Abstract
In this study, TiO2 nanocolloids were successfully fabricated in deionized water without using suspending agents through using the electric spark discharge method at room temperature and under normal atmospheric pressure. This method was exceptional because it did not create nanoparticle dispersion and the produced colloids contained no derivatives. The proposed method requires only traditional electrical discharge machines (EDMs), self-made magnetic stirrers, and Ti wires (purity, 99.99%). The EDM pulse on time (Ton) and pulse off time (Toff) were respectively set at 50 and 100 μs, 100 and 100 μs, 150 and 100 μs, and 200 and 100 μs to produce four types of TiO2 nanocolloids. Zetasizer analysis of the nanocolloids showed that a decrease in Ton increased the suspension stability, but there were no significant correlations between Ton and particle size. Colloids produced from the four production configurations showed a minimum particle size between 29.39 and 52.85 nm and a zeta-potential between −51.2 and −46.8 mV, confirming that the method introduced in this study can be used to produce TiO2 nanocolloids with excellent suspension stability. Scanning electron microscopy with energy dispersive spectroscopy also indicated that the TiO2 colloids did not contain elements other than Ti and oxygen.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Because of TiO2's characteristics, such as stable physical and chemical properties, corrosion resistance, and photocatalytic effect [1–4], it has been used for various environmental protection purposes including air purification and antibacterial or antifungal processes in recent years [5–7]. Because TiO2's photocatalytic effect increases as particle size decreases, TiO2 nanoparticles (NPs) have superior oxidation abilities in decomposing organic pollutants and killing bacteria [8–10]. TiO2 NPs have superior photocatalytic properties as well as antiseptic properties and can provide ultraviolet shielding [11, 12]. Because of these advantages, they have a broad range of applications, including photocatalysts, sunscreen, dye sensitized solar cells, biomedical materials, and anticancer medical treatments [13–18]. Although TiO2 can be used in cosmetics, medical treatment, and the remediation of environmental pollution, TiO2 NPs that contain other derivatives may cause environmental pollution as well as adverse effects to humans. Therefore, the search for a TiO2 NP fabrication method that is toxicity-free to humans and the environment has become a critical issue in nanomaterial research.
At present, TiO2 NP production methods mainly comprise physical and chemical methods. Although chemical methods are the most common, they require suspending agents during production. This creates derivatives, thus limiting the applicability of TiO2 NP in some domains [19–23]. For example, when TiO2 NPs with derivatives are used as a photocatalyst, they cause secondary pollution to the environment. In addition, this production method is subject to problems such as contamination, producing fabricated samples that are difficult to collect, and creating NP dispersion in the production environment. Many studies have indicated that exposure to NPs is harmful to humans; in particular, inhalation will lead to diseases such as lung lesions [24]. Therefore, solving the problem of NP dispersion and identifying methods to create green energy have become critical issues in the field of nanotechnology [25, 26]. A recent study on producing TiO2 nanocolloids with electrical discharge machines (EDMs) operating on the electric spark discharge method (ESDM) has been published [27]. This production method is physical method-based. Compared with chemical methods, the ESDM entails a simpler, faster, process, less complex material composition, easier method for collecting NPs, and favorable environmental friendly results. However, this method requires the use of pressure equalization and coolant circulation systems to maintain low temperatures and a vacuum pressure-based production process. In addition, an ultrasonic oscillator is required to rapidly condense vaporized Ti into nanoscale particles. Thus, this method is expensive. Reducing ESDM-related equipment costs, however, will make it more competitive as a TiO2 NP fabrication method. In this study, the ESDM was improved to produce TiO2 nanocolloids at room temperature and under normal atmospheric pressure. The new method requires only traditional EDMs and self-made magnetic stirrers for equipment, thus significantly reducing the production costs. EDM-related studies have demonstrated the considerable effect of time parameters (i.e., Ton and Toff) on material removal rates. Thus, in the present study, Ton and Toff were adjusted during the production process to determine how they are related to NP size and suspension stability [28].
