Abstract
Objective. To study electrical stimulation of the lacrimal gland and afferent nerves for enhanced tear secretion, as a potential treatment for dry eye disease. We investigate the response pathways and electrical parameters to safely maximize tear secretion. Approach. We evaluated the tear response to electrical stimulation of the lacrimal gland and afferent nerves in isofluorane-anesthetized rabbits. In acute studies, electrical stimulation was performed using bipolar platinum foil electrodes, implanted beneath the inferior lacrimal gland, and a monopolar electrode placed near the afferent ethmoid nerve. Wireless microstimulators with bipolar electrodes were implanted beneath the lacrimal gland for chronic studies. To identify the response pathways, we applied various pharmacological inhibitors. To optimize the stimulus, we measured tear secretion rate (Schirmer test) as a function of pulse amplitude (1.5–12 mA), duration (0.1–1 ms) and repetition rate (10–100 Hz). Main results. Stimulation of the lacrimal gland increased tear secretion by engaging efferent parasympathetic nerves. Tearing increased with stimulation amplitude, pulse duration and repetition rate, up to 70 Hz. Stimulation with 3 mA, 500 μs pulses at 70 Hz provided a 4.5 mm (125%) increase in Schirmer score. Modulating duty cycle further increased tearing up to 57%, compared to continuous stimulation in chronically implanted animals (36%). Ethmoid (afferent) nerve stimulation increased tearing similar to gland stimulation (3.6 mm) via a reflex pathway. In animals with chronically implanted stimulators, a nearly 6 mm increase (57%) was achieved with 12-fold less charge density per pulse (0.06–0.3 μC mm−2 with 170–680 μs pulses) than the damage threshold (3.5 μC mm−2 with 1 ms pulses). Significance. Electrical stimulation of the lacrimal gland or afferent nerves may be used as a treatment for dry eye disease. Clinical trials should validate this approach in patients with aqueous tear deficiency, and further optimize electrical parameters for maximum clinical efficacy.
Export citation and abstract BibTeX RIS
Introduction
In the United States alone, moderate and severe dry eye disease afflicts nearly 5 million individuals over age 65, and many more suffer from less-severe cases [1, 2]. Dry eye symptoms range from mild itchy irritation to severe eye pain. Symptoms arise due to an unstable tear film (aqueous, lipid, or mucus layers), originating from insufficient secretion of the lacrimal gland fluid or meibomian gland dysfunction, which prevents rapid evaporation of the tear film [3]. Tear production decreases following damage to any component of the neural feedback loop controlling the tear release from the lacrimal glands [4]. Damage to sensory fibers on the cornea reduces tear secretion, which can lead to inflammation and greater damage to the sensory fibers, thereby crippling the neural feedback circuit [5, 6].
Current treatments to increase tear volume at the eye surface include (1) artificial ointments and eye drops, which provide only short-term relief [7]; (2) punctal plugs, which reduce the tear drainage into the nasal cavity and may improve symptoms, but which also prevent clearance of the inflammatory proteins responsible for corneal desensitization [7, 8]; (3) tarsorrhaphy, a surgical procedure to restrict eyelid opening which also reduces the visual field of view; (4) cyclosporine, an anti-inflammatory drug that provides mild improvement in symptoms, but is plagued with irritating side effects [9, 10]; and (5) heat therapy, to clear blocked meibomian gland secretions [11, 12].
Early electrophysiological studies, aimed at identifying the nerves responsible for lacrimation, discovered enhanced tear secretion upon electrical stimulation of the efferent facial nerve [13–15], superior cervical ganglion neurons [16, 17], and afferent branches of the trigeminal nerve [14]. Acinar cell voltage–gated ion channels play a key role in tear secretion and are also electrically excitable [18]. Recently, Kossler et al [19] introduced electrical stimulation of the lacrimal nerve and gland to increase tear volume and treat dry eye disease.
We explore two approaches: (a) stimulation of the lacrimal gland, which may activate acinar cells directly, or the efferent neurons controlling tear secretion; and (b) stimulation of the sensory (afferent) nerves to activate a reflex tearing response. Using electrical stimulation in rabbits, we aim to (1) reveal the stimulation pathways, including any cells and nerves involved; (2) identify electrical parameters for maximum tear secretion; (3) determine acute tissue damage thresholds; and (4) show stimulation efficacy using a chronically implanted stimulator.
