Abstract
Left ventricular volume–time curves (VTCs) provide hemodynamic data, and may help clinical decision making. The generation of VTCs using echocardiography, however, is time-consuming and prone to inter-operator variability. In this study, we used a new non-invasive, operator-independent technique, the hemodynamic cardiac profiler (HCP), to generate VTCs. The HCP, which uses a low-intensity, patient-safe, high-frequency applied AC current, and 12 standard ECG electrodes attached on the thorax in a pre-defined pattern, was applied to five young healthy volunteers, five older healthy volunteers, and five patients with severe mitral regurgitation. From the VTCs generated by the HCP, the presence or absence of an isovolumetric contraction phase (ICP) was assessed, as well as the left ventricular ejection time (LVET), time of the pre-ejection period (tPEP), and ratio of the volumes of the early (E) and late (A) diastolic filling (EV/AV ratio), and compared to 2D transthoracic echocardiography (2D TTE) at rest. The reproducibility by two different operators showed good results (RMS = 5.2%). For intra-patient measurement RMS was 2.8%. Both LVET and the EV/AV ratio showed a strong significant correlation between HCP and 2D TTE derived parameters (p < 0.05). For tPEP, the correlation was still weak (p = 0.32). In all five patients with mitral regurgitation, the ICP was absent in the VTC from the HCP, whereas it was present in the 10 healthy volunteers, which is in accordance with pathophysiology. We conclude that the HCP seems to be a method for reproducible VTC generation, and may become a useful early screening tool for cardiac dysfunction in the future.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Numerous techniques have been developed for the screening and monitoring of patients with cardiac diseases. Most extensively used in clinical practice is the electrocardiogram (ECG), which provides a non-invasive and continuous measurement of the electric activity of the heart. However, it lacks information about the actual blood volume displacement. For a more comprehensive evaluation of the cardiac function, electrophysiological data need to be combined with hemodynamic data. The volume–time curve (VTC) displays the volume change of the left ventricle (LV) as a function of time during a single cardiac cycle. Deviations in the VTC may be useful in the screening and monitoring of patients with a cardiac disease. A general example from clinical practice is the impaired diastolic filling as the first sign of ischemia (Montalescot et al 2013); VTC-derived parameters for diastolic function have shown to be well correlated with the severity of ischemia (Nakae et al 2008). Therefore, early detection and regular monitoring using the VTC, may prevent progression of disease.
So far there has been no method available to easily obtain reliable VTCs in clinical practice. Former methods are either invasive or restricted by technical limitations or patient safety issues. For instance, contrast ventriculography (Hammermeister and Warbasse 1974), radionuclide angiography (Mancini et al 1983, Lavine et al 1985, Bonow et al 1988, Pace et al 1992) and single positron emission computed tomography (SPECT) (Nakae et al 2008) require catheterization or injection with radioisotopes. Technological advancements in non-invasive imaging techniques as cardiac magnetic resonance (CMR) imaging and real-time 3D echocardiography (RT3DE) have made them promising tools to acquire VTCs (Zeidan et al 2002, Shin et al 2006, Fei et al 2007, Rodríguez-Granillo et al 2012, Duvall et al 2013), although still requiring time-consuming, semi-automatic post processing. Furthermore, the time-resolution of CMR and RT3DE is limited and insufficient to detect short-lived phenomena, such as variations in the isovolumetric contraction time.
However, in previous publications, we have presented a new, operator-independent and non-invasive method for continuous measurement of ventricular stroke volume using a high-frequency electric current: the hemodynamic cardiac profiler (HCP). We have shown the ability of the HCP to measure the LV stroke volume in an animal study (Konings et al 2011), and have described the principle of operation of the HCP in mathematical detail (Konings et al 2012), pointing at the possibility to measure VTCs with the HCP. In the present paper the focus will be completely on the generation of VTCs by the HCP, and the cardiologic parameters that can be extracted from these VTCs. Since the HCP has a particularly high temporal resolution (200 Hz), we concentrate on the assessment of clinically relevant time intervals, such as the time of the pre-ejection period (tPEP), LV ejection time (LVET), which have been suggested to be important parameters of the systolic function of the heart (Reant et al 2010). Polak et al also showed that the LVET is correlated with the LV ejection fraction (LVEF), and thus strongly associated with prevalent heart failure (Polak et al 2015). Furthermore, the ratio of the volumes of the early (EV) and late (AV) diastolic filling (EV/AV ratio), provides a quantitative assessment of the diastolic function.
In the present paper the generation of VTCs by the HCP is studied in healthy volunteers and patients with severe mitral regurgitation (MR).
