Abstract
The criticality of raw materials has become an important issue in recent years. As the supply of certain raw materials is essential for technologically-advanced economies, the European Commission and other international counterparts have started several initiatives to secure reliable and unhindered access to raw materials. Such efforts include the EU Raw Materials Initiative, European Innovation Partnership on Raw Materials, US Critical Materials Institute, and others. In this paper, the authors present a multi-faceted and multi-national review of the essentials for the critical raw materials (CRMs) Co, Nb, W, and rare earth elements (REEs). The selected CRMs are of specific interest as they are considered relevant for emerging technologies and will thus continue to be of increasing major economic importance. This paper presents a 'sustainability evaluation' for each element, including essential data about markets, applications and recycling, and possibilities for substitution have been summarized and analysed. All the presented elements are vital for the advanced materials and processes upon which modern societies rely. These elements exhibit superior importance in 'green' applications and products subject to severe conditions. The annual production quantities are quite low compared to common industrial metals. Of the considered CRMs, only Co and REE gross production exceed 100 000 t. At the same time, the prices are quite high, with W and Nb being in the range of 60 USD kg−1 and some rare earth compounds costing almost 4000 USD kg−1. Despite valiant effort, in practice some of the considered elements are de facto irreplaceable for many specialized applications, at today's technological level. Often, substitution causes a significant loss of quality and performance. Furthermore, possible candidates for substitution may be critical themselves or available in considerably low quantities. It can be concluded that one preferred approach for the investigated elements could be the use of secondary resources derived from recycling. W exhibits the highest recycling rate (37%), whereas Co (16%), Nb (11%) and rare earths (~0%) lag behind. In order to promote recycling of these essential elements, financial incentives as well as an improvement of recycling technologies would be required.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Abbreviations
| C | Natural graphite |
| PGE | Platinum group metals (Ru, Rh, Pd, Os, Ir, Pt) |
| Rubber | Natural Rubber |
| LREE | light rare earth elements (see table 3) |
| HREE | heavy rare earth elements (see table 3) |
| PR | Phosphare rock |
| SW | Sapele wood |
| NC | Natural cork |
| NT | Natural teak |
| CC | Coking coal |
| Gyp | Gypsum |
| BT | Bentonite |
| Agg | Aggregates |
| LS | Limestone |
| Si sand | Silicon sand |
| DT | Diatomite |
1. Introduction
The present paper presents a 'sustainability evaluation' of selected critical raw materials (CRMs), by elucidating the availability, critical nature and analysis of production value chains and downstream processes of cobalt (Co), niobium (Nb), tungsten (W) and the rare earth elements (REEs). The selected CRMs are of specific interest as they are considered relevant for emerging technologies and will thus continue to be of increasing major economic importance.
The European Commission (EC) has acknowledged the importance of the accessibility of selected raw materials to the economy of the European Union (EU) (EC 2014). Globally, industrialised economies such as the United States (US) and Japan likewise consider the availability of raw materials strategically important for various industries and technologies, exemplified by the EU-US-Japan trilateral dialogue on CRMs, the regular EU-US-Japan Trilateral Conferences on Critical Materials, as well as the Critical Materials Institute (CMI) based at the US Department of Energy Ames Laboratory. The US National Intelligence Council requested a 2013 report from the RAND Corporation specifically on the topic of Critical Materials: Present Danger to US Manufacturing (Silberglitt et al 2013). One pioneering attempt of the EU to tackle this issue was the Raw Materials Initiative (RMI) in 2008 (EC 2008). It was recognized that several industrial sectors such as construction, chemicals, automotive, aerospace, machinery and equipment are strongly dependent on mineral raw materials. The major importance is emphasized by the fact that the above-mentioned industries provide jobs for at least 30 million people and result in a total value added of over 1 trillion euros (EC 2016). Therefore, the possibility to address CRMs as a priority can be based on strategic, scientific, industrial and economic importance.
In 2010 the EC established a list of economically significant raw materials, which exhibit a high risk of supply interruption (EC 2010c). Furthermore, the EC has published lists of CRMs, updated periodically, based on market developments; the first list included 14 CRMs (EC 2011a); the second 20 CRMs (EC 2014); and the third 27 CRMs (EC 2017c). From the environmental as well as market perspective, it is essential to characterize the scope of the problem and propose alternative solutions. It can be advantageous for industrialised high-technology countries to continually simultaneously develop scientific knowledge and know-how pertinent to CRMs, as applied advanced technology industries (including small-medium enterprises, automotive, aerospace, energy, and other sectors) ensure suitable innovation paths to higher technology readiness levels, from the laboratory scale to the market.
In assessing the merits of potential substitution materials and processes, it is essential to analyse pertinent materials and energy flows for any proposed processes. All input streams, recycling routes and emissions—gaseous, liquid and solid—should be taken into consideration. It is furthermore important to consider not only the quantity, but the quality (toxicity) of emissions. The aim is to minimize the consumption of resources and (non-renewable) energy as well as the negative impact on environment and health. Generally, such analysis is performed by scientists working in the area of life cycle assessment and/or value chain analysis.
In addition to material- and energy-efficiency, the economic viability of the proposed CRM substitution processes is essential to consider. As the goal of advanced research into CRM substitution is to design routes for new materials to enter the markets, it is helpful to cooperate with industry along the value chain. Environmental benefits of potential technologies can be realized in practice if they are viable from an economic point of view.
The RMI asserts that new strategies for closing the loop and minimizing the demand for virgin materials are essential to sustain high-technology industries and maintain economic progress (EC 2008). The analysis of the applications, usage, reserves and trends for various CRMs (Co, Nb, W, and REEs) as presented in this work, can form the basis for a long-term CRM strategy aligned with scientific trends and industrial practices. Such analysis is essential in formulating a science-based and economically-attuned strategy for the identified materials exhibiting high risks and the economic impact of supply shortage.
2. Criticality
Although it is evident that a shortage of certain materials could hinder economic development, it is quite difficult to find an unambiguous and quantifiable definition for criticality. The term 'criticality' itself is rather new for the raw materials field. The first evidence in the American Chemical Society database of bibliographic information is from 1941, when Croxton and Shutt (1941) reviewed the position of toluene in national defence.
In recent years, concern about the availability of raw materials has increased and one can observe a rapidly growing number of scientific papers and political reports (see table 1) in this field. Achzet and Helbig (2013) reviewed the available literature and concluded that there is a lack of consensus about indicators for criticality. As outlined by Frenzel et al (2017), the reason for the imprecise definition is based on the variability in definitions and assessment methodologies used and the vagueness of the definitions given in many studies. The authors also concluded that, independent of the variability of definitions, two points are crucial throughout most studies:
Table 1. Documents of the EC dealing with criticality of raw materials or resource-efficient use.
| Date | Title | Reference |
|---|---|---|
| 28 October 2010 | COM (2010) 614 final: An integrated industrial policy for the globalization era putting competitiveness and sustainability at centre stage | EC (2010a) |
| 3 March 2010 | COM (2010) 2020 final: A strategy for smart, sustainable and inclusive growth | EC (2010b) |
| 26 January 2011 | COM (2011) 21 final: A resource-efficient Europe—Flagship initiative under the Europe 2020 Strategy | EC (2011b) |
| 2 February 2011 | COM (2011) 25 final: Tackling the challenges in commodity markets and on raw materials | EC (2011a) |
| 24 June 2013 | COM (2013) 442 final: On the implementation of the Raw Materials Initiative | EC (2013a) |
| 24 July 2013 | COM (2013) 542 final: Towards a more competitive and efficient defence and security sector | EC (2013b) |
| 26 May 2014 | COM (2014) 297 final: On the review of the list of critical raw materials for the EU and the implementation of the Raw Materials Initiative | EC (2014) |
| 11 July 2017 | Methodology for establishing the EU list of critical raw materials | Blengini et al (2017) |
| 3 August 2017 | Assessment of the methodology for establishing the EU list of critical raw materials | EC (2017a) |
| 13 September 2017 | Study on the review of the list of critical raw materials—Critical raw materials factsheets | EC (2017b) |
| 13 September 2017 | COM (2017) 479 final: Investing in a smart, innovative and sustainable Industry—A renewed EU Industrial Policy Strategy | EC (2017c) |
- the vulnerability or economic importance: the importance of the raw material under consideration, and the consequent impact of supply shortfalls
- the supply risk: the likelihood of the occurrence of such disruptions
The situation can be addressed in a more detailed and complex fashion, as shown by Graedel et al (2012). The time scale of scarcity may be considered as 1–5 years (on the company level), 5–10 years (on the national level), or even up to 100 years (on the worldwide level). In parallel, the supply potential, technological change, geopolitical and social factors, environmental implications and intensity of competition may have different importance rankings in the overall assessment (Graedel et al 2012).
The evaluation of criticality itself is, however, not the subject of this communication. Criticality assessment will have different results, depending on the respective region. This communication will focus on the European point of view. As demonstrated by table 1, over the last year the EC has launched quite a large number of publications dealing with the supply of raw materials and the efficient use of resources.
