Abstract
Birds and mammals have evolved many thermal adaptations that are relevant to the bioinspired design of temperature control systems and energy management in buildings. Similar to many buildings, endothermic animals generate internal metabolic heat, are well insulated, regulate their temperature within set limits, modify microclimate and adjust thermal exchange with their environment. We review the major components of animal thermoregulation in endothermic birds and mammals that are pertinent to building engineering, in a world where climate is changing and reduction in energy use is needed. In animals, adjustment of insulation together with physiological and behavioural responses to changing environmental conditions fine-tune spatial and temporal regulation of body temperature, while also minimizing energy expenditure. These biological adaptations are characteristically flexible, allowing animals to alter their body temperatures to hourly, daily, or annual demands for energy. They exemplify how buildings could become more thermally reactive to meteorological fluctuations, capitalising on dynamic thermal materials and system properties. Based on this synthesis, we suggest that heat transfer modelling could be used to simulate these flexible biomimetic features and assess their success in reducing energy costs while maintaining thermal comfort for given building types.
Export citation and abstract BibTeX RIS
1. Introduction
Animals and buildings can be seen to have similar requirements for the regulation of internal temperature and energy use. Many species of animals have evolved insulation, physiological mechanisms for the control of body temperature and a range of behaviours that optimize energy use and heat exchange with the environment. The cost of energy and the need to reduce the burning of fossil fuels has brought about a renewed interest in looking to nature for methods of minimizing energy use in buildings. Thermal products with their origins in the hierarchal structure of animal insulation, particularly with respect to radiative or surface properties [1, 2], or from anatomical analogies that may improve the design of heat exchange systems [3] have been suggested for future development. However, when attempting to incorporate biomimetic solutions to buildings, engineers are faced with a vast number of species, highly adapted to a range of climates. Extracting useful and novel adaptations from nature requires knowledge of animal morphology, physiology and behaviour, but also requires a way of translating the main characteristics of animal thermoregulation into design principles [4].
The aim of this review is to synthesize current knowledge of biological adaptations involving regulation of body temperature, and to highlight principles that may be relevant to the temperature control of buildings, where daily and seasonal adaptations are required for the maintenance of thermal comfort and to minimize energy use. We have concentrated on the thermal adaptations of birds and mammals, for the main reason that many of them have comparable features relevant to temperature control and energy use in buildings (table 1). Birds and mammals are endotherms, meaning that their body temperature is mainly controlled by internal generation of metabolic heat and through waste heat as energy is converted to mechanical power in the muscles. Endotherms have a complex temperature control system that is precisely tuned to meteorological changes, as well as controlling regional variations in temperature in order to minimise energy use. Most birds and mammals are well insulated and have the ability to dynamically alter insulation according to conditions; finally, endotherms adjust their behaviour to control heat exchange, for example to adjust solar gain or create favourable microclimates. Coordination of these multi-dimensional adjustments of the organism in reaction to perceived environmental changes are integrated by a central place organ, the brain. We also refer to features of a small number of ectotherms (organisms whose body temperature depends on external sources of heat) in order to illustrate how solar gain is used for thermoregulation.
Table 1. Analagous features of endothermic organisms (birds and mammals) and buildings.
| Endotherm (birds and mammals) | Building | |
|---|---|---|
| Internal heat production |
|
|
| Temperature control |
|
|
| Morphology |
|
|
| Behaviour |
|
|
In this review we firstly examine the biomaterials used for insulation in animals, particularly with respect to how physiological and behavioural responses act to give insulation dynamic heat transfer properties. We then discuss the main physiological and behaviour adaptations that allow animals to control body temperature and minimize energy use in hot and cold environments. In conclusion, we suggest a modelling approach that may aid the translation of important features of animal thermoregulation for future work on biomimetic temperature control and energy use in buildings.
2. Heat transfer processes in animals
For an animal to maintain a constant body temperature, total heat production (Qtot) must be balanced by heat losses or gains through radiation (qrad), convection (qconv), conduction (qcond), evaporation (qlat), and through changes in heat storage (qstored), where heat fluxes are in watts (W) [5], given by:
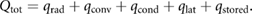
Qtot is derived from routine metabolic processes for organism maintenance (basal metabolic rate, or BMR), activity metabolism, and also waste heat generated through the conversion of energy to work, complemented if necessary by on-demand heat production (shivering or non-shivering thermogenesis).
Heat transfer within the body occurs by conduction, through internal tissues, skin, and external insulation (feathers or fur), and is further enhanced by the circulation of blood through the arterial-venous network and the transfer of heat through the respiratory system. For animals in air, heat exchange at the outer surface of the animal occurs largely by radiation and convection with the surrounding environment, while in water most heat is lost by convective heat transfer. Conduction of heat from the body also occurs through direct contact with solid surfaces (the ground or ice) when animals are standing or lying, or when swimming or diving in water. Latent or evaporative heat transfer occurs mainly through respiratory water loss, as air is expelled from the lungs; this is one of the most important avenues of heat loss for animals living in hot climates. Latent heat loss also occurs by diffusion of water through the skin and by sweating in mammals [6, 7]. Evaporative heat loss may also occur when a surface of the animal becomes wet from saliva or urine, following immersion in water or through interception of precipitation. Finally, in many circumstances animals are not in steady state conditions and therefore the heat storage term in the above equation accounts for transient heat transfer, dependent on the temperature gradient between body and environment, body mass, and specific heat capacity of tissues [8]. Models of heat transfer have been widely used to estimate the energy balance of animals [8–10].
3. Insulation
3.1. Animal integuments: complex structures for heat and water regulation
The skin and associated tissues (integument) provide multiple functions in vertebrates, principally providing a barrier to water diffusion and physical protection of underlying tissues. It also determines thermal properties and is covered by numerous nerve endings and an extensive network of blood vessels. The vertebrate integument consists of epithelial cells attached to an underlying fibrous and vascular dermis. Localized cellular growth and differentiation give rise to appendages such as claws, glands, hair or feathers, and patterned folds or scales formed from keratin, a family of fibrous structural proteins. Keratin is a common and important structural feature formed in the epidermis [11]. Amphibian skin is relatively permeable to water (see below) and gas exchange can take place through the skin (cutaneous respiration). Secretion of mucus from glands in the dermis keeps skin moist, and in some species mucosubstances and lipid compounds form an extra-epidermal layer, reducing water loss. Reptilian skin has a thickened epidermis with a relatively thin dermal layer in comparison to that of birds or mammals (see figure 1 in [11]). This provides a more effective barrier to water and gas exchange compared to most amphibians. Reptile skin is mechanically protected by scales or scutes, and in lizards and snakes the entire skin is covered by scales. However, avian and mammalian integuments have a thickened outer layer of the skin (stratum corneum) which can be highly keratinized to provide a strong mechanical barrier. Specialized epithelial cells form feather and hair follicles in these skins.
