europe GmbH, Germany).
2.3. Mechanical properties assessment
Mechanical properties were assessed via a ball burst test, using a Z005 Zwick/Roell (Leominster, UK) testing machine, loaded with 1 KN load cell, as has been described previously [17]. The samples (N = 5 per group; 150 mm × 150 mm each sample) were prepared as per ASTM D3787-15 guidelines. To ensure full hydration and relatively physiological assessment of the samples' mechanical properties, prior to testing, all samples were incubated overnight at RT in PBS and immediately prior to testing, tissue paper was used to remove excess PBS. The samples were placed between two layers of vulcanised rubber and subsequently placed between the appropriate sample grips and hand tightened around the circumference. The extension rate was 20 mm min−1. The following definitions were used to calculate mechanical data: stress at break was defined as the load at failure divided by the original cross-sectional area (engineering stress), strain at break was defined as the increase in scaffold length required to cause failure divided by the original length and modulus was defined as the ratio of stress to strain.
2.4. Cell culture
Primary adult dermal fibroblasts (DF), passage 6–9, were cultured in Dulbecco's Modified Eagle's Medium (high glucose, Sigma Aldrich, Ireland) with 10% foetal bovine serum (FBS, Sigma Aldrich, Ireland) and 1% penicillin streptomycin (PS, Sigma Aldrich, Ireland) at a cell density of 30 000 cells/well for 1, 3 and 7 d on Nunc™ non-treated flasks (Thermo Scientific, Ireland). The cells were maintained at 37 °C in a humidified 5% CO2 incubator. The meshes were cut into discs of ∼15 mm in diameter and then fixed into 24-well tissue culture plate wells with a silicone O ring (Ace O-rings, Sigma Aldrich, Ireland). Glass coverslips were used as control (CTRL) and also contained O rings. Sterilisation was conducted through immersion in 70% industrial methylated spirits for 24 h, then rinsed three times in Hanks balanced salt solution (HBSS, Sigma Aldrich, Ireland), followed by ultraviolet light for 1 h. The samples were kept in HBSS until immediately prior to cell seeding. All experiments were carried out in 4 replicates.
2.5. Cell viability assessment
Cell viability was assessed using quantification of lactate dehydrogenase using supplier's protocol (LDH; CytoTox 96®, Promega, MyBio Ltd.). In brief, a standard curve was prepared from 0 to 50 000 cells and samples media containing released LDH were transferred to a 96 well plate, to which the reaction mixture was added. After incubating for 30 min in the dark, at RT, the stop solution was added and the absorbance was read at 490 nm using a microplate reader (Varioskan Flash, Thermo Scientific, UK).
2.6. Cell proliferation assessment
Cell proliferation was assessed with DNA quantification through PicoGreen® (Invitrogen™, Bio-Science, Ireland) as per manufacturer's protocol. Briefly, the media was extracted from the samples and replaced with 200 μl of water which was frozen at the appropriate time points and freeze/thawed three times. Equal quantities of the samples and PicoGreen® dye were added to a 96 well plate and incubated in the dark at RT for 5 min. A standard curve of 0 to 500 ng ml−1 of DNA was utilised. Samples and standards curves were read on a microplate reader (Varioskan Flash, Thermo Scientific, UK) at 485/535 nm.
2.7. Animal study
The protocol was approved by the NAMSA Ethical Committee before the beginning of the study. Twenty-seven (9 per time point) adult male Sprague Dawley rats (Charles River Laboratories, France), weighting 291–332 g prior to implantation, were used for subcutaneous implantation procedure. Prior to implantation, the fur of the rats was clipped and the area of implantation was cleaned with 70% isopropyl alcohol (Laboratoire Pharmaceutique Galénique, France) and povidone iodine (Vetedine® savon, Vetoquinol, France). A neutral veterinary ophthalmic ointment (Ocrygel®, TVM, France) was applied to the eyes to prevent drying and the rats were placed in ventral recumbency. The rats were anesthetised by inhalation of an O2–isoflurane mixture (IsoFlo®, Axience, France). Four incisions (two per side) large enough to accommodate each scaffold (non-coated, BAT collagen coated and hR collagen coated electro-spun scaffolds) were made through the skin and parallel to the vertebral column. Pockets placed at appropriately spaced intervals were formed by blunt dissection of the subcutaneous tissues. COL coated or uncoated scaffolds that had been sterilised with ethylene oxide were introduced in each pocket and the skin was closed with stainless steel wound clips (supplementary figure S1 is available online at stacks.iop.org/BMM/14/055007/mmedia). The rats were observed until recovery from the anaesthetic procedure and then returned to their respective cages.
