Abstract
The variation in surface morphology and hardness of human deciduous teeth samples has been investigated after laser irradiation at different wavelengths and energies. Nd:YAG was employed as a source of irradiation for IR (1064 nm) and visible (532 nm) radiation, whereas an excimer laser was used as the source of UV (248 nm) radiation. Scanning electron microscope (SEM) analysis was carried out to reveal the surface morphological evolution of teeth samples. Vickers microhardness tester was employed to investigate the modifications in the hardness of the laser-treated samples. It is observed from SEM analysis that IR wavelength is responsible for ablation of collagen matrix and intertubular dentine. For visible radiation, the ablation of collagen along with hydroxypatite is observed. With UV radiation, the ablation of peritubular dentine is dominant and is responsible for the sealing of tubules. The decrease in hardness at lower energy for both wavelengths is due to the evaporation of carbon content. With increasing energy, evaporation of water along with carbon content, and resolidification and re-organization of inorganic content causes the increase in hardness of the treated dentine. SEM as well as microhardness analyses reveal that laser wavelengths and energy of laser radiation significantly influence the surface morphology and hardness of samples.
Export citation and abstract BibTeX RIS
1. Introduction
Laser ablation is significantly important in a wide range of applications in medicine, industry space technology, and material physics [1–5]. Laser ablation of dental hard tissues has been widely studied, and the use of laser radiation in the field of dentistry has been reported on for 25 years [2]. In dentistry, laser treatment is used for soft tissue surgery, sterilization, desensitization, etching, curing of composites, and endodontic treatment [3].The major aim of the use of lasers in dentistry is the more precise and painless removal of caries than with conventional methods. Ruby and CO2 were the first lasers used in dentistry [4] but now different lasers, such as CO2, Nd:YAG, Er:YAG, Ti:Sapphire, excimer, and diode are used, at wavelengths ranging from the far IR to the UV region [5–8]. The effect of laser wavelength and pulse energy on the surface morphology and hardness is significantly important, and has been investigated by various research groups [9–13].
Khalid et al [14] recently performed laser-induced breakdown spectroscopy analysis of human deciduous teeth using a Nd: YAG laser for the evaluation of plasma parameters as well as elemental analysis. The elemental concentration of Ca, Fe, Sr, Zn, and Pb was compared in three different parts of human teeth, i.e. enamel, dentine, and cementum. The concentration of trace elements was highest in the enamel, then in dentine, and lowest in the cementum. This shows that the enamel is most impacted part of the teeth.
Shahabi et al [15] have investigated the morphological changes of human dentine after irradiation with Er:YAG (2940 nm) and CO2 (10 600 nm) lasers and acid etching. Melting and cracking was observed after irradiation with CO2. With Er:YAG irradiation, open dentinal tubules and flakes without smear layer were observed. With acid etch, the surface was covered by a smear layer and debris. It was concluded that Er:YAG laser irradiation was the best of the three techniques for surface treatment. Turkman et al [4] have reported the effect of CO2 (10.600 nm), Nd:YAG (1.064 nm), and ArF excimer (193 nm) lasers on dentine hard tissue and rise in pulp temperature. SEM images revealed the melting and recrystallization of material, along with the presence of smear layer at some dentinal tubule orifices, with both CO2 and Nd:YAG irradiation. A smear layer was also observed with ArF excimer laser irradiation.
Laser technology is becoming popular in the field of dentistry. Therefore, to improve the clinical treatment of teeth fracture and predict susceptibility of teeth to fracture, the micromechanical properties of irradiated human teeth such as microhardness must be investigated for better understanding [16].
A variety of investigations have been reported regarding the hardness of dentine after irradiation with lasers at various parameters. Lee et al [17] have investigated the changes of hardness and elastic modulus of human dentine irradiated with a Nd:YAG laser at 1064 nm, and found that irradiation at laser energies of 100 and 150 mJ/pulse reduced the hardness and elastic modulus of human dentine. Celik et al [18] have reported compositional changes and microhardness of cavity floors prepared by Er,Cr:YSGG and Er:YAG lasers, and compared the results with the conventional method of bur preparation. Minimal thermal damage in the dentine tissue was observed after irradiation with both laser systems.
