Abstract
Biomechanical properties are key to many cellular functions such as cell division and cell motility and thus are crucial in the development and understanding of several diseases, for instance cancer. The mechanics of the cellular cytoskeleton have been extensively characterized in cells and artificial systems. The rigidity of the plasma membrane, with the exception of red blood cells, is unknown and membrane rigidity measurements only exist for vesicles composed of a few synthetic lipids. In this study, thermal fluctuations of giant plasma membrane vesicles (GPMVs) directly derived from the plasma membranes of primary breast and cervical cells, as well as breast cell lines, are analyzed. Cell blebs or GPMVs were studied via thermal membrane fluctuations and mass spectrometry. It will be shown that cancer cell membranes are significantly softer than their non-malignant counterparts. This can be attributed to a loss of fluid raft forming lipids in malignant cells. These results indicate that the reduction of membrane rigidity promotes aggressive blebbing motion in invasive cancer cells.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The plasma membrane of a cell is not only a cell's barrier to its outside world [1], it is also an important cell organelle involved in cell signalling, cellular motion, and the formation of numerous diseases such as cancer [2–6]. Several publications concerning the lipid composition of cancer and healthy cells provide further indications that cancer affects membrane rigidity [7, 8]. These studies have focused on the phospholipid content, i.e. the main lipid components of cellular membranes, and have already shown differences in lipid compositions between cancer and healthy cells. Barcelo-Coblijn et al reported that human cancer cells have markedly lower levels of sphingomyelin (SM) and higher levels of saturated and monounsaturated phospholipids than non-tumour cells [9]. These changes, linked to cellular functions, are essential for metastasis and are a distinguishing element of cancer cells [2].
Cancer is further related to changes in the biomechanics of tissues and cellular cytoskeleton, regulating the development and homeostasis in tumour progression [7]. The cytoskeleton and the cell membrane are the most important factors characterizing the biomechanical behaviour of cells. While mechanical changes of cancer cells' cytoskeleton have become a focus of the emerging field physics of cancer [7, 10, 11], the role of membrane rigidity has attracted less attention, even though the plasma membrane is one of the first elements of a cancer cell that protrudes the vascular wall in metastasis [12]. This disregard results from the difficult experimental access to the bending rigidity of physiologically relevant cellular membranes due to the dominating rigidity of the underlying cytoskeleton. Only giant plasma membrane vesicles (GPMVs) or cellular blebs are devoid of cortical actin assembly [13] and thus allow to probe the membrane rigidity directly decoupled from the cytoskeleton [14]. In contrast to artificially modelled bilayer spheres or supported bilayers, GPMVs contain a larger variety of lipids and membrane peptides and are closer to physiological membranes.
Fourier fluctuation analysis of thermally excited membrane undulations has the potential to study changes in bending rigidity between cell membranes of cancer and non-malignant cells [15, 16]. Initial approaches of membrane rigidity measurements using thermal fluctuation analysis were attempted in the 1970s and 1980s [17]. However, these measurements have been mostly restricted to pure single component vesicles and red blood cells [15, 18, 19].
In total, former studies support the assumption of causality between migratory potential and the mechanical properties of cells. This causality especially applies to the migration strategy of invasive cancer cells, in which membrane blebbing, and thus the mechanics of cell membranes, play a pivotal role [20]. Based on these investigations and the fact that lipid composition strongly affects membrane rigidity [15], we assume a correlation between the malignancy of cancer cell membranes and membrane rigidity. We can further speculate that changes, at the interface, given by the mutual influence of the plasma membrane, cell signalling and cellular motion go hand in hand with changes of the whole phenotype. This is summarized in concepts such as epithelial–mesenchymal transition [21, 22]. Biomechanics further offer information on the metastasis capability of cells in primary tumours [23].
In our work, we present a direct link between membrane rigidity and malignancy of cells obtained from primary tissue. To achieve this aim, we used cellular blebbing, viz. vesiculation [13] to isolate pieces of the membrane as GPMVs and analyzed their rigidity. Based on Fourier fluctuation analysis of GPMVs, we found a significant membrane softening in human cancer cells compared to their non-malignant counterparts. The results are compared with rigidity measurements of cellular membranes extracted from malignant (MDA-MB-231) and non-malignant (MCF-10A) cell lines. Subsequently, we used matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS) to characterize the lipid composition of the cell membrane [24] of the various samples. Furthermore, we correlate the MS results with bending rigidity measurements of cell membranes obtained from the same cell type. We attribute the softening of cancer cell membranes to a loss of SM 16:0 and an increased proportion of phosphatidylcholines (PC) with shorter fatty acyl chain lengths in malignant cells. This study combines established biomechanical techniques with clinical samples and reveals a new insight in cancer biology.