As criticality is also a function of time, the EU Commission is regularly accessing the criticality of raw materials. The first list was published in 2011 (EC 2011a) followed by a second list published in 2014 (EC 2014). Only recently has the EU commission released an update (EC 2017c). The number of CRMs has been increasing over time, which is due to the fact that the number of evaluated materials has increased. In 2011, baryte and vanadium were assessed, but not classified as critical (EC 2011a). Both elements are, however, critical according to the 2014 (EC 2014) and 2017 (EC 2017c) assessments. It is also interesting that tantalum is a cross-border commuter as the element was classified critical in 2011 (EC 2011a), moved into non-criticality in 2014 (EC 2014), but is again critical in 2017 (EC 2017c). It is therefore important to note that the EU list of CRMs is not permanent and may change in the coming years.
A direct comparison of data of the assessments is not possible. In the 2017 assessment, the criticality threshold value for the supply risk remained at 1.0. However, the criticality threshold value for the economic importance was moved to 2.8 due to the implementation of a revised methodology (Blengini et al 2017). Figure 1 plots the economic importance versus the supply risk according to the current EU document (EC 2017c).
Figure 1. EU criticality assessment (EC 2017c). Materials plotted with red circles and printed in bold letters are the focus of this paper.
Download figure:
Standard image High-resolution imageThe EU CRM assessment of 2017 not only states the criticality of REEs, but evaluates all individual elements, except Ho, Lu, Yb and Tm, which are treated as a group. As shown in table 2, the values for the economic importance (3.6–3.7) and the supply risk (4.8–4.9) are basically the same (EC 2017b). The only exception is scandium, which shows a supply risk of 2.9.
Table 2. Criticality evaluation of individual REEs (EC 2017b).
| REEs | Economic importance | Supply risk | |
|---|---|---|---|
| LREE | Scandium | 3.7 | 2.9 |
| Lanthanum | 3.6 | 4.9 | |
| Cerium | 3.6 | 4.9 | |
| Praseodymium | 3.6 | 4.9 | |
| Neodymium | 3.6 | 4.9 | |
| Samarium | 3.7 | 4.8 | |
| Europium | 3.6 | 4.9 | |
| HREE | Yttrium | 3.7 | 4.8 |
| Gadolinium | 3.7 | 4.8 | |
| Terbium | 3.7 | 4.8 | |
| Dysprosium | 3.7 | 4.8 | |
| Erbium | 3.7 | 4.8 | |
| Ho, Lu, Yb, Tm | 3.7 | 4.8 | |
Technological changes may significantly influence the economic importance of certain raw materials. In particular, the CRMs Co, Nb, W and REEs are expected to be of major importance for emerging technologies. The EU commission therefore expects a strong increase in demand as outlined in table 3. The strong growth will exert pressure on the supply and further increase the economic importance. It can thus be assumed that future criticality assessments will include Co, Nb, W and REEs and these elements have thus been chosen for a closer inspection within this communication.
Table 3. Emerging technologies and expected demand for the CRMs selected in this paper.
| CRM | Emerging technology (EC 2010c) | Forecast average demand growth to 2020 (% growth per year) (EC 2014) |
|---|---|---|
| Co | Lithium-ion batteries, synthetic fuels | Strong (6.1) |
| Nb | Micro capacitors, ferroalloys | Very strong (8.2) |
| W | Moderate (4.5) | |
| Nd |
Permanent magnets, laser technology | |
| LREE |
Strong (6.0) | |
| HREE |
Very strong (8.2) |
aBelongs to group of LREE. bSee table 4.
3. State of the art
3.1. Introduction
In this chapter, the available literature on Co, Nb, W and REEs is briefly introduced. According to the International Union of Pure and Applied Chemistry (IUPAC), the REEs comprise the lanthanides (La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu) as well as the IUPAC Group 3 elements Sc and Y (Connelly et al 2005). In some publications, REEs are further separated into light and heavy as outlined in table 4. However, this classification is not used consistently throughout the literature.
Table 4. Classification of REEs (EC 2014).
| REEs—light (LREEs) | REEs—heavy (HREEs) |
|---|---|
| Scandium (Sc) |
Yttrium (Y) |
| Lanthan (La) | Gadolinium (Gd) |
| CeriumCer (Ce) | Terbium (Tb) |
| Praseodym (Pr) | Dysprosium (Dy) |
| Neodym (Nd) | Holmium (Ho) |
| Promethium (Pm) |
Erbium (Er) |
| Samarium (Sm) | Thulium (Tm) |
| Europium (Eu) | Ytterbium (Yb) |
| Lutetium (Lu) |
aSc and Y are transition metals of IUPAC Group 3; all other elements are lanthanides. bAll isotopes of Pm are radioactive; there is no natural occurrence of Pm.
Table 5 summarizes the concentrations of the selected CRMs in the upper crust of the Earth. It can be re-emphasized here that criticality is not directly dependent on scarcity. For the selected CRMs, the EC substitutability index range is considerably high and is at least 0.79 (SIEI) or 0.82 (SISR) but is in several cases even 1 (EC 2017c) as outlined in table 5. This means that none of the materials is readily substituted. It is relevant to note that the end-of-life recycling input rate is quite low for these materials, with W having by far the highest reported value among the selected elements (42%).
Table 5. Prevalence within the upper continental crust, EC substitutability index and end-of-life recycling input rate for selected CRMs.
| Element | Concentration |
Substitutability index (−) |
End-of-life recycling input rate |
||
|---|---|---|---|---|---|
| SIEI | SISR | ||||
| Co | 17.3 | 1.0 | 1.0 | 0 | |
| Nb | 12 | 0.91 | 0.94 | 0.3 | |
| W | 1.9 | 0.94 | 0.97 | 42 | |
| LREE | Sc | 14 | 0.91 | 0.95 | 3 |
| La | 31 | 0.98 | 0.99 | ||
| Ce | 63 | 0.95 | 0.98 | ||
| Pr | 7.1 | 0.90 | 0.94 | ||
| Nd | 27 | 0.89 | 0.92 | ||
| Sm | 4.7 | 0.79 | 0.82 | ||
| Eu | 1.0 | 1.0 | 1.0 | ||
| HREE | Y | 21 | 1.0 | 1.0 | 8 |
| Gd | 4.0 | 0.90 | 0.94 | ||
| Tb | 0.7 | 0.83 | 0.93 | ||
| Dy | 3.9 | 0.90 | 0.95 | ||
| Ho | 0.8 | 1.00 |
1.00 |
||
| Er | 2.3 | 0.92 | 0.96 | ||
| Tm | 20.3 | 1.00 |
1.00 |
||
| Yb | 2.0 | 1.00 |
1.00 |
||
| Lu | 0.3 | 1.00 |
1.00 |
||
aAccording to Rudnick and Gao (2003). bThe 'substitutability index' according to the EC (2017a) is a measure of the difficulty in substituting the material, scored and weighted across all applications, calculated separately for both economic importance (SIEI) and supply risk (SISR) parameters. Values are between 0 and 1, with 1 being the least substitutable. cThe 'end-of-life recycling input rate' according to the EC (2017b) measures the proportion of metal and metal products that are produced from end-of-life scrap and other metal-bearing low-grade residues in end-of-life scrap worldwide. dHo, Lu, Yb and Tm have not been individually assessed.
3.2. Cobalt
3.2.1. General.
Co belongs to Group 9 of the IUPAC Periodic Table. The interest in Co is due to its industrially useful properties including ductility, malleability and monetisability. These characteristics, combined with strength and heat resistance (melting point 1495 °C and boiling point 2870 °C) make Co suitable for a wide variety of industrial and military applications (Hannis and Bide 2009).
Co has been known since ancient times, and the first evidence is attributed to blue glazed pottery found in Egyptian tombs dated at 2600 BC. For centuries, Co-containing materials have been used as pigment. Co as a pure metal was isolated by Georg Brandt in 1735 (Donaldson and Beyersmann 2005).
3.2.2. Deposits and mining.
Co is typically mined as a by-product of copper, nickel and iron and is widely distributed in different mineral deposits including sulphides, sulphosalts, arsenides, oxides and carbonates (Cheang and Mohamed 2016, Nancharaiah et al 2016). Significant Co concentrations are present in the sea floor, within Co-rich crusts and manganese nodules; considering available technologies, sea floor-based mining is not economically sustainable at present (Hannis and Bide 2009).
Table 6 summarizes important Co-bearing mineral groups. Irrespective of the starting mineral, cobalt is not naturally present in a pure metallic form, but is commonly found in +2 and +3 oxidation states. There are Co mines in different geographical areas and the EC estimated that the main exporters to European countries are Russia (96%) and the USA (3%), (EC 2014).
Table 6. Common cobalt mineral-associated worldwide deposits, (Cailteux et al 2005, Gál et al 2008).
| Mineral name | Mineral group | Formula | Deposits in the world |
|---|---|---|---|
| Erythrite | Arsenate | Co3(AsO4)2 · 8 H2O | CA, DE, MA, NO, US |
| Skutterudite | Arsenide | (Co,Ni)As3 | CA, MA, NO, US |
| Cobaltite | Sulphosalt | CoAsS | AU, CA, CG, NO, US |
| Carolite | Sulphide | Cu(Co,Ni)2S4 | SE, US, ZR, ZM |
| Linnaeite | Sulphide | Co2+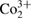 S4 S4 |
CIS, CG, DE; MA; RU, ZR |
| Asbolite (Asbolane) | Oxide | (Ni,Co)2-xMn4+(O,OH)4 · n H2O | NC |
| Sphaerocobaltite | Carbonate | CoCO3 | DE; ZR |
aAbbreviations of countries according to DIN ISO EN 3116-1 (ISO 2006).
