Abstract
Engineered adipose tissues are developed for their use as substitutes for tissue replacement in reconstructive surgery. To ensure a timely perfusion of the grafted substitutes, different strategies can be used such as the incorporation of an endothelial component. In this study, we engineered human adipose tissue substitutes comprising of functional adipocytes as well as a natural extracellular matrix using the self-assembly approach, without the use of exogenous scaffolding elements. Human microvascular endothelial cells (hMVECs) were incorporated during tissue production in vitro and we hypothesized that their presence would favor the early connection with the host vascular network translating into functional enhancement after implantation into nude mice in comparison to the substitutes that were not enriched in hMVECs. In vitro, no significant differences were observed between the substitutes in terms of histological aspects. After implantation, both groups presented numerous adipocytes and an abundant matrix in addition to the presence of host capillaries within the grafts. The substitutes thickness and volume were not significantly different between groups over the short-term time course of 14 days (d). For the microvascularized adipose tissues, human CD31 staining revealed a human capillary network connecting with the host microvasculature as early as 3 d after grafting. The detection of murine red blood cells within human CD31+ structures confirmed the functionality of the human capillary network. By analyzing the extent of the global vascularization achieved, a tendency towards increased total capillary network surface and volume was revealed for prevascularized tissues over 14 d. Therefore, applying this strategy on thicker reconstructed adipose tissues with rate-limiting oxygen diffusion might procure added benefits and prove useful to provide voluminous substitutes for patients suffering from adipose tissue loss or defects.
Export citation and abstract BibTeX RIS
Introduction
Tissue engineering is now recognized as a promising approach for tissue reconstruction and replacement. To ensure the survival, the integration and the maintenance of the functions of engineered substitutes, a fast and adequate vascularization must be achieved upon grafting [1, 2]. Angiogenesis can be stimulated by the use of various in vitro strategies that have been addressed in many reviews [3–8]. In particular, cells forming engineered tissues can be genetically modified to express angiogenic factors. Alternatively, angiogenic factors can be incorporated into the scaffold or encapsulated into microspheres that are combined with the constructs. Encapsulation strategies with biomaterials can also improve the angiogenic capacity of cells used for vascular regeneration. Hypoxic preconditioning on substitutes is also performed to stimulate angiogenesis. Another approach relies on the incorporation of endothelial cells within the constructs, a technique called prevascularization. Both human umbilical vein endothelial cells (HUVECs) or adult microvascular cells (from the endothelium of adult, postnatal small-caliber vessels), are able to undergo tubulogenesis and create a network of capillaries in vitro. After implantation, the preformed network of capillaries is expected to connect with the host vasculature to ensure a fast perfusion of the grafts [3–6]. This strategy can be applied to a multitude of substitutes such as reconstructed skin containing a microvascularized dermis [9, 10], a field in which the Laboratoire d'organogénèse expérimentale center has been a pioneer [11–13]. Our team also developed an adipose tissue model featuring a capillary network in vitro [14, 15], since the field of adipose tissue engineering would also greatly benefit from enhanced graft vascularization upon implantation.
Autologous fat grafting or lipotransfer is currently one of the most employed technique to fill soft tissue defects for patients affected by accidents, deep burns, tumor removal or congenital diseases. However, this approach is associated with a limited long-term volume retention ranging from 20%–80% of the initial volume after one year [16, 17]. This would be attributable to the fragility of mature lipid-containing adipocytes that can rupture during fat aspiration and injection, but also to the disruption of the native vascular network during these procedures [18]. Adipose tissue is now recognized as an active producer of many different hormones, growth factors and cytokines acting locally and systemically to impact key biological processes such as food intake, energy balance, blood pressure, angiogenesis, to name a few [19–21]. For example, leptin secretion is indicative of the adipocyte metabolic status, while the balance between secreted angiopoietin (Ang)-1 and Ang-2 modulates angiogenic processes [22–25]. The engineering of adipose tissue is therefore important as an alternative to fat lipotransfer and should aim at producing substitutes that will procure long-term structural and functional enhancement after grafting.
Nowadays, adipose tissue is known as a source of stromal/stem cells possessing an important multi-differentiation potential [26, 27], which is very useful for the engineering of various tissue types including adipose tissue itself [28–32], in addition to secretory functions beneficial in regenerative medicine [33]. Our method for adipose tissue engineering is the self-assembly technique [30, 34, 35]. This approach is based on the property of mesenchymal cells such as human adipose-derived stromal/stem cells (ASCs) to secrete and efficiently assemble their own extracellular matrix components upon stimulation with ascorbic acid and serum [36–38]. This results in the formation of matrix-rich cell sheets, without the use of thermoresponsive cultureware, that are superposed in the desired number to obtain thicker tissues. In parallel to matrix accumulation, an adipogenic induction step allows ASC differentiation into adipocytes and results in the production of adipose sheets [30, 36]. The seeding of human microvascular endothelial cells (hMVECs) onto adipose sheets during the reconstruction enables the production of human reconstructed adipose tissue substitutes containing a preformed network of capillary-like structures [14]. Indeed, the natural human cells and matrix provide a biologically relevant environment conducive to the migration, proliferation, and tubule formation by adult hMVECs, leading to a capillary network that can be modulated by external angiogenic modulators [15].
