Abstract
The discovery of χ(3)–nonlinear lasing by stimulated Raman scattering (SRS) in natural monoclinic crystal of petalite, LiAlSi4O10, is reported. All recorded Stokes and anti-Stokes lasing sidebands under picosecond laser pumping in the visible are identified and attributed to the two occurring SRS-promoting phonon modes with ωSRS1 ≈ 490 cm−1 and ωSRS2 ≈ 357 cm−1. A short review of known SRS-active crystalline minerals and observed manifestations of their χ(2)– and χ(3)–nonlinear interactions is given as well.
Export citation and abstract BibTeX RIS
1. Introduction
In the history of physics the study of natural crystals was the beginning of many of its modern directions. To a certain extent this is valid for crystals of optical silicates. The birth of spontaneous Raman scattering in solids was associated with α–quartz (SiO2) in 1928 [1, 2]. The start of nonlinear optics is also connected with natural α–quartz, when its χ(2)–nonlinearity of the second harmonic generation (SHG) of the ruby laser (Cr3+:Al2O3) radiation was discovered in 1961 [3]. The χ(3)–nonlinearity was manifested in the discoveries of recent years in the phenomenon of the stimulated Raman scattering (SRS) in natural silicates like α–quartz [4], topaz, Al2(F,OH)2SiO4 [5], phenakite, Be2SiO4 [6], and zircon, ZrSiO4 [7]. Up to now SRS–active synthetic silicate crystals e.g. Ca2ZnSi2O7 [8], Ca2Ga2SiO7 [8], Bi4Si3O12 [9], and Bi12SiO20 [10, 11] are also known, where the latter three are also laser-active doped with Nd3+ ions (see, e.g [10, 12, 13]). It is appropriate to mention here the particular role of crystalline silicates in the development and establishment of modern laser physics and nonlinear optics. This contribution is illustrated in table 1, which shows the currently known laser silicates with their generating ions of the trivalent lanthanides (Ln3+) and chromium. This work continues our search study for SRS-active natural silicates.
Table 1. Silicate laser crystals and their Ln3+ lasant ions
| Crystal |
Nonlinearity | Ln3+ lasant ions | ||||
|---|---|---|---|---|---|---|
| Nd3+ | Ho3+ | Er3+ | Tm3+ | Yb3+ | ||
| Ca2Al2SiO7 | χ(3) | + | + | + | + | |
| Ca2Ga2SiO7 |
χ(3) | + | ||||
| CaY4(SiO4)3O | χ(2) + χ(3) | + | + | + | ||
| CaLa4(SiO4)3O | χ(2) + χ(3) | + | ||||
| CaGd4(SiO4)3O | χ(2) + χ(3) | + | + | |||
| Sc2SiO5 | χ(3) | + | + | + | ||
| Sc2Si2O7 | χ(3) | + | ||||
| SrY4(SiO4)3O | χ(2) + χ(3) | + | + | + | + | |
| SrLa4(SiO4)3O | χ(2) + χ(3) | + | + | |||
| SrGd4(SiO4)3O | χ(2) + χ(3) | + | ||||
| Y2SiO5 |
χ(3) | + | + | + | + | + |
| (YGd)SiO5 | χ(3) | + | ||||
| (YLu)SiO5 | χ(3) | + | ||||
| La2Si2O7 | χ(3) | + | ||||
| 7La2O3–9SiO2 | χ(3) | + | ||||
| La3Ga5SiO14 | χ(2) + χ(3) | + | ||||
| Nd3Ga5SiO14 | χ(2) + χ(3) | + | ||||
| Gd2SiO5 | χ(3) | + | ||||
| Er2SiO5 | χ(3) | + | ||||
| Lu2SiO5 | χ(3) | + | + | + | + | |
| Lu2Si2O7 | χ(3) | + | ||||
| Bi4Si3O12 |
χ(2) + χ(3) | + | ||||
| Bi12SiO20 |
χ(2) + χ(3) | + | ||||
| Bi4(SiGe)3O12 | χ(2) + χ(3) | + | ||||
aTable is compiled on the basis data of survey publications [14–17]. bAlso known laser silicate crystals with generating chromium ions: Be3Al2Si6O18, Mg2SiO4, CaGd4(SiO4)3O, Y2SiO5, La3Ga5SiO14, SrGd4(SiO4)3O [15, 17]. cAs well as SRS-active.