At 10, 28 and 56 d post-implantation, the designated rats were anesthetised with an intra-muscular injection of tiletamine-zolazepam (Zoletil® 100, Virbac, France), weighed and sacrificed by a lethal intravenous injection of pentobarbital (Dolethal, Vetoquinol, France). The subcutaneous tissues were then macroscopically examined and excised, allowing a sufficient area around the site for proper histologic preparation. Any gross changes in tissues surrounding the implant were recorded using the following parameters: size, shape, colour, consistency, distribution, presence/absence of implant encapsulation, tissue integration, degradation and any other observations, as appropriate. Macroscopic pictures of each implanted site were taken. The sites were collected including epidermis, dermis, panniculus carnosus and the subcutaneous tissue surrounding the implant with at least 5 mm excess all around.
2.8. Histopathologic, histologic and histomorphometric analyses
The histopathologic evaluation scoring system is provided in supplementary table S1. Samples for histopathologic analysis from each site were fixed in 10% neutral buffered formalin (NBF, Sigma Aldrich, France), as was a spleen biopsy from one rat (control tissue marking to serve as a positive control for Picrosirius Red (PR) Staining). The draining axillary and inguinal lymph nodes were analysed for systemic inflammation. They were excised, macroscopically examined and any gross changes of the lymph nodes were recorded using the following parameters: size, shape, colour, consistency, distribution and any other observations, as appropriate. The lymph nodes were fixed in 10% NBF. After 24–72 h fixation in 10% NBF, the implanted together with a piece of each non-implanted sample were dehydrated in alcohol solutions of increasing concentration, cleared in xylene and embedded in paraffin. Two central longitudinal cranio-caudal cross sections (4–7 μm thickness) were prepared for each site using a microtome (MICROM®, France). Sections were stained with modified Masson's Trichrome (MT, Sigma Aldrich, France). Two additional serial central longitudinal cross-sections per sample were prepared for histomorphometric analysis: one section was stained with PR and the other one was stained with Feulgen and Rossenbeck (F&R) stain.
Histopathologic analysis focused on the following parameters, which were graded according to pathology report: inflammatory cells, necrosis, fibrosis, neovascularisation, fatty infiltrate, fibrin, haemorrhage, cell or tissue degeneration, fibroplasia, tissue integration, tissue ingrowth, encapsulation and scaffold degradation.
Histomorphometric analysis used a region of interest (ROI) (4 × 8 mm length × width) encompassing the scaffolds and located in the centre or near the centre of the site was determined to measure the tissue in growth rate and quality of the collagen. A light microscope (Nikon Eclipse 80i, Japan) equipped with an image analyser system (Tribun, France, IPS version 4.06) was used for the analysis.
2.9. Collagen quality and tissue ingrowth assessment
To measure the tissue in growth rate and quality of the collagen, the total collagen content percentage within the implanted scaffolds was determined with the PR stained sections via quantitatively analysis. Results were expressed in percent of total collagen area measured within the ROI. Collagen polymorphism (COL I and COL III), using PR stained sections, were assessed by cross-polarisation microscopy (CPM). Under CPM, a section of the rat spleen was used as the positive control to accurately set the angle of polarisation before measurement of the COL III area and COL I was confirmed with the spleen using the same angle of polarisation to analyses the quantitative histomorphometric. Results were expressed as the ratio of the COL I and COL III surface area. The F&R stained slides were used to determine the cell area density (%) corresponding to the cell area (μm2)/ROI area (μm2) × 100. The tissue in growth rate was defined by the total collagen content plus the cell area density (%).
