Abstract
A technology of laser engineering of microbial systems (LEMS) based on the method of laser-induced transfer of heterogeneous mixtures containing microorganisms (laser bioprinting) is described. This technology involves laser printing of soil microparticles by focusing near-infrared laser pulses on a specially prepared gel/soil mixture spread onto a gold-coated glass plate. The optimal range of laser energies from the point of view of the formation of stable jets and droplets with minimal negative impact on living systems of giant accelerations, laser pulse irradiation, and Au nanoparticles was found. Microsamples of soil were printed on glucose-peptone-yeast agar plates to estimate the LEMS process influence on structural and morphological microbial diversity. The obtained results were compared with traditionally treated soil samples. It was shown that LEMS technology allows significantly increasing the biodiversity of printed organisms and is effective for isolating rare or unculturable microorganisms.
Export citation and abstract BibTeX RIS
1. Introduction
The technology of laser engineering of microbial systems (LEMS) proposed in this work is based on the method of laser-induced forward transfer of a substance [1], which allows isolating proliferating cells of microorganisms. The relevance of this technology is due to the fact that more than 90% of bacteria from environmental samples remain uncultivable using standard cultivation methods on trivial media [2–4]. The expansion of the biodiversity of cultivated bacteria is extremely important for the development of biotechnologies, in particular, for the synthesis of new antibiotics and bioactive substances [5]. One approach that allows solving this problem in LEMS is to isolate micro-consortia containing single active cells of microorganisms [6, 7]. Unlike the dry soil printing procedure, described in [7], we use a liquid LEMS mixture to make laser printing of soil micro-aggregates more gentle and precise. This allows preserving native liaisons in microbial consortia and reduces concurrent interactions avoiding some major reasons for uncultivability.
In the LEMS technology, the transfer of a microdroplet of gel substrate with medium of micro-consortia occurs by rapid heating, under the action of a nanosecond laser pulse, of a thin absorbing film (usually metal) on which the substrate layer is located. Such heating leads to the evaporation of the absorbing film and the generation of a rapidly expanding bubble and liquid jet [1, 8, 9]. The jet transfers a microdroplet containing the medium of microbial systems micro-consortia to the acceptor glass plate.
The effectiveness of LEMS depends on many parameters (laser pulse energy, absorbing film, gel substrate, etc) and is determined by the possibility of transferring the required amount of cell-gel substrate with the medium of microorganisms without suppressing vital functions of living systems [6, 10]. A number of physical factors can affect the living systems in the process of microdroplet transfer with LEMS technology: (i) pulsed laser radiation transmitted through the absorbing film; (ii) shock waves; (iii) high temperatures; (iv) acceleration; and (v) nanoparticles of the absorbing film that can be transferred to the acceptor substrate together with the gel. Therefore, minimizing the side effects of such processes on living systems is an important problem in need of being resolved.
The present work is aimed at the development of LEMS technology as an effective method capable of achieving maximum spatial separation of soil microloci, minimum negative impact on living systems, and ensuring great biodiversity.
2. Materials and methods
2.1. LEMS technique
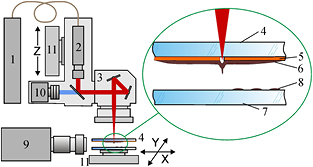
The experimental setup used to implement the LEMS procedure is shown in figure 1. Laser pulses of nanosecond ytterbium fiber laser YLPM-1-4x200-20-20 (wavelength λ = 1.06 µm, pulse duration τ = 8 ns, pulse energy E = 7–100 µJ) with a close-to-Gaussian beam intensity profile (M2 < 1.5) are focused onto a gold absorbing layer at the donor glass plate. The scanning of laser radiation is implemented by means of an Lscan H-10-1064 two-mirror Galvano scanning head (Ateko, Russia) with an SL-1064-110-160 F-theta objective (Ronar-Smith, Singapore), with a focal length of 160 mm that allows focusing of laser radiation into a spot 30 µm in diameter. Printing of microdroplets is carried out from the donor slide to the collector glass plate located at a distance of 1 mm. The donor glass plate is coated with a 50 nm thick gold layer covered with a layer of substrate (pure gel or gel with soil) with a thickness of 200 ± 30 µm. Homogeneous coverage with the gel substrate is produced using a blade coater. We used gel based on hyaluronic acid (2% aqueous solution). The gel viscosity determined with a Micro VISC viscosimeter (RheoSense, USA) amounted to 15.5 ± 0.05 mPa · s.
