Abstract
Here, we compared 3D-printed polycaprolactone/poly(lactic-co-glycolic acid)/β-tricalcium phosphate (PCL/PLGA/β-TCP) membranes with the widely used collagen membranes for guided bone regeneration (GBR) in beagle implant models. For mechanical property comparison in dry and wet conditions and cytocompatibility determination, we analyzed the rate and pattern of cell proliferation of seeded fibroblasts and preosteoblasts using the cell counting kit-8 assay and scanning electron microscopy. Osteogenic differentiation was verified using alizarin red S staining. At 8 weeks following implantation in vivo using beagle dogs, computed tomography and histological analyses were performed after sacrifice. Cell proliferation rates in vitro indicated that early cell attachment was higher in collagen than in PCL/PLGA/β-TCP membranes; however, the difference subsided by day 7. Similar outcomes were found for osteogenic differentiation, with approximately 2.5 times greater staining in collagen than PCL/PLGA/β-TCP, but without significant difference by day 14. In vivo, bone regeneration in the defect area, represented by new bone formation and bone-to-implant contact, paralleled those associated with collagen membranes. However, tensile testing revealed that whereas the PCL/PLGA/β-TCP membrane mechanical properties were conserved in both wet and dry states, the tensile property of collagen was reduced by 99% under wet conditions. Our results demonstrate in vitro and in vivo that PCL/PLGA/β-TCP membranes have similar levels of biocompatibility and bone regeneration as collagen membranes. In particular, considering that GBR is always applied to a wet environment (e.g. blood, saliva), we demonstrated that PCL/PLGA/β-TCP membranes maintained their form more reliably than collagen membranes in a wet setting, confirming their appropriateness as a GBR membrane.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Adequate bone volume is critical for the successful outcome of dental implants and prosthetic superstructures and to ensure satisfactory predictable aesthetics and long-term prognoses [1, 2]. To enhance the bone volume in the jaw, various methods such as distraction osteogenesis [3], osteoinduction [4], osteoconduction [5], and guided bone regeneration (GBR) [6, 7] have been introduced. Among these methods, GBR, a surgical procedure that uses a barrier membrane with or without grafting materials, has been recognized as the method that yields the most predictable results for new bone regeneration in peri-implant bone defect sites [8, 9].
During the GBR procedure, the bone defect is separated from the surrounding connective tissues to prepare a space for new bone formation. The barrier membrane plays important roles during this procedure [9, 10] and is required to exhibit properties such as biocompatibility, cell occlusivity, tissue integration, space maintenance, and manageability [11]. To date, both non-resorbable and resorbable commercially available membranes with unique properties have been used in the clinic because they have met consumer needs as GBR membranes [12, 13]. Most recently, resorbable membranes have been preferentially utilized because the non-resorbable forms inevitably require a surgical procedure for membrane removal, which can cause further patient discomfort, risk of tissue damage, and additional costs and duration of treatments [13, 14]. Moreover, non-resorbable membranes carry limitations such as a high membrane exposure rate and infection problems; thus, it is primarily used for specific indications in the form of titanium-reinforced expanded-polytetrafluoroethylene (e-PTFE) or as a titanium-mesh [1, 15].
In comparison, the collagen membrane, which is widely used as a representative resorbable GBR membrane, is mechanically malleable and adaptable, exhibiting excellent manipulability [16, 17]. Owing to its collagen property, it has advantages such as a hemostatic function, early wound stabilization, and semipermeablity [18], but it also has drawbacks such as unfavorable mechanical properties [19] and inadequate barrier stability over time [20–22]. Accordingly, studies on synthetic bioresorbable materials have been conducted for the fabrication of form-stable resorbable GBR membranes with a sufficient degradation rate [13, 23]. Bioresorbable materials such as polyglycolides (PGAs), polylactides (PLAs), and copolymers have been used for medical purposes [24]. The synthetic membranes prepared using those materials have been shown to be sufficiently biocompatible and biodegradable to also be used as barrier membranes [25–29].
Bioresorbable synthetic polymers are often used in tissue-engineering scaffolds that can be prepared through foaming/particulate leaching, phase separation, freeze-drying, and electrospinning [30]. However, these conventional fabrication techniques utilize toxic solvents that adversely affect cells and tissues if not completely removed. Furthermore, it is difficult to adjust the thickness, pore size, or external shape of the fabricated scaffold, and to maintain the architectural consistency [30, 31].
In contrast, three dimensional (3D) structures can be prepared without the aforementioned difficulties using the 3D printing method, which is based on computer-aided design/computer-aided manufacturing (CAD/CAM) and layer-by-layer processes [32, 33]. In particular, the multi-head deposition system (MHDS), an advanced type of extrusion-based 3D printing technology, has four dispensing heads for preparing hybrid scaffolds containing multiple biomaterials, and can prepare 3D microstructures precisely within a short time [34, 35]. For example, Shim et al [31] fabricated thin-membrane-type scaffolds using MHDS by blending polycaprolactone (PCL), poly(lactic- co-glycolic acid) (PLGA), and beta-tricalcium phosphate (β-TCP), and confirmed that the PCL/PLGA/β-TCP membrane prepared using the 3D printing technology promoted appropriate bone-formation in a rabbit calvaria bone-defect model [36–38]. This bioresorbable PCL/PLGA/β-TCP membrane has the biological and mechanical advantages of both PCL and PLGA, as well as the osteoconductivity of TCP. In addition, it can be prepared with diverse thicknesses and pore sizes via the 3D printing technology, and can be effectively used as a GBR membrane for peri-implant bone defects [39]. In a further Shim et al study [40], GBR procedures were conducted at the buccal open defects surrounding implants in the mandible of beagle dogs in designed clinical situations, and the bone generation effects of the PCL/PLGA/β-TCP membrane were compared with those of the non-resorbable titanium membrane. The results of this study confirmed that the PCL/PLGA/β-TCP membrane promoted sufficient new bone formation to serve as a GBR membrane [40].