Water loss through the integument occurs by the passive diffusion of water across cell membranes (not requiring metabolic energy) and also by active secretion in mucus (in amphibians) or from sweat (mammals). Water loss is therefore largely determined by the skin's resistance to water passage. For comparative purposes, skin resistance to evaporative loss may be negligible (~ 0 s cm−1) in many aquatic and terrestrial amphibians, 100–200+ s cm−1 in some frogs adapted to low moisture environments, and very high at over 1000 s cm−1 in desert reptiles, while most birds and mammals have skin resistances of 10–300 s cm−1 [11]. Only mammals may lose considerable quantities of water through sweating. Sweat is secreted to the skin's surface through pores from specialized glands, allowing cooling of the surface through latent heat transfer. Humans, horses and patas monkeys (Erythrocebus patas) are the only mammals reported to sweat profusely for thermoregulation. The compositions of their sweat fluids differ markedly; humans have high-salt, low-protein sweat, whereas horses have high-protein, low-salt sweat [12]. Sweating may appear to be maladaptive for species such as horses with a thick and waterproof pelt that would slow down the transfer of sweat from the skin to the outer hair surface for evaporative cooling. However, horses have evolved a surface-active detergent-like protein (latherin) that they secrete in their sweat. Latherin reduces water surface tension at low concentrations and most likely acts as a wetting agent to allow evaporative cooling through an existing waterproof coat [13]. Sweating is a unique physiological mechanism to only maximize latent heat loss, in comparison to other bi-directional heat transfer processes through adjustments to conduction, radiation and convection across the integument. Heat transfer occurs by a combination of conduction through the epidermis to the skin surface and latent heat transfer from passive diffusion of water and by evaporation of water on the skin surface. Conduction and latent heat transfer are strongly influenced by dilation and constriction of capillary networks through the epidermis that can bring about rapid changes in skin temperature [7].
3.2. Insulation types
Thermal insulation in animals is provided by layers of lipid-filled cells with low thermal conductivity (fat and blubber) or by a keratin matrix that traps still air (feathers and hair) (table 1). The thermal characteristics of different types of insulation are derived by a combination of physical structure and dynamic properties controlled by an animal's physiology and behaviour. Feathers and hair are hierarchical materials that derive their mechanical characteristics from the basic keratin unit (helical proteins), to individual element composition and their arrangement in plumage and fur [14].
3.2.1. Fat and blubber
Fat is distributed around the body, and functions both as a daily or seasonal energy reserve. Mammals have two functionally different types of fat: white adipose tissue comprising specialized cells (adipocytes) that are lipid-rich [23], and brown adipose tissue (BAT) that contains in addition to lipids a high concentration of mitochondria for the generation of metabolic heat. BAT has not been found in birds but some evidence suggests that birds are also able to generate heat by non-shivering thermogenesis [7]. Birds and mammals adapted to cold have relatively thick fat layers (thermal conductivity ≈ 0.2 W m−1 K−1 [8]) and have plumage or fur to increase total insulation.
White adipose tissue averages around 7% of the live body mass of free-ranging wild mammals but shows considerable variation, from > 0.5% to 50% [24]. White adipose tissue is partitioned into a few large and numerous small depots, especially in mammals and to a lesser extent in birds. The largest depots in mammals are inside the abdomen and between the skin and superficial musculature. Fat occurs under feather-bearing skin, but not normally beneath naked skin in birds. Only in diving species, particularly in penguins and some aquatic birds, does it functionally resemble the specialized adipose tissue in marine mammals [25].
The occurrence of large, naturally obese mammals living in seasonally cold regions has led to the idea that fat (superficial adipose tissue) is an adaptation for thermal insulation. Comparison of the ratio of white adipose tissue between superficial and internal depots, for example in mammalian carnivores of similar body shape but different body size in different climates, does not support this hypothesis [26]. Superficial fat deposition appears to be the optimal location for storing large quantities of lipid, where it can be mobilized rapidly. Therefore, it is the thick coat with superior thermal properties that provides most of the insulation for mammals in cold, not fat (table 2). The main exception to the evolution of fat as an insulator is found in marine mammals and diving birds where the evolution of internal insulation was required, as the air layer within external insulation cannot be maintained underwater [27].
Table 2. Examples of insulation thickness (mm), thermal conductivity (W m−1 K−1), thermal resistance (K m2 W−1) and U-value (W m−2 K−1) of animal coats compared to air, water and glass wool insulation.
| Species | Thickness mm | Thermal conductivity W m−h K−1 | R value K m2 W−1 | U-value W m−2 K−1 | Reference |
|---|---|---|---|---|---|
| Air (@ 20 °C) | 0.026 | [10] | |||
| Glass wool (ISG CWS36) | 50–150 | 0.036 | 1.39–4.17 | 0.72–0.24 | [15] |
| Feather (Adélie penguin) | 18 | 0.036 | 0.50 | 2.00 | [16] |
| Fur (polar bear) | 30 | 0.054 | 0.56 | 1.79 | [17] |
| Feather (passerine) | 5 | 0.069 | 0.07 | 14.3 | [18] |
| Blubber (bottlenose dolphin) | 16 | 0.147 | 0.11 | 9.09 | [19] |
| Blubber (harp seal) | 36–66 | 0.195 | 0.18–0.34 | 5.55–2.94 | [20] |
| Skin (human epidermis) | <1 | 0.209 | <0.005 | 200 | [21] |
| Muscle (human) |
38 | 0.560 | 0.07 | 14.29 | [22] |
| Water (@ 20 °C) | 0.600 | [10] |
Marine mammals possess a specialized densely vascularized layer of fat held together by structural collagen fibres beneath the skin, known as blubber [28]. Blubber thickness may range from a few centimetres in small fur seals to 0.5 m in the large whales. It is predominately composed of triacylglycerols providing thermal conductivities typically of around 0.1–0.2 W m−1 K−1 (table 2). These lipids have relatively low melting points and therefore the blubber layer can change phase. Blubber temperature changes with blood flow and water temperature, resulting in transient heat transfer due to heat of fusion when a phase change occurs [19, 28, 29]. Different features of blubber, including adipocyte size, thickness and lipid content, are reported to change with age in whales and dolphins (cetaceans). For example, in bottlenose dolphins (Tursiops truncatus) thermal insulation of young nutritionally dependent life stages are characterized by stable blubber quality (conductivity) and increased blubber quantity (thickness) with age, but in adults the blubber quantity remains relatively stable while quality is variable between individuals [19].