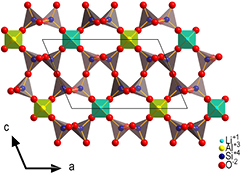
Since the first investigation with the aid of x-ray diffraction in 1930 [19] petalite, LiAlSi4O10, which is a commercially important source of lithium, has been subject of various studies focusing mainly on crystallographic and crystal chemical aspects [20–23]. This mineral crystallizes in the monoclinic space group P2/a with lattice parameters a1 = 11.754 Å, a2 = 5.139 Å, a3 = 7.630 Å, α2 = 113.04° at room temperature [22]. However, petalite exhibits a pronounced orthorhombic pseudo-symmetry, which is broken by the fully ordered distribution of Li+ and Al3+ cations [23]. The crystal structure can be classified as a 3D framework of corner-connected TO4 tetrahedra, where T = Li, Al or Si (figure 1). Alternatively, as there is perfect ordering of the cations on distinct crystallographic sites, petalite can be considered as a layer silicate. Following this interpretation, the structure is built up from folded [Si4O10] layers parallel to (0 0 1) which are linked by LiO4– and AlO4–tetrahedra. In a study to establish an internally consistent thermodynamic database for lithium-containing minerals the bulk modulus was measured in a multi-anvil cell and thermal expansion coefficients were determined using high temperature x-ray diffraction [24], which they combined with an earlier calorimetric study [25]. Poolton et al [26] carried out extensive studies on the IR stimulated luminescence behavior of this mineral. A sequently detailed study of the thermo–luminescence, the optical absorption, and electron paramagnetic resonance was performed by [27]. And most recently distinct crystal-physical properties were investigated like the elastic stiffness coefficients and thermal expansion [18]. In table 2 the physical properties at room temperature of LiAlSi4O10 are summarized.
Table 2. Selected known physical properties of petalite, LiAlSi4O10.
| Characteristics | |
|---|---|
| Space group [19, 22] | 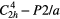 (No. 13) (No. 13) |
| Unit cell parameters cell, Å [18] | a1 = 11.724(2); a2 = 5.128(1); a3 = 7.622(2); |
| α2 = 113.07(2)° |
|
| Formula units per unit cell [22] |
Z = 2 |
| Density, g cm−3 [18] | ρx = 2.392 |
| Melting point, °C | ≈1400 |
| Hardness (Mohs scale) | 6–6.5 |
| Elastic constants (average), GPa [18] | c11 = 91.5(2); c22 = 93.04(4); c33 = 152.5(9); |
| c44 = 29.37(6); c55 = 69.7(2); c66 = 52.7(1); | |
| c12 = 0.1(1); c13 = 69.0(4); c23 = 6.5(2); | |
| c15 = −0.7(2); c25 = −1.5(4); c35 = −0.7(2); | |
| c46 = 2.4(1) | |
| Linear thermal expansion, 10−6 K−1 [18] | α11 = 0.974(1); α22 = 11.689(1); |
| α33 = −7.351(1); α13 = 0.244(1); | |
| UV-border of the optical transmission spectrum, μm | ≈0.2 |
| Linear optical character | Biaxial positive |
| Refractive indices |
nα = 1.504; nβ = 1.510: nγ = 1.516; |
| 2 V = 82°–84° | |
| Nonlinearity | χ(3) |
| Energy of SRS-promoting vibration modes, cm−1 [this work] | ωSRS1 ≈ 490; ωSRS2 ≈ 357 |
| Phonon spectra extension, cm−1d | ≈ 1150 |
| Atom | X | y | z | CN | SS | WN |
|---|---|---|---|---|---|---|
| Li | 0.25 | 0.2553 | 0 | 4 | C2 | 2e |
| Al | 0.25 | 0.7564 | 0 | 4 | C2 | 2e |
| Si1 | 0.9980 | 0.5128 | 0.2896 | 4 | C1 | 4g |
| Si2 | 0.1477 | 0.0099 | 0.2896 | 4 | C1 | 4g |
| O1 | 0 | 0.5 | 0.5 | Ci | 2b | |
| O2 | 0.25 | 0.9654 | 0.5 | C2 | 2f | |
| O3 | 0.0938 | 0.3012 | 0.2704 | C1 | 4g | |
| O4 | 0.3617 | 0.5358 | 0.1342 | C1 | 4g | |
| O5 | 0.0381 | 0.8011 | 0.2518 | C1 | 4g | |
| O6 | 0.2076 | 0.9779 | 0.1353 | C1 | 4g |
aAccording to [22]: a1 = 11.754 Å; a2 = 5.139 Å; a3 = 7.630 Å; α2 = 113.04°. bFractional position parameters, oxygen coordination number (CN), site symmetry (SS), Wyckoff notation (WN) [23]: cMineral Data Publishing (2001). dRRUFF Sample Data-Integrated Database of Raman Spectra, XRD and Chemistry Data for Minerals, Petalite RO40100.