2. Material and methods
Currently, many TiO2 NP production methods cause problems such as secondary pollution, fabricated samples containing other derivatives, and high production costs. To solve these problems, the present study utilized the ESDM as a basis for developing a green energy-saving process for producing TiO2 NPs. The method has low production costs and achieves excellent output efficiency. The TiO2 NP production principles, framework, and parameters are summarized in the following sections.
2.1. Principles of the ESDM
EDMs convert electrical energy into heat to melt and vaporize workpiece surfaces in order to remove unwanted materials. Workpieces that discharge periodically enable fine etching, making EDM suitable for precision machining [29, 30]. The use of EDMs to melt metal surfaces to obtain nanocolloids is called the ESDM (a.k.a. the electrochemical method) [31, 32], which involves immersing the upper and lower electrodes in a dielectric fluid charged with impulse voltage at frequencies ranging between several thousand times per second to several hundred thousand times per second. The upper and lower electrodes are the electrodes and workpieces of the EDM, respectively, and are non-nano metallic wires, the material of which is the same as the material of the nanocolloids to be fabricated. Subsequently, an EDM servo control system is used to control the distance between the electrodes, allowing the electrodes to form electric arcs. These arcs generate high temperatures that evaporate electrode surfaces. Finally, the dielectric fluid condenses the vaporized metal vapor into NPs. During Ton, the discharge circuit consists of fixed electrical resistance (Rfix; found in a circuit) and dielectric fluid electrical resistance (Rdie; found in electrode gaps) connected in series with a dc voltage source (VDC). The voltage (Vieg) and current (Iieg) between electrode gaps are expressed as follows:
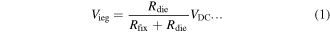
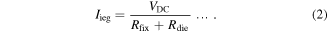
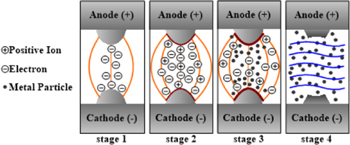
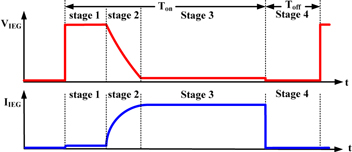
During Toff, no voltage is applied between electrodes. Thus, Vieg and Iieg equal 0. Figures 1 and 2 show the electrode discharge situations and the Vieg and Iieg when nanocolloids are fabricated using the ESDM. In the figures, Stages 1, 2, and 3 signify the early, middle, and late stages of nanocolloids fabrication during Ton, whereas Stage 4 denotes Toff. During Ton, dielectric fluid gradually ionizes because of the electric field effect, which causes Rdie to gradually decrease. This prompts a decrease in Vieg and an increase in Iieg. During Stage 1, dielectric fluid shows high electrical resistance; only a traceable amount of current is observed passing through the tips of the electrodes. During Stage 2, Rdie decreases, causing Iieg to increase. During Stage 3, Rdie approaches 0 Ω, resulting in Vieg becoming close to 0 V. When this occurs, Iieg reaches its maximum and electric arcs form between the electrodes. These arcs generate high temperatures that evaporate nearby electrode materials into metal vapor and vaporize dielectric fluids between the electrode gaps. The pressure created during the vaporization and expansion process forces metal vapor to scatter between dielectric fluids. Finally, the metal vapor is rapidly cooled by the relatively cooler dielectric fluids in the nearby environment before condensing into microparticles or NPs and suspending in the dielectric fluids. During Stage 4, Vieg and Iieg equal 0 and the relatively cooler dielectric fluid flows into the electrode gaps, washing away particles remain in the gaps and absorbing the residual heat. Therefore, the electrode gap will gradually return to an insulated state. Through implementation of the aforementioned periodic discharge process, nanocolloids can be produced. Figure 3 shows the discharge voltage and current between electrode gaps when using the ESDM to fabricate TiO2 nanocolloids with Ton–Toff values of 50–50 μs. The discharge failure is caused by the electrode gap does not return to an insulated state in the Stage 4 of the previous discharge cycle.