The aim of the present study is to evaluate the effect of laser wavelength on the surface morphology and hardness of teeth samples after irradiation using three different wavelengths Nd:YAG (1064 and 532 nm) and KrF (248 nm). The targets are exposed at four different energies of 70, 80, 90, and 100 mJ. The surface morphology of the irradiated dentine samples is examined using SEM, and their microhardness is evaluated for the wavelengths of 1064 and 248 nm. Then a correlation between surface modification and mechanical properties is established.
2. Experimentation
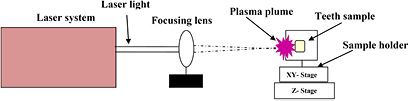
Twelve deciduous human teeth were obtained and preserved in saline solution. Samples were prepared by cutting them in a horizontal direction in the form of slices with dimensions of 7 mm × 2 mm × 2 mm. These samples were grinded and polished by SiC paper of 2000-grit under running water. The polished samples were autoclaved and ultrasonically cleaned in distilled water for 30 min before exposure. Three sets of experiments were performed. The teeth samples were placed on the sample holder mounted on an x–y–z manipulator. The laser light, after passing through the focusing lens of 50 cm focal length, was focused on the target material at an angle of 90° with respect to its surface. All experiments were performed in air environment with 500 pulses. A schematic of the experiment is shown in figure 1.
Figure 1. Schematic diagram of experimental setup.
Download figure:
Standard image High-resolution imageThe following three sets of experiments were performed by exposing the target at different wavelengths and energies of 70, 80, 90, and 100 mJ.
- (1)In the first set of experiments, four dentine samples were irradiated using Nd:YAG laser (CRF200: Big Sky Laser Technologies, Quantel, France) at 1064 nm, pulse duration of 10 ns, and repetition rate of 10 Hz as a source of IR irradiation.
- (2)In the second set of experiments, four dentine samples were irradiated using Nd:YAG laser (YG981C-10, Quantel France) with wavelength of 532 nm, pulse duration of 6 ns, and repetition rate of 10 Hz as a source of visible irradiation.
- (3)In the third set of experiments four dentine samples were irradiated using excimer laser KrF (GAM laser USA) with wavelength of 248 nm, pulse duration of 18 ns, and repetition rate of 20 Hz as a source of UV irradiation.
After irradiation of the samples, their surface morphology was examined using SEM (JEOL JSM-6480 LV). Vickers hardness tester (Zwick/Roell ZHV530) was utilized for the measurement of microhardness of the irradiated teeth samples with IR and UV radiation.
3. Results and discussion
3.1. SEM analysis
SEM images of figure 2 show the surface morphology of human dentine irradiated at IR radiation of 1064 nm at energies of (a) 70 mJ central ablated area, (b) 70 mJ peripheral ablated area, (c) 80 mJ central ablated area, (d) 80 mJ peripheral ablated area, (e) 90 mJ central ablated area, (f) 90 mJ peripheral ablated area, (g) 100 mJ central ablated area, and (h) 100 mJ peripheral ablated area.
Figure 2. Surface morphology of human dentine irradiated at wavelength of 1064 nm (IR region) for various energies of (a) 70 mJ central ablated area, (b) 70 mJ peripheral ablated area, (c) 80 mJ central ablated area, (d) 80 mJ peripheral ablated area, (e) 90 mJ central ablated area, (f) 90 mJ peripheral ablated area, (g) 100 mJ central ablated area, and (h) 100 mJ peripheral ablated area.