2.10. Statistical analysis
Statistical evaluation was conducted using Minitab® (in vitro data analysis) (Minitab® version 17, Minitab® Inc., USA) or SPSS (in vivo data analysis) (Software SPSS version 19.0, SPSS Inc.). One way analysis of variance with a Tukey's post-hoc test for multiple comparisons were employed after confirming the following assumptions: (a) the distribution from which each of the samples was derived was normal (normality test); and (b) the variances of the population of the samples were equal to one another (test for equal variances). Non-parametric statistics were used when either or both of the above assumptions were violated and consequently Kruskal–Wallis test for multiple comparisons or Mann–Whitney test for two-samples were carried out. The local tissue effects, integration and the degradation evaluations were based on qualitative macroscopic observation of the implanted sites at termination. Qualitative and semi-quantitative histopathologic evaluation of the mean score comparison of the semi-quantitative parameters and comparison of the qualitative histopathologic findings. Quantitative histomorphometric analysis of the collagen content, cellularity and tissue ingrowth rate. The histomorphometric individual data were presented in percentages. The histomorphometric individual data in percentages calculated based on the histomorphometric individual data in μm before being rounded-off. Numerical data is expressed as mean ± SD. Statistical significance was accepted at p ≤ 0.05.
3. Results
3.1. Structural and biomechanical assessment
Macroscopic analysis revealed that all scaffolds had the same macro-porosity, whilst microscopic analysis made apparent that the collagen coating, independently of the origin (BAT COL I or hR COL I), completely covered the fibrous topography and created a smooth layer (figure 1). COL coating, independently of the origin (BAT COL I or hR COL I), did not significantly (p > 0.05) affect the mechanical properties of the scaffolds (table 1).
Figure 1. Macro- and micro- scopic images of non-coated and collagen (bovine Achilles tendon (BAT) and human recombinant (hR)) coated electro-spun scaffolds. No particular differences were observed between the groups. Note: on the macroscopic images, the black circles are the pores, the rest is the electro-spun scaffold.
Download figure:
Standard image High-resolution imageTable 1. Ball burst test results of non-coated and collagen (bovine Achilles tendon (BAT) and human recombinant (hR)) coated electro-spun scaffolds. No significant difference was observed in any of the measured values.
| Group | Maximum strain (%) | Maximum stress (MPa) | Maximum force (N) | E modulus (MPa) | Thickness (mm) |
|---|---|---|---|---|---|
| E-spun (n = 5) | 4.53 ± 1.50 | 1.11 ± 0.17 | 47.61 ± 3.16 | 63.15 ± 7.61 | 0.48 ± 0.08 |
| BAT COL I (n = 5) | 6.08 ± 2.03 | 1.27 ± 0.15 | 40.02 ± 5.67 | 78.07 ± 4.10 | 0.34 ± 0.04 |
| hR COL I (n = 5) | 4.95 ± 1.65 | 1.14 ± 0.11 | 36.74 ± 3.69 | 67.06 ± 7.31 | 0.35 ± 0.04 |
3.2. In vitro assessment
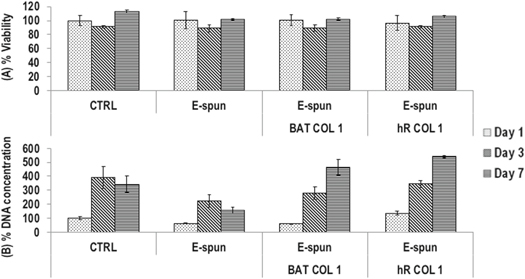
No significant (p > 0.05) differences were found in cell viability between the groups (figure 2(A)). At day 7, collagen coated groups exhibited significantly (p < 0.01) higher DNA concentration than their non-coated counterparts, although no significant (p > 0.05) difference was observed between the different (BAT COL I and hR COL I) collagens (figure 2(B)).