Figure 1. Electrode discharge when using the ESDM to fabricate nanocolloids.
Download figure:
Standard image High-resolution imageFigure 2. Vieg and Iieg when using the ESDM to fabricate nanocolloids.
Download figure:
Standard image High-resolution imageFigure 3. Discharge voltage and current when using the ESDM to fabricate TiO2 nanocolloids with Ton–Toff values of 50–50 μs.
Download figure:
Standard image High-resolution image2.2. TiO2 nanocolloid production framework for nanocolloids fabricated using the ESDM
The EDM and control panel for producing TiO2 nanocolloids are shown in figures 4(a) and (b), respectively. The servo knob (Servo) and sensitivity knob (Sens) in the control panel were used to control motor speed and speed sensitivity, respectively. The two knobs can be adjusted to maintain the electrode gaps at 30 μm. Ton and Toff were used to establish the pulse discharge and termination times. Increases in Ton caused the energy density at the electrode discharge location to decrease as the discharge column expanded [33], whereas increases in Toff allowed the electrode gaps to resume their insulated properties. The IP knob (IP) was set to control the size of the discharge current. Changes in the discharge current controlled the energy size of the discharge columns, thereby affecting the material removal effect. Capacitor parameters were set to control the energy levels at the moment of discharge.
Figure 4. (a) EDM (b) EDM control panel.
Download figure:
Standard image High-resolution imageFigure 5 shows the TiO2 nanocolloids production framework (when the nanocolloids were fabricated using the ESDM). The upper and lower electrodes were made using Ti wires with a diameter of 1 mm and purity of 99.99%. The dielectric fluid was 40 ml of deionized water (DW). The impulse power source needed by the discharge circuit comprised gate drive circuits, power transistors, and a dc voltage source. The gate drive circuit generates periodic gate signals on the basis of the Ton and Toff times, which are set by the user, whereas the power transistors are rapidly turned on and off according to the gate signals. Changing between the various power transistor statuses divides the input dc voltages into the impulse voltages that the electrodes required during the discharge process. The electrode discharge situations depend on electrode gaps. If the electrode gaps are too large, the discharge circuit acts like an open circuit and arcing will not occur between electrodes. In contrast, if the electrode gaps are too small, particles between the gaps cannot be expelled effectively from the gaps. Too many particles lingering in the gap can cause adhesion between the upper and lower electrodes. The servo control system was used to control the electrode gaps, which utilized the feedback signals sent by the electrode current in the electrode gaps to fine-tune the position of the upper electrode to ensure that the electrode gaps maintain favorable discharge spacing. At a width of approximately 30 μm, the electrode gap allowed the electrodes to discharge electric arcs. The high temperatures of the electric arcs melted electrode surfaces and forced metal vapor to scatter between the DW, causing the metal vapor to cool and condense into Ti NPs. The magnetic stirrer below the container (figure 5) was made of a permanent magnet fixed to a fan. When the fan rotated, the permanent magnet caused the magnetic stirrer to turn, enabling NPs to be dispersed evenly in the DW.
Figure 5. Framework of fabricating TiO2 nanocolloid using the ESDM.
Download figure:
Standard image High-resolution image2.3. Production parameter planning
When electrode gaps have sufficient discharge spacing, the electrode gap discharge effects are mostly influenced by Ton and Toff, in which the length of Ton affects the electric arc column size as well as the energy that can be sustained per unit area of electrode material during discharge. Thus, Ton affects the production process efficiency and properties of the produced TiO2 nanocolloid. Increases in Toff diminish production efficiency, whereas reduced Toff causes electrode gaps to contain excess residual heat and metal particles which cannot be washed away in time, preventing the electrode gaps from returning to an insulated state before the next pulse discharge. A markedly short Toff leads to electrode short circuits, causing the upper and lower electrodes to stick together. To understand the effect of production process time on TiO2 nanocolloid production performance, in this study, TiO2 nanocolloids were fabricated using four production configurations, which featured four pairs of Ton–Toff values (50–100 μs, 100–100 μs, 150–100 μs, and 200–100 μs). All production processes were conducted at normal temperature and pressure; the production time was 5 min. The EDM parameters employed during the production processes are shown in table 1.