In this study, we report on the fate of human engineered adipose tissue substitutes, preseeded or not with hMVECs in vitro, after their implantation in vivo. Extensive imaging analyses have been performed in order to describe the early kinetics of vascularization of the grafted tissues in nude mice (0–14 d). We hypothesized that preseeding hMVECs in vitro during the engineering of adipose tissue substitutes would favor anastomosis events upon implantation in vivo, and procure benefits to the grafted tissues by improving the extent of vascularization. Globally, our short-term in vivo experiments established the high compatibility of our constructs to integrate at the site of implantation, both in the presence and in the absence of hMVECs. The reorganization of the hMVECs in vivo led to an extended human capillary network that connected with the host vessels, as supported by the immunolabelings for human and murine CD31 and by the presence of murine red blood cells (mRBCs) within the human capillaries. Finally, the evaluation of the global extent of graft vascularization indicated a tendency towards an increase of the total capillary network surface and volume in the presence of hMVECs within the grafted adipose tissues substitutes.
Material and methods
Cell isolation
Human ASC populations were isolated from subcutaneous fat of two non-obese female donors undergoing cosmetic lipoaspiration procedures (44 years old, body mass index (BMI) 21.5; 35 years old, BMI 21.0). Cells were extracted as described previously using collagenase digestion [30] and were seeded at a density of 8.0–9.1 × 104 cells cm−2 in DH medium (1:1 Dulbecco's Modified Eagle Medium (DMEM): Ham's F12 medium (H); Life Technologies, Burlington, ON, Canada) containing 10% fetal calf serum (FCS) (HyClone, Thermo Scientific, Logan, UT, USA) and antibiotics [100 U ml−1 of penicillin (Sigma, Oakville, ON, Canada) and 25 μg ml−1 of gentamicin (Schering-Plough Canada Inc./Merck, Scarborough, ON, Canada)] in NUNC flasks (Thermo Scientific, Ottawa, ON, Canada). ASCs were batch frozen at passage (P) 0 and used at P3–P5 for tissue reconstruction. hMVECs were isolated from the dermis of a 59 years old female donor using a thermolysin digestion as described previously [14]. They were amplified in EGM-2MV BulletKit (Lonza, Walkersville, MD, USA) supplemented with 100 U ml−1 of penicillin and 25 μg ml−1 of gentamicin instead of the GA-1000 provided by the manufacturer, on gelatin-coated (Fisher Scientific, Ottawa, ON, Canada) T75 flasks (BD Falcon, BD Biosciences, Mississauga, ON, Canada) at a density of 5.8 × 103–9.3 × 103 cells cm−2. hMVECs were used at P6–P7 for seeding on human reconstructed adipose sheets. Both cell types were cultured at 37 °C with an 8% CO2 humidified atmosphere and were obtained after written informed consent of donors. Protocols were approved by the Ethics Committee of the CHU de Québec-Université Laval Research Center.
Reconstruction of human adipose tissue substitutes
Thawed ASCs were amplified at a density of 6.7–8.3 × 103 cells cm−2 in DH medium with 10% FCS and antibiotics. For sheet and tissue production [30, 36], ASCs were seeded at a density of 1.64 × 104 cells cm−2 in NUNC six-well plates (Thermo Scientific) containing a peripheral filter-paper anchorage device (Whatman, GE Healthcare, Ottawa, ON, Canada) to allow easier manipulation. The DH amplification medium was supplemented with 50 μg ml−1 (250 μM) of ascorbic acid (Sigma) prepared fresh with each medium change every 2–3 d, throughout the entire culture. After 7 d in culture, adipogenic differentiation was induced for 3 d using an adipogenic cocktail [DH medium containing 3% FCS, 100 nM insulin (Sigma), 0.2 nM triiodothyronine (T3) (Sigma), 1 μM dexamethasone (Sigma), 0.25 mM 3-isobutyl-1-methylxanthine (IBMX) (Sigma), 1 μM rosiglitazone (Cayman Chemical, Ann Arbor, MI, USA) and antibiotics]. Afterwards, the induction medium was replaced with an adipogenic maintenance medium (DH medium containing 10% FCS, 100 nM insulin, 0.2 nM T3, 1 μM dexamethasone and antibiotics). After a period of 21–22 d of culture and 14–15 d of adipogenic differentiation, evaluation of the cell sheets by microscopy indicated that approximately 75% of the cultures featured differentiated adipocytes. The hMVECs were then seeded on top of adipose sheets at a density of 1.2 × 104 cells cm−2 in 1:1 adipogenic: endothelial medium (adipogenic maintenance medium: EGM-2MV with antibiotics). Adipose sheets without hMVECs were cultured in the same medium. After 6–7 d of culture, adipose sheets were superposed in groups of 3 to produce the two types of adipose tissue substitutes (3 adipose sheets without hMVECs or 3 adipose sheets with hMVECs) and were kept in culture for an additional 6–8 d in 1:1 adipogenic: endothelial medium to allow cohesion between the sheets as well as hMVECs migration and reorganization when applicable. Phase contrast microscopy images were taken using a CKX41 microscope equipped with an E-620 camera (Olympus, Richmond Hill, ON, Canada) to confirm the presence of adipocytes within the tissues before implantation onto nude mice after a total of 34–36 d of culture, 27–29 d of differentiation and 13–14 d with or without hMVECs.