Methods
Acute experiments
Electrode fabrication
Bipolar electrodes were made of platinum foil (ESPI Metals, Ashland, OR) connected on one side to copper wire (CZ 1103, Cooner Wire, Chatsworth, CA) with medical grade conductive epoxy (H20E, Epoxy Technology, Billarica, MA). After embedding the platinum squares 10 mm apart in silicone rubber (Dragon Skin 20, Smooth-On, Easton, PA), 3 mm wide circular sections of silicone were removed above the platinum surface to create a pair of 3 mm disk electrodes. To stimulate the ethmoid nerve, a bared 36 gauge platinum wire (AS 770-36, Cooner Wire, Chatsworth, CA) was wrapped and tied at the end of a 1 mm (outer diameter) Teflon tube.
Surgery
All experimental procedures with animals were conducted in accordance with the Stanford University institutional guidelines and conformed to the guidelines of the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision research. New Zealand white rabbits (3.5–5 kg, Western Oregon Rabbit Co., Philomath, OR) were anaesthetized with ketamine (35 mg kg−1, IM) and xylazine (3 mg kg−1, IM). Buprenorphine (0.05 mg kg−1, IM) was administered for pain control. After placing a breathing tube, isofluorane (1–2.5%) was administered for the duration of the experiment. The animals' blood pressure, heart rate and temperature were monitored. Both eyes were surgically implanted with electrodes placed either under the lacrimal gland or near the ethmoid nerve. Seven animals were used for the stimulation optimization experiments and twelve for the neuro-blocking study.
Lacrimal gland electrode placement surgery proceeded as follows: (a) a vertical incision was made inferior to the medial canthus; (b) blunt dissection extended to the inferior orbital rim until the extraorbital portion of the inferior lacrimal gland was visualized; (c) blunt dissection continued over the orbital rim and along the anterior orbital floor to open a small pocket between the gland and the orbital floor; (d) the electrode was placed beneath the gland, within the orbit; and (e) the incision was closed with sutures. Figure 1(a) shows the electrode locations relative to the inferior gland within the orbit.
Figure 1. Electrode placement in acute stimulation experiments. (a) The inferior lacrimal gland rests beneath the globe, behind the orbital rim. A bipolar electrode was implanted within the orbit, adjacent to the lacrimal gland. (b) The ethmoid nerve courses deep within the orbit, exiting posterior and nasal to the globe. A monopolar electrode was inserted through the caudal supraorbital incisure and advanced, behind the globe, toward the ethmoid nerve foramen. Blue color corresponds to insulation.
Download figure:
Standard image High-resolution imageEthmoid nerve monopolar electrode stimulation surgery proceeded as follows: (a) an incision was made above the caudal supraorbital incisure; (b) the electrode was guided through the incisure and across the orbit, using a 30 gauge needle placed within the Teflon tube; (c) the electrode was advanced until it reached the rostral end of the frontal bone in the orbit, near the ethmoid foramen (figure 1(b)).
Stimulation and measurements
Baseline and stimulated tear secretion was measured using topical anesthesia of the eye (Schirmer test). Two minutes after administering proparacaine (0.5%, Bausch & Lomb), the tear lake in the inferior conjunctival sac was dried for 4 min using strips of filter paper. The Schirmer test (Eye Care and Cure Corp., Tucson, AZ) was performed immediately thereafter.
Due to depressed tearing with isofluorane anesthesia, we cut the Schirmer strips to 3 mm width to increase the sensitivity of the measurements. The 3 mm scores were then converted to the traditional 5 mm Schirmer score using the best-fit linear regressions of data obtained by pipetting saline at various rates onto 3 and 5 mm Schirmer strips in vitro.
During the 5 min Schirmer tests, a custom-made current source delivered bi-phasic, cathodic-first, symmetric square pulses of current with amplitude up to 12 mA, durations of 0.1–1.0 ms, and frequencies of 10–100 Hz.