Figure 1. Projection of the crystal structure of petalite along [0 1 0] which was determined at ambient conditions [18, 22].
Download figure:
Standard image High-resolution image2. SRS-spectroscopy
Colourless single crystals of natural petalite with gem quality were chosen for our SRS spectroscopy from a locality in Laghman, Afganistan. Its lattice parameters a1 = 11.724(2) Å, a2 = 5.128(1) Å, a3 = 7.622(2) Å, α2 = 113.07(2) obtained from x-ray diffraction experiments on a four-circle diffractometer equipped with graphite-monochromatized MoKα–radiation agree well with the literature values [21, 22] (see also table 2). Electron microprobe analyses revealed a nearly ideal composition with traces of Na, K, Mn, Mg, Ca, Fe, Ni, and Cr (in total less than 0.05 wt.%). The SRS properties reported in this work are referred to a Cartesian reference system (crystal-physical reference system) with basis vectors ei which are connected to the crystallographic basis vectors ai according to e1 || a1, e2 || a2, and e3 = (e1 × e2) ||  where
where  denotes the third basis vector of the reciprocal system. This non-standard setting was chosen to take advantage of the perfect cleavage plane parallel to (0 0 1)x-ray and of the pseudo-orthorhombic symmetry. For the SRS measurements a sample of LiAlSi4O10 with surface normals along e1, e2, e3 (faces (1 0 0)phys, (0 1 0)x-ray and (0 0 1)x-ray) respectively, and dimensions of 8.91 × 13.54 × 6.78 mm3 was prepared by cutting from a large petalite single crystal using a low-speed diamond wire saw and ground on a diamond disc (mesh 1200). All the sample faces were polished using 0.06 μm Al2O3 slurry but without an anti-reflection coating.
denotes the third basis vector of the reciprocal system. This non-standard setting was chosen to take advantage of the perfect cleavage plane parallel to (0 0 1)x-ray and of the pseudo-orthorhombic symmetry. For the SRS measurements a sample of LiAlSi4O10 with surface normals along e1, e2, e3 (faces (1 0 0)phys, (0 1 0)x-ray and (0 0 1)x-ray) respectively, and dimensions of 8.91 × 13.54 × 6.78 mm3 was prepared by cutting from a large petalite single crystal using a low-speed diamond wire saw and ground on a diamond disc (mesh 1200). All the sample faces were polished using 0.06 μm Al2O3 slurry but without an anti-reflection coating.