In vitro contraction and thickness determination
In vitro contraction of the tissues was evaluated by measuring the surface area of human adipose tissue substitutes before and after their detachment from the anchorage device, a step performed before implantation (N = 2, n = 17–18 substitutes per experimental group). Measurements were performed on macroscopic images using the ImageJ software (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA) and the percentage of contraction was subsequently calculated. In vitro thickness measurements were performed on histological cross-section images using the ImageJ software. Five images were analyzed for each human adipose substitute and 3 measures were taken on each image for a total of 15 measures (N = 2, n = 2–3 substitutes per group).
Secretion profile analysis
Conditioned media from the human adipose tissue substitutes were harvested on grafting day for two independent experiments (N = 2, n = 3–4 substitutes per experimental condition). Media alone incubated for the same period (24 h) without tissues were also harvested and used as controls. Ang-2 and leptin concentrations were determined by ELISA assays (Duoset, Bio-Techne, Minneapolis, MN, USA) according to the manufacturer's instructions. The baseline concentration in tissue-free controls was subtracted from human adipose substitute-conditioned media concentration when detected (Ang-2). The results are expressed as pg ml−1 per 24 h per tissue construct.
In vivo implantation
Two independent grafting experiments (N = 2, n = 18 human adipose tissue substitutes per experimental group) were performed on male athymic mice using human adipose tissue substitutes produced from two different ASC populations (Grafting experiment #1 = ASC population #1; Grafting experiment #2 = ASC population #2). Animals were housed and cared for according to protocols approved by the Institution Animal Protection Ethics Committee. At the time of grafting, tissues were rinsed in DH medium containing antibiotics but without serum. Mice were anesthetized with isoflurane. The reconstructed tissues were implanted subcutaneously on both flanks onto the muscle bed devoid of the panniculus carnosus and secured with monofilament Nylon sutures (Ethicon, Johnson & Johnson Medical, Markham, ON, Canada) [39]. Each mouse received one human adipose tissue substitute without hMVECs and one with hMVECs. Grafts were analyzed after 3, 7 and 14 d of implantation and harvested with the underlying muscle, when possible.
Macroscopic and histological analyses
At the time of implantation as well as before final tissue harvest, macroscopic images of the grafts were taken with an EOS Rebel XSi camera (Canon, Mississauga, ON, Canada). The tissues were then divided into samples that were used for the different analyses. Fresh samples were embedded in OCT compound (Tissue-Tek, Sakura Finetek, Torrance, CA, USA) and kept at −80 °C until analysis. Some samples were fixed in 3.7% buffered formalin for 24 h and embedded in paraffin for histological analyses. Masson's trichrome staining was performed on 5 μm-thick cross-sections to highlight the cells in pink and the matrix in blue. Images of stained tissue cross-sections were acquired using an Axio Imager.M2 microscope equipped with an AxioCam ICc1 camera and the AxioVision software v4.8.2.0 (Zeiss, Toronto, ON, Canada). Multiple images were merged to obtain mosaic images of entire tissue sections (Adobe Photoshop Software CS4 and CS5, Adobe Systems Incorporated, San Jose, CA). Adipocyte quantification (at 0, 3, 7 and 14 d after grafting) was performed on these histological sections using a customized ImageJ protocol script (threshold 245, circularity [0.4 < 100 μm2, and 0.3 when >100 μm2], scale 2.148). Data is expressed as the number of adipocytes counted per mm2 of graft tissue area (N = 1 experiment, ASC population #2, n = 3 substitutes per experimental condition per time-point, except for day 0 without hMVECs (n = 2)).
Finally, some 3.7%-buffered formalin-fixed samples were kept at 4 °C in phosphate buffered saline (PBS) for confocal imaging analyses. The graft surface was measured using the ImageJ software and macroscopic images taken at the time of tissue harvest (N = 2, n = 5–6 substitutes per experimental group). The surface of the grafts was subsequently compared to their initial tissue surface at time of implantation and data is expressed as percentages. Grafted substitute thickness was measured from histological cross-section images using the ImageJ software. Fifteen measures per graft were taken from separate images or merged mosaic images (N = 2, n = 2–3 substitutes per group). An estimation of the grafted substitute volume was then calculated by multiplying the thickness measured on histological cross-sections by the surface determined from macroscopic images (N = 2, n = 2–3 substitutes per group) and expressed as arbitrary units.