Neuro-blocking experiment
Neuro-blocking drugs included: prazosin HCl (2 mg kg−1, half dose both IV and SC; Sigma Aldrich); scopolamine (1.5 mg kg−1, SC; Sigma Aldrich); and hexamethonium bromide (10 mg kg−1, IV; Sigma Aldrich). Figure 2 details the role of each neural blocker: scopolamine blocks the efferent parasympathetic pathway; prazosin blocks the α1 efferent sympathetic pathway; and hexamethonium inhibits the nicotinic receptors in the ganglia, preventing efferent signals from the brainstem (i.e. the reflex tear pathway) from reaching the gland.
Figure 2. Schematic of neural pathways. Sympathetic and parasympathetic fibers innervate the lacrimal gland, signaling to receptors on the basal membrane of the acinar cells. Scopolamine blocks the efferent parasympathetic fibers. Prazosin inhibits sympathetic efferent fibers. Hexamethonium blocks synapses in ganglia and prevents afferent nerve inputs from reaching the acinar cells and generating tears.
Download figure:
Standard image High-resolution imageSchirmer tests with and without electrical stimulation began 20 min following drug delivery, when the heart rate was slowed with prazosin, and stimulated tearing decreased with scopolamine. The effects of hexamethonium occurred more quickly (within 10 min) due to only intravenous delivery. Tearing returned to normal in about an hour following scopolamine injection. To validate the ganglionic block from hexamethonium, we stimulated the cornea (6 mA, 500 μs, 30 Hz for 10 s) using two 0.16 cm2 platinum foil electrodes, and the ethmoid nerve (2–3 mA, 200 μs, 30 Hz for 5 min) using the monopolar electrode. Topical anesthesia (proparacaine) was omitted prior to corneal stimulation to ensure afferent nerve sensation. The second dose of hexamethonium was given when the corneal tear response recovered (approximately 45 min) and measurements continued. Administration of prazosin and hexamethonium reduced mean arterial blood pressure by about 10 and 7 mmHg, respectively.
At the conclusion of each experiment, the animals were euthanized through intravenous delivery of Beuthanasia-D (0.22 ml kg−1).
Chronic implantation experiments
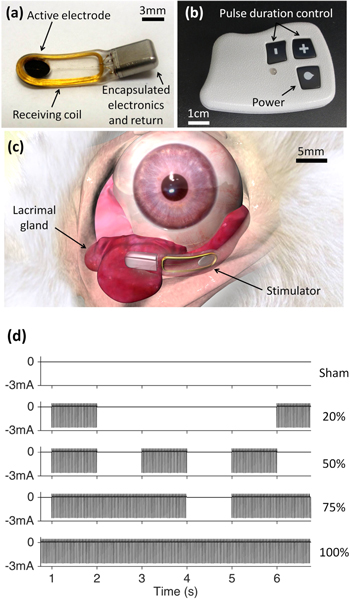
Using the same surgical procedure as the acute implantation, five rabbits were implanted with a lacrimal gland stimulator (Oculeve, South San Francisco, CA) in one eye (OD), as shown in figure 3. Animals were given buprenorphine (0.05 mg kg−1, IM) during recovery, housed in a temperature and humidity controlled environment, and given food and water ad libitum.
Figure 3. Chronic stimulator and placement. (a) Fully-implantable stimulator with a 3 mm diameter active electrode. (b) Wireless power transmitter with pulse duration control. (c) Stimulator within the orbit, adjacent to the inferior lacrimal gland. (d) Bi-phasic, cathodic-first charge-balanced asymmetric pulses of current with amplitude of about 2.8 mA, 170–680 μs duration repeated at 30 Hz were modulated with duty cycles of 0 (sham), 20, 50, 75 and 100% (continuous).
Download figure:
Standard image High-resolution imageOne week later, the stimulator pulses were set to the maximum duration with no observed distress (i.e. not pulling away sharply during initial stimulus or grinding teeth): 170 μs (3 animals), 500 μs (1 animal) and 680 μs (1 animal). The transmitter (figure 3(b)), placed gently against the skin, wirelessly powered the implant to deliver bi-phasic, cathodic-first, charge-balanced, asymmetric pulses of ∼2.8 mA at 30 Hz for 5 min.