3.2.2. Feathers and plumage
Birds are insulated by multiple layers of downy and contour feathers that form the plumage. The number of individual feathers on a bird is considerable, ranging in total from around 1000 to 10 000 for body mass of 10 g–1 kg [30]. The mean mass and total number of feathers show negative allometry, indicating that small species have relatively more feathers that are small and light in weight [30]. Down and contour feathers differ in their structure, with down trapping still air close to the skin surface while the contour feathers reduce wind penetration and provide waterproofing and mechanical protection [31]. Birds have the ability to increase or decrease plumage insulation by raising or depressing both contour and down feathers (ptiloerection). Feather structure and plumage depth is variable across the body, and both show considerable interspecific variation. As chicks grow, their downy feathers are progressively replaced by juvenile and adult plumages that contain both downy and contour feathers. As a rule, insulation is directly correlated with plumage thickness, but there is considerable interspecific variation in thermal conductivity between species, typically from around 0.069 W m−1 K−1 in a small song bird (passerine) to 0.036 W m−1 K−1 in penguins (table 2) [18]. There are also special adaptations depending on environment. For example, the rock ptarmigan (Lagopus muta) living in the Arctic has feathers with air-filled vacuoles within feather barbules that may increase insulation, and optimize their radiative properties [32]. Feathers are distributed into 7–8 distinct tracts (pterylae), with regions between them lacking feathers or with only a few feathers (apteria). In most species the apteria are not obvious externally because of overlapping feathers. In penguins there are only a few small apteria, so feather cover is almost complete [33]. Multi-layer insulation is a feature of seabirds that possess a subcutaneous adipose tissue layer underneath a thick plumage. The penguins show the most extreme adaptation to cold conditions. Most of the body fat (72%–82%) in emperor and king penguins is stored subcutaneously [34] and is distributed more uniformly under the skin, particularly around the legs, tail, and lower abdomen than in other species of birds [35]. Male emperor penguins (Aptenodytes forsteri) possess 20–30 mm of subcutaneous fat (mean girth/diameter around 750 mm) that disappears entirely at the end of the winter, but almost 90% of the insulation is provided by plumage (40–50 mm) alone [36]. Recent examination of emperor penguin plumage reveals that each contour feather has an attached afterfeather, and is surrounded by soft downy plumules. Contrary to initial thoughts, it is the plumules—not the downy afterfeather—that provides most insulation, forming a dense mat beneath the contour feathers, which are four times as numerous as other body feathers [37].
3.2.3. Hair, fur and wool
There are a number of different types of insulation in mammals but all are formed by the arrangement of primary hair and secondary fibres. Coat insulation in mammals is proportional to coat depth, with thermal conductivity averaging around 0.042 W m−1 K−1, but there are differences in the thermal conductivity of fur between species [8]. Hair density varies between coats [31]: moderately insulated coats may possess 100–200 hairs cm−2, consisting of coarse straight fibres that can be elevated; small mammals and Arctic species may have around 4000 hairs cm−2, increasing to the highest recorded density of 130 000 hairs cm−2 in sea otters [38]; and finally wool, which consists of dense, matted coats of crimped hair at a density of around 1000 hairs cm−2. Typically, hair length and density change with age, resulting in thicker fur in adults, allowing greater resistance to wind penetration [39]. In contrast to species that increase coat insulation with age, seals show the opposite trend as the young transition from fur to blubber insulation, resulting in decreases in hair density and the number of under hairs, and with reduced length and flattening of hairs with age [40]. Air-filled cavities within the guard hairs of Arctic species such as the polar bear (Ursus maritimus) and reindeer (Rangifer tarandus) may also provide additional insulative properties [41], but in polar bears these guard hairs represent only 10% of hair fibres and their contribution to total fur insulation is exaggerated [42].
Some animals living in hot climates also have thick and dense coats, as this protects them from absorbing high levels of solar radiation at the skin surface [42]. There appear to be no overarching physical features of the fur that separate mammals adapted to extreme heat and cold, but this perhaps warrants further investigation. This work does however indicate that as fur insulation decreases, colour increasingly influences heat gain from solar radiation. In contrast to the selective advantage of hair covering for insulation, a recent modelling study on African elephants (Loxodonta africana) has suggested that the combination of rough and creased skin surface and sparse hair cover (≈650 hairs m−2) enhances heat transfer by 10% at low wind speeds. This effect decreases as wind speed increases but results suggest that elephant hairs function as 'pin fins', allowing greater dissipation of heat [43].
3.3. Wind and waterproofing
Heat transfer through an animal's coat occurs by a number of processes, either by: (i) conduction, through trapped air and along feather or hair fibres; (ii) radiation from these fibres; (iii) free convection, and; (iv) forced convection when wind penetrates the coat [10]. Conduction along feather elements is the predominant mechanism of heat transfer, accounting for around 50% of heat flow through plumage [44]. Conversely, conduction in fur has been considered negligible as the cross-sectional area of hair represents approximately 0.01% of the skin's surface area [45]. Radiative heat transfer within bird and mammal coats is relatively small and represents around 4–10% of total heat flux due to efficient interception of radiation within the coat matrix [44, 46]. Free convection through plumage represents less than 15% of total heat transfer in still air but may be around twice this in fur because of its more open structure [44, 47]. In contrast, at high wind speeds, forced convection leads to the penetration of air inside the coat, decreasing coat conductance [18].
Wind penetration of the coat is influenced by wind direction relative to the coat axis, and there are large differences between species in the ability of wind to penetrate the coat [48]. Particularly wind-resistant plumages are found in penguins, where the thickened rachis (feather shaft) provides an effective barrier to wind, as well as providing hydrodynamic properties [36]. In Arctic mammals such as reindeer, stiff guard hairs and dense packing of hair elements prevents wind penetration [41]. In the rain, penetration of water into the coat also increases conduction or decreases insulation by displacing trapped air and mechanically disrupting the coat structure [49]. For diving animals, the pressure increase at depth compresses the coat's air layer and may allow water to penetrate and displace this air layer [27]. However, the coats of aquatic animals with stiffened rachis of contour feathers or primary guard hairs, combined with densely-packed coat elements, are especially efficient in reducing water penetration. This is further aided by the micro-structural topography of the coat and surface coatings of hydrophobic molecules that increase water shedding [50]. These lipids and waxes are produced by the uropygial (preen) gland and are coated onto feathers by preening. In mammals, lipids (sebum) secreted from the sebaceous gland at the base of the hair follicle provide the water-repellent coating [51]. Penguin body feathers also have air-infused micro- and nanoscale rough structures, giving hydrophobic and anti-adhesion properties that may prevent ice formation at the surface [52].