The vast majority of Co is mined in Congo. In 2016, the country accounted for 54% of global Co mining production. Furthermore, approximately half of the world's Co reserves are estimated to lie in Congo. Due to the dominance of Congo in terms of annual mining production and estimated reserves, the role of other countries is more limited with respect to supply risks, especially considering that other individual countries contribute less than 6% to the world's share of global Co mining. Figure 2 gives an overview of the geographical distribution of Co mining and reserves.
Figure 2. Global share of Co mining production and estimated Co reserves (Shedd 2017a).
Download figure:
Standard image High-resolution imageCo is a metal of historic importance, and world global Co mining production rapidly increased from 180 t in 1901 to 1450 t in 1909 (USGS 2017a). As shown in figure 3, there was a steady increase in global Co mining production from 1960 (14 000 t) until 1986 (50 000 t). In the mid-1990s, there was a production low of 18 000 t, which was followed by a seven-fold increase to 126 000 t in 2015.
Figure 3. World Co mining production, annually from 1960–2015 (USGS 2017a).
Download figure:
Standard image High-resolution image3.2.3. Uses.
Co is used for metallurgical applications in various industrial sectors for its several properties including high yield strengths, high work-hardening rates, resistance under cyclic stresses and the ability to absorb stresses, obtained thanks to its unstable face-centred cubic crystal structure and related low stacking-fault energy (Davis 2000). A typical use is in the aircraft industry, as a component of superalloys for aircraft engine turbines that operate under extreme temperature and pressure conditions. The application in this field started in the first half of 1900 and has continued combining the metal with additional elements (e.g. Ni, W, Al, Re, Cr, Ru) to obtain antioxidant and corrosion-resistant materials (Pollock 2016, Shen et al 2017). Further uses concern the chemical industry and the catalysts field. In this regard, Budiman et al (2012) describes a high-performance reforming process in which methane is adsorbed on the metal active surface and dissociated to produce hydrogen and hydrocarbon species. Co is also involved in the production of cemented carbides for cutting tools (CDI 2006). In this material, Co provides a ductile metal binder for carbide particles, allowing the achievement of high density thanks to its wetting or capillary action during the liquid phase sintering (Davis 2000). Additional applications include the industries of adhesives, pigments, ceramics, enamels, agricultural and medicinal chemistry. Figure 4 shows the share of prominent Co end uses.
Download figure:
Standard image High-resolution imageOne of the more common applications of Co is the production of lithium-ion batteries (LIBs), which can be used for the power supply of various electronic equipment. In LIBs, Co is present as lithium cobalt oxide (LiCoO2); this concentration is approximately 5%–20% higher than typical Co concentration in Co-containing minerals and may be attractive for recycling (Jha et al 2013a, Nancharaiah et al 2016). The EC considers LIBs as an emerging technology, which may strongly drive increased Co demand in the future (EC 2010c, EC 2017b).
3.2.4. Price.
As shown in figure 5, there have been significant price shifts for Co since 1960. A first peak occurred in 1979 with a price of 72 USD kg−1, equivalent to 250 USD kg−1 in 2017 currency. The most recent peak occurred in 2008 at 93 USD kg−1, which was followed by a steep drop to 25–35 USD kg−1 in subsequent years (Metalary 2016a).
Figure 5. Price of Co over time in USD kg−1 (red dots) and inflation-adjusted USD kg−1 (blue triangles) (Metalary 2016a).
Download figure:
Standard image High-resolution image3.2.5. Substitution and recycling.
As confirmed by table 7, the research is pushing towards the identification of materials suitable for Co replacement, in several applications. Nevertheless, the possible new strengths are often combined with potential reductions in product performance. In this regard, Baharuddin et al (2017) describes methods for the production of cobalt-free cathodes for solid oxide fuel cells, using perovskite structured materials, such as strontium ferrite oxide, combined with dopants. The study shows satisfying results, but this research is still new, with few applications. Consistent with the examples in the table, the literature reports several applications for the samarium cobalt magnet replacement by neodymium-iron-boron technologies with a consequent loss of performance at higher temperature and an increased corrosion tendency (Smith Stegen 2013, Binnemans and Jones 2015). Many compounds are also reported as cobalt substitute for paint applications, with different resulting disadvantages. In more detail, the manganese carboxylates are the most well known. Nevertheless, they show a lower drying ability than cobalt combined with the possible formation of brown compounds that makes it unsuitable for light colouring. On the other hand, iron carboxylates show poor activity at room temperature and a yellow-brown colour that limits their use (De Boer et al 2013). Table 7 lists some possible substitutes for Co.
Table 7. Possible substitutes for Co (Shedd 2017a).
| Application | Possible substitutes |
|---|---|
| Magnets | Barium or strontium ferrites, neodymium-iron-boron, nickel-iron alloys |
| Paints | Cerium, iron, lead, manganese, vanadium |
| For curing unsaturated polyester resins | Cobalt-iron-copper or iron-copper in diamond tools; copper-iron-manganese |
| Cutting and wear-resistant materials | Iron, iron-cobalt-nickel, nickel, cermets, ceramics |
| Lithium-ion batteries | Iron-phosphorous, manganese, nickel-cobalt-aluminium, nickel-cobalt-manganese |
| Jet engines | Nickel-based alloys, ceramics |
| Petroleum catalysts | Nickel |
As summarized in tables 8 and 9, during the last decade several publications have reported Co recovery efficiencies of 60%–100% from secondary resources, including recovery processes based on recycling mining residues, zinc plant scraps and end-of-life LIBs. In more detail, table 7 identifies solvent extraction as the most common technique selected for the first two streams, often simulated by synthetic solutions. Many organic solvents are tested for this treatment, which ends with the following stripping step, carried out with sulfuric acid. An electrochemical approach can be chosen either as the final stage, after the solvent extraction, to increase the product purity (Agatzini-Leonardou et al 2009, Katsiapi et al 2010), or as the main treatment for cobalt recovery from different solutions (Wen Min et al 2013, Cheang and Mohamed 2016). Bioleaching represents an interesting alternative, which combines the positive aspect of a lower environmental impact with lower efficiency and several days long processing time (Coto et al 2008, Ahmadi et al 2015).
Table 8. Recycling procedures for Co-containing waste streams.
| Waste | Treatment | Operative conditions | Co recovery efficiency | Reference |
|---|---|---|---|---|
| Synthetic solution of CoCl2 · 6H2O, CoSO4 · 5H2O and Co(NO3)2 · 6H2O | Electrogenerative process (NaCl as catholyte solution) | 90 min, room temperature, pH 5-7 | 99% | Wen Min et al (2013) |
| Synthetic solution of CoCl2 | Electrogenerative process (NH4Cl) | 240 min, room temperature, pH 7 | 95% | Cheang and Mohamed (2016) |
| Synthetic solution mimicking a laterite leach solution | Solvent extraction (Versatic 10 and Acorga CLX 50 in ShellSol 2046) | About 10 min, room temperature, pH 6.3 | 99% | Cheng et al (2015) |
| Stripping (H2SO4) | Room temperature | |||
| Synthetic solution of CoSO4 and NiSO4 | Use of solvent-impregnated resin and acid stripping (Lewatit® TP272 resin, H2SO4) | 15 min, room temperature, pH 5 | 50– 70% | Vaughan et al (2016) |
| Synthetic solution of CoSO4 and NiSO4 | Solvent extraction (TOPS 99 and TIBPS mixture in kerosene) | 1 min, room temperature, pH 1.1 | 99.5% | Reddy et al (2009) |
| Stripping (H2SO4) | ||||
| Synthetic solution of CoSO4 | Solvent extraction (NaCyanex 272, Cyanex 272, PC 88A) | About 10 min, room temperature, pH 6.85 | 100% | Devi et al (1998) |
| Stripping (H2SO4) | ||||
| Synthetic solution of CoSO4 and Li2SO4 | Solvent extraction (Cyanex 272, isodecanol in kerosene) | 5 min, room temperature, pH 5 | 99.9% | Jha et al (2013a) |
| Scrubbing (Na2CO3) | 5 min, room temperature, pH 4.98 | |||
| Stripping (H2SO4) | ||||
| Greek nickel oxide ores | Solvent extraction (Cyanex 272) Stripping (H2SO4) | 40 °C pH 5.5 40 °C | 93% | Agatzini-Leonardou et al (2009) Katsiapi et al (2010) |
| Precipitation (NaOH) | ||||
| Leaching (NH3 and (NH4)2CO3) (cobalt can be subsequently obtained by solvent extraction and electrowinning, or chemical precipitation) | 300 min, room temperature, pH 10.25 | |||
| Laterite tailings | Bioleaching (A. thiooxidans cultures growing on Sulphur) | 12–15 d, 30 °C, pH 1.5–3 | 80% | Coto et al (2008) |
| H2SO4 by A. thiooxidans | Few hours, 60 °C | 56% | ||
| Intermediate product of nickel production from laterite ores | Selective precipitation (magnesia) | 30 min, 50 °C, pH 5 | 100% | Harvey et al (2011) |
| Sulfidic tailing of Golgohar Iron Mine (Iran) | Bioleaching (A mixed culture of iron- and sulphur-oxidizing microorganisms, including leptospirillumferriphilum, acidithiobacillus caldus, sulfobacillus sp. and ferroplasma) | 30 d, 45 °C, pH 1.2 | 59.5% | Ahmadi et al (2015) |
Table 9. Recycling procedures for LIBs.