2. Materials and methods
2.1. Tumour dissociation and culture of primary cells
The use of mammary and cervical tumour samples obtained from the University Hospital Leipzig was approved by the ethics committee of the medical department of the Leipzig University. All patients in this study signed a consent document prior to surgery. After tumour classification by the pathology department, the remaining vital tissue was processed for cell isolation and culture.
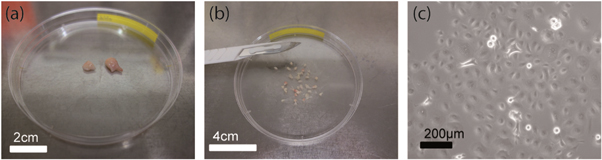
Tumour and healthy tissues were obtained from patients by surgical excision performed at Leipzig University Hospital. Figure 1 shows tissue samples of a human breast tumour and how cells were extracted.
Figure 1. Tissue sample preparation. (a) Pieces of a tissue sample (human breast tumour and the respective normal counterparts) obtained from a patient by surgical excision. (b) The tissue sample was cut into smaller pieces of about 1 mm3. (c) The tissue was digested with collagenase and DNase to obtain isolated epithelial cells.
Download figure:
Standard image High-resolution imageIn order to dissociate tissue samples (figure 1(a)) and form a cell monolayer, samples were cut into smaller pieces of about 1 mm3 (figure 1(b)). Digestion was performed in a gentleMACS C tube (Miltenyi Biotec, Germany) containing 5 ml Dulbecco's Modified Eagle/HAM's F-12 medium (DMEM/HAM's F12, Biochrom, FG 4815) supplemented with 20 μg ml−1 DNase I (AppliChem, A3778,0010). For breast tissue, 1.6 mg ml−1 collagenase P (Roche, 11213857001) was added. For cervical tissue, 0.25 mg ml−1 collagenase 1A (Sigma-Aldrich, C9722) and 0.25 mg ml−1 pronase (Roche, 10165 921001) was added. To enhance the dissociation process, gentleMACS C tubes were processed in a gentleMACS Dissociator (Miltenyi Biotec, Germany) and subsequently incubated at 37 °C and 5% CO2 in a humid atmosphere for 30–60 min. The last two steps were repeated until at least 80% of the tissue were dissociated.
The obtained cell suspension was centrifuged in two steps (at 40 g for 1 min and 300 g for 10 min) to separate epithelial cells from clusters, debris, and other cell types such as fibroblasts [25]. The remaining cell pellet was re-suspended in DMEM/HAM's F12 supplemented with 10% fetal calf serum (FCS, Biochrom, S 0615) and 1% antibiotic-antimycotic solution (Sigma-Aldrich, A5955) and cultured in six-well plates with the same cell culture medium (figure 1(c)). After 24 h, the medium was exchanged with serum-free HuMEC medium (Life Technologies, 12752-010) for breast cancer cells or with defined keratinocyte serum-free medium (Life Technologies, 10744-019) for cervical cells. Both media were supplemented with 1% antibiotic-antimycotic solution. Cell medium was renewed every 2 to 3 days.
Since passaging of cells can influence their biomechanical behaviour in vitro, primary cell membranes were obtained from cells which had not been passaged before and were only in culture for 5 to 10 days.
2.2. Culture of cell lines
Non-malignant MCF-10A breast epithelial cells (ATCC, CRL-10317) were cultured in 1:1 DMEM/Ham's F12 medium supplemented with 100 ng ml−1 cholera toxin (Sigma-Aldrich, C8052), 20 ng ml−1 human epidermal growth factor (Sigma-Aldrich, E9644), 10 μg ml−1 insulin (Sigma-Aldrich, I9278), 500 ng ml−1 hydrocortisone (Sigma-Aldrich, H0888) and 5% horse serum (PAA Laboratories, B15-021) and incubated at 37 °C and 5% CO2 in a humidified atmosphere. Cells were cultured in 75 cm2 flasks (TPP). Culture medium was changed every 2 to 3 days.