Diving animals such as otters show specialized morphology of the fur that reduces penetration by water. The cuticles of the thin under-hairs have sharply sculpted fins with deep grooves between them that provide an interlocking structure [53]. However, in contrast to the hydrophobic properties of many avian plumages, including most diving birds, the feathers of cormorants are partially wettable, allowing some reduction in buoyancy while retaining the insulative air layer. This is achieved by a loose outer section of the feather that is instantaneously wettable, with an inner section that remains dry. Microscopic examination indicates that this is due to regular and close interlocking feather elements that are resistant to water pressure [54].
Latent heat transfer is also an important heat transfer mechanism within animal coats. Water vapour transfer is influenced by feather or hair characteristics, particularly their hydrophilic properties, and by the humidity of air within the coat. Wool is highly hydrophilic, and in a sheep's fleece heat is released by condensation (equal to latent heat of vaporization) as water condenses on fibres and when water vapour is absorbed by the wool. Overall, these transient heating effects are trivial (typically less than 10 W m−2) because relative humidity changes slowly in the fleece and any increase in humidity near the skin is balanced by a decrease in humidity in the outer fleece [55]. Rain may increase relative humidity in the outer fleece, resulting in transient heat production, but prolonged rain will also increase heat loss by decreased insulation of the fleece and by evaporation.
3.4. Dynamic insulation
Animal insulation changes with time, responding to variation in the environment and longer-term seasonal climate patterns. Piloerection (mammals) and ptiloerection (birds) elevate hair or feather elements instantaneously to modify heat exchange. For example, contraction of muscles at the base of the hair raises the effective coat depth in horses by 16–32% or by a depth of 0.4–1.4 cm in new-born foals [56]. In birds, ptiloerection is controlled by muscles that result in fluffing or flattening of the plumage in response to air temperature or wind and may be responsible for increases in depth and insulation of 50% or more [57].
Species living in seasonal environments change their coat insulation in response to day length. Unlike the rapid change in insulation occurring over a few minutes with pilomotion, seasonal adjustments to insulation occur over a time scale of weeks to months. Many birds and mammals become obese before intense periods of fast (when migrating, breeding, or moulting) or in anticipation of the harsh season (combining food shortages and air temperatures far below body temperature). This will increase thermal inertia and will often be beneficial during the start of winter. For example, increases in fat stores have been attributed to increases in whole animal insulation in Arctic birds such as the rock ptarmigan [58]. As winter progresses, however, fat will be metabolised and any small benefit from this insulation will be lost. In contrast, the plumage will continue to provide effective insulation throughout this period.
Changes in coat insulation are controlled by the process of moult that brings about complete replacement of hair or feathers. This may occur as a gradual process over several months or relatively rapidly [33, 59, 60]. For example, Arctic foxes (Alopex lagopus), and Arctic hares (Lepus arcticus) undergo seasonal moults involving both colour change for camouflage and alterations to thickness and quality of insulation [61, 62]. The autumn moult replaces the thin summer coat with a thick, well-insulated coat that reduces heat loss [59]. Typically, changes to the density, diameter and/or length of hair result in higher insulation in the winter [63]. For example, in three species of sub-Arctic mammals, coat thermal resistance was greater in winter by as much as 27–87% compared to summer [64].
Similarly in birds, major differences in plumage thickness and insulation occur due to seasonal moult prior to the cold season. For example, the whole-body insulation of a small shorebird, the knot (Calidris canutus) was 35% lower in full breeding plumage compared to birds in winter plumage, due to a 37% reduction in feather mass [65]. A multi-species comparison of mostly passerine birds shows that larger species have fewer and heavier feathers, and the rate of feather loss is less in autumn and winter. During spring and summer the rate of feather loss remains constant, with a minimum number of feathers in summer prior to the start of moult [30].
3.5. Coat colour and heat transfer
In contrast to solid surfaces, animal coats are not simple surfaces with respect to radiative transfer. Coat reflectivity determines solar radiative gain, but how this contributes to overall heat transfer depends on how well radiation penetrates and is absorbed over a range of coat depths. These properties depend on the micro-optics and structure of the coat [66]. Light-coloured coats have a higher albedo, but solar radiation penetrates deeper into light coats as a result of forward radiative scattering. Animals with dark coats receive a greater heat load at the skin surface when wind speed is low, but at high wind speeds the heat load is less than for similar white-coated animals due to a decrease in conductance at the outer coat surface where radiation has not penetrated further [42, 67]. The polar bear remains white throughout the year but many Arctic species moult into cryptic white fur or feathers in winter. Any radiative advantages from a white coat are however offset by a combination of low winter solar radiation and thicker winter coats that prevent radiative penetration to the skin surface [42, 64]. There has been considerable interest in the radiative properties of polar bear fur, after it was reported that their fur has low UV reflectance. It was suggested that their hollow hairs facilitated internal reflection to their dark skin, thereby providing a thermal gain [68]. However, further evaluation of this model of fibre-optic transmission demonstrated that low UV reflectance could be attributed to the absorption of radiation by keratin that makes up hair [69]. Nevertheless, polar bear fur is extremely thick and has a high density of hairs that effectively intercept most radiation from the skin surface [70].
Colour change is common in animals for communication, camouflage, thermoregulation and protection from UV. In contrast to birds and mammals which moult at most twice a year, amphibians and reptiles have the ability to reversibly adjust skin colour over short periods of time, thanks to chromatophores within the integument. Morphological colour change over days or months occur due to changes in the amount of pigment and/or number of chromatophores, whereas rapid changes over seconds to hours occur by pigment migration within the chromatophores [71]. In some species of amphibians, colour change from dark to light is stimulated by increases in solar energy and anticipated changes in body temperature [72]. The ability to change colour is linked to the organisation of chromatophores in the dermis of the dorsal skin. Pale colouration is due to light-reflecting organelles (iridophores) comprising stacks of purine-containing reflecting platelets (a heterocyclic aromatic organic compound). However, the melanophores darken the skin by rearrangement of melanin in the cytoplasm [73]. Darkening melanosomes migrate from a basal position into cellular processes of the iridophores and obscure the reflective properties of the brightly coloured pigments they contain. When basking, however, melanosomes are found in the cell body below the iridiphores, allowing the light to be reflected by the crystals within the iridiphore and causing the skin to whiten. Recent studies on the bearded dragon lizard (Pogona vitticeps) showed that it exhibited an endogenous circadian rhythm in pigmentation that was entrained by light/dark cycle, decreasing reflectance in both the ultraviolet visible and near-infrared spectra (300–2100 nm) during the early part of the day to match solar input [71].
