Abstract
Gene electrotransfer (GET) is a proven and valuable tool for in vivo gene delivery to a variety of tissues such as skin, cardiac muscle, skeletal muscle, and tumors, with controllable gene delivery and expression levels. Optimizing gene expression is a challenging hurdle in preclinical studies, particularly for skin indications, due to differences in electrical conductivity of animal compared to human dermis. Therefore, the goal of this study was to develop an ex vivo model for GET using recellularized human dermis to more closely mimic human skin. Decellularized human dermis (DermACELL®) was cultured with human dermal fibroblasts and keratinocytes for 4 weeks. After one week of fibroblast culture, fibroblasts infiltrated and dispersed throughout the dermis. Air–liquid interface culture led to epithelial cell proliferation, stratification and terminal differentiation with distinct basal, spinous, granular and cornified strata. Firefly luciferase expression kinetics were evaluated after GET of recellularized constructs for testing gene delivery parameters to skin in vitro. Elevated luciferase expression persisted up to a week following GET compared to controls without electrotransfer. In summary, recellularized dermis structurally and functionally resembled native human skin in tissue histological organization and homeostasis, proving an effective 3D human skin model for preclinical gene delivery studies.
Export citation and abstract BibTeX RIS
1. Introduction
Gene electrotransfer (GET) uses pulsed electric fields to alter the plasma membrane of cells to facilitate intracellular delivery of DNA for gene therapy applications. Plasmid DNA delivery has been successfully accomplished in vivo for various tissues including skin, skeletal muscle, and cardiac muscle [1–8]. Skin is a very convenient target for gene delivery for various gene therapy applications due to ease of accessibility. GET to the skin can be used for clinical therapies such as DNA vaccination, wound healing or other systemic disorders. GET parameters such as pulse length, frequency, number of pulses, temperature and applied voltage all affect efficiency of gene transfer and, depending on the tissue and gene of interest, result in different expression profiles with often significantly different cellular effects. Therefore, it is important to modulate gene expression in gene therapy applications. Particular levels of the protein of interest can result in increased therapeutic benefits [9]. While higher expression is frequently desired, it is not always appropriate for therapeutic purposes, such as IL-12 gene therapy for melanoma. Higher levels of IL-12 resulted in no benefit to patients compared to a lower therapeutic range [10]. These dosage-related parameters are often determined empirically requiring a large number of treatment sites in a large numbers of animals [9].
Pre-clinical studies of GET-mediated delivery to the skin in vivo are further complicated by anatomical differences in skin structure of humans and animal models, such as rodents that are often used for these studies. Higher follicle abundance in rodents compared to low hair follicle abundance in human skin, results in differences in electrical conductivity [11, 12] and, in turn, electric field requirements between preclinical and clinical applications. There are several reported studies using spheroid 3D culture of tumor cells either alone or in co-culture systems for testing GET parameters for efficient transfection [13–18]. These models aim to mimic tumor microenvironment, but are not applicable for testing gene delivery to normal skin. A human recellularized skin model may prove highly useful for such studies; mimicking in vivo cellular and cell–matrix response, and more closely matching electrical conductivity.
Living dermal equivalents aim to approximate epithelial polarization, stratification and differentiation present in vivo, in particular cell–cell and cell–matrix interactions, and also mechanobiological related effects. There are numerous studies and a few commercial products available for culture of epithelial cells on different substrates, with or without supporting fibroblasts in co-culture. Many are based on the air–liquid interface principle for stimulating keratinocyte stratification and differentiation. Organotypic culture of keratinocytes is the most common and well-established 3D culture method for mimicking human skin. Organotypic culture consists of dermal fibroblasts embedded in collagen type I gel (usually rat tail collagen), with a superficial layering of dermal keratinocytes. After lifting the grafts to air–liquid interface, the keratinocytes are stimulated to stratify and differentiate, guided by signals from the embedded fibroblasts in co-culture within the gel [19–21]. This has been the standard skin model primarily due to the ease of construction, as well as the predictability with which epithelial cells morphologically resemble native epithelium after several weeks of culture.