Figure 1. Schematic of the LEMS setup. (1) Pulsed fiber laser; (2) beam shaper; (3) scanner with objective; (4) donor plate; (5) Au layer; (6) substrate layer; (7) acceptor plate; (8) microdroplet; (9) fast video camera; (10) digital camera; (11) translation stage.
Download figure:
Standard image High-resolution imageThe energy of the laser pulses was controlled using a S310C thermosensor for power measurement (Thorlabs, USA) and a S144C photodiode detector for power measurement in combination with a PM100D digital measurement control panel (all Thorlabs devices, USA). The samples were studied using an HRM-300 Series optical 3D microscope (Huvitz, Korea) and a PHENOM ProX scanning electron microscope (SEM) (Phenom World, the Netherlands) with an energy-dispersive detector unit.
Optical recording of the laser-induced transport processes was carried out using a Fastcam SA-3 video camera (Photron, Japan) at a rate of up to 60 000 frames per second. The illumination was implemented using a CW laser diode at 660 nm wavelength, shaped by a telescope to get a circular beam of 14 mm in diameter.
2.2. Soil mixture for LEMS technology
For the preparation of soil mixture, we used a 10 cm top layer of mollisol (Belgorod region, Russia); 0.2 g of the dry soil was mixed with 0.4 ml of water and 0.8 ml of hydrogel. The mollisol soil particle size distribution performed with a Microtrac S3500 particle size analyzer showed that most of the soil particles have sizes in the range of 5–50 µm. The mixture for the LEMS procedure was gently homogenized by a glass stick. In our experiments, we used a polysaccharide gel based on low-molecular hyaluronic acid (hyaluronic acid sodium salt from Streptococcus equi, Sigma). The gel preparation procedure included the addition of phosphate buffer solution to the dry hyaluronic acid in proportions of 2% and 4%. After that the gel was placed in a refrigerator at +4 °C for 48 h to reach full dissolution. For the cultivation of the microorganisms and their subsequent study, laser printing of microdroplets with the LEMS mixture was carried out directly onto a surface of glucose-peptone-yeast agar in Petri dishes, and in 96-well 'Eco-log' test plates.
In a separate series of experiments aimed at the analysis of the effect of printing processes on microorganisms, a reference Escherichia coli strain was used. The number of cells in the gel (2% aqueous solution of hyaluronic acid) was ~8 × 107 cells ml−1. The number of colonies formed was determined when the microorganisms were cultivated on the surface of glucose-peptone-yeast agar in Petri dishes. The obtained results were compared with traditionally treated samples.
2.3. Research techniques
2.3.1. Cultivation and identification of bacteria.
Direct laser printing on a surface of solid glucose-peptone-yeast media [11] in Petri dishes was performed to estimate bacterial growth ability after LEMS. Cultivation with the standard technique from vortexed soil slurry was also performed to estimate the effect of laser printing [11]. The dishes were incubated at a temperature of 28 °C for 5–7 d. The number of the grown bacteria colonies was counted with main morphotypes detection. The identification of bacteria cultures is carried out on the basis of phenotypical cultural, micromorphological, and physiological-biochemical signs as key factors for phylogenetic identification of soil bacteria growth [12] and determinants of bacteria types [13].
Identification of some strains using phenotypic characteristics is difficult and were identified by the analysis of nucleotide sequences of the 16S rRNA gene. gDNA extraction was provided using 'Probe-Express' kit ('Syntol', Russia) with the addition of 5% Triton X-100 ('AppliChem', Germany) followed by 10 min boiling at 100 °C with subsequent treatment by glass beads (50–200 µm) using a Mini-BeadBeater homogenizer (USA) at 5000 rpm for 60 s. Polymerase chain reactions (PCRs) were carried out using a 'ScreenMix' PCR-kit ('Evrogen', Russia) with 63f + 1100r and 63f + 537r primers [14] (Marchesi et al) for Actinobacteria and with 63f + 1387r primers [14] for other bacteria. The PCR products were purified and sequenced (Sanger dideoxy sequencing) by the 'Syntol' company (Moscow, Russia) using 537r and 1100r primers [15]. All the nucleotide sequences were manually verified using Chromas Lite 2.01 (www.technelysium.com.au). They were aligned and compared using the CLUSTALW2 (www.ebi.ac.uk/Tools/clustalw2/index.html) program and the closest relatives were found by using the BLASTn (NCBI) sequence search utility (www.ncbi.nlm.nih.gov/BLAST).