For this purpose we focus on three subgroups: (i) patients with severe MR on transthoracic 2D echocardiography (2D TTE); (ii) older, healthy volunteers (>40 years); and (iii) younger, healthy volunteers (18–40 years). It is known that in patients with severe MR, the isovolumetric contraction phase (ICP) is absent, because before the opening of the aortic valve blood already regurgitates from the LV back into the left atrium (Stouffer 2011). We hypothesize that the absence of the ICP in the VTC can be an indication of MR, thus distinguishing MR patients from healthy volunteers. Furthermore, we expect to find age-related differences in the shape of normal VTCs, as healthy individuals above age 40 typically show a slower filling rate during the early diastolic phase compared to younger individuals (Nagueh et al 2009). 2D TTE was used as reference method, because both 2D TTE and HCP were able to produce the tPEP, LVET, and the EV/AV ratio. These three parameters were studied to present a quantitative comparison between HCP and TTE. For the purpose of this study, several improvements were implemented in the former HCP system. In the methods section of this paper, these improvements and the underlying mathematical rationale are explained.
2. Methods
2.1. Principle of operation of the HCP method
The principle of operation of the HCP has been described in our previous paper (Konings et al 2012). In summary, a very weak, patient-safe, high-frequency AC current of frequency f (between 58 kHz and 90 kHz), with an amplitude of 8 mApp, was applied to the thorax, using standard ECG skin electrodes (Nutrode-P20M0 and Nutrode-P10M0 pre-gelled, GE Healthcare, United Kingdom) positioned on the legs and above the left and right clavicle. Simultaneously, 12 skin electrodes were placed at pre-defined positions on the thorax (see figure 1) in order to measure the amplitudes of the AC voltages between the various electrode pairs at the same frequency f. Each pair of measuring electrodes consists of one electrode at the top of the sternum near the neck, inside the solid rectangle D in figure 1, and an electrode outside the solid rectangle D. All voltage measuring electrodes are attached to a thin, flexible, transparent, soft plastic sheet, thus ensuring that these measuring electrodes are located at fixed distances to each other. A more detailed description of the principle of operation of the HCP is presented in the appendix.
Figure 1. Placement of the electrodes on the thoracic skin during VTC measurement by the HCP method. The current injection electrodes are not shown here. (A) Electrode group to be aligned along the vertical midsternal line. (B) V2 electrode of the ECG. (C) Electrode to be at the same height as (B). (D) Electrode group at top of sternum. (E) Electrode group located on lower left side of thorax.
Download figure:
Standard image High-resolution image2.2. Improvements of the HCP method
A number of improvements have been implemented over the last years to improve VTC measurements using the HCP technique. Most notably, we introduced an automated method to overcome artifacts due to inter-patient differences in (thoracic) anatomy. These differences cause small deviations in the spatial atrial and ventricular sensitivity patternsS(atr) and S(ven) (see the
A second improvement has been an additional guideline for an improved positioning of the electrode sheet on the thoracic skin. This guideline now included a reference to the fourth intercostal space, in order to ensure more robust and reproducible positioning of the electrodes. Following the new guidelines, the array of electrodes inside the dashed rectangle A in figure 1 should be aligned with the vertical midsternal line.
Secondly, while keeping the array of electrodes inside the dashed rectangle A aligned with the vertical midsternal line, the electrode marked by the single circle C in figure 1 should be at the same height (vertical position) as the V2 electrode of the standard ECG. The V2 electrode is located parasternal at the right, on the 4th intercostal space (double circle B in figure 1).
2.3. Selection of patients and volunteers
In this study we measured the VTC with the HCP in 15 human subjects: five healthy young volunteers, five healthy older volunteers, and five MR patients from the University Medical Centre of Utrecht (UMC Utrecht). The study protocol was reviewed and approved by the Research Ethics Board of the University Medical Centre Utrecht (METC UMCU, METC protocolnumber 15-129), and informed consent was obtained from all patients. The five healthy young volunteers were students at the University of Utrecht with a blank medical history. Symptoms and comorbidities were checked by anamnesis, and the absence of MR was confirmed by TTE. We asked five healthy older volunteers (>40 years) from the outpatient clinics of the UMC Utrecht. Data from the outpatient clinic regarding symptoms, comorbidities, exercise testing results and ECG results, were collected. The TTE was checked for valvular heart diseases in retrospective. Patients suspected for cardiovascular disease, in particular MR, valvular disease of at least moderate severity, and ischemia, were not included. The baseline characteristics of the 15 study subjects are depicted in table 1. Furthermore, patients with severe MR were asked at the outpatient clinics of the Cardiology department UMC Utrecht. TTE results were checked in retrospective to confirm presence of severe MR. Data regarding symptoms and comorbidities were collected. Four of these MR patients were also included in a prospective trial in the UMC Utrecht to determine the best treatment in asymptomatic patients with severe, organic MR and preserved LV function (Dutch AMR study). All HCP measurements were performed in supine position. Each subject underwent an HCP measurement session that took approximately 15 min: about 10 min for the position calibration protocol, and about 5 min for the VTC assessment.