The complex structural and optical properties of avian and mammalian coats in comparison to skin surface (above) provides a counter-intuitive selective advantage to dark-coloured birds and mammals in hot environments where heat transfer may also be modified by wind. This, combined with a thick and dense covering of fur or feathers, leads to relatively small heat loads at the skin surface [42]. To provide an extreme example, the fur surface temperatures of cape fur seal (Arctocephalus pusillus) pups were recorded reaching as much as 79.6 °C, while skin and rectal temperature were only 42.1 °C and 36.1 °C, respectively [74].
Studies into the thermal significance of colouration have generally focused on dark or light colouration, perhaps neglecting the thermal properties of animals with distinctive markings or stripes.
The adaptive significance of stripes in zebras may originate from social cohesion, predation evasion, avoidance of biting flies and/or thermoregulation [75, 76]. Black and white stripes have even been predicted to enhance heat loss through the formation of convection eddies under sun exposure, but this has yet to be investigated.
4. Physiology
4.1. Thermo-conformers and thermo-regulators
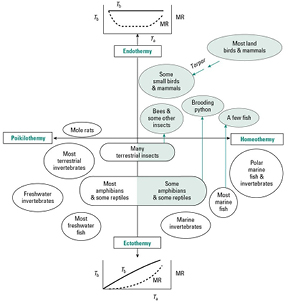
Vertebrate physiology can be broadly classified by stability in body temperature and source of body heat (figure 1). Ectotherms are thermo-conformers, i.e. their body temperature changes with environmental temperature. Ectotherm implies a reduced level of thermoregulation, as metabolism is driven by the thermal environment, whereby animals passively acquire their heat from the environment (e.g. by basking). It is only when they are warm enough that they can begin to undertake biological functions, such as foraging or mating. Actually, ectothermy is an ancestral state, and is still the prevailing thermoregulation system in invertebrates and the earliest evolved classes of vertebrates, including fish, amphibians and reptiles [77, 78].
Figure 1. Classification of body temperature regulation and source of body heat in animals. Graphs show the relationships between body temperature Tb and metabolic rate MR with air temperature Ta, for endo- and ectothermic animals. Copyright [7].
Download figure:
Standard image High-resolution imageEndotherms are thermo-regulators: they produce heat (through their metabolism) to maintain a high and relatively constant (homeothermic) body temperature. Normal core temperature ranges from 34–44 °C in birds, depending on species. Core temperature in mammals is usually maintained around 36–38 °C but can be as low as 30 °C in the monotremes (platypus and echidnas) and as high as 40 °C in some groups. However, many endotherms are regionally or temporally heterothermic, with variable endothermic heat production and body temperature [79]. The major benefit of endothermy is that it allows the uncoupling of internal thermal needs for maximal biological performance from external constraints, and particularly thermal constraints. It also permits permanent maintenance of high brain temperature for maximal cognitive performance (and therefore, integrated control of homeostasis) year-round.
One important challenge in applying animal models to the human environment is addressing the concept of 'thermal comfort', described as 'the condition of mind that expresses satisfaction with the thermal environment (ANSI/ASHRAE Standard 55)' [80]. This is based on the thermal aspects of a building that determine the physical and psychological perception of temperature and energy exchange, and which are influenced by metabolic rate, clothing insulation, air temperature, radiant temperature, air speed and relative humidity [81, 82]. For ectotherms, this could be equated with preferred body temperature, which is generally close to the 'optimal' temperature for many physiological processes, but this preferred temperature may vary for different activities. Thermo-preferences have been reported for endotherms, largely in the context of laboratory studies [83]. Often, 'thermal comfort' for endotherms is regarded as synonymous with 'thermal neutrality': the range of environmental temperatures over which metabolic rate and therefore heat production does not change [84]. An alternative approach is to derive a thermal index, the operative environmental temperature that combines microclimate (temperature, radiation, wind) with properties of an organism (size, shape, posture). This index indicates if the organism, given its current temperature, will gain or lose heat in that microenvironment [85].
4.2. Control theory applied to temperature regulation in animals
Control theory as used in engineering provides a framework for understanding temperature regulation in animals [86, 87]. On this basis, the regulated variable is body temperature, and temperature sensors throughout the body generate neural signals to a controller which is then compared to a reference signal or set-point (figure 2). The difference between body temperature and the set-point represents a load error which drives effector mechanisms for heat production or heat loss. Effector responses act in a direction opposite to the load error and therefore control it, typically by negative feedback. In this way, changes in heat production and heat loss are also proportional to the load error generated.
Figure 2. Animal thermoregulatory system according to control theory, showing regulated body temperature. Core body temperature is influenced by internal disturbances (e.g. waste heat from exercise or digestion) while external disturbances (e.g. cold or hot exposure) act primarily on the skin temperature (body shell). The actual temperature signal received by the controller is determined mainly from temperature sensation in the core (principally in the central nervous system) but also from skin temperature sensors. The difference between actual temperatures integrated throughout the body and the set-point generate a load error, driving effector mechanisms in the animal (skin blood flow, evaporation, behaviour, non-shivering thermogenesis (NST) in mammals and shivering) by negative feedback. These mechanisms control heat loss or heat production to maintain a stable core temperature. Redrawn from [87].
Download figure:
Standard image High-resolution imageEndotherms generally have a relatively stable internal or core temperature in comparison with variable skin temperature [88]. The regulated body temperature is therefore an internal temperature but it may also be an integrated temperature from different body regions. The function of the controller is to link afferent signals from temperature sensors with efferent drives to multiple effector mechanisms through the central nervous system (CNS). The preoptic anterior hypothalamus (POAH) of the brain appears to function as the main controller, in addition to some other CNS regions. Temperature sensors are not restricted to the CNS. They are found in the intestine, abdomen, skeletal muscles and trunk [87]. However, all thermoregulatory effector mechanisms may be activated or inhibited by skin temperature changes. Cold and warm thermoreceptors are found in the skin corresponding to the sensation of cooling or heating. These have different dynamic properties: cold receptors respond to rapid drops in skin temperature with a short-duration rise in activity, while warm receptors overshoot during warming and demonstrate transient inhibition while cooling quickly [86].