Schirmer tests with topical anesthesia were performed with 5 mm wide filter paper strips (Eagle Vision, Memphis, TN). Rabbits were placed in a restraint bag for Schirmer tests (J170, Jorgensen Laboratories, Loveland, CO). No more than two tests, at least 15 min apart, were performed per eye per day. Schirmer scores from continuous, 75%, 50% and 20% duty cycle waveforms (figure 3(d)) were compared to the sham (no stimulation but with a transmitter next to the animal) and baseline (no transmitter). In a period ranging between 4 and 7 months, the rabbits were euthanized under full anesthesia by an intravenous injection (ear vein) of Beuthanasia-D (0.22 ml kg−1).
Ex vivo assessment of tissue damage
Lacrimal glands harvested from fresh rabbit carcasses were immersed in Ames' medium (Sigma Aldrich) at room temperature. The glands were carefully dissected to 5 mm × 5 mm pieces and stimulated via a saline-filled pipette electrode (2 mm diameter aperture created using a 1 ml tuberculin syringe (Becton Dickenson, Franklin Lakes, NJ)). Each sample was stimulated for 5 min with 1 ms bi-phasic, cathodic-first, symmetric pulses at 30 Hz and amplitudes of 0 (control), 3, 6, 9, 12, 18, and 30 mA, using a custom built stimulator. Dead cells were detected using a fluorescent live-dead viability/cytotoxicity kit (Biotium, Hayward, CA). Images were captured before and after stimulation using an inverted fluorescence microscope (Nikon TE300). Increase in the total area (number of pixels) stained dead between the pre- and post-stimulation images were measured with ImageJ (NIH, Bethesda, MD). For probit analysis (MATLAB), the criterion of damage was a stained dead area at least two standard deviations above the control average.
Statistical analysis
Schirmer scores are presented as an increase in mm above the baseline value. In acute experiments, the tearing response varied greatly between both eyes in the same animal and target (either gland or nerve), so each eye was considered independent. Animals with chronically implanted devices received an implant in only one eye and each animal was considered independent. Error bars represent standard error of the mean (SEM). Statistical significance was determined using the two-tailed paired (or unpaired, when appropriate) Student's t-test.
Results
Stimulation pathway and neural blocker experiments
To determine the neural pathway(s) involved in enhanced tear secretion during lacrimal gland stimulation, we delivered scopolamine (efferent parasympathetic inhibitor), prazosin (efferent α1 sympathetic inhibitor) and hexamethonium (ganglionic inhibitor). Unlike prazosin or hexamethonium, scopolamine blocked the response to gland stimulation, which indicates that the efferent parasympathetic pathway facilitated the tear response, not the sympathetic pathway, a reflex (afferent) pathway or direct stimulation of the acinar cells (figure 4(a)). In anesthetized animals, gland stimulation did not produce afferent (reflex) tearing, as evidenced by the lack of change in the tear volume (∼4 mm) after the reflex pathway was inhibited with hexamethonium. On the other hand, reflex response to the afferent nerve stimulation (cornea and ethmoid nerve) was completely blocked by the application of hexamethonium, as shown in figure 4(b). Corneal stimulation induced a stronger response (11 mm) than the ethmoid nerve stimulation (3.8 mm), but both responses vanished upon application of hexamethonium.
Figure 4. Effects of neural blockers on stimulated tear volume. (a) Tear volume above baseline for gland stimulation (6 mA, 500 μs pulses repeated at 30 Hz for 5 min) with scopolamine (1.5 mg kg−1, n = 5), prazosin (2.0 mg kg−1, n = 5), hexamethonium (10 mg kg−1, n = 7) and no blockers (control, n = 10). Scopolamine blocked the efferent parasympathetic pathway. Hexamethonium did not affect tearing; suggesting afferent nerves were not involved. (b) Tear increase over baseline for afferent nerve stimulation with hexamethonium (10 mg kg−1) and without (control). Hexamethonium blocked tearing from corneal (6 mA, 500 μs pulses at 30 Hz for 10 s, n = 6) and ethmoid nerve (2–3 mA, 200 μs pulses at 30 Hz Hz for 5 min, n = 5) stimulations: *p ≤ 0.01, compared to control.