Immunological stainings
Immunolabelings were performed on formalin-fixed whole-mount samples (approximately 4–9 mm2) or 10 μm-thick frozen cross-sections of human adipose tissue substitutes harvested on the day of grafting and 3, 7 and 14 d after implantation. Whole-mount samples were stained for the human von Willebrand Factor (vWF) or were double-stained for these antigen combinations: human CD31 (hCD31) and perilipin, human and murine CD31, hCD31 and mRBCs. These whole-mount samples were clarified and immunolabeled with the antibodies listed in table S1 available online at stacks.iop.org/BMM/13/065013/mmedia according to our protocol described previously [14, 15, 40]. Confocal images were acquired with a LSM 700 confocal microscope and the Zen 2010 software (Zeiss). For labelings on frozen tissue cross-sections, double-staining for human and murine CD31 was performed using the antibodies listed in table S1. These cross-sections were first fixed and permeabilized with −20 °C acetone for 10 min and rinsed 3 times in PBS. They were next incubated with primary antibodies or isotypic controls for 60 min in a humidified chamber. After 3 washes in PBS, sections were incubated in the dark with secondary antibodies and 0.5 μg ml−1 Hoechst 33258 (Sigma) for nuclei counterstaining. Tissue sections were rinsed three times in PBS, once in H2O and were mounted in standard mounting medium. Images were acquired using an Axio Imager.M2 microscope equipped with an AxioCam HRm camera and the AxioVision software v4.8.2.0 (Zeiss).
Global microvascularization image analysis
Microvascularization analysis of the grafts was performed for two independent experiments (N = 2, n = 1–3 per experimental group). Analysis of CD31 double-stained (murine/human) frozen cross-sections was performed using the ImageJ software. Values corresponding to the area covered by either human or murine CD31-positive networks were quantified for 2–3 mosaic images of entire sections from each graft sample using the 'Analyze Particles' function. Graft delineation from the recipient bed was performed with corresponding phase contrast images using the same software. Results are expressed as the percentage of graft surface occupied by the total endothelial network (N = 2, n = 2–3 per group). Figure S1 shows the controls for the mCD31 and hCD31 co-labeling, as well as the threshold settings applied to ensure distinct quantification of the fluorescent signals while taking into account the low cross-reactivity (<5%) of the AF806 antibody with recombinant mouse CD31. In addition, CD31 double-stained whole-mount sample confocal images (1–3 per graft) were analyzed using the 'Surfaces' function of the Imaris software (Version 7.0.0, Bitplane, Concord, MA, USA). The human and murine CD31 networks were quantified and expressed as the percentage of tissue volume occupied by the total endothelial network (N = 2, n = 1–3 per experimental group).
Statistical analyses
Data are presented as mean ± standard deviation. Statistical analyses were performed using the unpaired t test with Welch's correction, the ordinary two-way ANOVA with Tukey's post test or the one-way ANOVA with Tukey's post test of the GraphPad Prism software versions 6.0 and 7.0 (GraphPad Software, La Jolla, CA, USA). Values of p < 0.05 were considered significant. # or Φ or *p < 0.05, ## or ΦΦ or **p < 0.01, ### or ΦΦΦ or ***p < 0.001, #### or ΦΦΦΦ or ****p < 0.0001.
Results
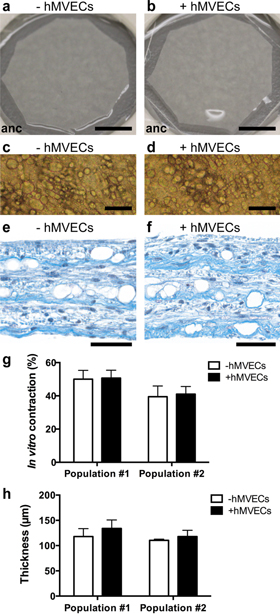
Adipose tissue substitutes composed only of human cells and matrix elements were produced in vitro by tissue engineering. Macroscopic and phase contrast microscopy images of these human adipose tissue substitutes revealed a semi-opaque whitish aspect (figures 1(a) and (b)) and the presence of adipocytes (figures 1(c) and (d)) for both types of tissues. Masson's trichrome staining on histological cross-sections also confirmed the presence of adipocytes (void spaces) and the presence of an abundant extracellular matrix (in blue) in both experimental groups (figures 1(e) and (f)). Quantification of the number of adipocytes on these histological sections indicated an average of 377 adipocytes per mm2 of human adipose tissue substitutes. Before grafting, the substitutes were detached from their peripheral anchorage device (figures 1(a) and (b), anc = anchorage device). The resulting tissue contraction (figure 1(g)) was not significantly different between experimental groups. The thickness of the substitutes, resulting from the superposition of three cell sheets, was also evaluated and no significant differences were measured as a function of hMVECs addition (figure 1(h)).
Figure 1. In vitro features of the human adipose tissue substitutes. (a), (b) Macroscopic, (c), (d) phase contrast microscopy and (e), (f) histological aspects of reconstructed adipose tissues seeded or not in vitro with human microvascular endothelial cells (hMVECs), as seen after a total of 34–36 d of culture, 27–29 d of adipogenic differentiation, 13–14 d with or without hMVECs, and 6–8 d of cell sheet superposition. (e), (f) Masson's trichrome staining of tissue cross-sections showing adipocytes (void spaces) and a rich extracellular matrix (in blue). (g) In vitro contraction and (h) tissue thickness measurements. Bars: (a), (b) 5 mm (anc = anchorage device); (c), (d) 100 μm; (e), (f) 50 μm.