3. Results and discussion
3.1. Laser printing in LEMS technology
An example of the distribution of soil microparticles in a gel after deposition of a layer of gel/substrate soil with a thickness of 200 ± 30 µm on a gold film of the donor plate is shown in figure 2. A comparison of 2D and 3D images shows that all soil microparticles and their agglomerates are located at the bottom of the gel/soil layer. They are at the maximum distance (~200 µm) from the gold film, which is explained by the higher density of particles compared to the density of the gel. This distribution of the particles is important from a practical point of view, since the maximum distance from the laser pulse impact site helps minimize the negative impact of a number of factors: high temperature, shock waves, laser light, and gold nanoparticles.
Figure 2. 2D and 3D images of a fragment of the donor plate with a gel/soil layer.
Download figure:
Standard image High-resolution imageThe impact of short high-power laser pulses leads to the formation of a thin substrate jet (figure 3(a)) that forms a microdroplet on the acceptor plate (figure 3(b)). It can be seen that there is a thickening—a bubble at the base of the jet. Similar thin jets with thicker bubbles at their bases were observed everywhere in the experimental study of the processes of laser-induced transport of liquid media [1, 6, 8–10, 16, 17]. At low energies, only a small bubble is formed. When the threshold (which is determined by the parameters of the liquid layer) is exceeded, a jet appears. We believe that the most important parameters affecting the threshold value are the viscosity and the coefficient of surface tension of the liquid (substrate).
Figure 3. Formation of gel (1) and gel/soil (2) jets (a) and microdroplets on the acceptor plate (b) under the action of laser pulses with different energies. B—bubble, J—jet. The recording rate is 10 000 frames per second. The dashed ellipse shows the region with stable microdroplet without spraying. The inset shows the optical images of the microdroplet on the acceptor plate.
Download figure:
Standard image High-resolution imageOur experiments on microsampling of the substrate have clearly shown that the addition of soil microparticles in a gel essentially changes the parameters of the microdroplet transport (figure 3(a)): the diameter of the jets increases, and their velocities decrease. However, the size of the droplets formed on the acceptor plate varies insignificantly (figure 3(b)). A significant difference in the droplet size of the gel and gel/soil is observed only at laser pulse energies of E > 25 µJ. With increasing energy E the droplet size on average monotonically increases and spraying appears gradually. As was shown in [18], such behavior can be explained by an increased laser-induced jet speed and violation of its laminarity at high laser pulse energies. At low energies E (the dashed ellipse on figure 3(b)), the droplets are stable without spraying. On the basis of the studies conducted with our experimental setup, the optimum conditions for the LEMS of gel/soil are realized using laser pulse energy in the range of 18–24 µJ.
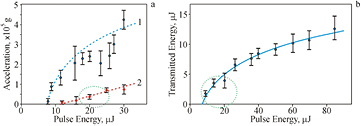
Dependences of the impact on living systems in the substrate of some negative physical factors—acceleration and laser irradiation on the laser pulse energy are shown in the figure 4. It can be seen that during the LEMS transfer, all living systems undergo significant dynamic loads, which on average increases with increasing of E (figure 4(a)). In the case of pure gel the acceleration is much larger and increases from 105 g to 4 · 105 G with increasing of pulse energy, where G is the gravitational acceleration (9.8 m s−2). The dashed ellipses on figure 4(a) show the region of E (16–25 µJ) with a stable jet and relatively low acceleration (2–7) · 104 g in the case of gel/soil printing.
Figure 4. Dependences of gel (1) and gel/soil (2) jet acceleration (a) and transmitted through the gold film laser radiation energy (b) on the pulse energy E. The recording rate is 60 000 frames per second. The dashed ellipses show: (a) the region with a stable jet and low acceleration; (b) the region with low transmitted laser energy.