| Treatment | Operative conditions | Co recovery efficiency | Reference |
|---|---|---|---|
| Reductive leaching (H2SO4 H2O2) | 10 min, 75 °C | 100 % | Shin et al (2005) |
| 1) Alkali leaching (NaOH) | 320 min, room temperature | 90 % | Chen et al (2011) |
| 2) Reductive acid leaching (H2SO4 H2O2) | 120 min; 85 °C | ||
| 3) Precipitation ((NH4)2S2O8, NaOH) | 120 min, 70 °C, pH 3 (Fe removal), pH 4 (Mn removal), pH 5 (Cu removal) | ||
| 4) Solvent extraction (P507 in kerosene) | 10 min, room temperature, pH 3.5 | ||
| 5) Stripping (H2SO4) | |||
| 6) Precipitation (C2H8N2O4) | Room temperature, pH 1.5 | ||
| 1) Reductive leaching (H2SO4 H2O2) | 180 min, 80 °C | >95 % extraction | Pagnanelli et al (2016) |
| 2) Precipitation (NaOH) | Room temperature pH 3.8 | ||
| 3) Impurities solvent extraction (D2EHPA in kerosene) | 10 min, room temperature, pH 3.8 | ||
| 4) Co solvent extraction (Cyanex 272 in kerosene) | 10 min, room temperature, pH 4 | ||
| 5) Precipitation (Na2CO3) | 30 min, room temperature, pH 9 | ||
| Reductive leaching (H2SO4 H2O2) | 60 min, 75 °C | 70 % | Jha et al (2013b) |
| 1) Reductive leaching (H2SO4 H2O2) | 60 min, 65 °C | 85 % | Dorella and Mansur (2007) |
| 2) Precipitation (NH4OH) | 50 °C, pH 5 | ||
| 3) Solvent extraction (Cyanex 272) | 50 °C, pH 5 | ||
| 4) Stripping (H2SO4) | 50 °C, pH 5 | ||
| Leaching (DL malic acid, H2O2) | 40 min, 90 °C | >90 % | Li et al (2010a) |
| 1) Reductive leaching (H2SO4, H2O2) | 180 min pH 3.5 | 90 % | Jian et al (2012) |
| 2) Solvent extraction (P507 in kerosene) | pH 3.5 | ||
| 3) Precipitation (C2H2O4) | pH 3.5 | ||
| 1) Leaching (HCl) | 60 min, 80 °C | 99 % | Wang et al (2009) |
| 2) Mn precipitation (KMnO4, NaOH) | 40-50 °C, pH 2 | ||
| 3) Ni complexation and precipitation (C4H8N2O2, NaOH) | 10 min, pH 11 | ||
| 4) Co precipitation (HCl NaOH) | pH 11 | ||
| 5) Li precipitation (Na2CO3) | 100 °C | ||
| 1) Alkaline leaching (NH4OH) | 60 min, 60 °C | 99 % | Nayl et al (2014) |
| 2) Reductive leaching (H2SO4, H2O2) | 120 min, 70 °C | ||
| 3) Precipitation (NaOH, Na2CO3) | Room temperature, pH 7.5 (Mn removal), pH 9 (Ni removal), pH 11 - 12 (Co removal) | ||
| 1) Leaching (NaOH) | 60 min, 30 °C | 97 % | Ferreira et al (2009) |
| 2) Reducing leaching (H2SO4, H2O2) | 60 min, 60 °C | ||
| 3) Crystallization | 60 °C | ||
| 1) Vacuum pyrolysis | 30 min, 600 °C | 99 % | Sun and Qiu (2011) |
| 2) Reducing leaching (H2SO4, H2O2) | 60 min, 80 °C | ||
| 1) Reducing leaching (H2SO4, H2O2) | 60 min, 60 °C | 92 % | Kang et al (2010) |
| 2) Precipitation (NaOH, CaCO3) | Room temperature, pH 6.5 | ||
| 3) Solvent extraction (Cyanex 272 in kerosene) | 30 min, pH 5.5-6 | ||
| 4) Stripping (H2SO4) | |||
| 1) Reducing leaching (H2SO4, H2O2) | 120 min, 60 °C | 95% | Zhu et al (2012) |
| 2) Co precipitation (NaOH, (NH4)2C2O4) | 60 min, 50 °C, pH 2 | ||
| 3) Ni precipitation (Na2CO3) | 60 min, 50 °C, pH 10 | ||
| 1) Cu and Al extraction (N-methylpyrrolidone) | 60 min, 100 °C | 90% | Li et al (2010b) |
| 2) Leaching (citric acid, H2O2) | 30 min, 90 °C | ||
| Supercritical reducing leaching (supercritical CO2, H2SO4, H2O2) | 5 min, 75 °C | 95.5% | Bertuol et al (2016) |
| 1) Reductive leaching (H2SO4, H2O2) | 120 min, 80 °C | Freitas et al (2010) | |
| 2) Multilayer electrodeposition | About 1 min, room temperature, pH 2.7, pH 5.4 | ||
| 1) Washing zinc (H2SO4) | 120 min, room temperature, pH 1 | 90% | Moradkhani et al (2014) |
| 2) Reductive leaching (H2SO4, H2O2) | 45 min, room temperature | ||
| 3) Cadmium cementation (Zn power) | 30 min, room temperature, pH 3.5 | ||
| 4) Cobalt and manganese separation (N-N reagent) | 30 min, room temperature, pH 1.5 | ||
| 1) Co-grinding with EDTA | 240 min | 98% | Wang et al (2016) |
| 2) Leaching (water) | 30 min | ||
| 3) Precipitation (NaOH, Na2CO3) | |||
| 1) Reducing leaching (H3PO4, H2O2) | 60 min, 90 °C | 99% | Pinna et al (2017) |
| 2) Precipitation (C2O4H2) | 60 min, 75 °C | ||
As confirmed by table 8, the recent literature about Co recovery from LIBs is vast. Irrespective of the optimized method, each of the described recovery processes begins with the leaching, carried out at alkali or acid pH, under different operative conditions, necessary for the cobalt extraction. In most of the cases considered here, the extraction treatment is carried out under reductive conditions with hydrogen peroxide, in order to reduce Co (III) to Co (II), which is soluble in water. Considering the composition of secondary resources, leach liquor contains many impurities, mainly copper, lithium, nickel, aluminium and manganese. To increase the Co percentage in the final product, further treatments including solvent extraction, precipitation, cementation, crystallization or electrochemical processes may be necessary. The majority of these additional treatments are readily reproducible and can be performed over short durations at moderate temperature, with the exception of vacuum pyrolysis at 600 °C, as described by Sun and Qiu (2011), and supercritical reducing leaching (carried out at 75 bar), optimized by Bertuol et al (2016).
3.2.6. Résumé.
Both the EC and the National Environmental Research Council (NERC) have confirmed the relevance of Co as a critical metal. The EC included Co in the list of CRMs of high supply risk and high economic importance, whereas the NERC included Co as an 'E-tech element' essential for technological development (Naden 2012, EC 2014)
Continued interest in this metal is based on its industrially useful properties including ductility, malleability and magnetizability. These characteristics, combined with strength and heat resistance (melting point 1495 °C and boiling point 2870 °C), make Co suitable for a wide variety of industrial and military applications (Hannis et al 2009, Nancharaiah et al 2016, Pazik et al 2016, Lison 2007). The critical value of this material is also confirmed by the 'EC substitutability index' of 1 (in a range between 0 and 1), attributed to Co by the EC, as a measure of the difficulty of substitution of individual CRMs (EC 2017c).
Considering its many uses and the recent growth in Co demand, it is important to increase Co production based on secondary resources, as a by-product from 'waste' of other industrial processes (Cheang and Mohamed 2016). As an estimate of the production of secondary Co, the EC has considered an 'end-of-life recycling input rate' of 16% for this metal (EC 2014).
3.3. Niobium
3.3.1. General.
Nb as well as Ta are transition elements of Group 5 of the IUPAC Periodic Table. Due to their properties such as high melting points, they are classified as refractory metals (Bauccio 1993). Nb and Ta have similar chemical properties, are co-located in natural deposits and are occasionally referred to as 'twins' (Schulz and Papp 2014). Both elements were discovered at approximately the same time. In 1801, English chemist, Charles Hatchett, derived a white oxide from a very heavy black stone, which originated from Massachusetts. Hatchett believed he had found a new element, which he named 'columbium', referring to the origin of the stone (Hatchett 1802). At the same time, Anders Ekeberg derived a white oxide from two minerals originating from Kimito, Finland and Ytterby, Sweden (Ekeberg 1803). As it was difficult to dissolve the oxide, Ekeberg assumed he had discovered a new metal and named it 'tantalum'. In 1809, English scientist, William Hyde Wollaston, concluded that 'tantalum' and 'columbium' were identical, even though he observed a distinct difference in the specific gravity of the oxides (Wollaston 1809). In 1844, Heinrich Rose found that 'tantalum' ores contain a second element, which he named 'niobium', after Niobe, the daughter of Tantalus in Greek mythology (Rose 1844). Two decades were required for the identities and naming practices of 'niobium', 'columbium', and 'tantalum' to be resolved (Deville and Troost 1865, Hermann 1865). Although the official IUPAC name is niobium (Nb), in North America the name columbium (Cb) is still sometimes used.