In the current study, the bone regeneration ability of the 3D-printed PCL/PLGA/β-TCP membrane was evaluated and compared with that of the collagen membrane, a widely used resorbable membrane, during the GBR procedure for peri-implant bone defects in the beagle mandible.
2. Experimental section
2.1. PCL/PLGA/β-TCP membrane generation using 3D printing technology
2.1.1. Preparation of blended PCL/PLGA/β-TCP
Collagen membrane (GENOSS, Suwon, Korea) and PCL/PLGA/β-TCP membrane were used as barrier membranes. The PCL/PLGA/β-TCP membrane was prepared using the thermal melting process by mixing PCL (19561-500G, 43 000–50 000 Mw; Polysciences Inc., Warrington, PA, USA), PLGA (430471-5G, 50 000–75 000 Mw; Sigma-Aldrich, St. Louis, MO, USA), and β-TCP (average diameter: 100 nm, Berkeley Advanced Biomaterials Inc., Berkeley, CA, USA). Briefly, PCL (0.4 g) and PLGA (0.5 g) granules were melted and mixed in a glass container for 10 min at 130 °C, and powder-type β-TCP (0.1 g) was added to the dissolved PCL and PLGA mixture, which was further mixed for five min. Then the PCL/PLGA/β-TCP mixture was put into the 10 ml steel syringe of the multi-head deposition system (MHDS) and maintained at 135 °C for dispensing.
2.1.2. Fabrication of PCL/PLGA/β-TCP membranes
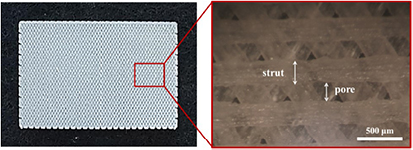
The MHDS, an extrusion-based 3D printing system used to prepare the PCL/PLGA/β-TCP membrane, was composed of four heads that could control the temperature, pneumatic pressure, and motion during extrusion. The system could simultaneously prepare four membranes [31]. Blended PCL/PLGA/β-TCP fibers were dispensed from the steel nozzle of the head at 135 °C and 650 kPa. In total, three layers were stacked to fabricate PCL/PLGA/β-TCP membranes using the layer-by-layer process. The PCL/PLGA/β-TCP membrane had a 3D-multilayer mesh-type structure similar to that of the resorbable collagen membrane. The 3D-printed membranes included a triangular pore structure. The overall dimensions were 30 mm × 20 mm × 0.25 mm (figure 1). The width of the strut was 300 µm and the pore size was 200 µm. Therefore, the calculated porosity was approximately 40%.
Figure 1. Photographs of the PCL/PLGA/β-TCP membrane.
Download figure:
Standard image High-resolution image2.1.3. Mechanical testing of collagen and PCL/PLGA/β-TCP membranes
A membrane-type specimen for a mechanical tensile test was fabricated using the 3D printing system. The experiment condition was based on ISO527 (Tensile test on plastics) and the dimensions of the specimen were determined according to Specimen Type 4 of ISO527-3. The tensile test of the membranes was performed using a single-column tensile test machine (Model 3345, Instron Co., Norwood, MA, USA) under dry and wet (soaking the membranes in a medium for 18 h) membrane conditions. The overall dimensions of the collagen and PCL/PLGA/β-TCP membranes were 30 mm × 10 mm × 0.3 mm. For the PCL/PLGA/ β-TCP membrane, the printed strut size was 300 µm, and the membrane pore size was 200 µm. The load and displacement were monitored at a constant cross-head speed of 5 mm min−1.
2.2. In vitro analyses
2.2.1. Cell culture and proliferation assay
Mouse fibroblasts (NIH3T3, ATCC #CRL-1658) and preosteoblasts (MC3T3-E1, ATCC #CRL-2593) were maintained in Dulbecco modified Eagle's medium (DMEM) and α-MEM containing 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin (all from Invitrogen, Carlsbad, CA, USA). For cell seeding on the membranes, all membranes (10 mm × 10 mm × 0.3 mm) were pre-wetted in culture medium for 2 h and then irradiated under UV for 30 min. Subsequently, 3 × 105 cells were seeded on each membrane and the rates of proliferation on the membrane were analyzed via a Cell Counting Kit-8 (CCK-8, Dojindo, Rockville, MD, USA) on day 2, 4, and 7.
2.2.2. Osteogenic differentiation and Alizarin red staining
The MC3T3-E1-seeded membranes were incubated in culture medium for 3 d, which was then replaced with osteogenic medium composed of α-MEM plus 20% FBS, 10-8 M dexamethasone, 0.2 mM ascorbic acid, 10 mM β-glycerol phosphate (all from Sigma-Aldrich) and 1% penicillin/streptomycin. The membranes were fixed with 4% paraformaldehyde on day 7 and 14, then stained with alizarin red to measure calcium deposition. For the quantification of staining, membranes were soaked in 10% cetylpyridinium chloride for 10 min at room temperature and the optical density was measured at 570 nm using a microplate reader (Epoch, BioTek, Winooski, VT, USA).