Sensation of temperature is dependent on both the absolute level of skin temperature and the extent and rate of change of temperature. For animals with fur and feather insulation, behavioural changes may be determined by the rate of change in skin temperature of bare skin areas. For skin temperature to activate or deactivate effector mechanisms, skin temperature must exceed an effector specific threshold. These thresholds are strongly defined by the extent of external insulation, such that for well-insulated animals a drop in skin temperature of 1 °C may initiate shivering, while for a bare-skinned animal such as a pig, shivering still does not occur even when absolute skin temperature is as low as 10 °C [89, 90]. Temperature changes of localised and small skin regions can activate effector mechanisms. For example, in humans, changes in face temperature produce a three-fold difference in sweating rate compared to a similar-sized region of the thigh [91]. However, small changes of body core temperature can produce large effector responses that are independent of skin temperature. For most free-living animals, core and skin temperature interact to influence effector mechanisms such as heat production, evaporative water loss and behavioural thermoregulation (figure 2).
4.3. Heat exchangers and heat sinks
Heat produced within the body is distributed by blood flow and through conduction from core to shell. In the cold, there is minimal flow of blood to the skin surface. Vertebrates have direct connections between arteries and veins controlled by valves (known as arteriovenous anastomoses, AVAs) that prevent blood from flowing to the fine blood vessels (capillaries) close to the skin surface [7]. In contrast, in the heat or when animals are active, peripheral blood vessels (arterioles) dilate, and AVAs allow blood to flow to the periphery. This system is particularly important in marine mammals, where the heat exchange shunt vessels are located beneath the blubber layer, allowing blood to bypass insulation when heat dissipation is required, or to restrict blood flow to the surface for heat conservation [28]. Heat distribution within the body is also facilitated by counter-current heat exchangers that are found in body extremities that are particularly well developed among cold-adapted species [92]. In this case, warm arterial blood runs parallel to venous returning blood to the core. This system may function together with vasomotor control, resulting in regional heterothermy of the body. Species such as antelope and deer have effective heat exchange systems to regulate brain temperature when excessive heat is produced during exercise [7]. However, more recent measurements on free-living animals have questioned the accepted view that brain cooling is a response to high body temperature, at least under moderate heat loads [93].
Vertebrates have the ability to precisely regulate skin surface temperature by vasoconstriction and dilation of peripheral blood vessels. However, certain regions of the body have particularly well-developed capillary networks, often on bare skin. These act as highly efficient 'thermal windows': when 'open' (vasodilated) they are used to dissipate excessive heat, and when 'closed' (vasoconstricted) they minimize heat loss. Their main function maximizes dissipation of excessive heat, e.g. the large external ears (pinnae) of elephants [94]. However, these are not necessarily confined to particular appendages but may also occur as isolated 'hot spots' across the body (e.g. in seals) that increase heat loss by convection or evaporation from a wet pelage [95]. Heat exchangers may potentially function to maximize solar heat gain. However, the extent to which 'thermal windows' are used by endotherms for heat gain is less well known than for ectotherms, where rewarming by basking is common [96].
Heat exchange occurs by passive latent heat loss through skin and by respiratory latent heat loss from lungs, which may be increased by panting in mammals or vibration of the throat (gular flutter) in birds. Evaporative water loss at high temperatures occurs from sweating or may be enhanced by the wetting of fur or feathers from bathing, etc. In cold climates, water can be replenished only by drinking cold water or ingesting snow, which costs energy along with the latent heat loss from the respiratory tract. For example, in reindeer, both latent heat and water losses are minimized by counter-current heat exchange in the nasal cavity [92]. The cavity contains an elaborate system of scrolled structures (conchae) which have a highly vascularized mucosal layer. Inhaled air becomes warmed and saturated en route to the lungs. This process cools the mucosa, and when air is returned from the lungs the warm humid air flows down a temperature gradient, allowing cooling and water condensation and resulting in the expiration of cold and dry air. This system is also seen in seals, with previous studies on grey seals (Halichoerus grypus) showing that more than 60% of heat and 80% of water added to inspired air is recovered at −20 °C, saving 10–30% of total water flux for the animal [97, 98].
4.4. Thermal flexibility: the best of endo and ectothermy
Most environments do not remain constant over time and therefore animals must cope with a range of conditions that vary across days, months and years. Thermal acclimation in an endotherm is a response to compensate for changes in resource availability and the thermal environment. Many of these may be gradual and involve daily, monthly or annual adjustments to behaviour, morphology, physiology or biochemistry [7].
Endotherms respond to changes in the thermal environment in multiple ways, involving initial behavioural (e.g. posture, activity or migration) and physical adjustments to alter their energy balance (insulation and energy storage). However, many vertebrates also respond to seasonal changes in climate or food availability by decreasing their metabolic rate (energy use) and down-regulating their body temperature. Metabolic rate and body temperature are controlled by endogenous signals entrained to the photoperiod (day length) and fine-tuned according to climatic conditions [99, 100]. Most endotherms show daily changes in body temperature, with diurnally active species generally having a lower body temperature at night and nocturnal species having a lower body temperature during the day.
There are generally four recognisable temperature control patterns in endotherms with progressively greater decreases in body temperature between activity and rest: strict homeothermy, daily heterothermy, daily torpor, and hibernation. Many species, including humans, show small decreases in body temperature related to rest/sleep-activity patterns. This confers only small energy savings when animals are inactive. However, some species use shallow heterothermia to cope with cold winter conditions. Large endotherms use hypometabolism as a strategy over the winter, and peripheral cooling may be the mechanism by which the down regulation of the resting metabolic rate occurs. For example, in red deer (Cervus elaphus), heart rate (as a measure of metabolic rate) has been shown to be endogenously lowered in winter. Rumen temperature (as a measure of core temperature) on average decreased by 0.5 °C in winter and was correlated with seasonal and periodic variation in heart rate, supporting the hypothesis that lowered body temperature controlled by peripheral cooling is also an important mechanism by which large mammals achieve hypometabolism during food shortage [101]. It appears that a reduction in BMR and the set-point of core body temperature are both required in order to achieve an overall decrease in thermoregulatory costs in cold temperatures [99].