3.3.2. Deposits and mining.
The abundance of Nb and Ta is linked due to their chemical similarity. In the metal extraction process, the challenge is not only to separate foreign elements, but to separate Nb and Ta. The most important minerals from an economic point of view are those of the columbite-tantalite group followed by the pyrochlore group (Crockett and Sutphin 1993).
The minerals of the columbite-tantalite group have the general formula AM206, where A = (Fe, Mn, and/or Mg) and M = (Nb in the case of columbite and Ta in the case of tantalite) (Cerny and Ercit 1989). For columbite, the Nb content is 78.2% and for tantalite the Ta content is 86.1%, according to Shaw and Goodenough (2011). However, it is common for Nb- and Ta-bearing minerals to be commingled.
Minerals of the pyrochlore group are the second most important source of Nb and Ta (Crockett and Sutphin 1993). These minerals comprise a series of oxides containing significant amounts of Nb, Ta and Ti, (either individually or in combination) and have the general formula A2−mB2O6(O,OH,F)1−n·pH2O, where B = Nb, Ta and/or Ti (Hogarth 1977). Other important minerals for Nb extraction are tapiolite: (Fe, Mn)(Ta, Nb)2O6, lueshite: NaNbO3 and euxenite: (Y, Ca, Ce, U, Th)(Nb, Ti, Ta)2O6 (Shaw and Goodenough 2011).The global share of Nb production and estimated Nb reserves are indicated in figure 6. The largest Nb deposits are situated in Brazil, which dominates global production. Canada had the second largest Nb reserves in the world in 2016, with world Nb mining production being 64 300 t, of which Brazil contributed a 90% share, or 58 000 t (Papp 2017a). A similar distribution can be noted for the world reserves of Nb, which are 4.3 Mt globally with a 95% share (4.1 Mt) concentrated in Brazil (Papp 2017a). At current usage levels, worldwide Nb reserves can be considered virtually inexhaustible (Schulz and Papp 2014), but Nb is classified as critical due to the high concentration of production and occurrence in Brazil (EC 2014).
Figure 6. Global share of Nb mining production and estimated Nb reserves (Papp 2017a).
Download figure:
Standard image High-resolution imageThe use of Nb in technological applications is a relatively recent trend, and before the late 1950s there was no significant Nb mining activity (Schulz and Papp 2014). Figure 7 plots the annual global mining production of Nb since 1965. From 1965–2000, a relatively linear trend of moderate increase can be observed. However, production more than doubled over the 6 year period from 2001 (approx. 26 000 t) to 2007 (approx. 60 000 t). Thereafter, the production remained relatively constant, slightly exceeding 60 000 t.
Figure 7. World Nb mining production, annually from 1965–2015 (USGS 2017b).
Download figure:
Standard image High-resolution image3.3.3. Uses.
As demonstrated in figure 8, 88% of the mined Nb is used to produce ferroniobium, an iron niobium alloy, which is important in the production of high-strength low-alloy steel (TIC 2016a). Ferroniobium is used in the form FeNb63 (containing 58%–68% Nb, EN steel number 1.4740) or FeNb65 (containing 63%–70% Nb, EN steel number 1.4745), whereas the Ta content must not exceed 0.5% or 1.0%, respectively (DIN 2004). Ferroniobium itself is mainly used as an alloying element for Cr-, CrNi- or CrNiMo steel grades with EN steel numbers beginning with 1.45 and 1.46, which are stainless and acid resistant (DIN 2004). Table 10 shows examples of steel grades containing Nb; across all table entries, the Nb content is not higher than 1%.
Table 10. Examples of steel grades containing Nb (DIN 2014).
| EN steel number | Short name | Nb content |
|---|---|---|
| 1.4509 | X2CrTiNb18 | (3 × C) + 0.30% up to 1.00% (C ⩽ 0.03%) |
| 1.4511 | X3CrNb17 | 12 × C up to 1.00% (C ⩽ 0.05%) |
| 1.4526 | X6CrMoNb17-1 | 7 × (C + N) + 0.10% up to 1.00% (C ⩽ 0.08%; N ⩽ 0.04%) |
| 1.4542 | X5CrNiCuNb16-4 | 5 × C up to 0.70% (C ⩽ 0.07%) |
| 1.4550 | X6CrNiNb18-10 | 10 × C up to 1.00% (C ⩽ 0.08%) |
| 1.4565 | X2CrNiMnMoNbN25-18-5-4 | Up to 0.15% |
| 1.4580 | X6CrNiMoNb17-12-2 | 10 × C up to 1.00% (C ⩽ 0.08%) |
| 1.4590 | X2CrNbZr17 | 0.35%–0.55% |
| 1.4595 | X1CrNb15 | 0.20%–0.60% |
| 1.4607 | X2CrNbTi20 | Up to 1.00% |
| 1.4621 | X2CrNbCu21 | 0.20%–1.00% |
| 1.4634 | X2CrAlSiNb18 | (3 × C) + 0.30% up to 1.00% (C ⩽ 0.03%) |
Figure 8. Share of the market for various applications of Nb as of 2014 (TIC 2016a).
Download figure:
Standard image High-resolution imageNb is also used in some grades of superalloys. These alloys are designed for high-temperature applications such as turbine blades for aerospace engines or power generation. Table 11 shows examples of nickel-based superalloys containing Nb, some with Nb concentrations up to 5.5%.
Table 11. Examples of superalloys containing Nb (DIN 2002).
| Material number | Short name | Content Σ Nb + Ta | Brands |
|---|---|---|---|
| 2.4600 | NiMo29Cr | Up to 0.40% | Nicrofer® 6629, Hastelloy® B-3 |
| 2.4619 | NiCr22Mo7Cu | Up to 0.50% | Inconel® G-3, Nicrofer® 4823hMo |
| 2.4660 | NiCr20CuMo | 8 × C up to 1.00% (C ⩽ 0.07%) | Nicrofer® 3620, Incoloy® alloy 20 |
| 2.4856 | NiCr22Mo9Nb | 3.15%–4.15% | Inconel® 625, Nicrofer® 6020 |
| 2.4868° | NiCr19Fe19Nb5Mo3 | 4.7%–5.5% | Inconel® 718, Nicrofer® 5219 |
3.3.4. Price.
As outlined in figure 7, the annual mining production of Nb increased significantly during the 2000s. As demonstrated in figure 9, the price of Nb followed a similar trend. Prices peaked around 1980 at around 15 USD kg−1, corresponding to the all-time price record of approximately 50 USD kg−1 when adjusted for inflation in 2017 currency. In the early 2000s, there occurred nearly a doubling of price, jumping from 19 USD kg−1 in 1998 to 42 USD kg−1 in 2010 (Metalary 2016c). Since then, the price of Nb has remained relatively constant around 40 US$ kg−1.
Figure 9. Price of Nb over time in USD kg−1 (red dots) and inflation-adjusted USD kg−1 (blue triangles) (Metalary 2016c).
Download figure:
Standard image High-resolution image3.3.5. Substitution and recycling.
According to the DIN standard (DIN 2002), the sum of Nb and Ta is specified, as shown in table 11, thus permitting substitution of Nb with Ta. In 2010, the EC classified Ta as a CRM (EC 2010c), but Ta is not defined as critical according to more recent EU documents (EC 2014). The annual mining production is significantly lower for Ta (2015 production 1100 t; Papp 2017b) than Nb (2015 production 64 300 t; Papp 2017a). In 2015, superalloys alone accounted for 2200 t Nb use (TIC 2016b), which twice exceeds the total production of Ta. It is thus evident that Nb cannot in practice be substituted by Ta. Furthermore, the price of Ta (128 USD kg−1; Metalary 2016b) is approximately three times higher than that of Nb (42 USD kg−1; Metalary 2016c).
According to Papp, Nb can be substituted by other metals or materials as shown in table 12, even if performance losses or higher costs are unavoidable and must be taken into account (Papp 2017a). The listed substitutes molybdenum (Mo), vanadium (V), tantalum (Ta), titanium (Ti), tungsten (W) and ceramics are associated with additional considerations. It has already been outlined that Ta is not a feasible substitute as the annual production of Ta is currently less than 1.7% of Nb. W is itself classified as critical and is therefore not a suitable substitute. V is not in the EC list of CRMs, but the economic importance of V is extremely high as demonstrated in figure 1. As the annual mining production of V (77 800 t in 2015 according to Polyak (2017)) is within the same range as that of Nb, a significant increase in the demand for V would most probably shift V into criticality. Thus, only Mo and Ti remain as potential substitutes for Nb in certain alloys. However, such substitution may decrease performance and there are several cases where Nb is essential. It is thus clear that Nb should be recycled to a high extent in order to decrease the demand for virgin Nb ores.