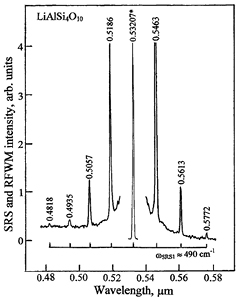
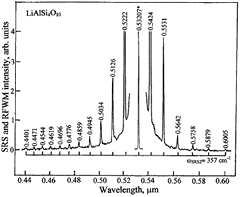
The investigations of Raman induced χ(3)–nonlinear interactions in monoclinic LiAlSi4O10 were carried out at room temperature in a cavity-free excitation scheme using an experimental setup based on a home-made picosecond Nd3+:Y3Al5O12 laser with an external KTiOPO4 doubler as the pumping source for λp = 0.53207 μm wavelength (see e.g [4, 28]). Two selected Raman-induced χ(3)–lasing spectra of petalite recorded at two different excitation geometries using a grating monochromater (McPherson Model 270) equipped with a Si–CCD line sensor (Hamamatsu S3924–1024Q) are shown in figures 2 and 3. They clearly demonstrate its two-phonon (ωSRS1 ≈ 490 cm−1 and ωSRS3 ≈ 357 cm−1) high-order Stokes and anti-Stokes generation in the UV and visible range. The results of their analysis are summarized in table 3. Due to the short sample length along e2 of the investigated natural LiAlSi4O10 sample, it was difficult to experimentally evaluate its steady-state Raman gain coefficient as it was usually possible in the comparative measurements of optically committed other inorganic SRS-crystals (see, e.g [5–8]). However, on the basis of the χ(3)–lasing behavior of the petalite and other silicates with the SRS-active tetrahedral SiO4 units (there are currently known about ten such silicates, among them are synthetic and natural crystals, see table 1 and table 4 [8]) it is safe to assert very roughly that the average gain coefficient of petalite is not below 0.2 cm · GW−1 in the visible spectral range.
Table 3. Spectral composition of high-order two-phonon cascaded Raman-induced Stokes and anti-Stokes χ(3)–nonlinear lasing of a single crystal of petalite, LiAlSi4O10, recorded at room temperature with a picosecond Nd3+:Y3Al5O12 laser pumping at the wavelength λp = 0.53207 μm (externally generation of SHG).
| Excitation geometry |
Stokes and anti-Stokes lasing components | SRS–active vibration modes, cm−1 | |||
|---|---|---|---|---|---|
| Wavelength, μm |
Lasing line | SRS and RFWM attribution |
ωSRS1 | ωSRS2 | |
| e2(e1, e1)e2 (see figure 2) | 0.4818 | ASt4-1 | ωp + 4ωSRS1 = [ωp + (ωp + 3ωSRS1) − (ωp − ωSRS1)] = ωp + ωASt3-1 − ωSt1-1] = ωASt4-1 | ≈490 | |
| 0.4935 | ASt3-1 | ωp + 3ωSRS1 = [ωp + (ωp + 2ωSRS1) − (ωp − ωSRS1)] = ωp + ωASt2-1 − ωSt1-1] = ωASt3-1 | ≈490 | ||
| 0.5057 | ASt2-1 | ωp + 2ωSRS1 = [ωp + (ωp + ωSRS1) − (ωp − ωSRS1)] = ωp + ωASt1-1 − ωSt1-1] = ωASt2-1 | ≈490 | ||