Download figure:
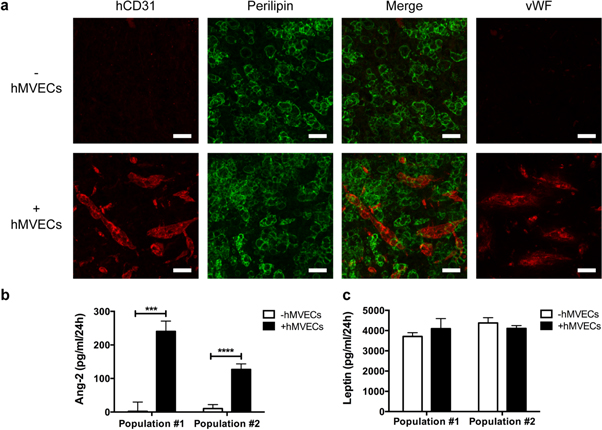
Standard image High-resolution imageNext, immunolabelings were performed on whole-mount samples of the human adipose tissue substitutes before grafting to assess the adipocyte and endothelial content of the tissues. It revealed numerous perilipin-positive adipocytes within each adipose tissue substitutes (figure 2(a)), with the additional presence of hCD31-positive structures for the substitutes preseeded with hMVECs only (figure 2(a)). The hMVECs structures were also positive for the endothelial marker vWF (figure 2(a)). The endothelial cell-secreted protein Ang-2 was detected in conditioned media from substitutes enriched with hMVECs while few to no expression was detected in absence of endothelial cells (figure 2(b)). Similar leptin levels were measured in conditioned media from both tissue types independently of the presence of endothelial cells (figure 2(c)).
Figure 2. Imaging of human microvascular endothelial cells (hMVECs) within human reconstructed adipose tissues in vitro and secretory profile analysis. (a) Confocal images of human CD31 (hCD31)/perilipin double immunolabelings of whole-mount tissue samples before grafting. Expression of von Willebrand Factor (vWF) was also detected. (b), (c) Secreted levels of (b) angiopoietin-2 (Ang-2) and (c) leptin in conditioned media prior to grafting. *** p < 0.001, **** p < 0.0001. Bars: (a) 50 μm.
Download figure:
Standard image High-resolution imageThe reconstructed adipose tissues were then implanted subcutaneously on the muscle of both flanks of adult male athymic mice (figure 3). Grafts were harvested after 3, 7 and 14 d of implantation for short-term behavior analysis. The gross appearance of the grafted adipose tissue substitutes was shiny and smooth (lipid-filled adipocytes), while the recipients bed pink color at all time-points suggests progressive neovascularization (figure 4(a)). Tissue remodeling occurred after implantation leading to a 38.7%–57.0% decrease in graft surface after 3 d when compared to the initial graft size at day 0 (figure 4(b), p < 0.0001). The initial graft surfaces were 130.4 ± 20.6 mm2 and 132.3 ± 23.1 mm2 (ASC population #1) and 198.7 ± 43.1 mm2 and 194.5 ± 38.5 mm2 (ASC population #2) for the adipose tissue substitutes preseeded or not with endothelial cells, respectively. For both experiments, graft surface measured on macroscopic images decreased between day 3 and 14 but no significant differences were associated with endothelial cell incorporation (figure 4(b)).
Figure 3. Schematic representation of the experimental design for the human adipose tissue substitutes grafting experiments. Adipose tissue substitutes preseeded with human microvascular endothelial cells (hMVECs) were engineered by the self-assembly approach. At the time of implantation, substitutes from both experimental groups were detached from their peripheral anchorage device. They were grafted subcutaneously onto the muscle devoid of the panniculus carnosus in nude mice (one substitute on each flank). Biopsies and analyses were performed after 0, 3, 7 and 14 d of implantation.
Download figure:
Standard image High-resolution imageFigure 4. Behavior of the grafted human adipose tissue substitutes. (a) Macroscopic aspect of adipose tissues comprising or not human microvascular endothelial cells (hMVECs) at day 0, 3, 7 and 14 of implantation. (b) Quantification of the graft surface in comparison to the initial graft size. Graft surface values observed at day 3, 7 and 14 were significantly different from the day of implantation (p < 0.0001). # symbols indicate significance in comparison to day 3 (# p < 0.05, ### p < 0.001, #### p < 0.0001). Φ symbols indicate significance in comparison to day 7 (ΦΦΦ p < 0.001). Bars: (a) 5 mm.