Table 1. Baseline characteristics.
| Patientgroup | Age | Sex | Height (cm) | Weight (kg) | BMI | Hyper-tension | Diabetes | Dyslipid-emia |
|---|---|---|---|---|---|---|---|---|
| Young healthy volunteers | ||||||||
| Young#1 | 25 | Male | 190 | 73 | 20.2 | No | No | No |
| Young#2 | 26 | Female | 163 | 53 | 19.9 | No | No | No |
| Young#3 | 26 | Female | 170 | 62 | 21.5 | No | No | No |
| Young#4 | 22 | Male | 180 | 65 | 20.1 | No | No | No |
| Young#5 | 24 | Male | 180 | 74 | 22.8 | No | No | No |
| Older healthy volunteers | ||||||||
| Older#1 | 49 | Female | 175 | 76 | 24.8 | No | No | No |
| Older#2 | 54 | Female | 160 | 79 | 30.9 | Yes | No | Yes |
| Older#3 | 66 | Male | 187 | 90 | 25.7 | No | No | No |
| Older#4 | 47 | Female | 172 | 68 | 23.0 | No | No | No |
| Older#5 | 43 | Female | 172 | 94 | 31.8 | Yes | No | Yes |
| Mitral regurgitation patients | ||||||||
| Patient#1 | 59 | Male | 181 | 79 | 24.1 | No | No | Yes |
| Patient#2 | 48 | Male | 196 | 105 | 27.3 | No | No | No |
| Patient#3 | 51 | Female | 168 | 75 | 26.6 | Yes | No | No |
| Patient#4 | 66 | Male | 175 | 75 | 24.5 | No | No | Yes |
| Patient#5 | 63 | Male | 175 | 60 | 19.6 | No | No | No |
2.4. Clinically relevant parameters measured by the HCP
Several clinically relevant parameters can be extracted from the VTC in general (figure 2). For our study, we used the following VTC key parameters in order to compare HCP with 2D TTE:
- (1)Ratio of the volume of the early (E) to the volume of the late (A) diastolic filling (EV/AV ratio), which is an indicator of diastolic function. The procedure to assess this EV/AV ratio was the following: First, within the diastasis time interval, which is the phase during the diastole that follows the early filling (E) and precedes the late filling (A), the point in time Tdias is established at which the slope of the VTC is closest to zero. Subsequently, the volumes EV and Av are defined as the volume of the filling that has taken place respectively before, and after, t = Tdias.
- (2)The presence, or absence, of an isovolumetric contraction phase (ICP) in the VTC from the HCP. The absence of an ICP in the VTC is associated with mitral valve regurgitation. The criterion for the presence of an ICP in the VTC is that, within the first 120 ms of the VTC (in which t = 0 coincides with the R-peak of the ECG), there must be a time interval of at least 40 ms duration during which the VTC is virtually horizontal, changing no more than 2% of the stroke volume.
- (3)Left ventricular ejection time (LVET), which is the duration of the time interval between the opening of the aortic valve and the aortic valve closure. This is measured in the VTC as the time interval between the end of the ICP and the point in time at which the VTC has reached its lowest point (i.e. the end of systole).
- (4)Time of the pre-ejection period (tPEP), which is a parameter reflecting cardiac contractility. This is measured in the VTC as the duration of the time interval between the R-peak of the ECG (i.e. t = 0), and the end of the ICP.
Figure 2. Schematic representation of a left ventricular volume–time curve (dotted line). Various parameters are depicted that are generally used to characterize the overall shape of the VTC. Tsystole: time duration from t = 0 (R-peak of ECG) to the end of the systole, Tdiastole: time duration from the end of the systole to the end of the diastole, ICT: isovolumetric contraction time, LVET: left ventricular ejection time, IRT: isovolumetric relaxation time, EV: Volume change during early filling phase, AV: Volume change during late filling phase (atrial kick), AoV: aortic valve, MV: mitral valve.
Download figure:
Standard image High-resolution imageSince the last two parameters, LVET and tPEP, can only be assessed from the VTC if an ICP is present, these two parameters have been measured only in the young and older volunteers.