Figure 2. Cell viability was not affected as a function of the groups (A). Collagen coating significantly increased DNA concentration at day 7 (B).
Download figure:
Standard image High-resolution image3.3. In vivo assessment
No clinical abnormalities or adverse events occurred during the post-operative period. All rats gained body weight between the surgery and the termination (up to 14% ± 3% at day 10, up to 34% ± 4% at day 28 and up to 46% ± 8% at day 56).
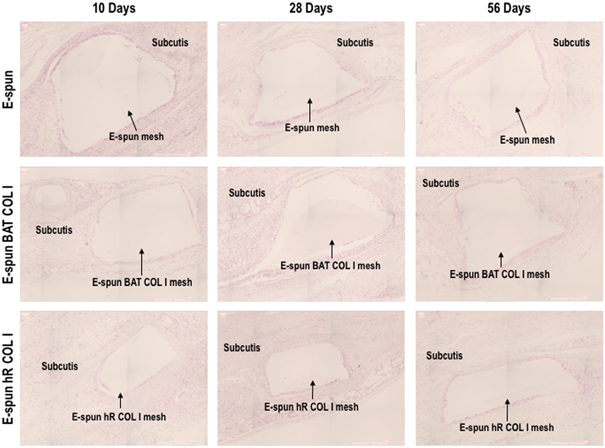
No major abnormalities were observed on the skin above the implanted sites or at the soft tissues surrounding the scaffolds for all groups at all time points. The macroscopic integration was equivalent for all samples per time point. At 10 d a premature tissue envelope was observed for all scaffolds with similar adhesion to the fascia muscle. After 28 d of implantation, the major part of the scaffolds was well integrated with the surrounding tissue with a thin and transparent to pinkish envelop. After 56 d of implantation, a thicker and transparent to whitish/pinkish envelop was developed (figure 3). Further, after 56 d of implantation, some inguinal lymph nodes had a slight atrophy, but no other abnormalities were observed (gross visual observations).
Figure 3. The macroscopic integration at 10 days post-implantation was equivalent for all samples. was equivalent for all samples. At 28 d post-implantation, the major part of the scaffolds was well integrated with the surrounding tissue. At 56 d post-implantation, a thick and transparent to whitish/pinkish envelop had been developed.
Download figure:
Standard image High-resolution imageAll groups showed slight to moderate vascularisation, which decreased over time post implantation. Further, there was no sign of scaffold degradation throughout the implantation period (table 2 and supplementary figure S2). Partial envelops (no envelope on the central part) were observed with an increased occurrence for the electro-spun and BAT COL I coated groups at day 28, but this observation was not significant according to semi-quantitative histopathologic analysis of the MT staining (figure 4, table 2 and supplementary figure S2) or quantitative morphometric and histomorphometric analysis of the F&R staining (figure 5 and supplementary figure S3) and PR staining (figure 6 and supplementary figure S4). However, BAT COL I and hR COL I samples had slightly higher integration mean scores in comparison to the non-coated scaffolds after 56 days of implantation (table 2 and Supplementary Figure S2). All scaffolds were well integrated, as evidenced by macroscopic and microscopic analyses, as well as all other macroscopic observations (e.g. adhesion and neovascularization) were considered normal and to be related with the surgical procedure or to the healing process.
Table 2. Semi-quantitative histopathologic analysis of non-coated and collagen (bovine Achilles tendon (BAT) and human recombinant (hR)) coated electro-spun scaffolds.