A disadvantage of organotypic culture is the absence of an extracellular matrix (ECM) that resembles the structure, composition and complexity of native ECM. Thus, while cell–cell interactions are present, native cell–matrix interactions are reduced to integrin binding to collagen I only, lacking integrin and other cell–matrix binding sites of a typical dermis ECM. Collagen IV, laminins, elastin, glycoproteins, proteoglycans and other components normally present in native ECMs (such as bound growth factors) are absent in the organotypic model, thus cell interactions dependent on these ECM components are also absent. As a result, there are significant differences in gene and protein expression between the organotypic model and normal human skin. Furthermore, polymerized collagen I used for embedding fibroblasts is different in structure to collagen I fibrils and bundles normally found in native dermis, providing a significantly different environment in terms of signaling and biomechanical cues. Gel structures have less mechanical integrity, and therefore cannot be manipulated and tested ex vivo for an intradermal injection or application of penetrating or non-penetrating electrodes for electrotransfer without destroying the constructs. Organotypic culture skin model thus has limited applicability when it comes to testing GET parameters.
Several studies indicate that decellularized dermis can be used for support of a 3D culture of epidermis in vitro [22]. There are different decellularization methods reported for this purpose, with variable outcomes [22]. Human dermis is rarely available for research in large quantities, thus porcine decellularized dermis is often used [23, 24]. There are notable differences in the ECM composition, density and stiffness, and therefore electrical impedance between human and porcine dermis [11, 12]. DermACELL® (LifeNet Health, Virginia Beach, VA), skin recovered from consenting human donors and decellularized, has been extensively used in clinical allograft applications for reconstructive surgical procedures, such as second and third degree burns, breast reconstruction, chronic non-healing wounds, and cosmetic reconstruction after traumatic burn injuries [25–30]. Wide clinical application of DermACELL® with evidence of positive therapeutic outcomes in humans is thus an attractive feature for its use in developing an ex vivo skin model.
Another important consideration for in vitro skin culture is the cell source. While ideally primary keratinocytes and primary fibroblasts from the same source could be used, it is often technically difficult to culture these cells and obtain the numbers necessary as well as accounting for passage and donor variability. Primary keratinocytes in particular are notoriously difficult to culture, requiring numerous media supplements and growth factors. For improving batch-to-batch reproducibility, immortalized keratinocyte cells lines are often used. HaCaT keratinocytes are human dermal keratinocytes that have been spontaneously immortalized, and are well known to organize into all layers of the epithelium when cultured at air–liquid interface and are often used to study epithelial differentiation [19, 31–33]. HaCaT cells, originate from a human, male donor, from histologically normal skin [33] and are commercially available (Cell Lines Service, Eppelheim, Germany). Using the HaCaT cell line eliminates short culture lifespan, passage variability, low cell number, and donor variability complications. Human dermal fibroblasts are commercially available from ATCC. They are relatively easy to culture without requiring esoteric culture conditions, growth supplements and exotic media formulations, making them perfect for co-culture experiments reconstituting biomimetic human skin.
In the current study, we investigated electrotransfer application to recellularized human dermis for gene delivery and measured expression levels. We first developed a biomimetic pre-clinical model of human skin replicating native cell–matrix and cell–cell interactions, including native biomechanical and inherent dermal growth factor cues. We then used commercially available equipment for GET to evaluate the feasibility of using our skin model for GET studies. We hypothesized that a decellularized human dermis can be populated with HaCaT keratinocytes and human dermal fibroblasts, resulting in a recapitulated dermis and epidermis, which can then be used for testing GET. The ability to optimize GET parameters in vitro in a recapitulated skin for in vivo applications would reduce the number of pre-clinical animal experiments and potentially reduce research and development time prior to translation of GET to clinical therapies.