Table 1. Production parameters for TiO2 nanocolloids.
| Voltage | 140 V |
| Current setting (IP) | 1 A |
| Capacitor | 0.1 μF |
| Pressure | 1 atm |
| SERVO. | 1/2 |
| SENS. | 1/2 |
3. Results and discussion
To identify the properties and compositions of the TiO2 nanocolloids produced in this study, precision instruments, such as a Zetasizer and field-emission scanning electron microscopy with energy dispersive spectroscopy (FE-SEM/EDX), were used to analyze their suspension stability, particle morphology, and composition, and examine the effects of Ton–Toff values on the properties of these nanocolloids from these results. A field-emission transmission electron microscope (FE-TEM) and x-ray diffraction (XRD), were used to investigate the microstructure and phase composition of fabricated colloid particles.
3.1. Particle size and suspension stability of the TiO2 nanocolloid
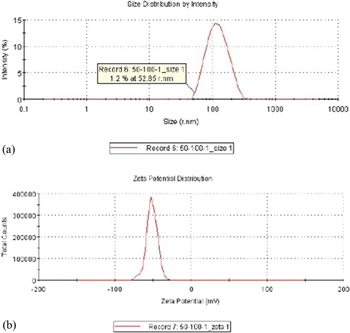
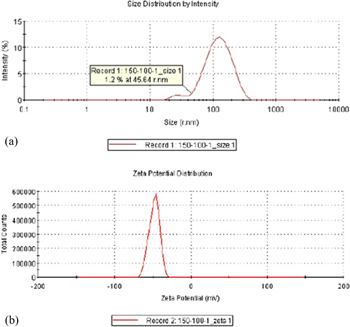
In this study, a Zetasizer (Malvern Zetasizer, Nano-ZS90, Worcestershire, UK) was used to analyze the particle sizes and suspension stability of the TiO2 nanocolloid. The analysis results are shown in figures 6–9 and table 2. Figures 6–9 show the zeta potentials obtained at Ton–Toff values of 50–100 μs (−51.2 mV), 100–100 μs (−50.5 mV), 150–100 μs (−48.0 mV), and 200–100 μs (−46.8 mV). The smallest particle sizes for the four production processes were 52.85, 39.41, 45.64 and 29.39 nm, respectively. All four production processes generated an average particle size of approximately 100 nm and contained a high ratio of NPs, validating the favorable suspension stability of the TiO2 nanocolloids developed in this study. Table 2 shows that at a Toff of 100 μs and Ton between 50 and 200 μs, changes in Ton exerted a nonsignificant effect on colloid particle size. However, the absolute value of the zeta potential decreased as Ton increased. Thus, increases in Ton decreased the suspension stability of the colloids.
Figure 6. (a) Particle size distribution and (b) zeta potential of TiO2 nanocolloids produced with Ton–Toff values of 50–100 μs.
Download figure:
Standard image High-resolution imageFigure 7. (a) Particle size distribution and (b) zeta potential of TiO2 nanocolloids produced with Ton–Toff values of 100–100 μs.
Download figure:
Standard image High-resolution imageFigure 8. (a) Particle size distribution and (b) zeta potential of TiO2 nanocolloids produced with Ton–Toff values of 150–100 μs.
Download figure:
Standard image High-resolution imageFigure 9. (a) Particle size distribution and (b) zeta potential of TiO2 nanocolloids produced with Ton–Toff values of 200–100 μs.