2.5. Clinically relevant parameters measured by echocardiography
2D TTE measurements were obtained in accordance with the guidelines of the American Society of Echocardiography (Vahanian et al 2012). Echocardiography was performed using the Philips iE33 ultrasound machine (Philips Medical Systems, Andover, Massachusetts, USA). M-mode, 2D PW-Doppler, continuous Doppler, and color Doppler echocardiographic examinations were performed with the patient in the left lateral position and in resting state. Offline analysis was performed using Philips Xcelera software (R3.3L1) and ImageJ software (National Institutes of Health, Bethesda, MD). Data analysis was performed by two observers. Mitral regurgitation was graded as recommended by the European Society of Cardiology (ESC) guidelines on the management of valvular heart disease. The ventricular time-interval parameters were acquired from PW Doppler recordings of the LV outflow tract (Reant et al 2010). In order to assess the ratio of the early (E) and the late (A) filling volume, the velocity–time- integral (VTI) of the E-wave and the A-wave was measured, respectively. A triangle-area method was used to determine EVTI and AVTI from standard MV-inflow PW-Doppler images in the apical four chamber view. The right side of the triangle was equal to the extrapolated deceleration slope. Echocardiographic measurements are known to suffer from inter- and intra-operator variability, caused by multiple factors, e.g. the angle dependency of the spectral Doppler measurement. However, 2D-TTE was used as a reference technique as it is currently the most widely used clinical standard. The Doppler measurements were performed by an experienced sonographer, and the results were checked by a cardiologist specialized in cardiac imaging.
2.6. Statistical analysis
Statistical analysis was done using SPSS software 23.0 software (SPSS Inc, Chicago, IL) and Microsoft Excel 2010 software (Microsoft, CA, USA). Inter-operator variations and reproducibility were assessed for each individual clinical parameter. Correlations were examined using Bland–Altman analysis, as well as using scatter plots and Pearson's correlation coefficient. Reproducibility of the VTCs was expressed as the root mean square (RMS) of the time series containing the standard deviation (SD) values between the different VTCs for each individual point in time during the cardiac cycle. Statistical significance was set at a 2-tailed P-value < 0.05.
3. Results
3.1. Reproducibility
A young healthy volunteer underwent repeated HCP measurement sessions. Each session started with the application of the HCP electrode sheet, and ended with the complete removal the sheet. The results of the seven repeated HCP measurement sessions, performed by two different operators, on the same young healthy volunteer, are shown in figure 3(a). The corresponding reproducibility of the parameters LVET, tPEP, Ev, Av, and EV/AV are rendered in table 2 ('two operator reproducibility'). For these VTCs the total RMS was 5.2%.
Table 2. Reproducibility of key parameters for one operator and for two operators.
| Type of measurement | Parameter measured | Mean ± SD | (SD as % of Mean) |
|---|---|---|---|
| Single operator reproducibility | LVET (ms) | 307 ± 7 | (2.2%) |
| tPEP (ms) | 60 ± 5 | (8.3%) | |
| EV (% of SV) | 73 ± 3 | (4.1%) | |
| AV (% of SV) | 27 ± 3 | (11.1%) | |
| EV/AV | 2.8 ± 0.3 | (10.7%) | |
| Two operator reproducibility | LVET (ms) | 315 ± 14 | (4.4%) |
| tPEP (ms) | 56 ± 6 | (10.7%) | |
| EV (% of SV) | 81 ± 4 | (4.9%) | |
| Av (% of SV) | 19 ± 4 | (21.1%) | |
| EV/AV | 4.2 ± 1.2 | (28.6%) | |
Figure 3. Multiple HCP measurements in a young healthy volunteer. Each graph shows the volume change in the left ventricle (vertical axis) during a single cardiac cycle as function of time in milliseconds (horizontal axis), in which t = 0 coincides with the R-peak from the ECG, and in which the volume change is defined as the change with respect to the volume at t = 0. (a) HCP measurements performed by different operators, at different time points (five times operator #1 and two times operator #2). The electrode sheet was removed after each measurement. The total RMS was equal to 5.2%. (b) HCP measurements (5 times) performed by one single operator without replacing the electrodes. The total RMS was equal to 2.8%.
Download figure:
Standard image High-resolution imageFigure 3(b) shows the variability of another set of five sequential measurements, performed by one single operator, on a different young healthy volunteer; this time however without removing the electrode sheet between measurements. The corresponding reproducibility of the parameters LVET, tPEP, EV, AV, and EV/AV are rendered in table 2 as well ('single operator reproducibility'). For these VTCs the total RMS was 2.8%.