2. Materials and methods
2.1. Cell culture
Human primary dermal fibroblasts from a male donor were purchased from ATCC (Manassas, VA). They were maintained in culture with DMEM, 10% fetal bovine serum, 1% Penicillin/Streptomycin at 37 °C and 5% CO2. Culture medium was replaced every 2 to 3 d. Passage 4 cells were used for cell seeding of the skin grafts. Human dermal keratinocytes, HaCaT cells, were provided as a generous gift from Dr Mark Jaroszeski from University of South Florida, College of Engineering. HaCaT cells were maintained in culture under the same conditions as the fibroblasts, including the same media formulation. HaCaT cells are spontaneously immortalized, thus original passage number was not known, but in general earlier passages from the original stock were preferred.
2.2. Dermis seeding and long-term culture
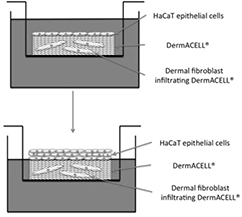
DermACELL® human allograft tissue with research authorization (provided as a generous gift from LifeNet Health, Virginia Beach, VA) was selected as the scaffold for in vitro skin graft modeling. DermACELL® grafts in the range of 0.7–1 mm in thickness were selected for the entire study as provided by the manufacturer. DermACELL® grafts were washed in PBS and cut to 12 mm disks with 12 mm biopsy punches (supplemental figures 1(A)–(D)) (stacks.iop.org/BMM/11/035002/mmedia). The disks were then placed into wells of 24 well tissue culture plates, reticular side up, and washed twice in DMEM. All manipulations of the dermal tissues were done under sterile conditions with sterile instruments in a tissue culture hood. Fibroblasts were then seeded on the reticular surface of the dermis at a density of 1 × 106 cells/disk [34, 35]. After the fibroblasts were attached (24 h), the grafts were moved to fresh wells, in order to culture only those cells that were attached to the dermis and not the bottom of the well plate. Fibroblasts were maintained on the dermis for one week, to allow cell infiltration throughout the matrix of the dermis, as confirmed histologically. The grafts were then flipped over with the papillary side up and HaCaT cells were seeded at 150 × 103 cells/disk. HaCaT Cells were allowed to attach overnight submerged, in the wells of the upper chamber of Transwell® Permeable Supports (Corning Incorporated, Life Sciences, Tewksbury, MA) with a 0.4 μm pore size and a 12 mm diameter. After cell attachment, the grafts were moved to a fresh upper chamber well in order to maintain only the cells attached to the grafts, and not those attached to the well plate. The bottom well was filled with the same media formulation used to maintain both fibroblasts and HaCaT cells, while the top well was left without media, effectively lifting the grafts to an air–liquid interface (figure 1, supplemental figure 1(J)). Recellularized dermis was cultured for up to 4 weeks with daily changes of media.
Figure 1. Diagram of air–liquid interface culture of recellularized dermis.