Download figure:
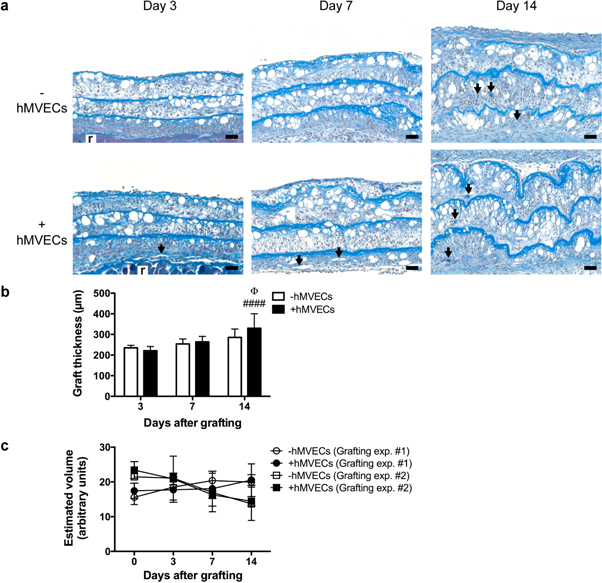
Standard image High-resolution imageThe persistence of adipocytes (void spaces) over 14 d and the presence of blood vessels (arrows) within the grafts were first evaluated on histological cross-sections (figures 5(a) and S2). As part of the tissue remodeling process, a gradual thickening of the grafted tissues was observed (figures 5(a) and (b)). The graft thickness values from day 3, 7 and 14 (figure 5(b)) were significantly different (p < 0.001 or p < 0.0001) from the day of grafting (figure 1(h)), but were not significantly different between experimental groups at the three time-points considered (figure 5(b)). Based on surface and thickness analyses, we calculated an estimated graft volume index (figure 5(c)), which indicates a rather stable graft maintenance over time for all series. Similarly, quantification of adipocyte numbers in the grafts was performed, supporting the persistence of the adipocytes for at least 14 d after implantation, for both types of human adipose tissue substitutes (figure S2).
Figure 5. Histological appearance of the grafted human adipose tissue substitutes. (a) Masson's trichrome staining on transverse sections reveals the presence of numerous adipocytes (void spaces) and an abundant extracellular matrix (in blue). Blood vessels are also visible within the grafts (arrows). (r = recipient bed). (b) Graft thickness quantification on tissue sections. # symbols indicate significance in comparison to day 3 (#### p < 0.0001). Φ symbol indicates significance in comparison to day 7 (Φ p < 0.05). (c) Estimated volume of the grafts. Bars: (a) 50 μm.
Download figure:
Standard image High-resolution imageIn terms of microvasculature development, a fast reorganization of the hMVECs was observed in vivo. Indeed, hCD31 staining revealed the presence of an organized human capillary network within the grafted adipose tissue substitutes containing hMVECs at day 3 of implantation (figures 6(a) and (a')). Connections between the human network and the host vessels were visible at the interface between the graft and it's recipient bed as early as 3 d after implantation (figure 6(b)) and were also observed after 14 d (figure 6(b')). Blood circulation within the human capillaries was also demonstrated following immunolabelings showing the presence of mRBCs within the capillary structures (figures 6(c) and (c')) after 14 d.
Figure 6. Characterization of the human capillary network upon grafting. (a), (a') Organized capillary network formed by human CD31 (hCD31)-positive human microvascular endothelial cells (hMVECs) 3 d after implantation. Examples from two different grafts are presented. (b), (b') Anastomosis events between human capillaries and the host microvasculature after (b) 3 and (b') 14 d post-grafting (arrows = connection sites). (c), (c') Detection of murine red blood cells (mRBCs) in hCD31-positive capillaries. Two different examples are presented at day 14 after grafting. Bars: (a), (a'), (b), (b'), (c), (c') 25 μm.
Download figure:
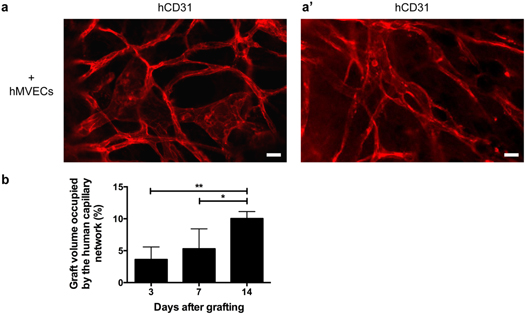
Standard image High-resolution imageA more detailed assessment of the murine and human capillary networks evolution within the grafts after 3, 7 and 14 d of implantation revealed a gradual sprouting of the host network (in green) from the recipient bed towards the grafted tissues for both experimental groups (figure 7(a)). Of note is the presence of the human capillary network (in red) within the microvascularized grafts (figures 6(c) and 7(a)). Indeed, confocal images of whole-mount samples showed that the human capillary network was robustly present with well-defined capillary structures even at 14 d after grafting (figures 8(a) and (a')). This is confirmed by the quantification of the graft volume occupied by the human capillary network showing a significant increase over time (figure 8(b)).
Figure 7. Analysis of vascularization during the first 14 d after implantation. (a) Detection of murine (green) and human (red) CD31-positive networks within sections from human reconstructed adipose tissues comprising or not human microvascular endothelial cells (hMVECs) after 3, 7 and 14 d of implantation. Nuclei were counterstained with Hoechst 33258 (blue). Graft localization (above the dotted line) on the recipient bed (r, below the dotted line) was determined using corresponding phase contrast images. (b) Tissue section-based quantification of the graft surface percentage occupied by the total capillary network (human and murine). (c) Confocal imaging-based quantification of the graft volume percentage occupied by the total capillary network (human and murine) on whole-mount samples. # symbols indicate significance in comparison to day 3 (# p < 0.05, ### p < 0.001, #### p < 0.0001). Φ symbols indicate significance in comparison to day 7 (ΦΦ p < 0.01). Bars: (a) 100 μm.
