Abstract
Bacteria typically swim in straight runs, interruped by sudden turning events. In particular, some species are limited to a reversal in the swimming direction as the only turning maneuver at their disposal. In a recent article, Großmann et al (2016 New J. Phys. 18 043009) introduce a theoretical framework to analyze the diffusive properties of active particles following this type of run-and-reverse pattern. Based on a stochastic clock model to mimic the regulatory pathway that triggers reversal events, they show that a run-and-reverse swimmer can optimize its diffusive spreading by tuning the reversal rate according to the level of rotational noise. With their approach, they open up promising new perspectives of how to incorporate the dynamics of intracellular signaling into coarse-grained active particle descriptions.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Living beings move. While plants may adapt their shape and position by growth, many animal cells and microorganisms can actively move from one place to another, a property commonly denoted as motility—the active movement of an energy consuming agent [1]. It serves a wide range of purposes, such as the foraging for food sources, the escape from a predator, or the search for a sexual partner. Given the vital role of motion for many organisms, different motility patterns have evolved that are adapted to the specific purposes they serve. The study of such motion strategies has in recent years developed into a vibrant field of research at the interface of physics and biology [2].
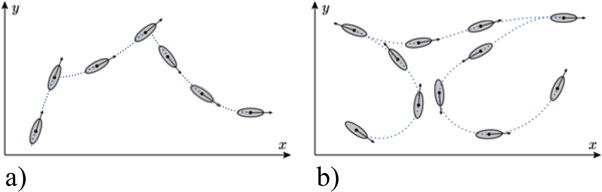
The most thoroughly studied paradigm of motility at the cellular scale is the swimming motion of Escherichia coli, a bacterium that lives in the intestine of warm-blooded animals [3]. E. coli moves in almost straight runs that are interruped by tumbles, abrupt changes in the swimming direction with an average angle of about 60° (run-and-tumble), see figure 1(a) [4]. Starting with the seminal work of Howard Berg in the 1970s, all aspects of E. coli's swimming motility have been studied down to molecular detail, including the force-generating molecular motor, the regulatory signaling pathways, and related sensing limits [3, 5, 6]. More recently, the swimming patterns of other bacteria have increasingly moved into focus, revealing a variety of different movement strategies. Among them, a large class of species relies on an alternating sequence of straight runs and reversals in the swimming direction (run-and-reverse), see figure 1(b). This includes many of the marine bacteria [7], where some of them also display more complex variants, such as the run-reverse-flick motion of Vibrio alginolyticus [8]. Similar run-and-reverse strategies are also found for soil bacteria, such as the swimming of Pseudomonas putida [9] or gliding motility of Myxococcus xanthus [10].
Figure 1. Schematical representation of bacterial swimming patterns. (a) Run-and-tumble pattern of E. coli. (b) Run-and-reverse pattern of P. putida.
Download figure:
Standard image High-resolution imageAlong with these experimental studies, a rich theoretical literature has evolved that analyzes the different motility patterns based on concepts from the theory of random walks, see for example [11]. These approaches are closely linked to the newly emerging research areas of active particles [12] and microswimmers [13]. The recent article by Großmann et al originates from this interdisciplinary field and presents an elegant theoretical framework to describe active particles that move following a run-and-reverse strategy [14]. Großmann and coworkers derive general expressions for the velocity correlation function, the mean squared displacement, and the diffusion coefficient of such particles. They then introduce a stochastic clock model, assuming that the intracellular biochemical pathway that triggers the directional reversals can be approximated by a sequence of random events. In this way, they find an expression for the diffusion coefficient that is maximized for a finite optimal level of rotational noise. This remarkable observation can be convincingly explained by a matching of time scales: particles spread most efficiently, if reversals occur at those times, when the particles have turned, on average, by 180° due to rotational diffusion. By applying their theoretical results to experimental data from Myxococcus xanthus and Pseudomonas putida, they indeed find a close agreement.
Two aspects are particularly remarkable about this work. Firstly, Großmann et al demonstrate how biological swimmers with only limited navigational degrees of freedom (running forward and turning back) can optimize their diffusive spreading by tuning their reversal rate according to the level of rotational noise. Secondly, they come to this insight by combining, for the first time, a coarse-grained active particle description with a model for the underlying intracellular signaling cascade, albeit a simplistic one. The dynamics of a biochemical reaction network that triggers reversals in the direction of motion will be admittedly much more complex than the stochastic clock proposed in this article. Also, we may expect strong variations from one organism to another. Nevertheless, Großmann's approach convincingly sketches a clear route towards merging random walk concepts from the field of active particles with mechanistic insight from the biological systems of interest.
We may thus expect that the work of Großmann et al will trigger manifold future developments that will push the frontiers of our field further along these lines. For example, variants of the stochastic clock model can be imagined that incorporate dynamical features of specific regulatory networks. This will become a more and more relevant perspective, as an ever increasing amount of detailed molecular knowledge of intracellular signaling pathways becomes available. In particular, modern fluorescence imaging techniques can now access dynamical processes inside living cells with unprecedented resolution. In combination with knockout mutations or drug-induced perturbations of signaling reactions and regulatory functions, live-cell imaging experiments thus reveal not only the topology of biochemical reaction networks but also their spatiotemporal dynamics. With this detailed mechanistic knowledge at hand, a modeling approch along the lines proposed by Großmann et al will prove particularly useful.
Interesting questions will also arise when integrating chemotaxis strategies into this framework. According to the classical picture of E. coli, temporal changes in chemoattractant concentration affect the tumble rate, a scenario that has also been observed for run-and-reverse swimmers and will thus directly interfer with the stochastic clock. We may also envision that other environmental cues are incorporated into this description, such as quorum sensing, or hydrodynamic interactions, keeping in mind that there are indeed examples, where the reversal rate changes under confinement [15]. Along these lines, even our understanding of collective effects in populations of swimmers may eventually benefit from this approach.