Download figure:
Standard image High-resolution imageDue to the Gaussian distribution of the laser pulse, the ablation spot is divided into two parts, i.e. central and peripheral ablated regions. The SEM image of figure 2(a) represents the central region of the ablation spot with lowest energy at 70 mJ, and is characterized mainly by the formation of wide, 44.2 µm cracks and re-deposition of debris. Melting and rippling of the collagen fiber of the intertubular dentine is observed at the boundary of the ablation spot, as shown in figure 2(b). By increasing the energy to 80 mJ, enhanced melting and porosity is observed at the central ablated region in figure 2(c), while the boundary region is dominated by fiber-like structures and micro-cracks as shown in figure 2(d). With further increase in energy up to 90 mJ, the growth of non- uniform porous island-like structures are formed at the central ablated region, as shown in figure 2(e). Spherical globular structures are observed at the boundary regions, as shown in figure 2(f). At the highest energy of 100 mJ, surface damage at the central region is dominated by the formation of a melted layer, pores, and unorganized and broken channels as shown in figure 2(g). Conical up-lifted structures are observed at the periphery, as shown in figure 2(h). The inset of figure 2(h) represents a magnified image of the cone. The diameter of the cone is calculated and found to be about 2.4 µm. Sub-structuring of the cone is observed due to ablation of the intertubular dentine and collagen fiber.
The SEM images of figure 3 show the surface morphology of human dentine irradiated at visible radiation of 532 nm: (a) 70 mJ central ablated area, (b) 70 mJ peripheral ablated area, (c) 80 mJ central ablated area, (d) 80 mJ peripheral ablated area, (e) 90 mJ central ablated area, (f) 100 mJ central ablated area, and (g) 100 mJ peripheral ablated area. In figure 3(a), the redeposition of particulates with non-uniform distribution of size and density is observed in the central ablated region at 70 mJ energy. Hollow globules and bumps in the form of re-solidified spherical mounts are observed at the periphery in figure 3(b). Small-scale melting and recrystallization of the dentine, along with a smear layer, is also observed. At 80 mJ, the melting is dominant and the intertubular structure is ablated and filled with debris, as shown in figure 3(c). Due to melting of the hydroxypatite, ablation pits are formed. In the outer boundary ablated at 80 mJ, the melting of bumps takes place and is responsible for the appearance of diffused channels in figure 3(d). At energy of 90 mJ, the central ablated area shows redeposition of ablated particles and scattered debris in figure 3(e). At the maximum energy of 100 mJ, the ablation of intertubular dentine and collagen fiber in the form of islands is observed in the central ablated area of figure 3(f). Figure 3(g) represents the peripheral ablated area at 100 mJ energy. The formation of channels with the growth of sub-grains and re-solidification of collagen is observed.
Figure 3. Surface morphology of human dentine irradiated at wavelength of 532 nm (visible region) for energies of (a) 70 mJ central ablated area, (b) 70 mJ peripheral ablated area, (c) 80 mJ central ablated area, (d) 80 mJ peripheral ablated area, (e) 90 mJ central ablated area, (f) 100 mJ central ablated area, and (g) 100 mJ peripheral ablated area.
Download figure:
Standard image High-resolution imageSEM images of figure 4 illustrate the surface morphology of human dentine irradiated at 248 nm: (a) 70 mJ central ablated area, (b) 70 mJ peripheral ablated area, (c) 80 mJ central ablated area, (d) 80 mJ peripheral ablated area, (e) 90 mJ central ablated area, (f) 90 mJ peripheral ablated area, (g) 100 mJ central ablated area, and (h) 100 mJ peripheral ablated area. At 70 mJ, ablation is dominant, and some porous and closed tubules are observed in figure 4(a). Periodic humps and valleys are also formed. In the valley regions, tubules are open while on humps, tubules are closed due to greater energy deposition. Also at 70 mJ, at the boundary of the ablated area, hollow globules are almost covered with the ablation debris and particulates, as shown in figure 4(b). At 80 mJ, the central ablated area exhibits micro-scale cracks and some pores, which are seen in figure 4(c), while organized hollow globules at the boundary well are observed in figure 4(d). This represents the uniform ablation of intertubular dentine and partial ablation of peritubular dentine, which is resolidified in the form of globules around the tubules. The magnified image of one tubule is shown in the inset. The diameter of the tubule of peritubular dentine is about 0.8 µm, which is almost close to the reported diameter of the tubule.