| 0.5186 | ASt1-1 | ωp + ωSRS1 = [ωp + ωp − (ωp − ωSRS1)] = ωp + ωp − ωSt1-1] = ωASt1-1 | ≈490 | ||
| 0.53207 | λp | ωp | |||
| 0.5463 | St1-1 | ωp − ωSRS1 = ωSt1-1 | ≈490 | ||
| 0.5613 | St2-1 | ωp − 2ωSRS1 = (ωp − ωSRS1) − ωSRS1 = ωSt2-1 | ≈490 | ||
| 0.5772 | St3-1 | ωp − 3ωSRS1 = (ωp − 2ωSRS1) − ωSRS1 = ωSt3-1 | ≈490 | ||
| e2(e3, e3)e2 (see figure 3) | 0.4401 | ASt11-2 | ωp + 11ωSRS2 = [ωp + (ωp + 10ωSRS2) − (ωp − ωSRS2)] = ωp + ωASt10-2 − ωSt1-2] = ωASt11-2 | ≈357 | |
| 0.4471 | ASt10-2 | ωp + 10ωSRS2 = [ωp + (ωp + 9ωSRS2) − (ωp − ωSRS2)] = ωp + ωASt9-2 − ωSt1-2] = ωASt10-2 | ≈357 | ||
| 0.4544 | ASt9-2 | ωp + 9ωSRS2 = [ωp + (ωp + 8ωSRS2) − (ωp − ωSRS2)] = ωp + ωASt8-2 − ωSt1-2] = ωASt9-2 | ≈357 | ||
| 0.4619 | ASt8-2 | ωp + 8ωSRS2 = [ωp + (ωp + 7ωSRS2) − (ωp − ωSRS2)] = ωp + ωASt7-2 − ωSt1-2] = ωASt8-2 | ≈357 | ||
| 0.4696 | ASt7-2 | ωp + 7ωSRS2 = [ωp + (ωp + 6ωSRS2) − (ωp − ωSRS2)] = ωp + ωASt6-2 − ωSt1-2] = ωASt7-2 | ≈357 | ||
| 0.4776 | ASt6-2 | ωp + 6ωSRS2 = [ωp + (ωp + 5ωSRS2) − (ωp − ωSRS2)] = ωp + ωASt5-2 − ωSt1-2] = ωASt6-2 | ≈357 | ||
| 0.4859 | ASt5-2 | ωp + 5ωSRS2 = [ωp + (ωp + 4ωSRS2) − (ωp − ωSRS2)] = ωp + ωASt4-2 − ωSt1-2] = ωASt5-2 | ≈357 | ||
| 0.4945 | ASt4-2 | ωp + 4ωSRS2 = [ωp + (ωp + 3ωSRS2) − (ωp − ωSRS2)] = ωp + ωASt3-2 − ωSt1-2] = ωASt4-2 | ≈357 | ||
| 0.5034 | ASt3-2 | ωp + 3ωSRS2 = [ωp + (ωp + 2ωSRS2) − (ωp − ωSRS2)] = ωp + ωASt2-2 − ωSt1-2] = ωASt3-2 | ≈357 | ||
| 0.5126 | ASt2-2 | ωp + 2ωSRS2 = [ωp + (ωp + ωSRS2) − (ωp − ωSRS2)] = ωp + ωASt1-1 − ωSt1-2] = ωASt2-2 | ≈357 | ||
| 0.5222 | ASt1-2 | ωp + ωSRS2 = [ωp + ωp − (ωp − ωSRS2)] = ωp + ωp − ωSt1-2] = ωASt1-2 | ≈357 | ||
| 0.53207 | λp | ωp | |||
| 0.5424 | St1-2 | ωp − ωSRS2 = ωSt1-2 | ≈357 | ||
| 0.5531 | St2-2 | ωp − 2ωSRS2 = (ωp − ωSRS2) − ωSRS2 = ωSt2-2 | ≈357 | ||
| 0.5642 | St3-2 | ωp − 3ωSRS2 = (ωp − 2ωSRS2) − ωSRS2 = ωSt3-2 | ≈357 | ||
| 0.5758 | St4-2 | ωp − 4ωSRS2 = (ωp − 3ωSRS2) − ωSRS2 = ωSt4-2 | ≈357 | ||
| 0.5879 | St5-2 | ωp − 5ωSRS2 = (ωp − 4ωSRS2) − ωSRS2 = ωSt5-2 | ≈357 | ||
| 0.6005 | St6-2 | ωp − 6ωSRS2 = (ωp − 5ωSRS2) − ωSRS2 = ωSt6-2 | ≈357 | ||
aNotation is used in analogy to that in [29]. Characters to the left and to the right of the square brackets denote the direction of the wave normal of the incident (pump) and the generated (χ(3)–lasing) light, characters within the brackets give the polarization directions of the incident and the generated light, respectively. bMeasurement accuracy ±0.000 3 μm. cIn square brackets three possibilities for nonlinear-laser components of the RFWM processes are given.