| Time period | Group | Macrophages | Lymphocytes | Plasma cells | Polymorphonuclear cells | Giant cells | Necrosis | Fibrosis | Neovascularization | Fatty infiltrate | Fibrin | Hemorrhage | Tissue degeneration | Fibroplasia | Tissue integration | Tissue ingrowth | Encapsulation | Material degradation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 10 | E-spun (n = 5) | 2.0 ± 0.0 | 1.8 ± 0.4 | 1.6 ± 0.5 | 2.2 ± 0.4 | 2.0 ± 0.0 | 0.0 ± 0.0 | 1.4 ± 0.5 | 2.4 ± 0.5 | 0.0 ± 0.0 | 0.6 ± 0.5 | 1.0 ± 0.7 | 0.2 ± 0.4 | 2.2 ± 0.4 | 2.0 ± 0.0 | 3.0 ± 0.00 | 1.0 ± 0.0 | 0.0 ± 0.0 |
| BAT COL I (n = 4) | 2.0 ± 0.0 | 2.0 ± 0.0 | 1.8 ± 0.5 | 2.0 ± 0.0 | 2.0 ± 0.0 | 0.0 ± 0.0 | 1.3 ± 0.5 | 2.8 ± 0.5 | 0.0 ± 0.0 | 0.5 ± 1.0 | 1.0 ± 0.8 | 0.0 ± 0.0 | 2.3 ± 0.5 | 1.8 ± 0.5 | 3.0 ± 0.0 | 1.0 ± 0.0 | 0.0 ± 0.0 | |
| hR COL I (n = 5) | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 0.0 ± 0.0 | 1.4 ± 0.5 | 2.2 ± 0.4 | 0.0 ± 0.0 | 0.4 ± 0.5 | 1.0 ± 0.0 | 0.2 ± 0.4 | 1.4 ± 0.5 | 1.4 ± 0.5 | 3.0 ± 0.0 | 1.0 ± 0.0 | 0.0 ± 0.0 | |
| Day 28 | E-spun (n = 5) | 2.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.2 ± 0.4 | 2.0 ± 0.0 | 0.0 ± 0.0 | 1.0 ± 0.0 | 1.8 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.6 ± 0.5 | 0.0 ± 0.0 | 1.2 ± 0.4 | 2.8 ± 0.4 | 3.0 ± 0.0 | 1.0 ± 0.0 | 0.0 ± 0.0 |
| BAT COL I (n = 5) | 2.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.2 ± 0.4 | 2.0 ± 0.0 | 0.0 ± 0.0 | 1.0 ± 0.0 | 2.0 ± 0.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.4 | 0.0 ± 0.0 | 1.6 ± 0.5 | 2.6 ± 0.5 | 3.0 ± 0.0 | 1.0 ± 0.0 | 0.0 ± 0.0 | |
| hR COL I (n = 5) | 2.0 ± 0.0 | 1.2 ± 0.4 | 1.0 ± 0.0 | 1.0 ± 0.0 | 2.0 ± 0.0 | 0.0 ± 0.0 | 1.2 ± 0.4 | 1.4 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.4 | 0.0 ± 0.0 | 1.2 ± 0.4 | 2.6 ± 0.5 | 3.0 ± 0.0 | 1.0 ± 0.0 | 0.0 ± 0.0 | |
| Day 56 | E-spun (n = 5) | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.2 ± 0.4 | 1.0 ± 0.0 | 3.0 ± 0.0 | 0.0 ± 0.0 | 1.2 ± 0.4 | 1.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.0 ± 0.0 | 3.6 ± 0.5 | 4.0 ± 0.0 | 1.2 ± 0.4 | 0.0 ± 0.0 |
| BAT COL I (n = 5) | 1.2 ± 0.4 | 1.0 ± 0.0 | 0.0 ± 0.0 | 1.0 ± 0.0 | 2.4 ± 0.5 | 0.0 ± 0.0 | 1.6 ± 0.5 | 1.2 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.6 ± 0.5 | 0.0 ± 0.0 | 1.2 ± 0.4 | 4.0 ± 0.0 | 4.0 ± 0.0 | 1.6 ± 0.5 | 0.0 ± 0.0 | |
| hR COL I (n = 5) | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.8 ± 0.4 | 1.0 ± 0.0 | 3.0 ± 0.0 | 0.0 ± 0.0 | 1.6 ± 0.5 | 1.2 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.4 ± 0.5 | 0.0 ± 0.0 | 1.2 ± 0.4 | 4.0 ± 0.0 | 4.0 ± 0.0 | 1.4 ± 0.5 | 0.0 ± 0.0 |
Figure 4. Representative Masson's Trichrome histological images at termination. No signs of local adverse tissue effect and no degradation were observed in any of the groups at any time point. All groups appeared to similarly integrate into the subcutaneous tissue at the time points tested.