Figure 4. Surface morphology of human dentine irradiated at wavelength of 248 nm (UV region) for energies of: (a) 70 mJ central ablated area, (b) 70 mJ peripheral ablated area, (c) 80 mJ central ablated area, (d) 80 mJ peripheral ablated area, (e) 90 mJ central ablated area, (f) 100 mJ central ablated area, and (g) 100 mJ peripheral ablated area.
Download figure:
Standard image High-resolution imageWith further increase in energy up to 90 mJ, a micro-crack of 41 µm size is observed, as shown in figure 4(e). On the lefthand side of the micro-crack, closed tubules are seen and diffused tubules with redeposition of irregular-shaped particulates are observed on the right. At 90 mJ, agglomerates with a large number of small-sized pores are observed in figure 4(f). At maximum energy of 100 mJ, self-organized structures and clusters are formed in the center, as shown in figure 4(g). Figure 4(h) shows the ablated tubules and self-organized structures in the peripheral ablated area at 100 mJ. The sealing of tubules is also observed at this highest laser energy.
3.1.1. Discussion.
In this study, irradiated dentine surface is evaluated through SEM. In order to understand the laser interaction mechanism with dentine, it is necessary to know the internal structure and composition of dentine and response of the biological tissues [19]. The main part of human teeth consists of dentine. Dentine encloses the pulp cavity. It basically consists of 70% hydroxypatite, 20% organic material, and 10% water. Filho et al [20] have reported two classes of water within the tooth structure, i.e. structural water and interstitial water. The organic material basically consists of 'collagen I' and 'collagen' like compounds. During dentine formation, dental tubules are formed. The tubules are small circles with a diameter of about 0.9 µm at the upper surface of dentine, and their size gradually increases in diameter up to about 2.5 µm near the pulp. Dentine tubules are surrounded by peritubular dentine, which is 40% more mineralized than the intertubular dentine [21, 22]. Hydroxypatite is an inorganic component of human dentine. Peritubular dentine is highly calcified, and the amount of hydroxyapatite is greater in peritubular dentine as compared to the intertubular dentine. Hydroxypatite consists of denser covalent bonds as compared to dentine matrix collagen [23]. Collagen is a complex polymer with covalent molecular bonds like C–H, C=O, C–N, and N–O. The energies of these bonds are 4.30, 5.15, 3.04, and 2.4 eV, respectively. Hydroxyapatite is a ceramic with ionic bonding and band gap of 5.4 eV [9]. Water has covalent bonding like H–O–H and O–H bonds with energy 5.12 and 4.75 eV, respectively [24].
Processing of dentine with Nd:YAG 1064 and 532 nm and KrF 248 nm excimer laser radiation leads to the formation of several types of surface structuring, depending on the composition and structure of the samples and also on the processing parameters.
The dentine surface processed with IR laser represents cracking, redeposition of debris, pores, melting of collagen fibers, and conical structures. At 70 mJ, cracks appeared as a result of contraction of the tissue after the loss of water and collagen matrix, which is the evidence of thermal damage [15]. The surface is also covered by small particles called debris, which are directly ejected from the material in the form of large clusters in the ablation plume due to the collection of atoms, molecules, and small clusters [9]. With the increase of energy to 80 mJ, ablation of collagen appeared in SEM (figures 2(c) and (d)) along with porous structure. With further increase in energy to 90 mJ, islands are formed. At the highest energy of 100 mJ, conical uplifted structures are observed. The development of cone-like structure is due to differential ablations of peritubular dentine, with preferential ablation of intertubular dentine. Cone development is due to the shadowing as well as diffraction effects and interference between the incident and reflecting light [25]. This shows that thermal damage and ablation of collagen matrix is dominant for the IR region. It also shows that this laser system is favorable for the ablation of intertubular dentine.