Table 4. Known SRS-active natural crystals (minerals) and their nonlinear laser properties.
| Natural crystals (minerals) |
Space group | SRS-active vibration modes,cm−1 |
Observed manifestations of χ(2)– and χ(3)–nonlinear laser interactions |
|---|---|---|---|
| LiAlSi4O10 (petalite) | 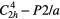 |
≈490,≈357 (present work) | SRS (present work) |
| Be2SiO4 (phenakite) | 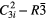 |
≈876 [6] | SRS, χ(3)–comb |
| C (diamond) | 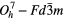 |
≈1332 [32] |
SRS [32] |
| Al2(F,OH)2SiO4 (topaz) | 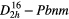 |
≈265 (≈268), | SRS, THG–SFG |
| ≈238 (≈239), | self–SFG(SRS) |
||
| ≈503e (≈507 |
χ(3)–comb, χ(3)–cr–casc |
||
| ≈27 |
com–phon |
||
| SiO2 (α-quartz) | 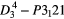 |
465.5 [4] | SRS, SHG, THG, |
| self–SFG(SRS), | |||
| χ(3)–comb [4, 33] | |||
| α–S8 (α-sulphur) | 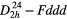 |
≈468, ≈216 [32] | SRS [32] |
| α–KAl(SO4)2·12H2O (potassium alum) | 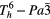 |
≈990 [34] | SRS, χ(3)–comb [34] |
| CaCO3 (calcite) | 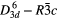 |
1086.5 [35], ≈282 [36] | SRS, SHG |
| FiHG |
|||
| χ(3)–comb, χ(3)–cr–casc [32, 35–41] | |||
| CaCO3 (aragonite) | 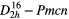 |
≈1087 (≈1096), | SRS, THG–SFG, |
| ≈152 (≈157), | self–SFG(SRS), | ||
| ≈205 [42] | χ(3)–comb, χ(3)–cr–casc [42] | ||
| SrSO4 (celestine) | 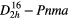 |
≈999 [43] | SRS, THG–SFG, |
| self–SFG(SRS), | |||
| χ(3)–comb [43] | |||
| ZrSiO4 (zircon) | 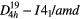 |
≈1008 [7] | SRS, χ(3)–comb, |
| χ(3)–cr–casc [7] | |||
| BaSO4 (barite) | 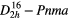 |
≈999 [44] | SRS, THG–SFG, |
| self–SFG(SRS), | |||
| χ(3)–comb [44] | |||
| PbCO3 (cerussite) | 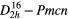 |
≈1054 [45] | SRS, χ(3)–comb [45] |
| Pb2CO3Cl2 (phosgenite) | 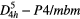 |
≈1062, ≈86 [46] | SRS [46] |
| PbSO4 (anglesite) | 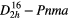 |
≈985 [44] | SRS, χ(3)–comb [44] |
aCrystals are listed by the 'alphabet' of the periodical table of elements.
bIn parentheses SRS-promoting vibration modes measured at ≈9 K are given.
cχ(3)–comb: spectrum of Stokes and anti-Stokes laser frequency components that span at least one octave (i.e. the highest frequency (energy) component must be at least double the lowest frequency component).
dMost probably natural crystals of diamond were used in this study [32].
eCom-phon: combined SRS-active phonon vibration arising as an interaction of two original SRS-promoting modes of crystal.
fTHG-SFG: third harmonic generation arising via parametric four-wave mixing (generation) under collinear coherent pumping at two fundamental wavelengths λf1 = 1.06415 μm and λf2 = 0.53207 μm (externally generated second harmonic generation of the fundamental wavelength λf1) of a picosecond Nd3+:Y3Al5O12 laser [5].
gself-SFG(SRS): sum-frequency generation arising from SRS-lasing components and pumping radiation.
hχ(3)–cr–casc: cascaded of one or many-step χ(3)–lasing with the involvement of the interaction of different SRS-promoting vibration modes of the crystal in the photon–phonon generation of high-order Stokes and anti-Stokes components.
iOr  jSHG: second harmonic generation.
kTHG: third harmonic generation.
lFiHG: fifth harmonic generation.
mFor aragonite a non-standard setting bca, corresponding to the space group symbol Pmcn, is frequently used in the literature.
jSHG: second harmonic generation.
kTHG: third harmonic generation.
lFiHG: fifth harmonic generation.
mFor aragonite a non-standard setting bca, corresponding to the space group symbol Pmcn, is frequently used in the literature.