2.2.3. Scanning electron microscopy (SEM)
To assess the morphology of the membrane surface alone or following cell seeding, we performed SEM a using high resolution SEM (HR-SEM) with acceleration voltage of 10 kV (FEI, Nova NanoSEM 450) as previously described [40].
2.3. In vivo analyses
2.3.1. Experimental animals
Three systemically healthy male beagle dogs aged 18 months and weighing approximately 10 kg were used in this study. All the experimental animals were fed a soft food diet to preserve their dentition with a healthy periodontium. The animal selection and management as well as the surgical procedures were approved by the Ethics Committee on Animal Experimentation of Chonnam National University (CNU IACUC-TB-2013-10). All the experiments were performed at the animal dental laboratory accredited by the Chonnam National University Animal Hospital.
2.3.2. Experiment design
A total of 12 implants (INNO Implant, Cowelmedi, Busan, Korea), 3.0 mm in diameter and 6.0 mm in length, were bilaterally placed in healed extraction sites (below). A total of four peri-implant dehiscence defects were observed per animal, and the following two therapeutic methods were randomly applied:
- Collagen group: Collagen Membrane (GENOSS, Suwon, Korea) with a deproteinized bovine bone grafting material (Bio-Oss, Geistlich Biomaterials, Wolhusen, Switzerland).
- PCL/PLGA/β-TCP group: PCL/PLGA/β-TCP membrane with a deproteinized bovine bone grafting material (Bio-Oss).
2.3.3. Surgical procedures
The first surgery was performed to extract the second and fourth premolars (P2 and P4) under general anesthesia induced by intravenous injection of atropine (0.05 mg kg−1 IV; Dai Han Pharm Co., Seoul, Korea) and intramuscular injection of a combination of xylazine (Rompun, Bayer Korea Co., Seoul, Korea), followed by inhalation of anesthesia with isoflurane (Choongwae Co., Seoul, Korea). At the surgical sites, dental infiltration anesthesia was used with 1 ml 2% lidocaine HCL and 1:100 000 epinephrine (Yu-Han Co., Gunpo, Korea). The targeted premolars were bilaterally extracted. The extraction site was sutured with 4-0 nylon (Mersilk, Ethicon Co., Livingston, UK) for healing. Oral prophylaxis was performed on the remaining teeth. The stitches were removed after 10 d, and the extraction sites were allowed to heal.
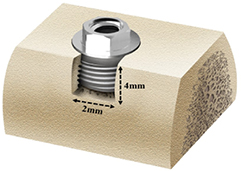
The second surgery was performed after the 8 weeks recovery period. General anesthesia and local infiltration anesthesia were performed as in the first surgery. A horizontal incision was made on the experimental site, and vertical incisions were made through the mucogingival junction that extended into the alveolar mucosa at the mesial and distal ends of each defect. The mucoperiosteal flaps were elevated to expose the edentulous alveolar ridge. Two identical dehiscence defects (4 mm apicocoronally and 2 mm mesiodistally) were surgically prepared on the buccal sides of the left and right partial edentulous ridges (figure 2). In each of the four defects, an implant was placed following serial drilling according to the standard protocol. The platform of the fixture was located at the level of the alveolar crest.
Figure 2. Schematic diagram of the peri-implant defect model. The implant (3.0 mm × 6.0 mm) was inserted in a standardized defect, 4 mm in height and 2 mm in width.
Download figure:
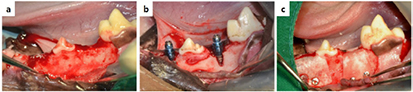
Standard image High-resolution imageNext, 0.1 mg deproteinized bovine bone grafting material (Bio-Oss) was quantified and dampened in sterile saline for 5 min, then positioned at the buccal defect. Upon completion of the grafting, collagen membranes (Collagen Membrane, GENOSS) or PCL/PLGA/β-TCP membranes were randomly positioned at the buccal defect (figure 3). All membranes were cut to a size that covered 2–3 mm of the adjacent alveolar bone to overlap the entire defect. To enhance the stability of the grafting material, titanium pins (Dentium Co., Seoul, Korea) were used for the fixation. To obtain primary wound closure, the buccal flaps were carefully released and sutured. All surgeries were performed by the same professionally trained operator.
Figure 3. Surgical operation procedures. (a) The alveolar ridge was trimmed for implant insertion. (b) Buccal defects were created and implants were inserted into the partial edentulous mandibular alveolar ridge. (c) All the peri-implant defects were filled with the grafting material. Then, all the membranes were placed randomly on the defect.
Download figure:
Standard image High-resolution image2.3.4. Post-operative care and sacrifice
Antibiotics such as penicillin G procaine and penicillin G benzathine were intramuscularly injected (1 ml/5 kg) immediately and 48 h after the surgery. Plaque was controlled using 2% chlorhexidine gluconate through daily flushing of the oral cavity until the end of the study. The animals were kept on a soft diet for 2 weeks, followed by a regular diet. Animals were sacrificed 8 weeks post-operatively through intravenous injection of concentrated sodium pentobarbital (Euthasol, Delmarva Laboratories Inc., Midlothian, VA, USA). The mandibles of the sacrificed beagles were harvested after cutting them to include the alveolar bones near the implant, as well as the membranes and surrounding mucosae. The 12 harvested mandible block sections were fixed using neutral buffered formalin (Sigma Aldrich).