Pronounced patterns of heterothermy in endotherms are characterized traditionally as two different types of hypometabolic states associated with low body temperature: daily torpor lasting less than 24 h and followed by foraging activity, and hibernation with periods of torpor occurring over consecutive days to several weeks, where animals do not usually forage but rely on food caches or body energy stores. Heterothermic birds and mammals are small (<1 kg; apart from bears) and hibernators are larger and found at higher latitudes (or altitudes) compared to most daily heterotherms. Basically, it seems that heterotherms are endotherms that evolved in environments where energetic constraints are too strong to be compensated by usual adaptations; they are too small to store internally a sufficient amount of energy, and their small size (high surface/volume ratio) means that they passively lose considerable amounts of heat. Heterothermic bouts are on average more than 25 times longer, and minimum body temperatures ~ 13 °C lower in hibernators [102]. In energy saving terms, the minimum torpor metabolic rate of ~ 35% of BMR in daily heterotherms compares with only 6% of BMR in hibernators, indicating the much greater energetic efficiency of hibernation over daily torpor. Daily heterotherms use a circadian system to control the timing of torpor, which allows foraging to continue following torpor bouts. In contrast, hibernators are 'uncoupled' from circadian control so as to facilitate long-duration periods of low metabolism; but they maintain a circa-annual clock that is necessary in order to time the termination of the hibernation period, and emergence from the winter burrow (or den), in the absence of direct cues about exterior environmental conditions. Interestingly, in the transition between summer and winter, hibernators have a period when they are daily heterotherms: they use torpor facultatively, flexibly, on a daily basis, depending on current needs and environmental constraints. It also seems that there is a need for a period of acclimation of the organism, when torpor is progressively used for longer periods of time, and increasingly lower body temperature [103].
Hibernation is an obvious seasonal adaptation of small mammals and birds to cold (or dry) climates, with predictable, long periods of low temperatures and/or drought. It is characterized by prolonged periods of inactivity and down-regulation of metabolism and body temperature. Therefore, suitable animal models for seasonal cold temperate climates may be better found amongst species that remain active during winter and are able to maintain energy use and control body temperature within set limits. Studies of species that are adapted to cold winter conditions may reveal obvious annual patterns in body temperature and energy use that provide a basis for seasonal adaptive control of temperature and energy use in buildings. New technology that records body temperature and energy expenditure has provided an annual picture of the dynamics of thermoregulation [79]. In cold environments, small diurnally active birds exhibiting rest-phase hypothermia during winter indicate an energy-saving strategy for animals that are inactive at night [104]. Nocturnal body temperature reaches a minimum in midwinter corresponding to the reduced period of daylight. However, body temperature also varies according to air temperature, suggesting that environmental stochasticity fine-tunes energy use. Daily heterothermia and torpor are physiological mechanisms that provide considerable energy savings for animals in cold climates. This combination of thermal adaptations may therefore be a useful model to explore energy savings for cold seasonal climates.
Applicable animal models that may provide insights into thermal aspects of building design for warm climates may come from large desert-dwelling mammals that tolerate extreme heat during the day and cool conditions at night. The key adaptive feature of these species is a thermoregulatory system based on dry (non-evaporative) heat exchange facilitated by daily heterothermia. For example, in the Arabian camel (Camelus dromedaries) during dehydration and heat stress, the amplitude of the daily body temperature rhythm in rectal temperature exceeded 6 °C [6]. More recent studies have shown that after a prolonged period of dehydration, when exposed to air temperatures of 38–46 °C in the day and 20–25 °C at night, the amplitude of the core body temperature rhythm averaged 3.8 °C (average minimum 35.4 °C in the morning and maximum 39.2 °C in the evening) [105]. By allowing body temperature to rise during the day, the gradient in temperature between body and environment is minimized, reducing thermal gain. The 'stored' heat offsets nocturnal heat loss, resulting in a minimum body temperature in the early morning. Camels also show morphological adaptations to cope with high solar gains. They possess thick fur on their dorsal surfaces and most adipose stores are distributed, particularly in the dorsal hump [24]. Fur is less thick on flanks and legs, which may enhance heat loss from these regions. The overall body shape may also be an adaption that minimizes solar gain. Small nocturnal heterotherms living in the tropics may also be useful models for understanding how patterns of heterothermia result in reduced energy costs during the dry season. There is considerable variation in the patterns of heterothermia between and within species. For example, in grey mouse lemurs (Microcebus murinus), energy savings (decreased metabolic rate) resulting from torpor are proportional to torpor depth (minimum temperature) and torpor duration. These savings increase with decreasing air temperature, indicating that these facultative thermoconformers can save more energy in colder conditions [106].
5. Behaviour
5.1. Behavioural thermoregulation
Behavioural and ecological factors allow endotherms to adapt to different climates, and these factors are actually more important than an animal's metabolic characteristics [107]. For species that are unable to avoid unfavourable thermal conditions by migration, there is a great diversity of behavioural adaptations for thermoregulation that reduce energy costs (see detailed review in [83]). These may be best described broadly as 'individual' or 'group' strategies. Individually, animals may change their posture to reduce their surface to volume ratio, and increase their effective insulative layer by adopting hunched or 'ball-like' postures such as those seen in small birds and rodents. Similarly, animals may bask to allow passive warming, or change posture or position to facilitate convective cooling from wind, or seek water to cool by evaporation. Group behaviours such as huddling and/or nest-sharing are principally used to reduce heat loss and decrease individual costs of heat production [108]. These behaviours function by reducing the surface area exposed to the environment, modifying the microclimate and sharing metabolic heat. Examples are common amongst species adapted to cold. The huddling behaviour of emperor penguins during the Antarctic winter is particularly effective in providing shelter from extreme winds, modifying the microclimate of the huddle and minimising exposed surface area. In contrast, huddling is highly beneficial for young birds and mammals before insulation has developed, allowing them to minimize surface area and increase the temperature of the sheltered and well insulated nest environment.