Download figure:
Standard image High-resolution imageFigure 5. Representative Feulgen and Rossenbeck histological images at termination. No signs of local adverse tissue effect and no degradation were observed in any of the groups at any time point. All groups appeared to similarly integrate into the subcutaneous tissue at the time points tested.
Download figure:
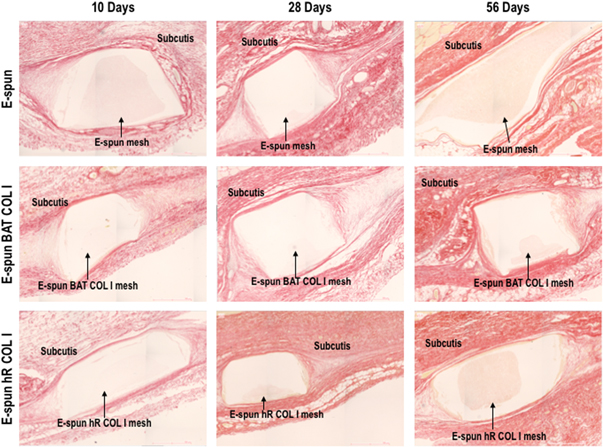
Standard image High-resolution imageFigure 6. Representative Picrosirius red histological images at termination. No signs of local adverse tissue effect and no degradation were observed in any of the groups at any time point. All groups appeared to similarly integrate into the subcutaneous tissue at the time points tested.
Download figure:
Standard image High-resolution imageStatistically significant differences were observed in the mean ratio COL I/COL III at day 28 between non-coated and BAT COL I coated scaffolds. However, it was not considered to be biologically relevant, as it was an isolated occurrence and the collagen polymorphism mean ratios for all three groups at day 56 were similar. The cell area density parameter did not show significant differences between the groups at each time point or over time (table 3 and supplementary figure S5).
Table 3. Quantitative morphometric and histomorphometric analysis. Total COL content (TCC)% and the ratio of COL I/COL III were obtained from PR stains, while the cell area density% and the tissue ingrowth rate (TIR)% were obtained from the F&R stains.
| Time period | Group | TCC% | Ratio COL I/COL III | Cell area density (%) | TIR% |
|---|---|---|---|---|---|
| Day 10 | E-spun (n = 5) | 14.1 ± 8.3 | 6.9 ± 2.8 | 4.7 ± 1.4 | 18.8 ± 9.3 |
| BAT COL I (n = 4) | 14.1 ± 3.6 | 3.6 ± 1.0 | 4.2 ± 0.4 | 18.3 ± 3.6 | |
| hR COL I (n = 5) | 19.7 ± 6.5 | 4.3 ± 2.5 | 4.3 ± 0.8 | 24.0 ± 7.0 | |
| Day 28 | E-spun (n = 5) | 15.3 ± 3.1 | 4.2 ± 1.3a | 3.2 ± 1.1 | 18.5 ± 3.5 |
| BAT COL I (n = 5) | 10.5 ± 3.8 | 2.1 ± 0.3a | 4.0 ± 1.5 | 14.5 ± 4.4 | |
| hR COL I (n = 5) | 17.1 ± 5.3 | 2.0 ± 1.1 | 3.3 ± 0.9 | 20.4 ± 6.0 | |
| Day 56 | E-spun (n = 5) | 17.6 ± 4.5 | 3.4 ± 3.5 | 3.4 ± 0.3 | 21.0 ± 4.8 |
| BAT COL I (n = 5) | 17.3 ± 5.5 | 2.9 ± 1.3 | 3.6 ± 2.4 | 21.0 ± 7.1 | |
| hR COL I (n = 5) | 19.4 ± 6.5 | 3.1 ± 1.0 | 3.9 ± 0.9 | 23.4 ± 7.3 |
4. Discussion
Porosity has been shown to modulate tissue integration and foreign body response (FBR). Small pores (0.1–0.6 mm [41]) have been associated with an elevated risk of encapsulation due to bridging scars and FBR [42] resulting in impeded abdominal movements [43]. Meshes with larger pores (1–2 mm [41]) have been shown to result in elevated tissue integration and correspondingly lower FBR [44]. In fact, macro-porous and light weight meshes have shown remarkable biocompatibility in subcutaneous mice models [45]. Traditional electro-spun meshes, although very light, they have small porosity, which results in FBR in preclinical models (e.g. incisional hernia rat model [46]). Although the use of macro-porous electro-spun meshes has been advocated with in vitro data, no in vivo data are currently available. Thus, herein, we ventured for first time to assess in vivo the influence of macro-porous electro-spun meshes without and with collagen (BAT and human recombinant) coating.