2.3.5. Micro-computed tomography (µCT) analysis
After fixation, 3D µCT images were generated to analyze the new bone density and new bone volume of the peri-implant dehiscence defect area. The specimens were wrapped with Parafilm M® (Bemis Company, Inc., Neenah, Wisconsin, USA) to keep them from drying during the scanning process. Then, all the samples were scanned with 130 kV energy, 60 µA intensity, and a 7.10 µm-pixel resolution using a bromine filter (0.25 mm) (Skyscan-1173, version 1.6, Bruker-CT, Kontich, Belgium). Reconstruction was performed using Nrecon software (version 1.6.10.1, Bruker-CT). All the applied scan and reconstruction parameters were identical for all the specimens. The overall augmented contour was evaluated at the 3D reconstructed view. Boundaries were set as follows to standardize the region of the augmented volume to be analyzed:
- Any augmented volume directly superior or lingual to the implant in its axial plane was excluded.
- Any augmented volume inferior to the buccal extension of the inferior border of the defect was excluded.
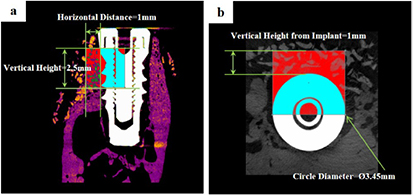
The region of interest (ROI) was 1 mm in width and 2.5 mm in height from the implant platform (figures 4 and 5).
Figure 4. The region of interest; 1 mm in width and 2.5 mm in height from the implant platform. (a) Cross-sectional view (b) occlusal view.
Download figure:
Standard image High-resolution imageFigure 5. 3D images obtained via micro-computed tomography. (a) 3D image of the mandible block section. (b) 3D image without the old bone. (c) 3D image of the region of interest.
Download figure:
Standard image High-resolution imageThe following parameters were calculated within the ROI:
- Total augmented volume (TAV; mm3)
- Area occupied by the total augmented volume within the ROI
- New bone volume (NBV; mm3)
- Area occupied by the new bone volume within the ROI
- Remaining bone substitute volume (RBV; mm3)
- Area occupied by the remaining bone substitute volume within the ROI
- Non-mineralized tissue volume (NMV; mm3)
- Area occupied by the non-mineralized tissue volume within the ROI
2.3.6. Histomorphometric analysis
Upon completion of the µCT analysis, the specimens were cleansed and dehydrated using an ethanol series with increasing concentration. After the dehydration was completed, infiltration was conducted using a mixture of ethanol and Technovit 7200 resin (Heraeus Kulzer, Hanau, Germany) while increasing the resin ratio. Then the specimen was fixed on an embedding frame to perform embedding using a UV embedding system (Exakt 520, Kulzer) to cure the resin for 1 d. The polymerized specimen block was longitudinally sectioned at each implant center using the Exakt diamond cutting system (Kulzer Exakt 300 CP), and attached to slides using an adhesive press system. The final slides were ground to have a thickness ranging from the initial 400 µm to the final 40 ± 5 µm using the Exakt grinding system (Kulzer Exakt 400CS). To observe the newly regenerated bone in the specimen, Goldner Trichrome staining was conducted before mounting the sample, then final slides were prepared. The final slide images were captured using a CCD camera (Spot Insight 2 Mp scientific CCD digital camera system, Diagnostic Instruments, Inc., Sterling Heights, MI, USA) with an adaptor (U-CMA3, Olympus, Tokyo, Japan) mounted onto a light microscope coupled to a computer (BX51, Olympus). For the analysis of the captured images, i-Solution ver. 8.1 (IMT i-Solution, Inc., Coquitlam, BC, Canada) was used. The general specimen images were observed at ×12.5 magnification; for the histometric analyses, ×40 magnification was used. For the histometric analysis, a single, professionally trained and blinded investigator measured the following items.
The area of interest (AOI) was 1 mm width and height from the implant platform to the old bone (OB) (figure 6).
Figure 6. Parameters measured in the histologic specimens. Red box = area of interest. Blue arrow = platform of the implant. Yellow arrow = most coronal point of the new bone. White arrow = most coronal point of the osseointegration site. Green arrow = most coronal point of the old bone.
Download figure:
Standard image High-resolution imageThe following measurements were analyzed and recorded within the AOI.
- New bone area (NBA; %)
- Area occupied by the new bone/AOI × 100 (%)
- Remaining bone substitute area (RBA; %)
- Area occupied by the remaining bone substitute/AOI × 100 (%)
- Bone-to-implant contact (BIC; %)
- Length of the contact with the new bone/total length of the exposed threads × 100 (%)
- Bone crest (BC)-OB (%)
- Distance from the BC to the OB/distance from the implant platform to the OB × 100 (%)
- Coronal point of osseointegration (CO)-OB (%)
- Distance from the most CO to the OB/distance from the implant platform to the OB × 100 (%)
2.3.7. Statistical analysis
All the experimental data were expressed as the means, standard deviations, and medians. All the statistical analyses were conducted using software R (version 3.1.3). The treatment group (Collagen group and PCL/PLGA/β-TCP group) was set as an independent factor, and the dog number was set as a random factor. To compare the radiographic and histomorphmetric parameters of the groups, a non-parametric mixed model was used [41]. The statistical significance level was 5% and post hoc analyses were performed.