Download figure:
Standard image High-resolution imageThe experiments have shown that under the action of laser pulse on the absorbing Au layer on the donor plate a part of the laser energy passes through this layer. The value of the transmitted energy increases on the average with increasing of E (figure 4(b)). The dashed ellipse on figure 4(b) shows the region of E (16–25 µJ) with the lowest values of transmitted energy.
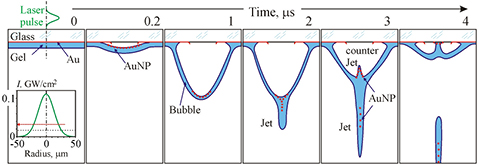
Sketches depicting the essential stages of the gel transfer dynamics in the LEMS technology under the action of a laser pulse are shown in figure 5.
Figure 5. Sketches depicting the essential stages of the gel transfer dynamics in the LEMS technology under the action of laser pulse. Inserts show the intensity distribution of the laser radiation in the focal plane and the considerably stretching in the vertical direction part of the donor plate. The red arrows show the direction of the movement.
Download figure:
Standard image High-resolution imageAt first, a portion of the energy of a short laser pulse is reflected (nonlinear [19]) and another portion is absorbed (~20% [6]) in the gold layer and heats it and the thin neighboring layers of the glass and gel to high temperatures. An estimate in [6] showed that at E = 20 µJ the temperature of such a system can reach 104 K at the end of the laser pulse. It is well known that if the temperature exceeds (0.9–1) · Tc, where Tc is the critical value, explosive boiling occurs. The critical temperature of water and Au are, respectively, 647.1 and 7250 K. As a result, during the action of the laser pulse (t = 0.01 on figure 5), an expanding bubble in the direction of the gel/soil [20] forms. When the bubble expands, the temperature and pressure inside it quickly fall and the gold vapor condenses on its walls and the glass surface. Then, the bubble with newly formed and transferred gold nanoparticles (AuNPs) in a thin layer of gel on its surface expands (t = 1). At t = 2, the expansion of the bubble is due to the inhibitory effect of atmospheric pressure stops and a thin jet in its base forms. One part of the AuNP passes into the jet and the other part remains on the bubble wall. At t = 3, because of the difference in the external (atmospheric) and internal pressures, the bubble begins to contract, and the jet breaks away from it. The region of the bubble near the optical axis moves faster, because it is far from the stationary walls, and as a result a counter jet is formed [1, 16, 17]. At t = 4, the anti-jet reaches the surface of the glass and subsequently the bubble finally collapses. The jet reaches the surface of the acceptor plate and forms a microdroplet that contains microparticles of soil and AuNP. As was shown in [6], for t = 8 ns the content of AuNPs SAu in the microdroplet (the ratio of their area in the SEM images to the droplet area in %) demonstrates almost linear dependence on E: SAu (%) ≈ 0.01 · E (µJ) in a wide range of energies of the laser pulse (5 µJ E 100 µJ). Therefore, from the viewpoint of minimizing the negative impact of the nanoparticles of the material of the absorbing layer on living systems in microdrops [21–23] it is preferable to carry out the transfer with minimum energy values (at E < 25 µJ and SAu < 0.25%).Thus, we revealed the optimal range of energies from the point of view of the formation of stable jets and droplets with minimal side effects on living systems of giant accelerations, laser irradiation, and AuNPs, is the range of E = 16–25 µJ.
3.2. Microbial growth after laser printing
Figure 6 shows optical images of gel/soil microdroplets on an acceptor plate, soil microparticles distribution in microdroplets, and the result of soil printing onto agar plates. On the size distribution chart (figure 6(b)) single microparticles (possible carriers of microorganisms) with a size of 8–12 µm are clearly observed. Laser printing of soil microparticles on a solid surface of nutrient media (glucose-peptone-yeast agar) in Petri dishes resulted in regular colony growth patterns (see figure 6(c)).
Figure 6. Gel/soil microdroplets on an acceptor plate (a), soil microparticles distribution in microdroplets (b) and colonies as the result of microbial growth after gel/soil printing of gel/soil microdroplets onto agar plates (c) with E = 20 µJ.