The dentine processes with the visible laser system show the formation of irregular-shaped droplets, presence of smear layer, melting of hydroxypatite, and channel formation.
At 70 mJ, the formation of droplets of melted dentine indicates thermal effects on the dentine [26]. In dentine, these micrometer-size particles are produced due to resolidification of the droplets in the form of liquid as a result of hydrodynamic instabilities [27]. Increasing the laser energy up to 80 mJ at the central ablated region, the removal of the material causes the development of ablation pits and melting of hydroxypatite. As the laser energy is further increased up to 90 mJ, redeposition of ejected spherical particulates and droplets are observed. These are mainly formed due to the decomposition of hydroxypatite [28]. At the highest energy of 100 mJ, melting of material is resolidified in the form of islands and channels. Micro-channels are formed on the surface due to recoil pressure [29]. Sub-structuring inside the channels can be also observed due to the non-homogenous structure of dentine and the non-uniform ablation of collagen fibers. For visible laser as compare to the IR laser system, ablation of hydroxypatite is dominant. Collagen fibers are also ablated but their ablation is not as dominant as in the case of the IR laser system. This indicates that, in the case of the visible laser system, ablation of both intertubular and peritubular dentine is observed.
In the case of the UV laser system, the surface processed with excimer laser shows sealing of tubules and formation of agglomerates and self-organized hollow structures. At 70 mJ, hump-like structures and sealing of most tubules is shown. With the increase in laser energy up to 80 mJ, sealing and micro-cracking is observed at the central ablated area but open tubules are seen at the peripheral ablated area. The diameter of the tubules is calculated and found to be about 0.8 µm, which is equal to the reported tubule diameter value. With further increase in energy up to 90 mJ, cracking and sealing of tubules and agglomerates are observed. For the peripheral ablated region at 90 mJ, it is observed that formation of protruding agglomerates is accompanied by the formation of holes and dips. This indicates that material relocation at the micro-scale is responsible for the formation of agglomerates [30]. Sub-structuring on the agglomerates represents the ablation of intertubular dentine. With further increase in laser energy up to 100 mJ, the explosive boiling of inorganic material due to hydrokinetic forces is responsible for such hollow structures [15]. Both the intertubular and peritubular dentine are ablated, and this is responsible for the formation of bumps. A comparison between the IR, visible, and UV results reveals that the ablation of peritubular instead of intertubular dentine is dominant with UV laser irradiation.
In order to understand the various structure formation and ablation mechanisms of hard dental tissues, we have calculated the energy of a single photon of three different lasers. The single-photon energies of 1, 2, and 5 eV correspond to the wavelengths of 1064 nm (IR), 532 nm (visible), and 248 nm (UV), respectively.
With the IR laser, thermal ablation is dominant. In thermal ablation, optical energy is converted into heat energy, causing the evaporation of the water content present in human dentine. The single photon of 1 eV corresponding to the IR laser does not have sufficient energy to break the covalent bonding of the chemical composition of dentine. Multiple photons are required for the ablation of hard dental tissues; this is called plasma-mediated ablation [31]. As in the SEM images of dentine, samples treated with IR laser show fiber-like structures due to the ablation of collagen. As the binding energy of the bonds in collagen are 4.30, 5.15, 3.04, and 2.4 eV, the energy of IR photons corresponding to 1 eV causes more ablation in collagen. This also reveals that intertubular dentine is more ablated for IR radiation because the content of collagen in peritubular dentine is greater as compared to the hydroxypatite. For ablation with photons of energy 2 eV, corresponding to visible laser radiation, the generation of plasma causes the formation of acoustic shock waves; this mechanism is called photodisruption [31]. However, thermal processes always dominate for nanosecond lasers operating at the infrared and visible wavelengths. SEM micrographs reveal the ablation of both hydroxypatite and collagen matrix. This leads to the ablation of both intertubular and peritubular dentine.