3. Results
3.1. SEM analysis of the PCL/PLGA/β-TCP membrane
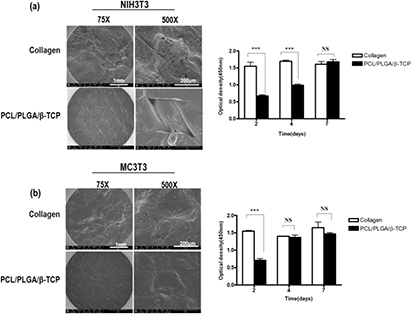
Representative SEM images of the collagen and PCL/PLGA/β-TCP membranes are shown in figure 7. The surface of the collagen membrane was smooth and dense (figures 7(a)–(c)), whereas the PCL/PLGA/ β-TCP surface was rough owing to the β-TCP particles. In particular, the PCL/PLGA/β-TCP membrane showed a triangular and uniform pore architecture that was fully interconnected (figures 7(d)–(f)).
Figure 7. SEM images of collagen ((a)–(c)) and PCL/PLGA/β-TCP ((d)–(f)) membranes. Magnification, ×75 in ((a) and (d)), ×200 in ((b) and (e)) and ×1000 in ((c) and (f)). The collagen membrane showed a smooth surface without visible pore-like fabric, while the PCL/PLGA/β-TCP had a rough surface and a fully interconnected porous architecture.
Download figure:
Standard image High-resolution image3.2. Mechanical testing of collagen and PCL/PLGA/β-TCP membranes
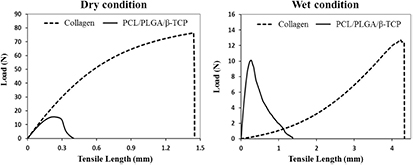
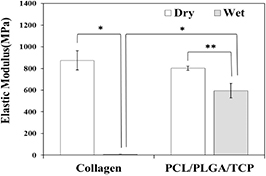
To compare the mechanical properties of the PCL/PLGA/β-TCP membrane with those of the collagen membrane, a tensile test was performed under dry and wet conditions. The tensile test results of the collagen and PCL/PLGA/β-TCP membranes are shown in figures 8 and 9, and table 1. From the load-displacement curve (figure 8), the maximum load of the collagen membrane in the dry condition was approximately 4-times higher than that of the PCL/PLGA/β-TCP membrane. In contrast, in the wet condition, the maximum load of collagen (12.8 N) was 24.3% higher than that of PCL/PLGA/β-TCP membrane (10.3 N). On the other hand, as shown in figure 9, a considerable decrease of the elastic modulus (the slope of first linear portion of the stress–strain curve reflecting membrane rigidity) between the dry (874.9 ± 87.3 MPa) and wet (7.9 ± 0.1 MPa) conditions was measured in the collagen membrane (P < 0.001). Specifically, the elastic modulus of the collagen membrane in the dry condition fell by 99.1%. In contrast, that of the PCL/PLGA/β-TCP membrane in the dry condition decreased by 26% when the membrane was wetted (P < 0.01). Thus, the decreasing rate of the elastic modulus of the PCL/PLGA/β-TCP membrane in the wet condition was much less severe than that of the collagen membrane.
Figure 8. Tensile testing results (load-displacement curve) of collagen and PCL/PLGA/β-TCP membranes in the dry and wet conditions.
Download figure:
Standard image High-resolution imageFigure 9. Elastic modulus of collagen and PCL/PLGA/β-TCP membranes in the dry and wet conditions (*P < 0.001, **P < 0.01). A substantial decrease of elastic modulus between dry and wet conditions was seen for the collagen membrane.
Download figure:
Standard image High-resolution imageTable 1. Maximum tensile load and elastic modulus of collagen and PCL/PLGA/β-TCP membranes under dry and wet condition (means ± SDs; n = 3).
| Group | Maximum tensile load (N) | Elastic modulus (MPa) | ||
|---|---|---|---|---|
| Dry | Wet | Dry | Wet | |
| Collagen | 68.8 ± 15.3 | 12.8 ± 1.4 | 874.9 ± 87.3 | 7.9 ± 0.1 |
| PCL/PLGA/β-TCP | 15.7 ± 0.9 | 10.3 ± 0.9 | 803.0 ± 17.8 | 594.7 ± 66.7 |
3.3. In vitro results
To evaluate the cytocompatibility of the membrane, we first observed the morphology and proliferation of fibroblasts (NIH3T3) and preosteoblasts (MC3T3-E1 cells) seeded onto the membranes. On day 2, the number of attached cells on the collagen membrane was approximately 2.6-times higher than that on PCL/PLGA/β-TCP for both cell types. However, proliferation of the MC3T3-E1 cells on PCL/PLGA/ β-TCP increased and had reached similar levels as that on the collagen membrane by day 4 (P > 0.05). In parallel, SEM images showed that the MC3T3 cells completely covered the surface of the collagen and PCL/PLGA/β-TCP membranes on day 7 (figure 10(b)). On the other hand, the optical density of NIH3T3 cells did not differ between membrane type (P > 0.05) at day 7 even though it had retained a significant difference up to 4 d (P < 0.001) (figure 10(a)). These results suggested that the PCL/PLGA/β-TCP membrane provide an appropriate environment for cell proliferation.