For passaging or extraction of lipids for MS, cells were rinsed with phosphate buffered saline (PBS, Life Technologies, 18912-014) and detached by applying 1 ml 0.025% trypsin/EDTA solution (Biochrom, L2143). Afterwards, 5 ml culture medium was added to inhibit trypsin. The trypsin containing medium was removed by centrifugation at 100 g for 4 min and the cell pellet was re-suspended in 1 ml culture medium. The cell suspension was either extracted according to the Bligh and Dyer method to extract the lipids [26], or cultured again in a new flask with fresh medium under the same conditions as already described above.
Malignant MDA-MB-231 (ATCC, HTB-26) breast epithelial cells were cultured in 90% DMEM, supplemented with 10% FCS at 37 °C and 5% CO2 in humidified atmosphere. Cells were cultured in 75 cm2 flasks. Cell medium was renewed every 2 to 3 days. Passaging and lipid extraction were performed in the same way as described above for MCF-10A cells.
2.3. Giant plasma membrane vesicles
GPMVs are bilayer spheres (1–100 μm) which are produced from biological cells by the vesiculation mechanism. During vesiculation, the membrane dissociates from the actin cortex and small pieces of the plasma biomembrane separate from the cytoskeleton. A higher hydrostatic pressure inside the cells, induced by a contraction of the cytoskeleton, leads to the formation of GPMVs [27–29].
Cells were grown to approximately 90% confluency in 75 cm2 tissue culture flasks prior to the vesiculation process. After removal of the cell culture medium, cells were rinsed twice with GPMV buffer. The GPMV buffer, composed of 150 mM NaCl (Sigma-Aldrich, S7653), 10 mM 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES, Sigma-Aldrich, H3375) and 2 mM CaCl2 (Sigma-Aldrich, C5080) was prepared with ultra-pure water (Milli-Q system, Integral 5, Merck Millipore, r > 18 MΩ cm).
In order to trigger vesiculation, 2.5 ml GPMV buffer including 25 mM PFA (Sigma-Aldrich, P6148) and 4 mM 1,4 di-thiothreitol (DTT, Roth, 6908) was added to the washed cell monolayer [13, 30]. Mono- and divalent cations, disulfide reducing agents such as DTT and hypertonic medium potentiate the vesiculation process [14]. The pretreatment of cells, e.g. with chemical agents that destroy the cytoskeleton, is not necessary. Disrupting the microfilaments and microtubules is not accompanied by detectable effects on vesiculation [31, 32]. Membrane blebbing occurred during shaking for 120 min at 37 °C, 5% CO2 and 60 cycle min−1. Afterwards, the content of the flask was decanted into a 15 ml centrifuge tube containing 4 ml GPMV buffer solution and cooled on ice for 45 min The upper ¾ of the solution in the centrifuge tube contain most of the GPMVs and were transferred directly onto a microscope slide and immediately imaged via phase contrast microscopy (Leica, DM IRB, Leica Microsystems, Germany) with a 100× immersion objective.
2.4. Fourier analysis of thermally excited membrane fluctuations
Based on vesicle edge R(θ, φ, t) and displacement u(θ, φ, t) from its spherical form, bending rigidity κ can be calculated by Fourier analysis of the thermal shape fluctuation [15, 30, 33–35].
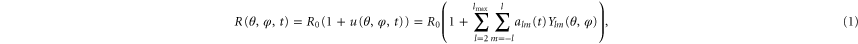
R0 is the average radius and R(θ, φ, t) the current radius of the vesicle. The displacements are decomposed in spherical harmonic eigenfunctions Ylm(θ, φ) and their time-dependent amplitudes alm(t). l denotes the azimuthal, m the magnetic quantum number, θ the polar and φ the azimuthal angle. States with l = 0 do not conserve the volume and states with l = 1 correspond to translations of the sphere (equation (1)).