Download figure:
Standard image High-resolution image2.3. Immunofluorescence staining
Samples were fixed in 4% paraformaldehyde at 1, 2, 3 and 4 weeks post cell seeding then shipped to Virginia Commonwealth University Histology Core (Richmond, VA) for paraffin embedding, sectioning and glass slide mounting. Rehydration was accomplished using standard protocols, briefly, two washes in xylene, and subsequent washes in gradient alcohol (100, 95, 75, 50, and 25%), followed by rehydration in RO water. Samples were then boiled for 15 min in 10 mM citric acid to expose epitopes. Cell permeabilization was performed for 20 min in 0.25% Triton X-100 in phosphate buffered saline (PBS). Blocking was performed with 4% bovine serum albumin in phosphate buffered saline with 0.01% Tween 20 (PBST) for 1 h at room temperature. Samples were then exposed to primary antibodies diluted 1:200 in blocking buffer overnight at 4 °C. Primary antibodies were monoclonal mouse antibodies against human collagen IV (Affymetrix, Santa Clara, CA), human Ki67 (Affymetrix, Santa Clara, CA), human involucrin (ThermoFisher Scientific, Waltham, MA) and Pan keratin (ThermoFisher Scientific, Wltham, MA) as well as polyclonal rabbit antibodies against human vimentin (Sigma-Aldrich Corp., St. Louis, MO). After incubation with primary antibodies, samples were washed 5 times for 15 min with PBST, and incubated with corresponding secondary antibodies diluted 1:200 in blocking buffer for one hour at room temperature and washed again with PBST. Samples were then counterstained with DAPI and mounted with VECTASHIELD® HardSet™ mounting medium (Vector Laboratories, Burlingame, CA) and allowed to set for 15 min and room temperature. The samples were then stored in −20 °C until imaging. Secondary antibodies used for this study included goat anti-mouse IgG-AlexaFluor®488 for Ki67, Collagen IV, Keratin, and involucrin, and goat anti-rabbit IgG-AlexaFluor®565 (Life Technologies, Grand Island, NY) for vimentin. Negative controls for the staining included incubation with secondary antibodies, without primary antibodies.
2.4. Gene electrotransfer
Recellularized dermis samples cultured for 2 weeks were split into four groups (n = 4 per group). Samples from one group received an intra-dermal injection of 50 μl of 2 mg ml−1 plasmid DNA encoding firefly Luciferase, pGwiz™Luc (Aldevron, Fargo, ND; abbreviated to pLuc) and were then placed into electroporation cuvettes with a 2 mm gap, filled with culture medium to the fill line, and pulsed with 60 V for 8 pulses with a 150 ms pulse width, which is similar to our electrotransfer conditions typically used in vivo [5, 36–40]. Intradermal injections were considered successful if there was a white bubble completely contained within the sample that formed at the site of the injection. This is the same appearance as an intradermal injection in human patients, for example for a conventional PPD test. An intradermal injection of green food coloring was performed for visualizing the technique (supplemental figures 1(E)–(I)). Samples from a second group were placed into cuvettes with 2 mm gap, filled with culture medium containing 0.1 mg of plasmid DNA surrounding the samples, and pulsed with 60 V for 8 pulses with a 150 ms pulse width (supplemental figure 1(K)). Electric pulses were delivered using the BTX ECM 830 (Harvard Apparatus, Inc. Holliston, MA). Samples from the third and fourth group received an intradermal injection or were exposed to plasmid DNA in the media respectively as the first two groups, but received no electric pulses. Disks were returned to air–liquid interface culture conditions after pulsing.
2.5. Bioluminescence imaging
Luciferase gene expression was measured on days 1, 2, 4 and 7 after GET according to a previously described protocol [41]. Briefly, old media was removed and replaced with fresh media containing 150 μg ml−1 Luciferin. Whole samples were incubated in the dark for 5 min and then imaged with a Caliper IVIS Spectrum whole body imaging system (PerkinElmer, Massachusetts, USA). Luciferase expression for each sample was quantified as total flux measured in photons per second.
2.6. Metabolic activity assay
PrestoBlue® Cell Viability Assay (Life technologies, Grand Island, NY) was used to evaluate metabolic activity of the constructs following the manufacturer's instructions. Briefly, each construct was incubated with the PrestoBlue® reagent for two hours, the media was removed and fluorescence intensity was measured with a fluorescence plate reader. Metabolic activity analysis was performed 1, 2 and 3 d after gene electrotransfer (n = 4).
2.7. Statistics
All quantitative data (luciferase expression and metabolic activity) was compared using one-way analysis of variance to determine if differences exist between the experimental groups. The Tukey-Cramer multiple comparison test was used to determine significance of differences if such were found. A p-value less than 0.05 was considered significant.