3.2. Volume–time curves
The volume–time curves for the three subgroups are represented in figure 4. Each graph shows the volume change in the LV during one cardiac cycle, as function of time (in milliseconds). This volume change is defined with respect to the end-diastolic volume at t = 0. Hence, by definition, at t = 0 the volume change is 0 ml in each curve. The VTCs of the five young healthy volunteers (age 24.4 ± 1.7 years) in figure 4(a) shows the presence of an ICP. In addition, a strong early filling phase with respect to the late (atrial) filling phase (high EV/AV ratio) is visible. Figure 4(b) represents the older healthy volunteers (age 51.8 ± 8.9 years). In these curves, the ICP is present directly after t = 0, but in contrast to the younger volunteers, there is a larger contribution of the atrial filling phase (lower EV/AV ratio). In figure 4(c) (five patients with mitral regurgitation) the ICP is absent. Furthermore, the curve of the symptomatic MR patient shows a relatively large duration of the systole (LVET), distinctively different from duration of the systole in the asymptomatic patients.
Figure 4. (a) Volume–time curves generated by the HCP in five young healthy volunteers, age 24.4 ± 1.7 years. ICP: isovolumetric contraction phase. (b) Volume–time curves generated by the HCP in five older healthy volunteers, age 51.8 ± 8.9 years. (c) Volume–time curves generated by the HCP in five patients with severe MR, age 57.4 ± 7.7 years. In these patients, the ICP is absent; hence the X printed over the label 'ICP'.
Download figure:
Standard image High-resolution image3.3. Isovolumetric contraction phase
The presence or absence of an isovolumetric contraction phase in the VTC of the HCP was determined for all the subjects. This phase was present in all 10 healthy subjects without MR, while this phase was lacking in the VTC of all 5 mitral regurgitation patients.
3.4. Time parameters
Figure 5 shows the results of the LVET and tPEP assessed from the VTC's of the HCP, compared to the LVET and tPEP from the LVOT-PW Doppler images. For LVET, bias was −38.9 ms, upper limit of agreement was 17.9 ms, lower limit of agreement was −95.9 ms, and Pearson's R was 0.67 (p = 0.038).
Figure 5. (a) Left: Plot of the LVET measured using the HCP (vertical), as function of the LVET determined by the LVOT PW-Doppler (horizontal) for young (n = 5) and older (n = 5) volunteers. Squares: young volunteers. Triangles: older volunteer. Right: Bland–Altman plot of the same results. Bias was −38.9 ms, upper limit of agreement was 17.9 ms, lower limit of agreement was −95.9 ms. (b) The same plots, but now for tPEP. Bias was −9.6 ms, upper limit of agreement was 23.5 ms, lower limit of agreement was −42.7 ms.
Download figure:
Standard image High-resolution imageFor tPEP, the results were relatively poor, with bias, upper limit of agreement, lower limit of agreement equal to −9.6 ms, 23.5 ms, and −42.7 ms, respectively, and with Pearson's R equal to 0.35 with a p-value that was not significant (p = 0.32).
The LVET and tPEP, as measured by the HCP, both show a negative bias with respect to the results obtained using Doppler.
In mitral regurgitation patients, the isovolumetric contraction phase is lacking, and therefore the LVET cannot be established by the HCP in this specific group of patients. Hence the mitral regurgitation patients were not included in this specific graph.
3.5. EV/AV ratio
In figure 6, the results of the EV/AV ratio assessed using the HCP are rendered for all 15 subjects, as function of the EVTI/AVTI ratio according to echo PW Doppler of the LV outflow tract. Bias was −0.66, upper limit of agreement is 0.51, lower limit of agreement was −1.84.
Figure 6. Top: Results of the EV/AV ratio assessed using HCP, as function of the EVTI/AVTI according to echo PW Doppler of the LV outflow tract, for all 15 subjects. Squares: young volunteers. Triangles: older volunteers. Dots: mitral regurgitation patients. Bottom: Bland–Altman plot of the same results. Bias was −0.66, upper limit of agreement was 0.51, lower limit of agreement was −1.84.
Download figure:
Standard image High-resolution imageThe Pearson's R was 0.82 (p < 0.001). The EV/AV ratio, as measured by the HCP, shows a negative bias with respect to the results obtained using Doppler.