5.2. Nest building
Animals have the ability to construct structures that enhance the thermal and ventilation properties of their nests. The most well-known nests in bioinspiration studies are termite mounds, which appear to promote cooling and gas exchange by some combination of enhanced convection due to heat derived from metabolism (termites and fungal colonies) and ventilation driven by vertical wind speed profiles (Bernoulli's principle) [109]. Magnetic or compass termites (Amitermes meridionalis) endemic to northern Australia build wedge-shaped mounds many metres in height. They are composed of a hard outer shell (with connections between chambers) that is aligned with the north–south axis. This has been interpreted to provide a thermal function, allowing the early morning sun to warm the nest while its narrow width minimizes solar gain when the sun is overhead. Such explanations have however been questioned, and there may be multiple environmental factors influencing mound shape and structure [110–112]. Indeed, recent measurements of air flow within mounds of the species (Odontotermes obesus) in south Asia show that 'a simple combination of geometry, heterogeneous thermal mass and porosity allows the mounds to use diurnal temperature oscillations for ventilation' [112]. The outer structure of the mound containing 'flutelike conduits' rapidly heats up during the day relative to the internal 'chimneys', forcing air to move down the chimneys in a closed convection cell. The direction of flow is reversed at night as the exterior of the mound cools, and these cyclic flows allow CO2 to be flushed out and air to be replenished to the termite colony. Construction is also widespread amongst many ectotherms to maintain humidity within nests and cocoons. Silk produced by the hornet (Vespa orientalis) and several similar species also has thermoelectric properties [113]. Measurements taken on strips of hornet silk showed rises in electric charge when temperature increased from 20 °C to 33 °C and at more than 90% relative humidity [114]. In the warm daytime hours, hornet silk transports water, raising relative humidity, thus providing the evaporation needed for nest thermoregulation, and at night electric current and heat flows along the silk.
Amongst vertebrates, a variety of nests and dens are constructed underground to avoid either excessive heat or cold (as well as to provide a hiding-place from predators). These provide favourable microclimates and are insulated by collected material [115] or from snow [62, 116]. Maximising insulation has the potential to compromise ventilation. The paired and elevated entrances to burrows constructed by prairie dogs (Cynomys sp.) in arid zones of North America facilitate wind-induced ventilation [117]. Measurement of air flow and temperature within the burrow systems of small rodents shows that even at low wind speeds, the unpredictable penetration of eddies into a burrow through its openings can maintain CO2 concentration within physiologically appropriate limits [118].
Avian nest construction is highly specialized, providing enhanced insulation in cold environments and protection from wind and rain. Successful nests are often associated with the choice of suitable microclimates with regard to orientation to the sun and shelter from wind and precipitation [119]. Nesting materials vary but feather down provides the best insulation while some grasses are relatively poor insulators [120]. In general, the insulation properties of nests are poorer in warmer low latitudes and this may involve changes in morphology (wall thickness) and composition (dry grasses) [121]. Many nests incorporate a variety of materials including mud, plant stems and branches to provide additional structural support. However, there are direct thermal benefits from the choice of nest material. For example, in Australia, the malleefowl (Leipoa ocellata) constructs a large nest mound containing buried plant material that maintains incubation temperature from the heat of decomposing vegetation [122]. Many birds also occupy man-made structures, including customised nest boxes or buildings that provide favourable microclimates for nesting or roosting [123].
6. Conclusion: translating animal models to buildings
Translation from biology to engineering may be aided by modelling that incorporates aspects of animal insulation, physiology and behaviour into heat transfer models for buildings (see suggestions of principles to be transferred in table 1). Concentrating on function rather than biological characteristics per se may be most effective for biomimetics [124]. Suitable models for seasonal cold temperate climates may be based on groups of organisms that remain active during winter and are able to control body temperature within set limits. These would incorporate temporal changes in insulation and body temperature that provide a basis for seasonal adaptive control of temperature and energy use. Similarly, a range of strategies used by animals to manage solar heat gain through surface reflectivity and insulation, combined with daily temperature changes driven by thermal inertia, may provide solutions for buildings in hot climates. A number of incremental modelling steps could involve the following:
- (1)Building a heat transfer model: the aim of the first modelling stage would be to develop a transient heat transfer model of a simple building type that is heated internally and exchanges heat with the environment. The characteristics of the building would be based on typical specifications for resistances and capacitances of materials and 24 h simulation of solar radiation and environmental temperature. At this stage, the model could include temperature control features by incorporating daily requirements for the building's temperature analogous to the circadian rhythms of body temperature in animals. Outputs from such a model would include the internal temperature of the building and the total energy required to heat it within set thermal comfort limits.
- (2)Simulating animal features: this modelling step would begin to incorporate a number of features considered to be analogous to animal thermoregulation. These could include, for example: (i) 'insulated core': an insulated zone within the building; (ii) 'spatial heterothermy': regional differences in temperature and heating between zones; (iii) 'core to shell blood flow': transfer of heat between zones depending on temperature within a zone, and; (iv) 'pilomotion/ventilation': to represent the bypass of an animal's external insulation to exchange heat rapidly with the exterior. At this stage the model would incorporate bioinspired features of insulation and physiological control of blood circulation and heat distribution. Each of these adaptations could be simulated to examine their individual or combined contribution to energy use and thermal comfort.
- (3)Incorporating annual and geographical variation in climate: the models could be tested over a year using environmental data recorded in different geographical regions for the building type. At this stage it may be appropriate to incorporate behavioural aspects such as basking and shade-seeking to change solar gain based on thermal comfort within the building.
- (4)Sensitivity analysis: this step would test the sensitivity of model outputs (temperature, thermal comfort index and energy use) to changes in inertia, thermal resistances, and other thermal characteristics of the given building type.
- (5)Design: The final stage of the exercise would be to determine how the most effective biomimetic features could be realised in terms of choice of structural materials or incorporation of control systems for the heat management of the given building.
In summary, thermal adaptations that allow animals to cope with hot or cold climates are characterized by: (i) structurally complex insulation that dynamically responds to changes in environmental conditions; (ii) sophisticated neural control and sensing systems; (iii) dynamic control of blood flow and circulation; (iv) flexible patterns of temperature control and heat production, and; (v) highly responsive patterns of behaviour to avoid extra energy costs. Rather than seeking a particular species that best fits the building design, the alternative approach for bioinspiration would be to bring together the most efficient combination of biomaterials, physiological and behavioural mechanisms to explore optimal models for temperature control and energy management.
Acknowledgments
We thank Caroline Gilbert (École Nationale Vétérinaire, d'Alfort, UMR 7179 MECADEV) and Jeremy Terrien (MNHN, UMR 7179 MECADEV) for useful discussions on thermoregulation, and Mike Hansell (University of Glasgow) for sharing expertise on animal architecture. We are grateful to SGI (Rantigny) and MECADEV (CNRS.MNHN) staff for supporting interdisciplinary cooperative work on this project. The work was funded by SGI/MNHN research contract #573-15.