3. Results
3.1. Development of 3D skin constructs using DermACELL
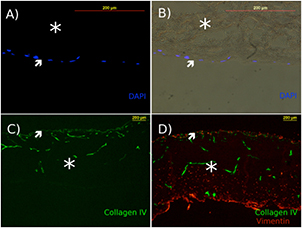
Initial assessment of DermACELL® yielded evidence of an intact basement membrane, with positive staining for Collagen IV, observed on the papillary surface of the grafts, as well as outlining the original blood vessel networks in the decellularized allograft (figure 2(C)). DermaACELL® grafts were seeded with fibroblasts either on the papillary or the reticular side of the dermis to compare cell infiltration and attachment between both sides and whether the polarity of the decellularized dermis was maintained. After one week of culture, fibroblast infiltration and attachment were confirmed histologically (figure 2). Fibroblast cell seeding of the reticular surface of DermACELL® resulted in cellular attachment when left overnight. Subsequent infiltration and population of the entire thickness of the dermis (figure 2(D)) occurred after one week of culture. Fibroblast cell seeding on the papillary surface resulted in cell attachment, but no cell infiltration past the basement membrane (figures 2(A) and (B)), suggesting that the basement membrane still maintained its barrier function.
Figure 2. Decellularized dermis maintains its basement membrane and supports fibroblast attachment and infiltration from reticular seeding surface only. (A) and (B) Fibroblasts seeded on the papillary surface remained on papillary surface, (DAPI stain for cell nuclei), (B) brightfield image merged with DAPI. (C) DermACELL® without cells is stained for collagen IV (green). (D) Fibroblast infiltration into decellularized dermis after seeding on the reticular surface and one week of cell culture, with fibroblasts stained with vimentin (red). Scale bar = 200 μm. Stars indicate the location of the dermis, while arrows point to the papillary surface and basement membrane.
Download figure:
Standard image High-resolution imageGrafts with fibroblasts seeded on the reticular side were turned papillary side up for HaCaT cell seeding. Keratinocytes attached overnight, and the grafts were transferred to fresh wells for air–liquid interface culture (figure 1). Recellularized dermis was cultured for up to 4 weeks with daily media changes. Seeding keratinocytes on the papillary surface resulted in cell attachment. After the cells were allowed to attach for 24 h, the grafts were lifted to air–liquid interface. This resulted in cell proliferation and stratification after one week of culture. Figure 3 demonstrates the structure of the recellularized dermis over 4 weeks of culture. Cells positive for keratin were stratified, but were confined to the papillary surface and did not appear to cross the basement membrane highlighted by collagen IV immune-reactivity. As visible in H&E stained samples, the morphology of the keratinocytes was polarized and stratified with distinct basal, spinous, granular and cornified strata visible after 3 weeks of culture.
Figure 3. Recellularized dermis supports keratinocyte polarization and stratification forming structured epithelium. Serial sections were stained either with hematoxylin and eosin (top row), pan keratin (middle row) for HaCaT keratinocytes, or collagen IV for basement membrane (bottom row). Scale bar = 20 μm.
Download figure:
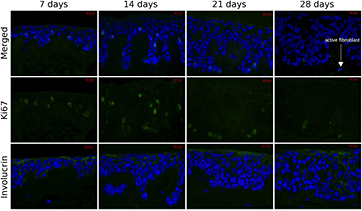
Standard image High-resolution imageFurther culture for up to 4 weeks at continuous exposure to air resulted in additional polarization and stratification of the epithelium. Proliferating cells, expressing Ki67, appeared to be confined to the basal strata. Differentiation and even terminal differentiation of the keratinocytes was notable after 2 weeks with expression of involucrin. Cell nuclei flattened and even disintegrated completely towards the stratum corneum, while terminal differentiation became more prominent in respective layers (figures 3 and 4). Stratum corneum appeared thin and no stratum lucidum is discernable, consistent with thin skin covering the majority of the human body and in contrast to thick skin; which is prominent in the palms and soles.