The assumption that single oscillating modes are independent (hydrodynamic theory) allows for the calculation of time-dependent amplitudes alm from each spherical harmonic. According to the equipartition theorem, mean square amplitudes of the spherical harmonic modes behave as described by Gracià et al [33]:
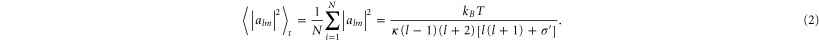
σ' = σeff R2/κ introduces the effective tension σeff, κ denotes the bending rigidity, T the temperature and kB the Boltzmann constant. The sum in equation (2) depends only on l, allowing the study of vesicle membrane fluctuations by observing only the equatorial plane (θ = π/2). By employing Fourier transformation, displacements u(φ,t) of the vesicle can be expressed as amplitudes Vq(t) [33]:
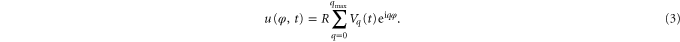
Substituting equations (1) and (2) into (3) yields mean square values of the fluctuation amplitudes:
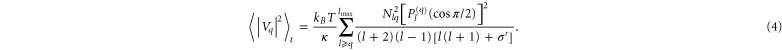
In equation (4), q is the mode number and Nlq P(q)l (cos(π/2)) are fully normalized associated Legendre functions. The sum in equation (4) is rapidly converging for moderate tensions and was calculated up to lmax = 400 [33].
As seen from equations (2) and (4) three regimes depending on q can be observed in the experimentally measured variance. Low-wave number modes are dominated by non-linear tension effects and are ignored in our fluctuation analysis. Higher order modes are noise dominated and were also not considered. The intermediate regime is bending dominated and was used to determine the bending rigidity κ in our experiments. In this regime the measured κ is nearly constant, viz. independent of the mode number q. Here the statistical variance (equation (S3)) of the fluctuation amplitude Vq scales ∼q−3 in agreement with Gracià et al [33].
In our experiments, vesicles and their contours were observed by phase contrast microscopy (figure 2(a)). Representative sequences of n images (≈10 000) per vesicle were recorded with an iXon DV887 EMCCD camera (Andor Technology, UK) with frame rates between 90 and 150 fps, depending on the binning size. An acquisition time of approximately 1 min was used, which guaranteed that GPMVs were able to move through the majority of their available configurations [33, 36]. A self-written MATLAB (The MathWorks, Natick, MA, USA) gradient based edge detection algorithm was employed to detect the contours of individual vesicles with subpixel resolution (figure 2(b)). After applying the edge detection, each point of the edge R(φi) was rescaled using the average radius.
Figure 2. Determination of the bending elastic modulus. (a) Phase contrast image of a GPMV obtained from isolated primary cells. (b) Contour of a GPMV extracted from a self-written edge detection algorithm.
Download figure:
Standard image High-resolution imageThe Fourier coefficients Vq from equation (4) were obtained via a fast Fourier transformation and their mean square values were calculated by averaging |Vq|2 over the number n of taken images (ensemble average).
The mode number independent κ (figure 3(a)) and the  ∼ q−3 behaviour (figure 3(b)) demonstrate that our analyzed modes (7 ≤ q < 16) are bending dominated in agreement with [37, 38]. Following Pecreaux et al and newer publications of Yoon et al and Gracià et al [33, 37, 38] we used
∼ q−3 behaviour (figure 3(b)) demonstrate that our analyzed modes (7 ≤ q < 16) are bending dominated in agreement with [37, 38]. Following Pecreaux et al and newer publications of Yoon et al and Gracià et al [33, 37, 38] we used
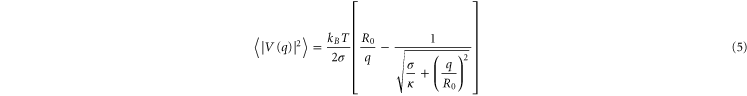
to fit our data and verify the  ∼ q−3 behaviour and thus bending rigidity domination (figure 3(b)).
∼ q−3 behaviour and thus bending rigidity domination (figure 3(b)).
Figure 3. Bending dominated regime for a GPMV obtained from primary breast cancer cells. (a) Bending elastic modulus κ (rigidity) of a GPMV shown as a function of the mode number q. Modes 7 ≤ q < 16 (red box) illustrate the intermediate regime employed to determine the bending elastic modulus of vesicles. The regime is characterized by practically mode number independent bending moduli (dashed line). Error bars are given only for the intermediate regime used to estimate the mean value of κ. The bending rigidity obtained from equation (4) neglecting tension is κ = (1.37 ± 0.14) × 10−19 J. (b)  shown as a function of the mode number q. The solid line corresponds to the fit shown in equation (5). The values obtained from the fit for bending rigidity and tension are κ = (1.46 ± 0.28) × 10−19 J and σ = (8.69 ± 1.76) × 10−7 N m−1.
shown as a function of the mode number q. The solid line corresponds to the fit shown in equation (5). The values obtained from the fit for bending rigidity and tension are κ = (1.46 ± 0.28) × 10−19 J and σ = (8.69 ± 1.76) × 10−7 N m−1.