In contrast to IR and visible radiation, for UV lasers, photochemical effects of the interaction of UV light with organic materials are dominant as compared to thermal and other dynamic (i.e. ponderomotive) effects. Because of this, direct bond dissociation takes place in the material [32]. Given photon energy of 5 eV, which corresponds to UV laser energy, the ablation of dentine is due to direct disruption of chemical bonds through high-energy photons. This ablation mechanism is considered to be photochemical [9]. SEM micrographs reveal absence of fibrous structures. The presence of agglomerates and Laser Induced Periodic Surface Structures (LIPSS) confirms the ablation of collagen matrix and hydroxypatite having binding energy 5.4 eV. This reveals the ablation of peritubular dentine because, in peritubular dentine, the hydroxypatite content is greater compared to collagen matrix. It also reveals that UV lasers produce a little thermal damage, and closings of tubules are obtained. The occlusion of tubules is helpful for reducing damage to soft tissues in the pulp cavity and the hypersensitivity of dentine [33]. Sivakumar et al [34] have reported that UV lasers are considered the best tool for treatment of dental hypersensitivity.
Ablation also affects the pulp temperature. The heat produced during laser irradiation causes the structural changes and increase in pulp temperature. The temperature of the pulp with irradiation of Nd: YAG 1064 nm is 28 °C and, with ArF 193, nm 1 °C [4]. The reported value of threshold safe temperature is 5.5 °C [15]. Therefore, the excimer laser is also more suitable for maintaining a safe pulp temperature rise. For shorter wavelengths, optical penetration depth, laser energy coupling, as well as mass ablation rate is greater as compared to longer wavelengths. However, for longer wavelengths, thermal penetration depth and thermal ablation are dominant. The electron density is higher for the UV wavelength of 1.0 × 10−18 cm−3 than the visible (532 nm, 9.5 × 10−17 cm−3) and IR (1064 nm, 9.3 × 10−18 cm−3) wavelengths, whereas the electron temperature is higher for the IR wavelength (1064 nm, 15; 201 K) compared to the visible (532 nm, 14; 399 K) and UV wavelengths (13, 717 K) for metals [35]. The increase in electron temperature for the IR wavelength causes an increment in pulp temperature as compared to the UV region. This shows that chemical ablation is more dominant at 248 nm and thermal ablation is more dominant at 1064 nm.
3.2. Hardness analysis
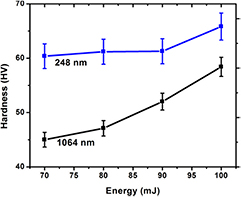
Figure 5 shows the variation in hardness for irradiated dentine samples, at different energies, for both IR and UV lasers. For untreated dentine, the value is 70 HV. After irradiation, the hardness of the treated dentine decreases for both wavelengths. However, with increasing energy, the value of hardness increases for both lasers. The value of hardness for the UV laser, i.e. 248 nm, is greater than the IR laser.
Figure 5. Variation of microhardness of human dentine irradiated with IR and UV laser systems.
Download figure:
Standard image High-resolution image3.2.1. Discussion.
Dentine is a composite of water and organic and inorganic components. Collagen is the main content of the organic components and hydroxypatite comprises most of the inorganic component. The strength and hardness of dentine is provided by the hydroxypatite, but stiffness and elasticity are obtained from collagen matrix and water [36]. The response of the different components of dentine to laser irradiation is different for different wavelengths and energies. According to our results as shown in figure 5, a decrease in microhardness in the irradiated dentine sample is observed at the initial energy of 70 mJ for both lasers. This decrease is due to the vaporization of the organic component collagen, leaving behind pores and voids. It is reported that 20% of dentine consists of collagen matrix. Collagen bundles help increase the resistance of dentine to crack propagation and revealing stress. The high temperature of the laser vaporizes the organic matrix, including collagen, leaving pores and voids and reducing the hardness of the dentine [16]. The decrease in microhardness is more pronounced in IR radiation compared to UV radiation, due to enhanced thermal effects of IR radiation.