Figure 2. SRS and RFWM spectrum of a single crystal of LiAlSi4O10, recorded at room temperature under picosecond pumping at λf = 0.53207 μm wavelength in excitation geometry e2(e1, e1)e2. The wavelength of all lines (pump line is asterisked) are given in μm, their intensities are shown without correction for the spectral sensitivity of the analyzing system with a Si-CCD line sensor. The spectral spacing between Stokes and anti-Stokes lines of the cascaded χ(3)–lasing is related to the SRS-promoting vibration mode with ωSRS1 ≈ 490 cm−1 of the studied crystal is indicated by the horizontal scale brackets. The assignment of all recorded nonlinear-lasing lines is given in table 3.
Download figure:
Standard image High-resolution imageFigure 3. SRS and RFWM spectrum of a single crystal of LiAlSi4O10, recorded at room temperature under picosecond pumping at λf = 0.53207 μm wavelength in excitation geometry e2(e3, e3)e2. The spectral spacing between Stokes and anti-Stokes lines of the cascaded χ(3)–lasing is related to the SRS-promoting vibration mode with ωSRS2 ≈ 357 cm−1 of the studied crystal is indicated by the horizontal scale brackets. Notations are analogous to those used in figure 2.
Download figure:
Standard image High-resolution imageNow we will discuss the vibronic nature of the observed SRS-promoting modes of the natural crystal of LiAlSi4O10. Its primitive unit cell contains two Li+, two Al3+, eight Si4+ and twenty O2− ions (see table 2). Lithium and aluminium ions occupy the 2e position of the C2 symmetry and silicon ions occupy the 4 g positions of the general C1 symmetry. In the primitive cell there are three different oxygen atoms that differ in their symmetry. Two oxygen atoms occupy the 2b sites of the Ci symmetry, two other oxygen atoms lie on the 2f sites of the C2 symmetry, and eight oxygen atoms occupy the 4 g sites of the general symmetry 1(C1). The structure of the petalite originates from the orthorhombic pseudo-symmetry, which is broken by the fully ordered distribution of Li+ and Al3+ cations [23]. The 3D framework of the petalite structure is built up from the LiO4 and AlO4 tetrahedrons which link the SiO4 tetrahedra constituting the Si4O10 layer. Such crystal structure of this mineral strongly influences its physical properties, which was considered in [18]. The basis for the calculations of phonons in the unit cell of LiAlSi4O10 is the above-described structure (see also section 2). The 32 atoms in the primitive cell of petalite (Z = 2) give rise to 3NZ = 96 zone-center degrees of freedom described by the irreducible representation: Γ96 = 21Ag + 24Bg + 24Au + 27Bu. Three of them, ΓT = Au + 2Bu, describe the acoustic phonons and remaining, ΓO = 21Ag + 24Bg + 23Au + 25Bu, correspond to the optical modes. The optical modes can be further subdivided among the vibrations of components of the primitive cell:
- Translations of the Li+ ions: ΓT' (Li+) = Ag + 2Bg + Au + 2Bu,
- Translations of the Al3+ ions: ΓT' (Al3+) = Ag + 2Bg + Au + 2Bu,
- Translations of the Si4+ ions: ΓT' (Si4+) = 6Ag + 6Bg + 6Au + 6Bu,
- Vibrations of the O(1) atoms: Γv (O1) = 3Au + 3Bu
- Vibrations of the O(2) atoms: Γv (O2) = Ag + 2Bg + Au + 2Bu
- Vibrations of the O(3–6) atoms: Γv (O3 ÷ O6) = 12Ag + 12Bg + 12Au + 12Bu.