Download figure:
Standard image High-resolution imageFigure 8. Presence and development of the human capillary network after grafting. (a), (a') Confocal images of the human CD31 (hD31) network within human reconstructed adipose tissues incorporating human microvascular endothelial cells (hMVECs) after 14 d of implantation. Examples from two different tissues are presented. (b) Confocal imaging-based quantification of the graft volume occupied by the human capillary network within whole-mount samples. * p < 0.05, ** p < 0.01. Bars: (a), (a') 25 μm.
Download figure:
Standard image High-resolution imageIn terms of global vascularization (human and murine networks), the evaluation of the graft surface occupied by the total capillary network on tissue cross-sections revealed an increase over time for both experimental groups with a tendency towards a higher percentage for human adipose tissue substitutes preseeded with hMVECs (8.0 fold, 2.6 fold and 1.6 fold for day 3, 7, and 14, respectively) (figure 7(b)). The quantification of the graft volume occupied by the total network (human and murine) was next performed using confocal images of whole-mount samples (figure 7(c)). Similarly to the surface analysis, when expressed as tissue volume occupied by the networks, a higher percentage of capillary network coverage was measured for human adipose tissue substitutes preseeded with hMVECs after 3, 7 and 14 d of implantation (11.3 fold, 4.0 fold, 2.4 fold, respectively), although it did not reach statistical significance (figure 7(c)).
Discussion
In order to achieve a successful implantation of engineered tissues, a timely perfusion of the grafts is required. The incorporation of an endothelial component to engineered tissues has become a widely studied strategy to favor rapid vascularization after grafting [3–6, 41, 42]. In the field of soft tissue engineering in particular, various models of prevascularized substitutes are being developed, comprising either differentiated adipocytes (adipose tissue substitutes) [14, 43–49], or adipogenic-induced or non-induced human ASCs (connective tissue substitutes) [50–55]. These human cell-based reconstructed tissues, which differ in cell types, scaffolding elements, formulations, and size, are used as tools for in vitro studies or as potential tissue fillers in preclinical studies.
In this study, we evaluated the impact of the in vitro incorporation of adult microvascular endothelial cells on the vascularization dynamics of our adipose tissue model after transplantation. These engineered adipose tissues represent highly relevant substitutes as they are densely populated with adipocytes but are still easily manipulated (using or not a peripheral anchorage device) and then sutured in vivo. They are engineered using ascorbic acid supplementation to promote matrix production and assembly by the ASCs while supporting adipogenic differentiation, leading to adipose cell sheets that are then combined into thicker tissues [36]. The production of these adipose tissue substitutes does not rely on the use of thermoresponsive plates or exogenous scaffolds/supporting devices also used for the engineering of cell sheet-based substitutes [56, 57]. Under our culture conditions, the extent of adipogenic differentiation was also similar in presence or absence of hMVECs, as observed in our previous work [14]. Perilipin staining revealed the presence of numerous viable adipocytes in the substitutes. Perilipin is a lipid-droplet associated protein and loss of perilipin content is associated with adipocyte cell death [58–61]. Quantification of leptin secretion confirmed the presence of functional adipocytes in our human adipose tissue substitutes. The incorporation of hMVECs did not impact leptin secretion under these engineering conditions, which were previously optimized to favor both adipogenesis and angiogenesis [14]. Maintaining functional adipocytes in vascularized adipose tissue models is important and can be a challenge considering that the timing of endothelial cell incorporation, the seeding density and the choice of cell culture media and supplements can clearly negatively impact adipose tissue engineering [14, 62–64]. This explains why many soft tissue models do not actually comprise differentiated adipocytes at the time of implantation, and rely on in vivo adipogenesis for adipose tissue formation. Our human adipose tissue substitutes are mainly designed to provide a living and functional adipose layer after grafting. They are not injectable and could find applications for various reconstructive surgery procedures such as maxillofacial defects and injuries in order to promote healing and restore contour and function. They could be used as an alternative or a complementary approach to autologous fat grafting. It would also be interesting to evaluate their capacity to stimulate the in situ formation of adipose depots during long-term grafting experiments.
Immunolabelings for hCD31 and vWF confirmed the endothelial nature of the structures formed within the human adipose tissue substitutes when hMVECs were seeded on adipose sheets featuring already differentiated adipocytes (14–15 d). The detection in conditioned media of Ang-2, secreted mainly by endothelial cells [65], confirmed the presence of metabolically active hMVECs in the tissues. The survival and proliferation of hMVECs are well-supported within our engineered tissues. This is likely due to the endogenous human matricial elements that are produced and assembled by ASCs in our self-assembly model [14, 15]. Indeed, the importance of extracellular matrix composition, quality and density on the modulation of angiogenic processes is now recognized [66, 67]. The stability of the capillary networks in our tissues is particularly valuable for the in vitro evaluation of angiomodulatory molecules over many days [15].
After implantation, the grafted human adipose tissue substitutes presented a smooth appearance with the presence of blood vessels surrounding the grafts, independently of their prevascularization status. For both groups, the graft surface area decreased sharply during the first three days after implantation but slowed down afterwards, as evaluated from macroscopic pictures. This was accompanied by a thickening of the grafts measured on histological cross-sections, supporting the expected tissue contraction and remodeling. The reorganization of the hMVECs into fine capillaries observed three days after grafting confirmed their capacity to form an extended network that persisted and even developed throughout the 14 d period we analyzed. The human capillary network was able to connect with the host microvasculature as soon as three days after grafting and was also able to convey blood supply to grafted adipose substitutes, as supported by mRBCs found inside human capillaries.