4. Discussion
In this paper, we present the assessment of VTCs by the hemologic cardiac profiler (HPC) in healthy volunteers and patients with severe mitral regurgitation (MR), and a pilot study on the accuracy of these curves, by comparing parameters derived from the VTCs from the HCP to the corresponding parameters derived from 2D echocardiography. Based on experiments in five young healthy volunteers, five older healthy volunteers and five patients with MR, we demonstrate that the HCP can produce VTCs with a high time resolution (200 points s−1), so that short-lived phenomena (e.g. presence or absence of an isovolumetric contraction phase) are clearly detectable. Furthermore, we have shown that, from the VTCs measured by the HCP, other important parameters can be distilled, such as e.g. the ratio of the volume of the early diastolic filling to the volume of atrial diastolic filling (EV/AV ratio, which provides information about diastolic function), and the left-ventricular ejection time (LVET, which provides information about systolic function). The HCP-derived parameters LVET and EV/AV ratio showed a significant correlation with the corresponding parameters obtained with 2D TTE (p-values < 0.05). There was no significant correlation between tPEP measured by HCP and measured by echocardiography. However, we should take into consideration that PW-doppler images have a frame-rate of 50 Hz, resulting in a measuring-interval of 20 ms (4 times larger than the HCP). This can cause a significant measurement error when measuring a short-lived phenomenon such as the tPEP (50–90 ms).
The VTC's as produced by the HCP were consistent with known phenomena from pathophysiology: in all VTC's of patients with MR the ICP was absent, as result of the regurgitant blood flow from the LV into the left atrium even before the opening of the aortic valve (i.e. before the end of the ICP). Furthermore, the EV/AV ratios as determined by the HCP in the healthy young volunteers, were significantly higher than the EV/AV assessed by the HCP in the older healthy volunteers. This is in accordance with the previously described phenomenon that diastolic function (and hence EV/AV ratio) is diminished in older people (Nagueh et al 2009). From the viewpoint of electrophysiology, the natural definition of the starting point of a cardiac cycle is the Q-peak of the ECG. Due to limitations in our current equipment however, we have taken the R-peak of the ECG as the origin (t = 0) in the VTCs in this paper.
We used echocardiography as a reference method for the assessment of the ICT, LVET, tPEP and EV/AV ratio. Echocardiographic measurements are known to suffer from inter- and intra-operator variability, caused by multiple factors. However, 2D-TTE was used as a reference technique in this study as it is the most commonly used technique in clinical practice nowadays.
In contrast to echocardiography, the HCP does not need an operator during the actual measurements; once the thorax-electrodes have been placed correctly, the HCP produces VTCs every 20 s continuously and automatically. However, a small amount of inter- or intra-operator variability can still be caused during the act of placing the electrodes on the skin. In order to test the reproducibility of the HCP, as well as the inter- and intra-operator variability due to differences in electrode placement, we performed multiple measurements in a healthy volunteer with and without replacing the electrodes. The reproducibility of the LVET is good (2.2% for single operator, and 4.4% for two operators, see table 2). The reproducibility of the EV is reasonably good as well (4.1% for single operator, and 4.9% for two operators), which however does not translate into equally favourable reproducibility data for the EV/AV ratio, because of the small values of AV in the denominator in EV/AV. Based on our results, we can assume that the HCP measurements are consistent, with a reasonably low inter- and intra-operator variability. This is especially important for accurate follow-up and monitoring of patients. Larger studies are needed to confirm our results and assess the exact extent of the inter- and intra-operator variability.
4.1. Applicability of the HCP
Nowadays, screening for cardiac diseases is increasingly performed in the general population (e.g. to prevent sudden cardiac death), and in several patient populations to prevent or predict cardiac involvement. Cardiac function screening is often restricted to ECG analysis, because other diagnostics, e.g. echocardiography, are too expensive and time-consuming. For a more comprehensive evaluation of the cardiac function however, electrophysiological data need to be combined with hemodynamic data. The HCP provides hemodynamic data in the form of VTCs, and, furthermore, the HCP is designed to work in tandem with the ECG: as has been explained in the method (section 2.1), the HCP electrodes are compatible with the ECG electrodes, and ECG and HCP signals are recorded simultaneously. As a result, the HCP technology could, potentially, be able to screen more intensively for cardiac diseases in situations were precise screening is desirable; such as in preoperative screening, sport screening, or cardiac function screening in patients before administration of chemotherapy.
In preoperative screening, the challenge for anesthesiologists is that patients with significant cardiovascular disease often do not present preoperative abnormalities in vital signs, ECG, or physical examination. In the future, HCP could, potentially, be used to easily pick out patients with silent cardiovascular disease of significance to anesthesia.
In athletes, hypertrophic cardiomyopathy is a leading cause of sudden cardiac death. ECG screening seems to introduce a large number of false-positive tests. Combining HCP with ECG however may have a role in improving sport screening in the future.