Biomechanical analysis made apparent that the collagen coating, independently of the source, did not affect the structural and biomechanical properties of the scaffolds. Similar results have also been reported when collagen was used to coat, through plasma treatment, random and aligned poly(L-lactic acid) electro-spun scaffolds [47] or, through simple coating, random poly(3-hydroxybutyric acid)-gelatin electro-spun scaffolds [48]. Despite these data, previous studies have shown composite scaffolds to have superior mechanical properties than their single component counterparts [49, 50], this was not observed here, possibly due to the fact that the collagen was not co-extruded or that the collagen layer was too thin to induce any notable difference in mechanical properties. One should also note that ECM materials, such as collagen, elastin, gelatin, hyaluronic acid, are not used for mechanical integrity, but to improve biological response. Indeed, herein, collagen coating, independently of the source, improved in vitro cell response by day 7. Electro-spun coated or composite synthetic polymers and collagen [32, 47, 48, 51–53], gelatin [54, 55], collagen/gelatin [56], elastin [57] and hyaluronic acid [37] scaffolds have been previously shown to have enhanced cell attachment, proliferation and growth, when compared to synthetic polymer alone scaffolds.
Post-implantation macro- and micro- scopic analysis of the scaffold integration suggests minimal differences between the groups. By day 28, the majority of the scaffolds for all groups were well integrated, with full integration by day 56. Qualitative analysis revealed that all other macroscopic observations were indicative of a normal post-operative healing process. Collagen coated and uncoated samples showed no relevant differences in terms of local tissue effects. The inflammatory cell response was consistent with that of a normal healing response, whereby basal inflammatory response was observed at day 10 and dissipated almost completely by day 28 and 56. The exception to this was the presence of giant cells, which were heavily infiltrated by day 56 in all groups. The presence of giant cells around the electro-spun PCL meshes after 28 and 56 d is typical of FBR and is consistent with the literature [58, 59]. The amount of giant cells has been shown to be significantly lower in electro-spun PCL meshes compared to solid PCL constructs (melted until smooth electro-spun PCL meshes), which indicates initially elevated M1 macrophages with subsequently M1/M2 macrophages modulation and upregulation of pro-healing cytokines compared to the pro-inflammatory cytokines of the solid construct [60]. Similar results for inflammation have been reported in a porcine hernia model, with heavy macrophage inflammation after 90 d, including giant cell infiltration with fibrosis around individual mesh macro-fibres and periodic bridging across mesh interstices [11]. Electro-spun porous PCL sheets (aligned electro-spun mates were fabricated, plasma etched, collagen coated, six layers stacked together and laser cut to yield a porous scaffold with 150 μm in diameter pores at 15% pore area) have also been shown to elicit minimal inflammatory response in a rodent ligament reconstruction model; although high number of monocytes and macrophages were observed at 2 weeks, their number peaked at 6 weeks and significantly decreased from week 6 to week 12 post-implantation [61]. The limited inflammatory response is consistent with finding comparing solid PCL films to electro-spun PCL meshes, where a significantly thicker encapsulation layer formed around films compared to electro-spun PCL meshes [58]. While the presence of new vascularisation around the implant was not observed inside the scaffold, mature vascularisation was evidenced around the implant from the termination site images for all time points. Previous studies have shown that rolled PCL electro-spun scaffolds with 300 μm pores to significantly improve vascular ingrowth within 14 d post-implantation in a rat abdomen model [62], whilst neovascularisation in knitted meshes has been seen within the mesh, without crossing through the interstices of the mesh after 90 d [11], suggesting that longer time points may have been needed. This can be further advocated by considering that no degradation was observed in vivo, which is consistent with reports of 1%–7% mass loss after 6 months [63] and with complete degradation after 30 months [18] for PCL electro-spun meshes. A tubular PCL/PTMC electro-spun scaffold has also been reported to remain intact for up to 4 weeks post-implantation and its micro-porous structure allowed for infiltration of autologous cells, whilst, although at the periphery giant cells were present, there was no obvious fibrous encapsulation [64].