Download figure:
Standard image High-resolution imageOptimal electrical parameters for secreted tear volume
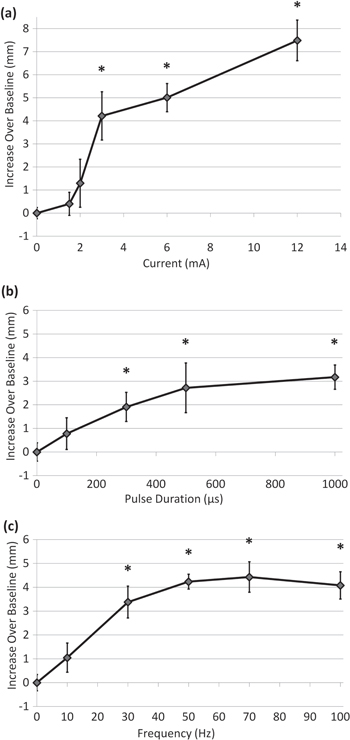
Figure 5 shows how each parameter of the electrical waveform (pulse amplitude, duration and repetition frequency) impacts tear secretion. Tearing increased with stimulation amplitude: at 3 mA the increase over baseline was 4.2 mm (210%), which grew to 7.5 mm (375%) at 12 mA (figure 5(a)). Tear secretion also increased with increasing pulse duration, as shown in figure 5(b) for 3 mA current. Pulses of 300 μs and above provided statistically significant improvement in tear volume, but pulse durations above 500 μs provided little added benefit (<1 mm increase above the 500 μs response) (figure 5(b)). Similarly, tear response increased with pulse repetition rate, and all frequencies in the 30–100 Hz range provided at least a 3 mm improvement over baseline (figure 5(c)). Increase in frequency beyond 30 Hz provided minor additional benefit. Tearing returned to the baseline level (2 mm) within 5 min (the minimum time needed to set up a second Schirmer test). Response to stimulation varied between groups of animals. For example, with 3 mA, 500 μs, 30 Hz, tearing increased in different groups by 4.2 mm (figure 5(a)), 2.7 mm (figure 5(b)) and 3.4 mm (figure 5(c)). In summary, an approximately 3 mm increase in tear secretion required 3 mA, 500 μs pulses at rates of 30 Hz, and it could reach as much as 8 mm above the baseline at higher settings.
Figure 5. Tear production from gland stimulation with various waveform parameters. (a) Tear volume increases with stimulation amplitude for 500 μs pulses applied at 30 Hz (*p ≤ 0.01 compared to control; n ≥ 6, except 12 mA where n = 3). (b) For constant amplitude and frequency (3 mA pulses at 30 Hz) tear volume increases with pulse duration (*p ≤0.05 compared to control, n = 6). (c) Tear secretion increases with pulse repetition frequency up to 70 Hz (3 mA, 500 μs) (*p ≤ 0.01 compared to control, n = 5).
Download figure:
Standard image High-resolution imageTear secretion with chronically implanted stimulator
Chronically implanted stimulators allowed measurement of the tear response to stimulation without the effect of anesthesia. Anesthesia reduced the baseline Schirmer score from 10 to 2 mm, and probably impacted reflex tear production from gland stimulation. In non-anesthetized animals continuous (non-modulated) stimulation in a wide range of pulse durations (170–680 μs) enhanced tearing as much as gland stimulation (500 μs) in anesthetized animals (∼4 mm).
Modulating the duty cycle, a ratio of the stimulation time to the total time period (the sum of the 'on' and 'off' times; see figure 3(d)), improved the tear secretion compared to continuous 5 min long stimulations (figure 6). With a 50% duty cycle (1 s of stimulation repeated every 2 s) tear secretion peaked at nearly 6 mm above the baseline (57% increase), while with continuous stimulation (100% duty cycle), the enhancement was only 3.7 mm (37% increase), compared to baseline (10 mm), similar to the 20% duty cycle (3.5 mm, 35% increase).
Figure 6. Tear secretion as a function of duty cycle with chronically implanted stimulator. Rabbits received baseline (no transmitter), sham (transmitter held in place but no stimulation) and electrical stimulation (∼2.8 mA, 170–680 μs pulses at 30 Hz) treatments. Duty cycle of 20% was implemented by 1 s of stimulation every 5 s, 50% by 1 s of stimulation every 2 s and 75% by 3 s of stimulation every 4 s. *p ≤ 0.05, compared to sham, and p < 0.01, compared to baseline, (n = 5).