With increasing laser energy, an increase in hardness is observed for both lasers. This is due to the vaporization of water and organic components. Annealing of the dentine surface occurs with this increase in energy, and the surface becomes harder due to the loss of water content and carbon. The evaporation of water and carbon are responsible for increasing the microhardness of dentine [18, 37]. This increase in hardness with increasing energy is also due to the melting and resolidification of dental hard tissues [16].
Dentine apatite consists of hydroxypatite carbonate associated with other components. Structural reorganization of the apatite crystal and chemical and mineral changes are another cause of the increase in hardness [38], as it causes crystal defects in the apatitic structure due to the increase in temperature [18]. The graph shows a significant micro-hardness increase in the dentine sample treated with the IR laser and also a pronounced increase for the sample treated with UV laser light. The binding energy is 4.30, 5.15, 3.04, and 2.4 eV for collagen; 5.4 eV for hydroxypatite; and 5.12 and 2.98 eV for water.
For IR radiation, as the energy of a single photon is 1 eV, hardness decreases at lower energy due to the vaporization of collagen matrix, keeping in mind that the binding energy of some collagen bonds is low compared to that of other materials. In figure 2, SEM images for the IR laser depicts the ablation of collagen and pores at low energy. With the increase in laser energy, however, photothermal ablation is dominant and causes the evaporation of water and carbon content, resulting also in an increase in microhardness [37]. At the highest energy of 100 mJ, the evaporation of water and carbon content in the dentine causes an increase in internal pressure and destruction of inorganic components before the melting point of teeth tissue, leading in turn to the ejection of micro-fragments [16]. The SEM image for 100 mJ energy shows the resolidification of the uplifted conical structures.
For the UV laser (248 nm), the energy of a single photon is 5 eV, and at lower energy hardness decreases due to the vaporization of collagen matrix. Photochemical ablation is dominant with the UV laser; this causes the bond disassociation of water and carbon content in dentine which in turn causes the increase in hardness. The increase in hardness at higher energies is also due in part to the ablation of hydroxypatite (which has binding energy of 5.4 eV), causing an amorphous change in crystal structure and resolidification of the material [16]. SEM images reveal that, at the highest energy, self-organized structures are developed which cause the increment in hardness.
It is concluded from the SEM images and hardness results that ablation of intertubular dentine is observed with IR laser irradiation but peritubular dentine is more resistant to IR laser energy as compared to UV. SEM images also reveal that less thermal damages occurs with UV as compared to IR radiation. Nowadays, laser energy absorbed by the hydroxypatite crystal is more preferable for teeth treatment [18].
4. Conclusions
The effect of IR, visible, and UV laser irradiation at different wavelengths on human dentine samples is investigated. The comparison of the effect of three different wavelengths on human dentine has been evaluated at four different energies. SEM results reveal that, for IR (1064 nm) radiation, intertubular dentine and especially collagen matrix is ablated, and cracking, melting, and conical structures are formed. For visible (532 nm) radiation, resolidification of ablated dentine, orifices of melted hydroxypatite, and channel formation are found. Fibrous-like structures due to the melting of collagen matrix are also observed. For UV (248 nm) radiation, the sealing of tubules at the central ablated areas and self-organized structures are observed. From the comparison of the three wavelengths, it is concluded that UV radiation (i.e. 248 nm) is more suitable for teeth treatment due to the observed sealing of tubules, which reduces the hypersensitivity of the dentine. Furthermore, the pulp temperature is very low with UV radiation compared to IR and visible radiation, and is also less than the reported threshold safe temperature value.
The microhardness of dentine after IR and UV laser irradiation is also evaluated and compared with that of non-irradiated dentine. A reduction in dentine hardness is observed after laser treatment generally, but this decrease is more pronounced in the case of IR radiation. The hardness is largely influenced by laser energy and increases with increasing energy with both types of radiation. However, this increase is more pronounced in the case of UV radiation.
Acknowledgment
We acknowledge the Higher Education Commission of Pakistan for funding the project Strengthening of Laser Facilities at GC University Lahore.