In cancer patients, baseline cardiac function screening with echocardiograms is frequently performed before administration of anthracycline and monoclonal antibodies chemotherapy, due to the concern for potential cardiotoxicity. Moreover, advanced heart failure could be averted with better and earlier detection using other techniques than just the ECG. However, at a population level, these additional cardiac screening tests (e.g. radionuclide imaging, cardiac MRI) can add up to significant costs for both patients and the health care system. We anticipate that the HCP technology is lower in costs compared to current cardiac function screening tests, and could therefore potentially be used on a wider scale in cancer patients, provided that future clinical trials confirm the added diagnostic value of the HCP in cancer patients.
4.2. Comparison to competing techniques
In current clinical practice, mitral regurgitation is often detected by the presence of a murmur during physical examination. This however has two major drawbacks:
First, the diagnostic accuracy of cardiac auscultation varies widely among clinicians. Mangione et al (1993) found that the diagnostic accuracy of cardiac auscultation ranged from 0% to 56.2% for cardiology fellows (median 21.9%) and from 2% to 36.8% for medical residents (median 19.3%). Furthermore, studies in family practitioners, academic internists, and general internists all demonstrate a proficiency of no more than 40% in recognizing basic heart murmurs (Roy et al 2002, Ommen and Nishimura 2004).
Secondly, a systolic murmur is not always observed in the presence of MR, and the intensity of a murmur is not used to indicated severity of disease (Maganti 2010). Barron et al (1988) compared auscultation to Doppler ultrasound for the detection of MR, and found that a murmur was absent in 13 of the 24 patients with isolated MR (sensitivity 53%). In addition, Varadarajan et al (2006) showed that grade 3 MR or grade 4 MR is common and present in patients with congestive heart failure; however, MR was silent on physical examination in 75% of these patients.
The HCP may be able to detect silent MR in a screening setting, because the HCP is based on direct volumetric measurements, whereas detection of MR on the basis of a murmur relies on the presence of a 'secondary' phenomenon (i.e. the production of a murmur) that may or may not be produced in case of MR.
4.3. Limitations and future work
Given the small number of patients (5 patients in each group), the study presented in this paper is merely a first pilot study on a new technique (the HCP).
Furthermore, the present HCP hardware still has sub-optimal noise suppression properties. An improved, portable, lightweight, and miniaturized version of the HCP equipment is currently being built. In this portable version, all electronics of the HCP are contained in a small flat box that is attached to the waist belt.
In the literature, the ratio tPEP/LVET is mentioned as an important parameter; e.g. Reant et al (2010) indicate that the ratio tPEP/LVET could prove to be relevant in the selection of patients requiring cardiac resynchronization or cardiac implantable defibrillator, especially when precise LVEF evaluation by echocardiography is difficult. In our study, the assesment of the tPEP is still poor using the current version of the HCP. We expect this to improve when, in the future, a small portable version of the HCP will be used. This however needs to be subject of a future study.
In order to assess the reproducibility of parameters like LVET and tPEP (as measured by HCP) properly, it needs to be placed in the context of data about reproducibility in echocardiography. Using two experienced observers, Reant et al (2010) found that the mean intra-observer variability was 2% for tPEP and 4% for LVET (calculated by averaging the differences between 10 measures). Based on two different acquisitions in 20 patients, mean intra-observer variability was 7.6% for PEP and 4.3% for LVET. Inter-observer variability was 3% for PEP and 4.5% for LVET (in data obtained for 10 patients). In an other study (Polak et al 2015), the inter-reader reproducibility for measuring LVET was determined by blinded rereadings of 14 cases by a second reader and gave a correlation coefficient of 0.78 (95% CI, 0.43–0.93). The intra-reader reproducibility was estimated by blinded readings in a separate group of 24 patients and gave a correlation coefficient of 0.94 (95% CI, 0.88–0.98). With respect to the reproducibility data for echocardiography above, the reproducibility data for the HCP are relatively weak. However, it will depend on the specific application of the HCP in a screening context to what extend the reproducibility of the HCP will prove to be sufficient.
Given the limitations of this pilot study, various future studies will need to be performed in order to assess the actual clinical value of the HCP. For example, a statistically powered diagnostic accuracy study is needed, as well as a clinical utility study for various applications of the HCP as a screening tool.
5. Conclusion
The HCP seems to be a method for reproducible VTC generation with low operator- dependency, although according to some studies, its reproducibility is still inferior to that of echocardiology. However, in the future, further study is needed to improve the following two aspects: (i) reproducibility of the parameters estimated by HCP, and (ii) agreement between HCP and echocardiography. The ability of the HCP to assess changes in cardiac function may lead to the use of the HCP as an screening and monitoring tool in patients with cardiac diseases, and may facilitate accurate diagnosis and easy VTC comparison during follow-up. Furthermore, in the future, the high temporal resolution, light-weight nature and expected low costs of the system, could potentially make the HCP a suitable tool for early detection of cardiac diseases, and possibly also for prolonged and continuous (tele)monitoring in large numbers of patients.