Download figure:
Standard image High-resolution imageEx vivo assessment of tissue damage
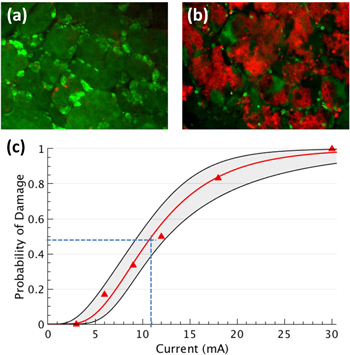
We observed no damage in lacrimal glands stimulated ex vivo with currents of 3 mA, 1 ms per phase (0.9 μC mm−2 per pulse), and up to 9000 pulses (figure 7(a)). With 6 mA current and 1 ms pulses (1.9 μC mm−2 per pulse), 16% of the samples exhibited some damage to the acinar cells. Pulses of 30 mA with 1 ms duration (9.5 μC mm−2 per pulse) always damaged gland tissue (figure 7(b)). The threshold for acute lacrimal gland damage, defined as 50% probability of damage, corresponded to 11 mA with 1 ms pulses (3.5 μC mm−2 per pulse), as can be seen in figure 7(c).
Figure 7. Assessment of acute tissue damage. Probability of damage in excised pieces of lacrimal gland treated for 5 min with pulses of 1 ms duration at 30 Hz (2 mm diameter pipette electrode). (a) and (b) Show tissue stimulated with 3 and 30 mA, respectively. The color red marks cells with damaged cell membrane. (c) Probit analysis generated the best-fit curve and confidence intervals (68%, n = 6). Dashed lines indicate the threshold for acute lacrimal gland damage (50% probability of damage) to be 11 mA (3.5 μC mm−2 per pulse).
Download figure:
Standard image High-resolution imageDiscussion
It has been observed previously that electrical stimulation of the afferent [14, 19] and efferent [13–15, 20, 21] nerves proximal to the lacrimal gland can elicit a tear response. Other pathways (i.e. acinar cell voltage–gated ion channels, VIP, β-adrenergic, and other α-adrenergic varieties) play roles in tear secretion [4, 16, 18], but we found that electrical stimulation of the lacrimal gland engages primarily the efferent cholinergic pathway to enhance tear secretion.
Although isofluorane anesthesia should not affect efferent stimulation, it could inhibit a reflex tear response originating from the sensory portion of the lacrimal nerve within the gland. This may explain why, in some animals without anesthesia, much shorter pulses (170 μs) increased tear secretion as much as gland stimulation with 500 μs pulses in animals with anesthesia (∼4 mm, continuous stimulation).
Stimulating the afferent ethmoid nerve pathway increased tearing nearly as much as gland stimulation (∼4 mm), but with less current and less than half the pulse duration (0.08 μC mm−2 compared to 0.4 μC mm−2 per pulse). Therefore, ethmoid nerve stimulation (or another sensory branch of the ophthalmic nerve) may be more productive for eliciting tearing, and future experiments will attempt afferent nerve stimulation without general anesthesia.
Increase in tear secretion with higher stimulus amplitude might be due to stronger neural activation, or recruitment of the nerves from a larger volume of tissue, or it might be due to engagement of smaller diameter axons. Kossler et al [19] elicited tearing with stimulation levels (1.6 mA, 100 μs pulses) below our observed threshold; however they stimulated a different target (lacrimal nerve in the superior orbit), maintained closer proximity of the electrode to the lacrimal nerve and superior lacrimal gland, and performed stimulation and measurements without general anesthesia.
Our optimal frequencies (50–70 Hz) differ from those obtained in several previous studies (10–20 Hz) with stimuli amplitudes many times above the threshold [13, 14, 20, 21]. Our results resemble those of Botelho et al [15], who stimulated the lacrimal nerve in cats with pulse amplitude near threshold and found 20–50 Hz optimal. The efficacy of stimulation near threshold appears to improve with higher frequency. Tearing decreased at 100 Hz, probably due to the depletion of acetylcholine at the pre-synaptic terminal [22]. Because of this depletion, shorter treatments (below 5 min) may comparably increase tear secretion.