In our study assessing the early dynamics of the reconstructed adipose grafts, no impact of the presence of hMVECs was observed on their estimated volume over 14 d. The incorporation of hMVECs translated into the presence of a developed and functional network of capillaries leading to an increased global vascularization after implantation, although statistical significance was not reached when compared to non-microvascularized tissues. Indeed, the vascularization analyses were performed on a limited number of samples. However, we used two approaches to quantify the capillary networks, namely on frozen cross-sections (% surface) and on whole-mount samples (% volume), which independently support the conclusions.
In a study conducting a side-by-side comparison of constructs devoid or preseeded with HUVECs, Verseijden and collaborators evaluated their model 7 d after transplantation into nude mice [54]. Their prevascularized fibrin constructs contained a 1:5 ratio of human ASCs (induced towards adipogenesis for 7 d) and HUVECs. Similarly to our results, no differences were seen for the total graft vascularization (human and murine) after 7 d. However, the contribution of the human-specific vascularization seemed much lower for these constructs than for our human adipose tissue substitutes [54]. In our study, the absence of a significant impact on graft volume and the extent of global vascularization in presence of hMVECs is likely due to the rapid vascularization of the tissues averaging 120 μm in thickness on histological sections before grafting. Despite the large surface of our constructs (130–200 mm2), the incorporation of hMVECs might not provide a robust stimulation to an already effective neovascularization of the non-microvascularized tissues. It will therefore be important to evaluate the impact of hMVECs on the vascularization of thicker tissues in which the oxygen rate is limiting and to extend the follow-up period after implantation. The challenge of accurately determining the volume and shape of grafted adipose substitutes is always present when performing preclinical studies. In our previous work, thicker human adipose tissue substitutes that were not microvascularized in vitro (∼1 mm-thick) were successfully implanted into nude mice and followed in vivo using magnetic resonance imaging (MRI). Dynamic contrast-enhanced (DCE)-MRI studies were also performed, showing signal enhancement indicative of the graft perfusion after 14 and 21 d. Graft volume retention was followed up to 6 weeks, showing a gradual resorption to 44% of the substitutes initial volume on average [39]. Therefore, determining the influence of prevascularization on thicker adipose tissue substitutes will be particularly informative using these non-invasive imaging modalities in longitudinal comparative studies.
Other teams have evaluated the impact of HUVEC co-transplantation over a longer period of time after grafting and found a rather limited impact of prevascularization. In their study, Frerich and collaborators compared two different types of constructs, namely microparticles (on collagenous carriers) and larger constructs (13 mm in diameter) for which the microparticles were embedded in a fibrin gel [55]. After 12 days, 4 weeks or 4 months post-implantation, the mean explanted weight of the fibrin constructs was similar whether or not they were preseeded with HUVECs. Quantifications performed on histological cross-sections revealed a significantly smaller number of acellular/necrotic areas after four weeks and four months for HUVECs-enriched experimental groups. However, no long-term benefits could be measured in terms of adipose tissue formation over four months [55]. Globally, this highlights that more research is needed to understand the potential benefits of prevascularization and the dynamics of fat grafting, both for native and reconstructed substitutes since many parameters can influence the outcomes of grafting such as the anatomic site of the recipient bed, the size and delivery of the constructs, as well as the choice of an animal model (immunocompromised or not) [68].
Conclusions
Successful reconstruction of adipose tissue requires efficient strategies, such as in vitro prevascularization, to stimulate angiogenesis after transplantation. The short-term behavior of the entirely human adipose tissue substitutes we produced, preseeded with human microvascular endothelial cells, indicated the formation of human capillary networks that developed and even expanded after transplantation in mice. Our model displays many advantageous features: our biomimetic, scaffold-free human substitutes are built from adipose-derived stem cells, which efficiently produced and assembled extracellular matrix in addition to their recognized capacity to secrete therapeutic molecules. The lack of exogenous biomaterials could reduce the risks of immunogenic reactions in some patients. We used adult microvascular cells that are more representative of autologous therapy. Therefore, it will be relevant to evaluate the potential benefits of preformed human capillary networks in thicker human adipose tissue substitutes in order to engineer optimal replacement tissues for patients suffering from adipose tissue loss or defects.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (CIHR) grants #84368 and #111233. We acknowledge the support of the CHU de Québec-Université Laval Research Center of the Fonds de Recherche du Québec-Santé (FRQS) and of the Quebec network for cell, tissue and gene therapy-ThéCell (a thematic network supported by the FRQS). MP received studentships from National Science and Engineering Research Council of Canada (NSERC), FRQS and Canadian Federation of University Women (CFUW). JF received a FRQS career award. The confocal imaging system was obtained through the Fonds des leaders program from Canada Foundation for Innovation (CFI) to JF. The authors are grateful to Amandine Maux for technical assistance and experimental input. We thank Stéphane Chabaud and Véronique Moulin for providing human skin microvascular endothelial cells as well as Anne-Marie Moisan and Michel Fortin for excellent assistance with the animal studies.
No conflicts of interest.