Table 12. Possible substitutes for Nb (Papp 2017a).
| Substitute elements/materials | Application |
|---|---|
| Mo and V | Alloying elements in high-strength low-alloy steels |
| Ta and Ti | Alloying elements in stainless- and high-strength steels |
| Ceramics, Mo, Ta and W | High-temperature applications |
The typical recycling process for Nb does not involve the recovery of Nb as a pure element; rather, Nb-containing scrap is remelted into the same alloy (Cunningham 2004). In particular superalloys with high Nb content (e.g. EN steel number 2.4868 with up to 5.5% Nb), recycling rates of up to 70% have been reported (Phillips 1990). However, only a small fraction (approximately 4%) of Nb is used for superalloys (TIC 2016a) and lower recycling rates are reported for the total Nb market; 50% in the year 1998 (Cunningham 2004) and 56% more recently (Birat and Sibley 2011). However, according to newer figures from Papp, the amount of Nb recycled is unavailable and may be approximately 20% (Papp 2017a). The Scholz company, a large scrap dealer processing 1.4 Mt steel scrap annually, has indicated that only alloying elements higher than 1% are evaluated for recycling; this implies that Nb is not considered at all (Hodecek 2017). This does not mean that Nb ends up in a landfill, but rather that it is heavily diluted into secondary products where its unique positive characteristics are underutilised. According to Graedel et al, this practice is called 'non-functional recycling'; recycling in which metal is collected as old metal scrap and incorporated into large-magnitude material streams as 'tramp' or impurity elements (Graedel et al 2011).
3.3.6. Résumé.
A separate collection of Nb-containing alloys would be a viable option to increase the recycling rate of Nb. In addition to alloys of higher Nb concentration, the importance of recycling alloys with Nb content below 1% (e.g. EN steel numbers beginning with 1.45 and 1.56) should be stressed, as these lower-concentration alloys account for 88% of the Nb market. Unfortunately, in current practice most industrial materials are recycled only to the extent that they can be separated from other waste materials at a cost less than that of new primary materials (Ayres and Peiró 2013). Recycling companies have not currently found a viable business case for recycling low-concentration Nb alloys, and the logistics cost for the separate collection of Nb-containing scrap currently overshadows the additional revenue that could be gained from the recovered Nb.
The EC has classified Nb as a CRM (EC 2014), but other sources have come to different conclusions. According to Ayres and Peiró (2013), several elements are labelled as 'hitchhikers', meaning that they are mined unintentionally with other metals. A representative calculation helps to emphasise the magnitude of these unintentionally mined 'hitchhiker' elements. In the Bayan Obo mines in China, the Baosteel company annually intends to mine 69 Mt iron ore (with 35% Fe content), to produce 24 Mt iron per year (Qi 2011). As the iron ore of the Bayan Obo mines exhibits a Nb content of 0.13% (Drew et al 1991), the described annual ore mining corresponds to 89 000 t Nb mined inadvertently, which exceeds the 64 300 t annual global production of Nb, which is mined intentionally.
In conclusion, more accurate data about Nb recycling would be necessary to fully characterize the situation. The recycling rates reported in the literature can be quite high (Phillips 1990, Birat and Sibley 2011), whereas other sources draw other conclusions (Papp 2017a, Hodecek 2017). It is conceivable to significantly reduce the supply risk, considering the large amounts of Nb available in iron ores in China. From a longer perspective, more sophisticated collection schemes for Nb-containing alloys are required to decrease the demand for virgin Nb, even if they may not appear to be economically advantageous in the short term.
3.4. Tungsten
3.4.1. General.
Tungsten (W) is one of the materials included in the EU list of CRMs (EC 2017c, EC 2014). The name 'tungsten' originates from the Swedish language meaning 'heavy stone' (Andrew 1955).
W and W-containing materials exhibit unique properties, which make them highly attractive for specific applications, in European and global industries. Tungsten is the only viable option for a variety of applications, due to certain distinguishing features including the highest melting point of all metals (3422 °C), the lowest vapour pressure of all metals, a high density (19.3 g cm−3) similar to gold and very high hardness of certain W compounds (such as tungsten carbide, which is close to diamond) (Zeiler et al 2007).
3.4.2. Deposits and mining.
Table 13 summarizes the most important W-containing minerals (Schubert and Lassner 2006, Pitfield and Brown 2011).
Table 13. Principal W-containing minerals (Schubert and Lassner 2006, Pitfield and Brown 2011).
| Mineral | Chemical name | Formula | WO3 content, % |
|---|---|---|---|
| Scheelite | Calcium tungstate | CaWO4 | 80.6 |
| Stolzite | Lead tungstate | PbWO4 | 50.9 |
| Wolframite (ferberite and hübnerite) | Solid solution of iron tungstate and manganese tungstate | (Fe, Mn)WO4 | 76.3–76.6 |
The principal economically interesting tungsten ores are the scheelite and wolframite-group minerals. However, scheelite remains the most important raw material for the manufacturing of tungsten chemicals and pure W metal.
The content of W in the Earth's crust is 0.007%. According to US Geological Survey data (Shedd 2017b), the global W reserves account for approximately 3.1 Mt. Most global W resources are in the form of scheelite and wolframite-group minerals (Anthony et al 2005). The right-hand chart in figure 10 outlines the share of W resources by country, based on data from the US Geological Survey (Shedd 2017b). However, this global distribution does not include data for the USA, Bolivia and Rwanda. The largest deposits of W ores are in China (1.9 Mt; i.e. 60% of worldwide reserves), and other countries lag far behind, with the second largest claim of 290 000 t (i.e. 9% of worldwide reserves) located in Canada. According to Argus Media Ltd, China accounts for the majority of global W production (Seddon 2016).
Figure 10. Global share of W mining production and estimated W reserves (Shedd 2017b).
Download figure:
Standard image High-resolution imageThe left-hand chart in figure 10 indicates the 2015 global distribution of W mining production. China plays a predominant role, accounting for 82% of global production (i.e. 73 000 t). Based on 2015 data, Vietnam was the second largest producer (i.e. 5600 t), accounting for only 6% of the world market.
Figure 11 plots the annual production of W over time, which has generally exhibited a trend of moderate increase between 1960 and the early 2000s, fluctuating between production values of 25 000 and 50 000 t. However, from 2004–2015, a significant increase was observed, culminating at 89 400 t according to the US Geological Survey (USGS 2017c) and at 80 900 t according to Brown et al (2017).
Download figure:
Standard image High-resolution imageModern mining techniques for W-containing ores can be divided into underground and surface mining categories (Schmidt 2012). The selection of mining technique depends on the mineral properties and the physical peculiarities of the ore body (MSP-REFRAM 2016). Due to low W content in ores (0.3%–1.5% of WO3), an enrichment of the ore is necessary in order to produce a concentrate containing 60%–75% WO3. Ore enrichment processes include gravity concentration and flotation processes for scheelite, and magnetic separation for wolframite-group minerals. Additional processes are implemented after enrichment; the hydrometallurgical approach (Gaur 2006) for the production of W compounds, and pyrometallurgical methods (Gostishchev and Boiko 2008) for the production of pure W powder (or ferrotungsten). W-containing scrap is an important secondary source for pure W, as it usually contains more W than ore materials.
3.4.3. Uses.
A combination of unique properties including a very high melting point, high wear resistance, low coefficient of expansion and others, makes W an essential material for technological applications under extreme conditions. According to recent data from the British Geological Survey (Brown et al 2017), modern applications of W can be classified into four main branches as sketched in figure 12. The main application areas of W, including annual production is in 1000s of tonnes. The respective share is in percent units (Pitfield and Brown 2011, USGS 2017c). Specialised applications of tungsten comprise the production of catalysts, electrical and electronic components (Anthony et al 2005). The manufacture of cemented carbide tools is the most widespread use of W, accounting for 61% of global average W consumption (Hayashi et al 2016). Specialised applications of W-containing materials include photocatalysis based on WO3 and Bi2WO6 nanomaterials (Kumar and Rao 2015) and electrocatalytic applications for fuel cells (Christian et al 2007).
Figure 12. Main application areas of W, including annual production in 1000s of tonnes. Respective share is in percentage (Pitfield and Brown 2011, USGS 2017c).
Download figure:
Standard image High-resolution image3.4.4. Price.
Phases of industrial activity in China along with reduced demand for tungsten carbide have influenced the volatility of W prices. EU directives (EC 2009, 2012) on phasing-out W filament and incandescent light bulbs have also affected the demand for W used in lighting devices. Figure 13 shows a dramatic fluctuation of W prices after 2005, including peaks in 2006 (37 USD kg−1) and 2012 (57 USD kg−1). However, over recent years the price of W has steadily decreased to reach the current value of 35 USD kg−1 (Metalary 2016d).
Figure 13. Price of W over time in USD kg−1 (red dots) and inflation-adjusted USD kg−1 (blue triangles) (Metalary 2016d).
Download figure:
Standard image High-resolution image3.4.5. Substitution and recycling.
Novel approaches to cemented carbides and cermets can enable substitution possibilities for W. The main substitution routes are directed to the replacement of W by molybdenum and titanium (Ishida 2011). Possible substitutes for tungsten in technical applications are listed in table 14 (MSP-REFRAM 2016).
Table 14. Tungsten applications and possible substitution routes (MSP-REFRAM 2016).
| Tungsten applications | Possible substitution |
|---|---|
| Cemented carbides | Molybdenum, titanium, hardened steels, niobium carbide |
| Lighting devices | Carbon nanotubes, LEDs |
| High-speed steel for tools | Molybdenum alloyed with chromium, vanadium and nickel |
| Superalloys | Molybdenum, and carbide/nitride ceramic matrix composites reinforced with rhodium fibres |
Cemented carbides are among the most demanding W-containing materials for recycling. There are several processes to recycle cemented carbide to form ammonium paratungstate (5(NH4)2O·12WO3·5H2O) (Itoh 2014). The conventional process used by European industries consists of multiple technological stages as sketched in figure 14.