In the chronically implanted animals, modulating the stimulation waveform (1 s ON, 1 s OFF) generated the strongest response (more than 5 mm above the baseline). A sustainable 5 mm increase in Schirmer score could shift a subject from severe to moderate, or possibly mild, classification of dry eye disease [2].
Increasing the modulation duty cycle beyond 50% eventually reduced the response to about 2.5 mm above the baseline for continuous stimulation. However, one animal in the cohort followed a different trend: the response peaked at 20% duty cycle (9.7 mm) then monotonically decreased with increasing duty cycle until reaching no response (<1 mm) to continuous stimulation. Since continuous stimulation effectively engages efferent pathways (acute studies), the high response to the 20% duty cycle and no response to continuous stimulation suggest that this implant may have activated only the afferent pathway.
Interrupted stimulation may improve secretion by avoiding accommodation along an afferent (reflex) pathway in the central nervous system, among other possibilities. Comparing continuous and modulated stimulation in non-anesthetized animals with an inhibited tear reflex pathway (i.e. hexamethonium, or another ganglionic blocker) could confirm whether duty cycle modulation exploits a reflex pathway; however, safely doing this would require live blood pressure and heart rate monitoring, among other safety interventions not readily at our disposal.
The damage threshold of the lacrimal gland acinar cells (3.5 μC mm−2 per pulse with 1 ms pulses) was similar to intramuscular stimulation (no damage observed at 2 μC mm−2 per pulse, at the same pulse duration) [23] but higher than previous measurements with chorioallontoic membrane of chicken embryo (0.3 μC mm−2 per pulse) and retinal stimulation (1.3 μC mm−2 per pulse), both at same pulse duration [24]. Comparing charge density damage thresholds with stimulation thresholds at different pulse durations should be done cautiously since the damage threshold varies with pulse duration differently than does the neural stimulation threshold [24].
The 5 mm Schirmer score increase in chronically implanted rabbits was achieved with charge densities between 0.06 and 0.3 μC mm−2 per pulse—12 times below the damage threshold. McCreery et al [25] also found no tissue damage after stimulating the feline cortex with 0.1 μC mm−2 per pulse for several hours with similar waveform parameters.
Some forms of dry eye disease involve dysfunctional lacrimal glands from aging, autoimmune diseases (i.e. Sjögren's syndrome) or graft-versus-host disease (due to hematopoietic stem cell transplantation) [26]. Gland innervation in animal models of diseased glands was unaltered [27], which may allow experiments with stimulation of diseased glands.
Besides the tear volume, dry eye disease is also associated with changes in tear osmolarity, protein composition, inflammatory markers, and lipid content. Further studies should examine the effect of electrical stimulation of the lacrimal gland on these characteristics of the secreted tear, and compare tear volume and quality from afferent and efferent pathways to determine the best stimulation target.
In summary, we demonstrate that electrical stimulation of the lacrimal gland can safely and significantly increase tear secretion, and this effect is mediated by efferent parasympathetic nerves. Stimulation of the afferent nerves also enhances tear production, and both targets could be explored in clinical tests. Additional studies will explore the quality of the electrically-enhanced tear and its impact on dry eye disease in animal models. With a successful therapy, millions of patients with dry eye disease could benefit from electrical enhancement of tear secretion [28, 29].
Acknowledgments
We would like to thank Dr Michael Ackermann and Oculeve Inc. for providing the implantable stimulators and Professor Christopher Ta for discussions and encouragement. The experiments were conceived by all authors. M Brinton, A Kossler, J L Chung and K H Hoon performed the experiments and surgical procedures. M Brinton and D Palanker analyzed data and prepared figures. All authors contributed to writing of the manuscript. This work was funded by the National Institutes of Health (NIH) through a National Eye Institute grant EY023259.
Conflicts of interest
M Brinton (Consultant, Oculeve Inc.), J L Chung (none), K H Kook (none), A Kossler (none), M Franke (patent; employee, Oculeve Inc.), J Loudin (patent; employee, Oculeve Inc.), D Palanker (patent). Oculeve is now part of Allergan.