Figure 4. Recellularized dermis supports keratinocyte proliferation and differentiation forming functional epithelium. Serial sections were stained for Ki67 for proliferating cells in top and middle rows (merged DAPI and FITC channels or FITC channel alone respectively), or for involucrin for terminal differentiation in bottom row. Scale bar = 20 μm.
Download figure:
Standard image High-resolution imageThe dermal layer of the recellularized construct appeared to have both active and inactive fibroblasts, or fibrocytes and fibroblasts respectively. The majority of the fibroblasts were in their resting inactive state, as evident in H&E images with presence of long flat nuclei and low cytoplasmic content (figure 3). Other fibroblasts were in their active state, evident by the presence of oval nuclei, by more abundant cytoplasmic content visible in H&E, and occasional positive Ki67 staining (figure 4, 28 d panel), consistent with human dermis in vivo, which contains largely inactive fibroblasts/fibrocytes with a small number of active fibroblasts remodeling the extracellular matrix.
3.2. Gene electrotransfer to DermACELL
Four groups were compared for expression of firefly luciferase with and without applied voltage: GET with plasmid DNA intradermal injection, GET with plasmid DNA added to culture medium, plasmid DNA intradermal injection without electrotransfer and plasmid DNA added to culture medium without electrotransfer. GET significantly enhanced expression of luciferase in both GET groups compared to their respective controls without electric pulses. Higher expression in GET groups persisted for at least 7 d (figure 5, supplemental figure 2). While plasmid DNA intradermal injection group without electrotransfer did result in higher luciferase expression levels than adding DNA to the media, this elevation was not statistically significant. Also there was no significant difference in luciferase expression between two GET groups.
Figure 5. Gene electrotransfer of recellularized dermis enhances luciferase expression. GET + pLuc groups have significantly higher expression than no GET groups for each time point (p < 0.05).
Download figure:
Standard image High-resolution image3.3. Metabolic activity following gene electrotransfer
PrestoBlue® metabolic activity assay was performed on the samples from day 1 through day 3 after gene electrotransfer as an indicator of viability. While gene expression was significantly enhanced, metabolic activity of recellularized dermis graft exposed to electric pulses was significantly reduced one day after GET when compared to corresponding controls (figure 6). The difference in metabolic activity was maintained for the subsequent days, indicating no further decrease in viability after the initial drop. This was consistent with other GET experiments, indicating the need to optimize GET parameters to optimize expression and viability.
Figure 6. Metabolic activity of recellularized dermis after gene electrotransfer is reduced compared to no electrotransfer groups for each time point (p < 0.001). There was no significant difference in metabolic activity between GET groups at any time point. There were no significant differences in metabolic activity between no electrotransfer control groups at any time point.
Download figure:
Standard image High-resolution image4. Discussion
Delivery of molecules to the skin, particularly for the purpose of gene transfer, is a complex approach due to multiple cell types and varying properties within the different layers. For GET, this is further complicated by anisotropy within the skin and variation between skin of different species [11, 12]. Structure of human and animal skin is drastically different in terms of paucity and abundance of fur respectively; thus optimal electrotransfer conditions in an animal model, may not translate to optimal electrotransfer conditions in clinical applications. Development of equivalent in vitro models can facilitate development of appropriate delivery approaches and reduce the use of animals.