Figure 10. The cell morphology and proliferation assays of NIH3T3 (a) and MC3T3-E1 (b) cells on the collagen and PCL/PLGA/β-TCP membranes showed that the attached cells grew well. (***P < 0.001, NS = no significant difference).
Download figure:
Standard image High-resolution imageWe further investigated the osteogenic differentiation of MC3T3-E1 cells on collagen and PCL/PLGA/β-TCP membranes. The MC3T3-E1-seeded membranes were placed in osteogenic media for 7 and 14 d and then fixed with 4% paraformaldehyde. Osteogenic differentiation of the MC3T3-E1 cells on the membranes was assessed by alizarin red S staining, which revealed increased calcium deposition on the membranes (figure 11(a)). To quantify the staining, stained dyes were extracted from the membranes using 10% cetylpyridinium chloride in 10 mM sodium phosphate. On day 7, the level of osteogenic differentiation of MC3T3-E1 cells on the PCL/PLGA/β-TCP membrane was less than that on the collagen membrane. However, no significant difference between membrane type could be determined on day 14. The quantitative analysis results were consistent with the staining results (figure 11(b)). Taken together, the in vitro experiments demonstrated that the PCL/PLGA/β-TCP membrane exhibited similar efficacy as a GBR membrane with respect to cytocompatibility and osteogenic differentiation as compared to collagen.
Figure 11. Alizarine red S staining (a) and quantification (b) indicated that no difference was observed in osteogenic differentiation between collagen and PCL/PLGA/β-TCP membranes at day 14.
Download figure:
Standard image High-resolution image3.4. In vivo results
3.4.1. Clinical findings
All experimental animals survived the surgical procedures, and the 12 implant sites healed without noted problems. No implant failure or dropout was observed, and no membrane exposure or separation occurred during the healing period. No complication was reported from either experimental group during the study period.
3.4.2. Volumetric analysis using µCT
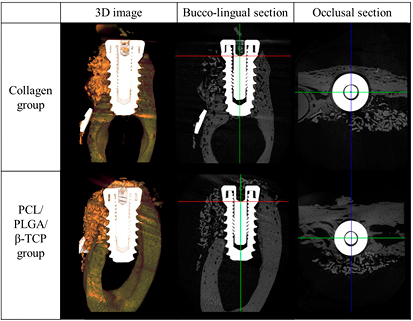
In the collagen group, some specimens were observed wherein the grafting materials were scattered in the peri-implant dehiscence defect area. In comparison, in the PCL/PLGA/β-TCP group, the grafting materials formed a shape in the peri-implant dehiscence defect area in most specimens (figure 12).
Figure 12. Micro-computed tomography images of the collagen and PCL/PLGA/β-TCP groups.
Download figure:
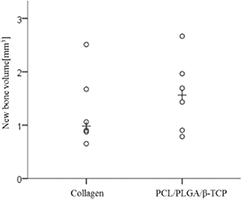
Standard image High-resolution imageThe volumetric measurements are summarized in table 2 and figure 13. The PCL/PLGA/β-TCP group showed higher levels of new bone volume (mm3) and remaining bone substitute volume (mm3) than the collagen group, but the differences were statistically insignificant (NBV (mm3), P = 0.339; and RBV (mm3), P = 0.295). Conversely, the collagen group showed higher levels of total augmented volume (mm3) and non-mineralized tissue volume (mm3) than the PCL/PLGA/β-TCP group, but the differences were also statistically insignificant (TAV (mm3). P = 0.127; and NMV (mm3), P = 0.185).
Table 2. Volumetric analysis within the area of interest (n = 6; mm3).
| NBV (mm3) | TAV (mm3) | RBV (mm3) | NMV (mm3) | |
|---|---|---|---|---|
| Collagen | ||||
| Mean ± SD | 1.28 ± 0.70 | 14.27 ± 0.19 | 2.69 ± 1.97 | 10.29 ± 2.71 |
| Median | 0.98 | 14.27 | 1.88 | 11.42 |
| PCL/PLGA/β-TCP | ||||
| Mean ± SD | 1.57 ± 0.70 | 14.04 ± 0.31 | 3.95 ± 1.97 | 8.52 ± 2.47 |
| Median | 1.56 | 14.05 | 4.15 | 8.23 |
| P | 0.339 | 0.127 | 0.295 | 0.185 |
Note: NBV, new bone volume; TAV, total augmented volume; RBV, remaining bone substitute volume; NMV, non-mineralized tissue volume. No significant difference between the two experiment groups (P > 0.05).
Figure 13. Scatter plot and median (plus sign) representing the new bone volume (mm3).
Download figure:
Standard image High-resolution image3.4.3. Histologic findings
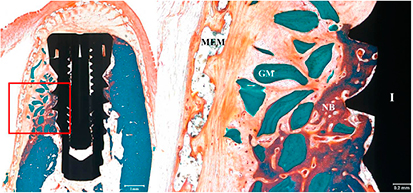
In the collagen group (figure 14), a small amount of new bone was formed in the implant in the buccal peri-implant dehiscence defect area. In some specimens, the membrane was not observed because it was absorbed. In the specimen whose membrane was completely absorbed, fibrous tissues were observed around the implant, and the grafting materials were observed only because they were scattered in the peri-implant dehiscence defect area.