With respect to animal extracted versus human recombinant collagen, no particular differences were observed in in vitro and in vivo setting, further corroborating a previous suggestion that a niche area should be identified for recombinant collagens to justify their cost [65], although at the low amounts used in this study, still makes it a viable option. Several studies have shown scaffold functionalisation to improve host response. For example, PCL and PCL/gelatin electro-spun fibres loaded with metronidazole have been shown to evoke a less severe inflammatory response after 8 months of subcutaneous rabbit implantation than pure PCL nanofibers [66, 67]. Further, PCL/bFGF electro-spun scaffolds exhibited minimal inflammatory response 16 weeks post-implantation in an athymic rat model for anterior cruciate ligament reconstruction [68]. Electro-spun PCL/collagen scaffolds facilitated penetration of tissue cells into the fibrous scaffolds at day 7, whilst no such cell penetration was noticed in the PCL alone scaffolds [69]. In our case, non-coated and collagen coated scaffolds exhibited similar host response. Although the collagen cross-linking method employed has been shown to be crucial in FBR [70], carbodiimide cross-linking has been used extensively in the literature with acceptable in vivo results [71–73], although in vitro data have shown less efficiency in comparison to other cross-linking methods [39, 40]. We therefore believe that the fibrous architecture of the electro-spun meshes is responsible for this acceptable in vivo host response, even without collagen coating, which is in agreement with other studies in numerous clinical indications [74–76].
5. Conclusion
Macro-porous, compressed and collagen (BAT and human recombinant) coated poly-ε-caprolactone electro-spun scaffolds were fabricated and their biomechanical, biological and host response properties were assessed. Collagen coating, independently of the origin, did not affect the biomechanical properties of the scaffolds. Although cell viability was not affected as a function of the collagen coating, DNA concentration was increased after 7 d in culture. Implantation studies showed no differences between the groups. Electro-spun macro-porous and compressed scaffolds have been produced that even without collagen coating demonstrate suitable properties for biomedical applications.
Acknowledgments
This work was supported by the European Union Seventh Framework Programme (FP7/2007-2013), under grant agreement number 263289 (Green Nano Mesh); College of Engineering and Informatics at NUI Galway, Postgraduate Scholarship Scheme; Irish Research Council, Enterprise Partnership Scheme, under grant agreement number EPSPG/2011/64; Health Research Board, Health Research Awards Programme, under grant agreement number HRA_POR/2011/84; Science Foundation Ireland, Career Development Award, under the grant agreement number 15/CDA/3629. This publication was also supported from Science Foundation Ireland and the European Regional Development Fund, under grant agreement number 13/RC/2073. The authors would also like to acknowledge the Centre for Microscopy & Imaging of the National Centre for Biomedical Engineering Science (NCBES) at NUI Galway, which is funded by NUI Galway and the Irish Government's Programme for Research in Third Level Institutions, Cycles 4 and 5, National Development Plan 2007–2013.
Disclosures/conflict of interest
Oded Shoseyov is Founder and Chief Scientific Officer of CollPlant Ltd., Weizmann Science Park, Ness-Ziona, Israel.