Download figure:
Standard image High-resolution imageTable 2. Particle size and zeta potential analysis of nanocolloids (using Zetasizer) fabricated using the four production processes.
| Ton–Toff (μs) | Average particle size (nm) | Minimum particle size (nm) | Zeta potential (mV) |
|---|---|---|---|
| 50–100 | 111.9 | 52.85 | −51.2 |
| 100–100 | 107.4 | 39.41 | −50.5 |
| 150–100 | 104.4 | 45.64 | −48.0 |
| 200–100 | 105.5 | 29.39 | −46.8 |
3.2. Morphology, structure and composition analysis of TiO2 nanocolloids
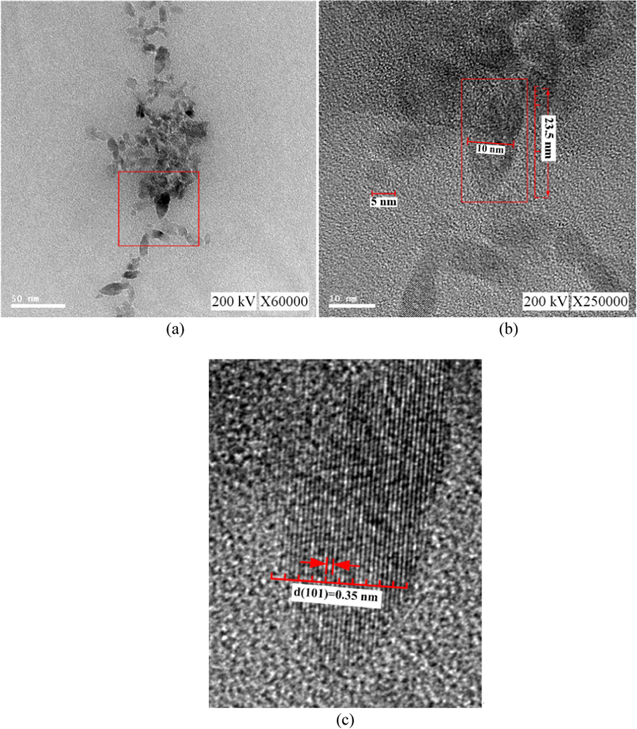
The morphologies and compositions of colloid particles were studied through FE-SEM (Hitachi, S-4700/JSM-6510LV, Tokyo, Japan)/EDX (51-ADD0076). Because smaller particle sizes produced more blurry SEM images, NPs with relatively larger minimum particle sizes were selected for SEM imaging. The Zetasizer analysis showed that TiO2 nanocolloids fabricated with Ton–Toff values of 50–100 μs and 150–100 μs produced larger minimum particle sizes. The surface morphologies of the two samples are shown in figures 10(a) and (b), respectively. According to figure 10, the colloid particles were spherical in shape. Because the colloid particles were processed using a centrifuge before the SEM images were taken, the centrifuged NPs aggregated to form larger particles. Therefore, the particle sizes of the colloid particles in figure 10 are larger than those shown in figures 6 and 8. To determine the composition of the colloid particles, colloid samples produced with a Ton and Toff of 150 and −100 μs (respectively) were analyzed through FE-SEM/EDX (figure 11). The EDX spectra showed that the composition of the TiO2 nanocolloids consisted of Ti, C, and O, in which the carbon was the carbon glue used during SEM imaging and the O was derived from water. This result indicated that the colloid fabricated was TiO2. The microspheres of fabricated colloid particles with Ton–Toff values of 50–100 μs were studied through FE-TEM (JEOL, JEM-2100F, Tokyo, Japan) at an accelerating voltage of 200 kV (figure 12). Figure 12(b) is the magnified view of subregion indicated by the red squares in figure 12(a). It shows that the fabricated colloid particles with sizes between 5 and 23.5 nm. Figure 12(c) is the magnified view of subregion indicated by the red squares in figure 12(b). It shows that the lattice distance of 0.35 nm is a good agreement with (101) lattice distance of anatase TiO2. The phase composition of fabricated NPs with Ton–Toff values of 50–100 μs were studied through XRD (Empyrean, PANalytical, the Netherlands, CuKα radiation at 45 kV). The XRD patterns of the samples (figure 13) showed that the fabricated NPs were anatase (Ti4O8), titanium (Ti) and titanium oxide (TiO, Ti2O3 and Ti7O13). The peak positions (2θ) and Miller indices of the largest 5 intensities for each component of the sample are listed as shown in table 3. The obtained anatase TiO2 NPs are suitable for photocatalytic reactions due to their high specific surface and structural properties [34].