Figure 14. Histological sections of the collagen group. A small amount of new bone was formed. NB, new bone; GM, grafting material; MEM, membrane; I, implant (Goldner Trichrome stain; magnification ×12.5 (a) and ×40 (b)).
Download figure:
Standard image High-resolution imageIn the PCL/PLGA/β-TCP group (figure 15), a comparatively large amount of new bone was formed in the implant in the buccal peri-implant dehiscence defect area. The membranes survived in most of the specimens, and a large amount of the grafting material that formed a shape was observed in the peri-implant dehiscence defect area.
Figure 15. Histological sections of the PCL/PLGA/β-TCP group. A large amount of new bone was formed. NB, new bone; GM, grafting material; MEM, membrane; I, implant (Goldner Trichrome stain; magnification ×12.5 (a) and ×40 (b)).
Download figure:
Standard image High-resolution image3.4.4. Histometric analysis
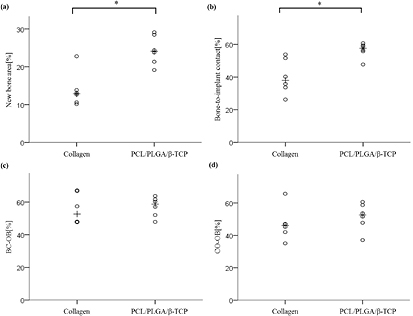
The histometric measurements are summarized in table 3 and figure 16. The PCL/PLGA/β-TCP group showed significantly higher levels than the collagen group. In terms of the new bone area and the bone- to-implant contact; (NBA (%), P = 0.000; and BIC (%), P = 0.000). The PCL/PLGA/β-TCP group also showed higher levels of the collagen group in terms of the remaining bone substitute area (%), BC-OB (%) and CO-OB (%), but the difference was insignificant (RBA (%), P = 0.256; BC-OB (%), P = 0.759; and CO-OB (%), P = 0.185).
Table 3. Histometric analysis within the area of interest (n = 6; %).
| NBA (%) | RBA (%) | BIC (%) | BC-OB (%) | CO-OB (%) | |
|---|---|---|---|---|---|
| Collagen | |||||
| Mean ± SD | 13.84 ± 4.60 | 16.08 ± 3.25 | 40.22 ± 10.71 | 55.81 ± 9.41 | 47.03 ± 10.19 |
| Median | 12.86 | 15.69 | 38.00 | 52.70 | 46.15 |
| PCL/PLGA/β-TCP | |||||
| Mean ± SD | 24.36 ± 3.90 | 19.19 ± 5.28 | 56.48 ± 4.68 | 57.11 ± 6.08 | 51.59 ± 8.51 |
| Median | 24.07 | 18.37 | 57.63 | 58.69 | 52.62 |
| P | 0.000 |
0.256 | 0.000 |
0.759 | 0.185 |
aIndicates statistical significance between the two experiment groups (P < 0.05). Note: NBA, new bone area; RBA, remaining bone substitute area; BIC, bone-to-implant contact; BC-OB, distance from the bone crest to the old bone; CO-OB, distance from the most coronal point of the osseointegration to the old bone.
Figure 16. Scatter plot and median (plus sign) representing (a) the new bone area, (b) bone-to-implant contact within the defect, (c) distance from the bone crest to the old bone, and (d) distance from the most coronal point of the osseointegration to the old bone (n = 6). *Significantly different (P < 0.05).
Download figure:
Standard image High-resolution image4. Discussion
GBR maintains the defect area after the extraction of a tooth before an implant, enhancing the migration of surrounding bone tissue and preventing ingrowth of soft tissue [42]. GBR is removed approximately 3–4 months after surgery when the bone is reconstructed and ready for an implant. For these reasons, first, GBR membranes must be biocompatible to allow migration and proliferation of the surrounding tissue. In addition, GBR membranes must be able to protect the wound area from mechanical disruption and salivary contamination and must be naturally resorbed so that an additional surgery is not required for their removal [43–45]. Collagen membranes have gained popularity as a GBR membrane as they facilitate cell attachment and proliferation in the defect area, resulting in effective bone formation, are bioresorbable within 4 weeks after the application, which obviates a second surgery for removal, and have little immunogenicity [18, 22, 46, 47]. Hence, for comparison with collagen membranes, this study fabricated 3D-printed PCL/PLGA/β-TCP membranes using a combination of TCP, a biodegradable material known to induce bone formation by releasing calcium, and two biocompatible polymers, PCL and PLGA [37, 38].
Synthetic biomaterials such as PCL, poly lactic acid, poly glycolic acid, and PLGA have been used to prepare the scaffolds for enhanced tissue regeneration [36]. In particular, PLGA is used for the reconstruction of various tissues owing to its high cytocompatibility [36, 48, 49].