Spheroid in vitro models have been prominently used to model 3D organization of tissues across many disciplines [42]. While for some applications such as embryoid body formation for stem cell differentiation, spheroid culture may be a perfectly desirable model [43]. Nevertheless, there are key organizational elements missing in this type of model for in vitro modeling of mature adult tissues such as skin. Primarily, the appropriate extracellular matrix and polarized cell–cell/cell–matrix organization cannot be replicated in spheroid culture. However, spheroid culture has been the main in vitro model for testing delivery of molecules via electrotransfer, in particular for testing GET to cells in 3D [13–15]. Therefore, there is a need for more tissue specific ex vivo model, especially for human skin delivery, which the present skin model addresses. Our recellularized dermis is a major step toward developing more efficient parameters for GET to skin, and possibly for additional ex vivo dermal model testing strategies, including but not limited to surface disinfection strategies (e.g. cold plasma), pharmacotoxological and related efficacy and safety testing (therapeutic, cosmetic), dermal cancer metastases and transformation models and drug efficacy studies and 3D biomimetic culture, irritancy, genotoxicity, skin metabolism, penetration (mechanical testing), and wound healing.
We demonstrated that decellularized human dermis can be rapidly recellularized while exhibiting characteristic cell–cell, cell–matrix interactions and histological features present in native human skin. Our recellularized epithelium is polarized and stratified, containing all layers of human epithelium present in thin skin, which covers the majority of the human body. These layers are stratum basale, stratum spinosum, stratum granulosum, and stratum corneum [44] as can be seen in figures 3 and 4. In normal human skin, as keratinocytes in stratum basale divide and differentiate, they move towards the outer surface of the skin [44]. In our model, proliferation marked by Ki67 expression is prominent in basal epithelial strata, while differentiation and terminal differentiation marked by involucrin are notable in granular and cornified strata (figure 4). This is consistent with normal skin epithelium.
The dermis is fully populated with dermal fibroblasts in various stages of activity (figures 3 and 4). Both active and inactive fibroblast phenotypes can be observed histologically, with the large majority of the fibroblasts resting in their inactive state. Human dermis in vivo is normally populated by inactive fibroblasts, with a small number of active fibroblasts contributing to maintenance of the dermal ECM, unless wound healing or inflammation promote transition to the active state at the site of injury [44]. The structure of the decellularized dermis seems to be intact to the point that the majority of the re-introduced fibroblasts remain inactive, with only a small number of cells participating in reorganization of the matrix and proliferation. The basement membrane, at the epidermal–dermal junction, performs its barrier function, preventing the epithelial cells from growing in the dermis. Degradation of the basement membrane in vivo correlates with migration of malignant cells from the epithelial layer to distant sites and further carcinogenesis, thus an intact basement membrane is vital for normal skin function [45]. The presence of a functional basement membrane in our reconstituted dermis, further validates is utility as a human skin model.
Mechanical integrity of the grafts allowed for their retrieval from culture wells with sterile forceps, and further manipulations such as intradermal injections of plasmid DNA, as well as transfer both to and from the electroporation cuvette. Therefore, our ex vivo skin model could be used for other possible applications, where mechanical integrity is essential.
5. Conclusions
Recellularized dermis encompasses functional epidermis and dermis for recapitulating human skin ex vivo, with native human cellular and matrix components. This skin model can be used as a 3D culture model for a variety of applications, in particular for in vitro evaluation of gene expression following gene electrotransfer. We show that our recellularized DermACELL® skin graft model for GET development readily expresses luciferase following GET. Intradermal injections without electrotransfer resulted in slightly elevated luciferase levels, though expression levels were significantly lower than levels achieved in electrotransfer-mediated groups. The drop in metabolic activity between GET and controls occurred as expected, thus optimization will be required to improve viability, while maintaining desired expression levels. These findings are consistent with previously reported expression profiles of gene delivery in vivo, further validating this 3D model. Future studies will entail optimizing delivery parameters using various electrodes designed for in vivo gene delivery to human skin, which this ex vivo model system uniquely makes possible. Furthermore, as recellularized dermis is mechanically stable it can also further be surgically implanted in vivo following in vitro manipulation as a model for either cell and/or gene therapy.
Acknowledgments
The authors would like to thank Gary Walters of LifeNet Health, for the generous gift of DermACELL® grafts for this research. We would also like to thank Dr Mark Jaroszeski for the generous gift of HaCaT cells.