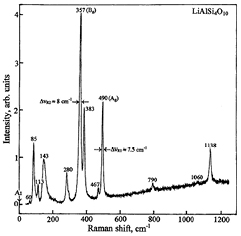
It should be noted that in the Si4O10 layer some of the oxygen atoms form the terminal Si–O bonds, other atoms participate in Si–O–Si bridges, and the next atoms form the Si–O–Al and Si–O–Li bridges. Therefore, 78 internal vibrations of the 2 × Si4O10 units are described by the representations: 19Ag + 20Bg + 20Au + 19Bu. The internal vibrations of the layer are strongly coupled with the translations and librations of the SiO4–tetrahedra, therefore all the observed IR and Raman bands have a mixed character. The Ag and Bg modes are Raman-active and those of Au and Bu are IR-active. The first-order spontaneous Raman scattering spectrum of LiAlSi4O10 is shown in figure 4. The wave numbers of the bands observed in this spectrum will be used in the analysis of the observed SRS components that are related to phonons with ωSRS1 ≈ 490 cm−1 and ωSRS2 ≈ 357 cm−1. This spectrum is typical for the polymeric silicates containing the internal and external phonons originating from the vibrations of the tetrahedral SiO4 units joined through Si–O–Si oxygen bridges. The following assignment of the bands of a given spectrum to the respective normal vibrations could be proposed [30, 31]:
- The bands at 1138 cm−1 and 1060 cm−1 correspond to the symmetric and asymmetric stretching vibrations νs(SiO4) and νas(SiO4) coupled with the νs(Si–O–Si) vibration,
- The band at 790 cm−1 corresponds to the stretching ν(Si–O–Si) mode,
- The strong band at 490 cm−1 originates from the symmetric bending δs(SiO4) vibrations of the silicate tetrahedrons. The satellite of this band at 467 cm−1 corresponds to the Bg–mode of this vibration,
- Raman lines observed in the range between 300–400 cm−1 correspond to the bending vibrations of the SiO4 and AlO4 tetrahedra. Therefore the very strong intensity band at 357 cm−1 was described by the δ(SiO4/AlO4) vibrations of the Big–symmetry,
- Other lines of the lattice modes should be assigned to the lattice modes corresponding to the translations of the Li+ and Al3+ ions.
Figure 4. The unpolarized first-order spontaneous Raman scattering spectrum of a monoclinic LiAlSiO4O10 crystal (petalite) excited at room temperature by laser emission of an Ar-laser at a wavelength of 0.514 μm (indicated by a vertical arrow) using a spectrometer U1000 (borrowed from the index of the Raman spectroscopy data set PETAL1.S.00). The energy of Raman shifted lines is given in cm−1.
Download figure:
Standard image High-resolution imageBased on the foregoing, we conclude that the observed SRS-promoting phonon modes ωSRS1 ≈ 490 cm−1 and ωSRS2 ≈ 357 cm−1 can be attributed to the symmetric Ag–bending δs(SiO4) vibration and asymmetric Bg–bending δas(SiO4/AlO4) vibration, respectively.
3. Conclusion
We have discovered and investigated the χ(3)–nonlinear optical potential of monoclinic natural petalite, LiAlSi4O10, as a novel material for Raman laser converters in the visible spectral range. All recorded Stokes and anti-Stokes lines of picoseconds χ(3)–lasing were identified and attributed to two SRS-promoting modes (ωSRS1 ≈ 490 cm−1 to the symmetric Ag–vibration and ωSRS1 ≈ 357 cm−1 to the asymmetric Big–vibration) of the tetrahedron SiO4 units of the studied silicate. It would be highly desirable from a practical point of view, if our investigation spurs the growth of large single crystals with the high optical quality of LiAlSi4O10. Petalite can enrich modern laser physics and nonlinear optics and is a valuable material for their applications. The studies also confirm that obtaining detailed knowledge of the physical properties of natural optical crystals is a persuasive tool in the development of new optical materials for modern laser physics and nonlinear optics and their applications. This is confirmed by the data in table 4, which shows the χ(2)– and χ(3)–nonlinear laser properties of the known natural SRS-active crystals. Here it is appropriate to add that some of their synthetic analogues have already been used. Given the heuristic value of such investigations, we will continue our nonlinear-laser search experiments with other natural optical crystals.
Acknowledgments
This study was supported by research programs of the Institute of Crystallography of the Russian Academy of Sciences, by the Deutsche Forschungsgemeinschaft (Project HA-5137/3), by the Institute of Optics and Atomic Physics of the Technical University of Berlin, by the Institute of Low Temperature and Structure Research of the Polish Academy of Sciences, by the Institute for Laser Science of the University of Electro-Communications in Tokyo, and by the Photon Frontier Network Program of the Ministry of Education, Culture, Sport, Science, and Technology, Japan.