Download figure:
Standard image High-resolution image2.5. MALDI-TOF MS lipid characterization
The MALDI-TOF soft-ionization MS technique allows analysis of samples without significant fragmentation of the analytes of interest [24]. About 600 μl of the obtained cellular lipid extracts (∼5 × 106 cells) were dried under vacuum and subsequently re-solubilized by addition of 10 μl matrix solution (2,5-dihydroxybenzoic acid (DHB, 0.5 M in methanol, Sigma-Aldrich, 39319)) [39]. The lipid moiety of cells was extracted according to the Bligh and Dyer method [26]. Then, 1 μl of the sample/matrix mixture was transferred onto a gold-coated MALDI target. To overcome assignment problems (interferences between differences in the fatty acyl compositions of lipids and the simultaneous generation of H+ and Na+ adducts), selected analyses were repeated with the same DHB matrix solution that has been saturated with caesium chloride (CsCl, ICN, 150589) as described in [40]. All MALDI-TOF mass spectra were recorded on a Bruker Autoflex MS device (Bruker Daltonics, Germany). The system utilizes a pulsed nitrogen laser emitting at 337 nm. All measurements were performed in the reflector mode using delayed extraction conditions. Positive ion spectra were exclusively recorded. Raw data were processed using the software 'Flex Analysis' version 2.2 (Bruker Daltonics). The intensities of the observed peaks are given relative to the intensity of the most intense peak. Further information regarding MALDI MS analysis of lipids is available in [24].
3. Results
3.1. Increased membrane softening in human breast and cervical cells
Tumour samples of breast and cervical cancer patients were analyzed and compared with measurements from two different breast epithelial cell lines (non-malignant MCF-10A and malignant MDA-MB-231). For both tumour types, corresponding normal epithelial cells from the same patient were used as reference. This approach allows a comparison of plasma membranes obtained from cancer and non-malignant cells. Most of the observed GPMVs had diameters between 10 μm and 30 μm. Based on the vesicle edges observed via phase contrast microscopy, bending rigidities were calculated by Fourier fluctuation analysis. For our calculation, only flaccid vesicles with a low excess area compared to the minimal spherical shape were analyzed to avoid membranes under mechanical tension.
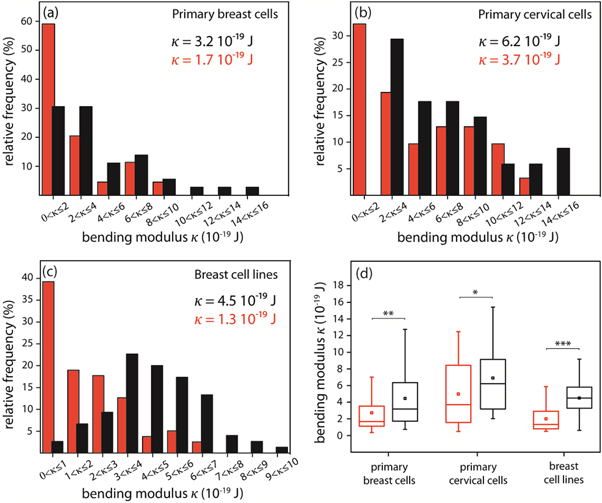
Figures 4(a) and (b) show bending rigidity measurements of GPMVs obtained from primary breast and cervical cells, presented as relative frequency count over κ. Vesicles obtained from two breast epithelial cell lines, non-malignant MCF-10A and malignant MDA-MB-231, were investigated as references. These cell lines shown in figure 4(c) constitute an established model system to compare the behaviour of cancerous and healthy cells [41]. Vesicles from healthy (N = 36) and malignant (N = 44) primary breast cells as well as healthy (N = 34) and malignant (N = 31) ones from primary cervical cells were measured. For primary breast and cervical cancer cells we found a significantly increased number of GPMVs with lower bending rigidities in contrast to GPMVs obtained from normal tissue of the same patient. These results are confirmed by the established model cell lines (figure 4(d)). Here 75 vesicles from MCF-10A and 79 from MDA-MB-231 were analyzed.