Figure 14. Conventional carbide processing scheme (based on data from Itoh 2014).
Download figure:
Standard image High-resolution imageA new process developed and launched in 2011 by the Japanese corporation, Sumitomo Electric Industries Group, comprises fewer stages compared to the standard process and is sketched in figure 15 (Hayashi et al 2016).
Figure 15. Modernized cemented carbide processing scheme as developed by Sumitomo Electric Industries Group (based on data from Hayashi et al (2016)).
Download figure:
Standard image High-resolution imageAccording to the International Tungsten Industry Association data of 2013, the percentage of recycled W-containing materials in Europe and the USA was approximately 50%, compared to only 30% in Japan (Hayashi et al 2016). The effectiveness of recycling secondary W strongly depends on the origin of the feed materials. For example, the recycling of W-containing high-speed steel is more favourable for recycling than W used in lamps and electrodes, due to the higher W content in the former. At the same time, processes for W recycling require less energy compared to raw mineral processing (Leal-Ayala et al 2015). Recently, a low-cost hydrometallurgical process for W recycling was suggested by Ishida et al (2012). Moving towards a circular economy, Shishkin et al (2012) investigated a recycling of industrial residues that consisted of structural composite reinforced by tungsten filaments into aluminium and copper matrix composites suitable for polishing applications (figure 16).
Figure 16. (a) Industrial tungsten-containing composite, (b) milled tungsten filaments in aluminium matrix, (c) milled tungsten filaments in copper matrix. Photos taken at Riga Technical University by the authors.
Download figure:
Standard image High-resolution image3.4.6. Résumé.
Distinctive mechanical and chemical resistance position tungsten as a very important material for use under extreme conditions. There are several major application areas for W and its compounds: the production of cemented carbides, production of catalysts, W-containing alloys, electrical and electronic components, and many other specialised applications. However, an efficient application and recycling of tungsten-containing materials remain a key point of supply risk minimisation for technological innovation in modern circular economies (Leal-Ayala et al 2015, Ku et al 2017).
3.5. REEs
3.5.1. General.
The REEs are scandium (Sc), yttrium (Y) and the 15 lanthanide elements of the IUPAC Periodic Table (Connelly et al 2005). This group of 17 elements share chemical and physical similarities: cerium (Ce), dysprosium (Dy), erbium (Er), europium (Eu), gadolinium (Gd), holmium (Ho), lanthanum (La), lutetium (Lu), neodymium (Nd), praseodymium (Pr), promethium (Pm), samarium (Sm), scandium (Sc), terbium (Tb), thulium (Tm), ytterbium (Yb) and yttrium (Y). As already outlined in table 4, REEs can be subdivided into LREEs and HREEs.
The name 'rare earth element' is somewhat misleading, as these elements are actually not rare, but can be quite abundant. As outlined by Klinger (2015), the name is mainly indicative of certain assumptions made at the time of their discovery. As there is no natural occurrence of promethium (Pm), and all isotopes of Pm are radioactive, the majority of this section will deal with the other 16 REEs, for which there is relevant information on markets, applications, recycling and substitution.
3.5.2. Deposits and mining.
The average concentration of REEs in the Earth's crust is higher than the concentration of some metals mined industrially on a large scale (see table 5). The abundance of REEs in the Earth's crust is for example higher than the abundance of copper (55 ppm) or zinc (70 ppm). The main sources of REEs are alkaline igneous rocks and carbonatites. Pegmatites, iron-oxide copper-gold deposits, placer deposits, residual deposits and marine phosphates (Long et al 2010) are considered to be potential sources as well, since the concentration of REEs is sufficiently high from an economic point of view. However, bastnäsite, monazite, xenotime and ion-adsorbed clays are the typical sources from which REEs are recovered on the commercial level. Monazite and xenotime occur in heavy mineral sand deposits. For those minerals, the beneficiation processes are already developed and they need minimal or no comminution, which make monazite and xenotime economically advantageous. Bastnäsite has been processed on industrial scale in Mountain Pass, USA and Bayan Obo, China (Jordens et al 2013). Ion-adsorbed clays require limited or even no beneficiation.
Figure 17 shows the world mining production of REEs, annually from 1960–2015. In recent times, China has been the dominant global supplier of REEs, although excess supply has caused a decrease in price. China's Rare Earth Industry Association has forecast that the use of REE oxides in China will increase to 149 000 t by 2020, while it was only 98 000 t in 2015. Magnets, abrasives and catalysts were the main applications of REEs in China in 2015. Approximately 35% of REEs were used for magnet production, 18% for abrasives and 15% for catalysts. During 2015, the export of REEs from China increased by up to 20% in comparison to 2014 (USGS 2016).
Figure 17. World mining production of REEs, annually from 1960–2015 (USGS 2017d).
Download figure:
Standard image High-resolution imageIn addition to China, India also has significant sources of REEs. In India, the main reserves are based on heavy mineral sand deposits and are located along the coastline. Only three government-owned companies extract those minerals: Tamil Nadu (Manavalakurichi), Kerala (Chavara) and Odisha (Zepf 2013, Roskill 2015). In Russia, there are two important deposits at Khibiny and Lovozero (Walters et al 2011). Within the EU, the Norra Kärr mine in Sweden is a potential source of REEs, which could last for 20 years at a mining rate 1.15 Mt yr−1, with a stripping ratio of 0.73:1 (Schreiber et al 2016).
Table 15 summarizes essential data of the individual 17 REEs. Ce occupies the most important position, as it covers 42% (i.e. 24 000 t) of the tonnage produced followed by La with 22% (i.e. 12 500 t). Among the other REEs, only Y (8900 t corresponding to 16 %) and Nd (7300 t corresponding to 13%) are notable amounts. All the other REEs show a share of less than 5% and may be as low as 400 kg per year, as is the case for Sc.
Table 15. Concentration, estimated reserves, production and time to depletion for REEs.
| REE | Concentration |
Estimated resources |
Production by 2008 | Time to depletion |
|
|---|---|---|---|---|---|
| Amount (t) |
Share (%) | ||||
| Sc | 14 | n.a. | 0.4 | 0.001 | n.a. |
| La | 31 | 22 600 | 12 500 | 21.8 | 1800 |
| Ce | 63 | 31 700 | 24 000 | 41.9 | 1300 |
| Pr | 7.1 | 4800 | 2400 | 4.2 | 2000 |
| Nd | 27 | 16 700 | 7300 | 12.7 | 2300 |
| Sm | 4.7 | 2900 | 700 | 1.2 | 4100 |
| Eu | 1.0 | 240 | 400 | 0.7 | 600 |
| Y | 21 | 9000 | 8900 | 15.5 | 1000 |
| Gd | 4.0 | 3600 | 400 | 0.7 | 9100 |
| Tb | 0.7 | 570 | 10 | 0.02 | 57 000 |
| Dy | 3.9 | 3 000 | 100 | 0.2 | 30 000 |
| Ho | 0.8 | n.a. | 10 | 0.02 | n.a. |
| Er | 2.3 | 1900 | 500 | 0.9 | 3700 |
| Tm | 20.3 | 330 | 50 | 0.1 | 6700 |
| Yb | 2.0 | 1900 | 50 | 0.1 | 38 000 |
| Lu | 0.3 | 400 | n.a. | n.a. | n.a. |
| Σ (rounded) | 100 000 | 57 000 | 1700 | ||
aConcentration in the upper continental crust according to Rudnick and Gao (2003). bAccording to Haque et al (2014).
Table 15 also demonstrates the relation between the estimated reserves and the annual production. The 'time to depletion' was calculated under the assumption of constant consumption and not considering the potential discovery of new deposits. Based on this calculation, it could be estimated that humankind will not run out of REEs in the short term. The shortest time until depletion was determined for Eu, which would last for 600 years, given these assumptions. Under these assumptions, Ce and La as the most relevant REEs in terms of volume, would not expire before 1300 and 1800 years, respectively.
3.5.3. Uses.
Catalysts, glass production, fluorescent lamp production and metallurgy consume 59% of the total production of REEs, whereas the remaining 41% is used for ceramics, alloys in batteries, and permanent magnets. Approximately 80% of Ce and La is used for catalysts, glass, fluorescent lamp production and metallurgy. This sector consumes 45% of cerium oxides, 39% of lanthanum oxides and 8% of yttrium oxides. About 85% of Nd, Pr and Dy is applied in batteries, ceramics and magnets (Charalampides et al 2015). Neodymium, gadolinium, praseodymium and dysprosium oxides and other REE oxides represent 7% of the total consumption of REE oxides (Goonan 2011, Charalampides et al 2015). Ce is applied as the oxygen storage promoter, while La and Nd are used as promoters or stabilizers. The most significant property of Ce is the oxygen storage capacity (Cuif et al 2005). Cerium oxide possesses cubic structure similar to a fluorite type. This structure of cerium oxide enables fast diffusion of oxygen as a result of the oxygen vacancies, because the atoms of the oxygen in the structure are arranged in plane. The increased number of vacancies promotes movement of the oxygen, which leads to the reduction and oxidation (Dahle and Arai 2015). The use of lanthanum originates from the fact that the trivalent lanthanum generates Brønsted acid sites by hydrolysis of the water (which improves the activity of the catalyst), but at the same time modifies the electronic environment and causes the change of the silanol (ceramic support) acidity strength (Dalla Costa et al 2014). In the glass industry, Ce is used for glass discoloration, since tetravalent cerium oxide oxidizes the iron as the impurity, which results in the colour change from blue to light yellow (Cuif et al 2005). Due to the hardness and chemical reactivity, cerium oxide is largely applied for glass polishing. Cerium oxide reacts with the glass silica at the interface forming the silicate stratum, which makes glass more fragile and not so resistant to physical modification (Cuif et al 2005). Fluorescent lamps are another important application of REEs. Those lamps use the different mixes of green, red and blue REE phosphors in order to produce visible light (Tunsu et al 2014). Tb3+ is a promising activator for phosphors applied in fluorescent tubes, plasma display panel devices, etc. Tb3+ shows a strong VUV/UV excitation characteristic due to its spin-allowed 4f–5d transition, and an intense visible emission can be achieved in an appropriate host lattice upon the VUV/UV excitation (Liu et al 2016). The red emission is obtained by Y2O3:Eu. Emission occurs within the f-levels of the Eu3+ ions (Ronda et al 1998). Eu3+ activated Y2O3 exhibits sharp red emissions at ~610 nm arising from the 5D0 f 7F2 intra-4f electronic transitions of the Eu3+ ions, upon UV excitation into the charge transfer band at ~254 nm (Li et al 2008).