However, scaffolds composed only of PLGA do not maintain their shapes either in the in vitro or in vivo environments owing to their brittle property and rapid resorption [34, 50]. In contrast, PCL has comparatively less cell affinity than PLGA, but it has excellent mechanical properties that prevent early fractures of the scaffold [39] and its degradation rate is slower than the rate of bone regeneration [51]. Thus, blended PCL/PLGA scaffolds exhibit biological and mechanical advantages obtained during the PCL and PLGA mixing processes that complement their respective weaknesses [36]. In addition, as β-TCP was added to the blended PCL/PLGA for the fabrication of the PCL/PLGA/β-TCP membrane, their elastic modulus was enhanced and their surface roughness was increased [1, 52, 53]. In the SEM images of the PCL/PLGA/β-TCP membranes prepared in this study, the rough surfaces generated by the incorporated β-TCP were confirmed (figure 7). During cellular testing with fibroblasts and preosteoblasts, early cell attachment and differentiation were higher in collagen, but the difference of proliferation and differentiation had disappeared when examined on day 7 and day 14, respectively. In contrast, fibroblasts aggregated and were detached from the membrane when maintained until day 14 (data not shown). This was in line with the results of a previous paper [40] and is an indication of the membrane's ability to inhibit the ingrowth of soft tissues.
We next applied the GBR to an in vivo implant model utilizing beagle dogs and performed histological analyses after sacrifice at 8 weeks. The histologic and histomorphometric analyses in this study showed that the PCL/PLGA/β-TCP group did not differ significantly from the collagen group; furthermore, in particular among the histometric measurements, the new bone area (%) and bone-to implant contact (%) of the PCL/PLGA/β-TCP group were significantly higher than those of the collagen group (P < 0.05). These findings indicated that the group receiving PCL/PLGA/β-TCP membranes showed levels of new bone formation and regeneration in the defect area comparable with those induced by collagen membranes, confirming that the biocompatibility of the PCL/PLGA/β-TCP membranes as well as their ability to maintain migration and proliferation of surrounding tissue and to support osteogenic differentiation to ultimately induce bone formation are comparable to those of collagen.
Rakhmatia et al [9] suggested that a non-resorbable titanium mesh could be an excellent solution when special mechanical support is required for bone defect treatments. According to Hämmerle & Jung [13], a titanium-reinforced e-PTFE membrane can be used in GBR for large defects; on the other hand, in casual dehiscence defect cases, the resorbable membrane is the current material of choice. However, in response to a report that resorbable membranes such as collagen have disadvantages as barriers owing to inferior generation of implant space caused by their poor mechanical properties [12], we performed mechanical property testing for PCL/PLGA/β-TCP membranes under biomimetic dry and wet conditions. As shown in figures 8 and 9, and table 1, the elastic modulus of collagen markedly decreased in dry and wet conditions whereas that of the PCL/PLGA/β-TCP membrane was relatively stable. Furthermore, in the volumetric analysis using µCT, the PCL/PLGA/β-TCP group showed a greater new bone volume (mm3) than the collagen group. This might have been due to the stability of the PCL/PLGA/β-TCP membrane based on its superior mechanical properties and slower degradation rate. This finding shows that PCL/PLGA/β-TCP membranes are stable in maintaining their structure and during the space-making required for bone regeneration in defect areas exposed to blood, saliva, and irrigation, which would be a beneficial property for use as a GBR membrane.
In addition, the PCL/PLGA/β-TCP membrane can be prepared quickly and economically using 3D printing technology to generate diverse shapes, thicknesses, pore sizes, pore geometries, and porosities. Upon loading antibiotics, growth factors, or adhesion factors, this synthetic membrane can be used as a delivery device for specific agents [38, 54]. In addition, its mechanical properties and degradation rate can be modified according to the component ratio. Actually, in the previously study, we combined PCL/PLGA/β-TCP in a ratio of 2:6:2, to manufacture a membrane that would reflect the stiff properties of PLGA with the focusing physical strength like titanium membrane [39, 40]. In the present experiment, PCL/PLGA/β-TCP was combined in a ratio of 4:5:1. Increasing the proportion of PCL aims to make the membrane flexible, like a collagen membrane, while also acquiring a little space maintaining ability. Therefore, the membranes used in this study were much softer than those used in the previous study [40], and we could bended and shaped membranes to fit the patient's defect, ultimately maintaining the space for bone regeneration. It will be also helpful to maintain the bone grafting materials within defect area. We concluded from this study that this membrane is more effective in handling and maintaining the space while it also embraces the advantages of collagen membrane such as resorption, biocompatibility and barrier function.
Overall, our results demonstrate that the 3D-printed PCL/PLGA/β-TCP membrane is useful as a GBR membrane. In addition, it can be prepared in the form of a patient-specific membrane from personal CT scan data, and it can be fabricated as a drug- or growth factor-releasing bioactive membrane. Further studies will likely be required to define and optimize these and other potential future uses.
5. Conclusions
In this study, the 3D-printed PCL/PLGA/β-TCP membrane showed a bone regeneration performance similar to that of collagen membranes during a GBR procedure performed in peri-implant defects. Therefore, the 3D-printed PCL/PLGA/β-TCP membrane was confirmed to have substantial efficacy as a resorbable GBR membrane for peri-implant defect treatment. Considering the higher stability of membrane, the 3D-printed PCL/PLGA/β-TCP membrane is expected to become a feasible alternative to the commonly utilized collagen membrane.
Acknowledgments
This research was supported by a grant from the Korean Health Technology R&D Project through the Korean Health Industry Development Institute (KHIDI), funded by the Korean Ministry of Health & Welfare (grant number: HI14C3309).
Conflicts of interest
The authors declare no conflict of interest.