Download figure:
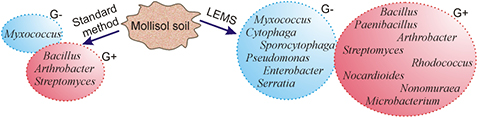
Standard image High-resolution imageAbout 400 colonies germinated after LEMS were compared on the genera level with 250 grown by the standard inoculation method. All colonies were described and identified on the basis of phenotype analysis and a potassium hydroxide test. Gram-positive (G+) and gram-negative (G−) bacteria are identified (figure 7).
Figure 7. Cultivated and identified groups of G+ and G− bacteria from the mollisol soil using the standard method and LEMS technology.
Download figure:
Standard image High-resolution imageApplication of the LEMS method significantly increased the diversity of G+ bacteria (from 3–8) and G− bacteria (from 1–6). It is well-known that G− bacteria have a thinner cell wall compared to that of G+ bacteria which explains the higher sensitivity of the G− bacteria to external influences. The observed bacteria abundance results demonstrate that the LEMS technology is friendlier for both for the G+ and G− bacteria compared to the standard method. It is important that all bacteria that are cultured by the standard method also give colonies after laser printing. The very significant result is the segregation of the rare genus Nonomuraea by the LEMS technology.
A separate series of experiments was aimed at testing the effect of microprint processes in the LEMS technology on highly sensitive, hard-to-breed microorganisms. For this purpose, a gel substrate with a reference E. coli strain was used. The experiments have shown that when this culture is cultivated by a standard method, the colonies of microorganisms on the surface of the nutrient medium do not arise in Petri dishes. At the same time, the use of the LEMS technology led to the fact that all the microdroplets transferred to the nutrient medium formed colonies during cultivation.
The reason why the LIMS technology allows obtaining a greater variety in comparison with the standard method can be understood from the diagram shown in figure 8.
Figure 8. A diagram illustrating the main differences between the LEMS and standard method, leading to an increase in biodiversity in the isolation of microorganisms from soil. The numbers indicate microbes that, with the standard cultivation method: 1—easy to flush out of their microenvironment, 2—most actively multiply, 3—separate from those with which they exist in symbiosis, 4—remain in the 'sleeping' state.
Download figure:
Standard image High-resolution imageWith the standard method of cultivation, all particles are mixed together. At the same time, a part of the microbes is separated from its microenvironment (1 in figure 8) and dies. The other part of the existing symbiosis (3) is separated from each other and also dies. Some (4) that are in the 'sleeping' state are not activated. As a result, only the strongest remain (2). In the LEMS technology, microorganisms are neatly transferred with their microenvironment by separate carriers. Therefore, microorganisms 1 and 3 multiply in this case. Pulse action during the transfer leads to the activation of 'sleeping' microorganisms (4). As a result, using LEMS technology, biodiversity significantly increases, which was confirmed experimentally (figure 7).
4. Conclusions
A high throughput LEMS technology for direct isolation of pure microbial cultures and microbial consortia from soil has been developed. This technology is based on laser printing of soil microparticles by focusing near-infrared laser pulses on specially prepared samples of a LEMS mixture, spread onto a gold-coated glass plate with direct transfer to an agar surface or into a microplate well. Different transfer modes of soil microparticles have been described and the experimental protocol of laser printing of LEMS droplets has been optimized. It was found that the most optimal range of energies from the point of view of the formation of stable jets and droplets with minimal negative impact on living systems of giant accelerations, laser pulse radiation, and AuNPs is the range of 16–25 µJ with the laser focal spot of 30 µm in diameter.
On the basis of tests with the mollisol soil, the LEMS method has been confirmed to be suitable for printing soil microparticles while maintaining sufficiently high viability of microbial cells. A representative of the rare genus Nonomuraea was isolated due to the LEMS technology without application of any complex selective media and addition of antibiotics cocktails.
In our opinion, LEMS could be a new promising technology to achieve a higher level of rare or unculturable strain isolation. We believe that this method will allow the discovery of an isolate rare and possibly new species of microorganisms. It is extremely important for the development of biotechnologies, in particular, for the synthesis of new antibiotics and bioactive substances
Acknowledgments
This work was supported by the Russian Foundation for Basic Research, Grant No. 16-02-00955 (in the microbiological studies); the Russian Science Foundation, Grant No. 18-15-00277 (in the development of the laser bioprinting technique); the Federal Agency of Scientific Organizations, Agreement No. 007-GZ/C3363/26 (in the development of a new setup for 3D laser printing technologies).