Figure 10. FE-SEM image of the TiO2 nanocolloids with Ton–Toff values of: (a) 50–100 μs (b) 150–100 μs.
Download figure:
Standard image High-resolution imageFigure 11. EDX spectra of the TiO2 nanocolloids with Ton–Toff values of 150–100 μs.
Download figure:
Standard image High-resolution imageFigure 12. (a) TEM images of fabricated colloid particles with Ton–Toff values of 50–100 μs (b) partial magnification of figure 12(a) as indicated in the red square. (c) Partial magnification of figure 12(b) as indicated in the red square.
Download figure:
Standard image High-resolution imageFigure 13. XRD pattern of the fabricated colloid with Ton–Toff values of 50–100 μs.
Download figure:
Standard image High-resolution imageTable 3. The peak positions (2θ) and Miller indices of the largest 5 intensities for each component of the sample.
| Compound name | Chem. formula | The positions 2θ and Miller indices (h k l) of the largest five intensities | ||||
|---|---|---|---|---|---|---|
| Anatase | Ti4O8 | 25.2° (1 0 1) | 37.5° (0 0 4) | 47.9° (2 0 0) | 53.5° (1 0 5) | 54.9° (2 1 1) |
| Titanium | Ti | 40.4° (1 0 1) | 35.3° (1 0 0) | 38.4° (0 0 2) | 53.2° (1 0 2) | 63.2° (1 1 0) |
| Titanium oxide | TiO | 43.2° (2 0 0) | 37.2° (1 1 1) | 62.7° (2 2 0) | 75.2° (3 1 1) | 79.2° (2 2 2) |
| Titanium oxide | Ti2O3 | 35.0° (1 0 0) | 32.7° (1 0 4) | 23.8° (0 1 2) | 53.2° (1 1 6) | 62.7° (3 0 0) |
| Titanium oxide | Ti7O13 | 29.7° (−1 2 5) | 28.4° (1 −2 1) | 26.5° (0 2 6) | 23.5° (1 0 1) | 35.8° (1 0 13) |
4. Conclusions
In this study, TiO2 nanocolloids were fabricated using EDMs under normal temperature and pressure. This production method did not create NP dispersion and the produced colloids contained no derivatives, making the TiO2 NP production process green and suitable for energy conservation. The TiO2 NPs produced by the proposed method do not contain chemical agents. Therefore, when they are used in photocatalysis to decompose organic compounds, secondary pollution of the environment can be avoided. The colloids produced from the four production configurations had minimum particle sizes of 29.39–52.85 nm, an average particle size of approximately 100 nm, and a zeta potential between −51.2 and −46.8 mV, confirming that the proposed method is suitable for developing TiO2 nanocolloids with excellent suspension stability. The surface morphology images obtained through FE-SEM showed that the colloid particles were spherical in shape, whereas the SEM-EDX analysis indicated that the TiO2 colloids contained only Ti and O. The study results are summarized as follows:
- (1)TiO2 nanocolloids with excellent suspension stability were successfully produced in DW by using EDMs at normal temperature and pressure. In addition, no surfactants were added during the production process.
- (2)The fabricated TiO2 nanocolloids contained no other derivatives, signifying that the production of these nanocolloids will not create secondary pollution when applied to various fields, and that these nanocolloids are not harmful to humans.
- (3)When using EDMs, setting Toff to 100 μs and Ton to 50–200 μs increases the suspension stability of the TiO2 nanocolloids as Ton decreases. However, no significant correlations were observed between Ton and particle size.
- (4)The proposed method features such advantages as a simpler production process and easier method for collecting NPs. In addition, only an EDM and a magnetic stirrer are required for production, thus reducing production costs while improving output efficiency.
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.