Figure 4. Biomechanical behaviour of human carcinoma cell membranes (red) in contrast to membranes obtained from non-malignant cells (black). Distribution of bending elastic moduli κ of GPMVs obtained from (a) primary breast epithelial cells, (b) primary cervical epithelial cells and (c) breast epithelial cell lines MDA-MB-231 and MCF-10A. (d) Boxplots of bending elastic moduli κ of GPMVs obtained from primary cells and cell lines displaying upper quartile, median, mean value, lower quartile, and a 10% to 90% whisker range. (Mann–Whitney U test, * p < 0.05, ** p < 0.01, *** p < 0.001.)
Download figure:
Standard image High-resolution imageThe rigidity of primary mamma and cervix cell membranes are characterized by a twofold decreased median for cancer cells compared to normal cells (figures 4(a) and (b)). We found κ = 3.2 × 10−19 J for GPMVs from healthy and κ = 1.7 × 10−19 J for GPMVs from malignant primary breast cells (mean deviation from the median MD = 2.5 × 10−19 J and MD = 1.6 × 10−19 J, respectively), κ = 6.2 × 10−19 J for GPMVs from healthy and κ = 3.7 × 10−19 J for GPMVs from malignant primary cervical cells (MD = 3.3 × 10−19 J and MD = 3.1 × 10−19 J, respectively). The same behaviour was observed for membranes obtained from breast cell lines resulting in membrane softening by a factor of 3. A median rigidity of κ = 4.5 × 10−19 J was measured for vesicles from MCF-10A cells and κ = 1.3 × 10−19 J for GPMVs from MDA-MB-231 cells (MD = 1.4 × 10−19 J and MD = 1.2 × 10−19 J, respectively).
3.2. MALDI-TOF analysis of lipid composition
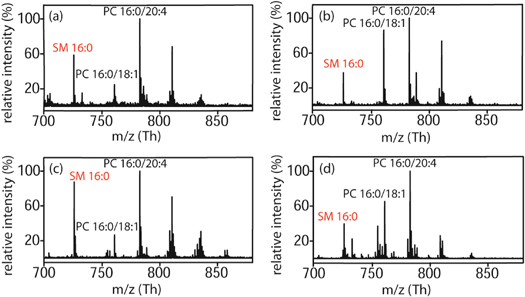
To investigate a potential correlation between membrane rigidity and lipid composition of cancer cells compared to their non-malignant counterparts, positive ion MALDI-TOF MS was employed for all investigated samples. Relative amounts of the individual lipids can be easily determined by comparison of the peak intensities. Two significant effects were found. First, we observed a 30%–40% decreased level of SM 16:0 in primary cancer cells in contrast to normal ones. Changes were normalized to the base peak corresponding to the most abundant membrane lipid, PC (16:0/20:4), see figure 5.
Figure 5. Positive ion MALDI-TOF mass spectra of primary cells. Mass spectra show the mass ranges of most common phospholipids such as phosphatidylcholines PC 16:0/18:1 and PC 16:0/20:4 as well as sphingomyelin 16:0. m refers to the molecular mass number and z to the charge number. Mass spectrum of (a) normal primary mammary cells and (b) primary mammary carcinoma cells obtained from the same patient. Mass spectrum of (c) normal primary cervical cells and (d) primary cervical carcinoma cells.
Download figure:
Standard image High-resolution imageSecond, we measured an increased proportion of PC with shorter fatty acyl chain lengths in malignant cells compared to non-malignant ones. Apart from the chain lengths of fatty acids, an increased proportion of fatty acids with a higher degree of desaturation was detected in non-malignant compared to malignant cells of the same patient (figure 5).
4. Discussion
In our work GPMVs were studied via two established and reliable methods, Fourier fluctuation analysis and MS, in order to examine the assumption of causality between migratory potential and the mechanical properties of cells.