Acknowledgments
The authors would like to thank T Mulder, MD of the UMC Utrecht for her kind advice and assistance.
: Appendix
Since blood is a better electric conductor than the surrounding tissues, the displacement of blood inside the heart during the cardiac cycle produces small changes in the measured amplitudes. These changes oscillate in sync with the rhythm of the beating heart, and differ for each combination of two measuring electrodes. For instance, the electrodes on the upper part of the sternum near the solid rectangle D in figure 1, are more sensitive to volume changes in the atria, whereas the electrodes on the lower part of the thorax (inside the ellipse E in figure 1) are more sensitive to volume changes in the ventricles. This is caused by the lower location of the ventricles inside the thorax compared to the atria. Consequently, the location of the 'ventricular epicenter' of the changes in the applied current field, due to the ventricular volume changes, differs from the location of the 'atrial epicenter' corresponding to atrial volume changes. Let x be an enumeration (x running from 1 to 8) of all electrodes below (outside) the solid rectangle D in figure 1, i.e. let x be an index for each measuring electrode pair (because the upper electrode of each electrode pair is invariantly located inside the solid rectangle D). For each value of x, there is a specific voltage change ('sensitivity'), resulting from a volume change of exactly 1 ml in exclusively the atria. We refer to the combined set of these atrial sensitivity values for all eight values of x as the 'spatial atrial sensitivity pattern'. This pattern can be written as one single vector S(atr) with 8 slots. Similarly, a 'spatial ventricular sensitivity pattern' S(ven) is defined, referring to the specific voltage change ('sensitivity') that results from a volume change of 1 ml in exclusively the ventricles.
Since the volumes of the atria and the ventricles change simultaneously during the cardiac cycle, and in addition extracardial changes in blood volume occur as well, the actual set of measured voltage changes during the cardiac cycle of a patient or healthy volunteer can be considered as a linear superposition of three different patterns:
- (i)the 'spatial atrial sensitivity pattern' S(atr) (which is known on the basis of modelling), multiplied by an unknown amount ΔV(atr)(t) of milliliters volume change in the atria, and
- (ii)the ' spatial ventricular sensitivity pattern' S(ven) (which is also known on the basis of modelling), multiplied by an unknown amount ΔV(ven)(t) of milliliters volume change in the ventricles, and
- (iii)a pattern Pextracardial due to extracardial effects, such as diameter changes in large blood vessels outside the heart.
This linear superposition of the three spatial patterns is represented in the following equation (Konings et al 2012):
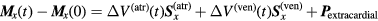
in which M represents the 'measured vector' with eight slots, and in which Mx(t) is the contents of the xth slot of M at time t, which equals the (normalized) voltage difference measured between electrode pair number x at time t. Furthermore, Mx(0) equals the (normalized) voltage difference measured between electrode pair number x at the moment of the R-peak of the ECG, and ΔV(atr)(t) and ΔV(ven)(t) are the volume changes (with respect to the volume at the moment of the R-peak of the ECG) in the atria and the ventricles, respectively.
We need to establish the volume changes in exclusively the ventricles to define a ventricular VTC. Total heart volume variation is a poor indicator of the ventricular stroke volume, and therefore not appropriate. An algorithm has been designed to distill the ΔV(ven)(t) (i.e. the ventricular VTC) from the equation above, using the measured M(t) − M(0) in combination with the known S(atr) and S(ven) as the input (Konings et al 2012). The HCP system performs this calculation automatically and continuously.
Furthermore, the HCP has been designed to work in tandem with the ECG: its electrodes are compatible with the ECG electrodes, and ECG and HCP signals are recorded simultaneously, in order to combine the electrophysiological data form the ECG with the hemodynamic (volumetric) data from the HCP.
The HCP can be used in two modes: (i) VTC assessment mode, in which the data from four measurement periods of 20 s each are combined to assess the VTC of a human subject in rest, and (ii) a monitoring mode, in which every 20 s an update is provided of the left ventricular stroke volume (as a percentage of the initial stroke volume), using a moving median over the last 2 or 3 periods. This latter mode can be used to monitor the stroke volume (and cardiac output) of a patient over several hours during daily life activities. In this paper however, only the VTC assessment mode (i) has been used.