We found a direct correlation between the rigidity of cellular membranes and the malignancy of the corresponding cells. Fourier fluctuation analyses of primary mamma and cervix cancer cell membranes show a twofold decreased median rigidity compared to normal ones. These results are in accordance with observed membrane softening in the established reference system consisting of the breast cell lines MDA-MB 231 and MCF-10A. The greater statistical dispersion in membrane rigidity obtained from primary cells (MD = 1.6 × 10−19 J to 3.3 × 10−19 J) in contrast to cell lines (MD = 1.2 × 10−19 J and 1.4 × 10−19 J) can be explained by naturally induced higher heterogeneity in these cell populations. Earlier work on the mechanics of lipid bilayers was typically carried out on synthetically produced vesicles composed of purified lipids [15, 33]. The new aspect of this work is that the used membrane vesicles obtained by surgical excision have a more physiological composition. This allows drawing direct conclusions on the membrane mechanics of living cells. Artefacts in the evaluation of mechanical differences caused by the physical condition are avoided using malignant and normal tissue of the same patient obtained within one biopsy.
Soft cell membranes are a necessary condition for alternative modes of cell movement such as blebbing motion [42]. The latter is associated with pathological mutations such as cancer [43, 44]. Blebbing motion facilitates the migration of cancer cells through the extracellular matrix or connective tissue [45]. The present study indicates that the role of the plasma membrane for cancer cell migration might be underestimated. This is in accordance with a recent study on migration of cancer cell lines [46].
MS measurements indicated a decreased level of SM 16:0 in primary cancer cells. This result is consistent with Barcelo-Coblijn et al [9]. A higher amount of SM in cellular membranes decreases the mobility [47], since SM constitutes a more condensed state of fluid membranes, which was predominantly found in lipid rafts [48]. Therefore, we assume that the SM content is important for the lower bending modulus of GPMVs obtained from malignant cells. Further, an increased level of PC species with shorter fatty acyl chain lengths was found. A shorter chain length reduces the tendency of the hydrocarbon chains to interact with each other by Van der Waals forces and decreases the rigidity of the membrane. Short lipid chains could act like a hinge and are easier to bend in a membrane. Thus both, chain lengths and SM contents, result in a more bendable membrane. In contrast to this, the higher amount of PCs with a lower degree of desaturation measured by MALDI-TOF should facilitate a dense packing and yield higher rigidity [49]. Based on the correlation measured between malignancy and softening it is concluded that chain length and SM content have a stronger influence on membrane rigidity than the double bond content of PC species. Our results, indicating a stronger influence of chain length to membrane rigidity compared to the degree of desaturation, are consistent with [46]. Moreover, Gracià et al assumed that transmembrane peptides do not alter the membrane stiffness significantly [33] and peptides could, therefore, not be responsible for such drastic changes in membrane rigidity.
The changes in lipid compositions of cancer cells not only influence the bending properties of cell membranes, but also impact cell signalling by reducing lipid rafts [50]. The reduction of raft forming lipids should lead to faster cell signalling. Both effects might have a decisive role in the progression of cancer and are therefore potential oncology targets.
5. Conclusion
The present study which comprises the combination of clinical samples along with biomechanical techniques to determine the rigidity of cell membranes, offers a new perspective in cancer biology. For the first time bending rigidity measurements of GPMVs obtained from primary cancer cells were performed and these measurements reveal a direct correlation between malignancy and biomechanics. In summary, we measured a significant softening in primary human breast and cervical cancer cell membranes. These results were confirmed with malignant and non-malignant cell lines. The reduced rigidity could be a significant advantage for tumour propagation and invasion when a cancer cell 'squeezes' towards a vascular wall. These results can further lead to a better understanding of other non-neoplastic diseases such as cardiovascular problems and diseases connected to abnormalities of the lipid metabolism in which changes of lipid composition are relevant.
Acknowledgments
Graduate students Sebastian Schmidt (Project KA1116/7-1) and Steve Pawlizak (Project KA1116/9-1) were financed by the DFG (Deutsche Forschungsgemeinschaft). The graduate student Anatol Fritsch was paid by SMWK (Project 4-7531,60/30/14). We would like to thank the graduate school 'BuildMoNa' for supporting Chris Händel, Steve Pawlizak, Tobias Kießling and Anatol Fritsch. Further we would like to thank Professor Dr Ana-Suncana Smith and Daniel Schmidt for fruitful discussion. We would like to thank Patricia Warne, Laura-Kaisa Maijala and Astrid Abel for proofreading. Finally, this work was supported by the special research unit (SFB 1052/B6). We acknowledge support from the German Research Foundation (DFG) and Universität Leipzig within the program of Open Access Publishing.