Usually, BaMgAl10O17 with the addition of Eu2+ is applied as blue phosphor. In this substance, the photons are absorbed by the Eu2+ ions and the absorption is the result of 4f → 5d transition (Ronda et al 1998). The metallurgy consumes 45% of cerium oxides, 39% of lanthanum oxides and 8% of yttrium oxides (Charalampides et al 2015) for mischmetal production. Mischmetal is an alloy of REEs with inexactly defined composition. In general, mischmetal contains 50% Ce and 25% La, together with a small concentration of Pr, Y and Nd. Mischmetal binds the impurities in the metal and forms solid compounds and thus reduces the effect of the impurities on metal properties (Dahle and Arai 2015). About 85% of Nd, Pr and Dy is applied in batteries, ceramics and magnets (Charalampides et al 2015). The electrode material of NiMH batteries uses REEs in a form of metal hydride (MH). In MH, the REEs are present as A2B7 (LaCePrNdNiCoMnAl plus Mg) or AB5 (LaCePrNdNiCoMnAl) or as AB2 (VTiZr-NiCrCoMnAlSn), where the 'ABx' represents the ratio of the A metals (LaCePrNd or TiZr) to B metals (VNiCrCoMnAlSn) (Ruetschi et al 1995). Neodymium and dysprosium are applied in the production of powerful magnets. Those magnets are based on the intermetallic material, which in general consists of Nd, Fe and B with the addition of Dy. The boron is used to increase the interatomic distance from Fe, which fixes the orientation of the magnetic moment. The origin of the magnetic moment of REEs is the spin and the orbital momenta of unpaired 4f electrons (Jensen and Mackintosh 1991). The orbital magnetic moment is well preserved, since the electrons in the inner 4f shell are shielded by the 5s25p6 shell (Shirsath et al 2014). Dy is used to enhance the performance of the magnets. The magnet's coercive force is improved by replacing the Nd on the periphery of crystalline particles with Dy (TechJournal 2011, Smith et al 2012), which have potential for utilization in refrigerator applications (Shah et al 2014).
Higher demand for REEs can be expected due to the development of new materials and alloys for various applications. For example, newly developed alloys such as Gd5(Si2Ge2), Gd5(SixGe1−x)4, La(FexSi1−x)13Hx and MnFeP1−xAsx have large magnetocaloric effects (Smith et al 2012), which have potential for utilization in refrigerator applications (Shah et al 2014).
3.5.4. Price.
Figure 18 shows the price of REE oxides from 1960–2015 in USD kg−1 (red dots) and inflation-adjusted USD kg−1 (blue dots). No significant price jumps can be observed until 2010, after which there was a major increase and temporary peaking in 2011–2012, and after 2013 prices dropped to the pre-peak level. The peak in the price between 2011 and 2012 may be attributed to export quotas, duties and licenses introduced by the Chinese government; these export restrictions were introduced in 2006 and gradually tightened. The quotas have become increasingly detailed (targeting LREEs and HREEs separately), with specific local regulations on reserves and the number of licensed exporters (ERECON 2015).
Figure 18. Historical price of REE oxides over time; price was estimated for the United States by a weighted average of imports and exports. Price is given in USD kg−1 (red dots) and inflation-adjusted USD kg−1 (blue triangles) (USGS 2017d).
Download figure:
Standard image High-resolution imageThe prices of individual REEs vary, but show a similar trend. Figure 19 plots the trend for selected REEs (Ce, Ce–La mischmetal, La, Nd), which are in the range of the low price level. For all the mentioned REEs, there has been a price drop since 2010. By analogy to the trend shown in figure 17, Nd showed a jump in price during 2011, peaking at almost 200 USD kg−1. The current price level is 40 USD kg−1, i.e. only one fifth compared to 2011. For La, Ce and mischmetal there has been no peak within the investigated time period. However, the price decreased massively, and current prices are only at 4% (La) and 5% (Ce) compared to 2010.
Figure 19. Price for selected 'low-price' REE oxides according to Gambogi (2015) and Gambogi (2017).
Download figure:
Standard image High-resolution imageAs shown in figure 20, the same trend occurred for selected REEs, which are in the range of the high price level. Dy (1400 USD kg−1), Tb (2800 USD kg−1) and Eu (3800 USD kg−1) peaked in 2011. Since then, prices have dropped tremendously. The Eu price in 2016 was 2% (66 USD kg−1) compared to 2011.
Figure 20. Price for selected 'high-price' REE oxides according to Gambogi (2015) and Gambogi (2017).
Download figure:
Standard image High-resolution image3.5.5. Substitution and recycling.
Due to the widespread application of REEs, there are a substantial number of end-of-life products that are considered to be a valuable source of those metals. Among them, NiMH batteries, fluorescent lamps and permanent magnets are the most common REE-based commodities that are being recycled, or there are a lot of efforts to develop recycling technologies for REEs recovery from these sources.
3.5.5.1. Spent NiMH batteries.
The use of hybrid and electric cars continues to increase and is accompanied by higher demand for more effective recycling methods of the batteries used in those cars. Spent portable NiMH batteries have been recycled using high-temperature processes to recover the Ni and Co. However, REEs are typically not recycled from the slags, which exhibit low REE concentrations compared to other potential sources (Binnemans et al 2013). The companies Umicore and Rhodia have designed a hydrometallurgical technology for REE recovery from the slag coming from the pyrometallurgical processing of spent batteries (Binnemans et al 2013). Several hydrometallurgical technologies were developed to recover metal from spent NiMH batteries on a laboratory scale. Inorganic reagents such as hydrochloric acid (Zhang 1998) and sulphuric acid (Pietrelli 2002, Pietrelli et al 2005) or sulphuric acid with the addition of hydrogen peroxide as a reduction agent (Mantuano et al 2006, Nan et al 2006) were used for the dissolution of the black mass. Preferably, acidic extractants such as bis(2,4,4-trimethylpentyl) phosphinic acid (Cyanex 272), 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (PC-88A), di-(2-ethyl-hexyl) phosphoric acid (D2EHPA), or Acorga M5640 as well as solvating extractants such as Cyanex 923 or tributyl phosphate (TBP) were used in the processes for metal recovery. Precipitation was applied in the metal recovery, but liquid–liquid extraction was preferred, since it allows for higher purity of the final products. A study (Provazi et al 2011) was done to compare the effectivity of the precipitation and liquid–liquid extraction. It was concluded that the precipitation was not sufficient, since the purity of the final products was low due to metal co-precipitation. In another work, the combination of precipitation and liquid–liquid extraction was also used (Nan et al 2006). Only a few processes were optimized in the counter-current system. In a study by Wu and co-workers, REEs were first obtained as precipitate by leaching with sulphuric acid (Wu et al 2009). To recover residual REEs, 20% PC-88A was used in the counter-current way. In another process, the mixture of solvating extractant was used to recover REEs, Al, Co, Fe, K, Mg, Mn and Ni (Larsson et al 2012, 2013). The process was performed in a mixer-settler. In the extraction process, 70% Cyanex 923 together with 10% TBP, 10 % 1-Decanol diluted in 10% kerosene was used. Despite the development of hydrometallurgical technologies, the majority of NiMH batteries are processed using a pyrometallurgical approach and REEs are not recovered.
3.5.5.2. Fluorescent lamps.
There has been considerable effort devoted to the recovery of REEs from spent fluorescent lamps (Tunsu et al 2015, Tunsu et al 2016b, Tunsu et al 2016a), since they are potential secondary sources of Y, Eu, Ce, Tb and Gd, where they are present as phosphors. Several hydrometallurgical technologies have been developed to selectively recover REEs. In general, organophosphorus extractants such as di-(2-ethylhexyl)phosphoric acid also called D2EHPA, 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester commercially called PC-88A or dialkyl phosphinic acid known as Cyanex 272 are commonly used for the extraction and separation of REEs, since they provide a high extraction rate and also selectivity for REEs. Several hydrometallurgical routes have been tested to recover those metals from those secondary streams (Tunsu et al 2015). Novel extractant Cyanex 572 was used in the process for the recovery of Y and Eu from fluorescent lamps from the nitric media, followed by precipitation with oxalic acid and calcination (Tunsu et al 2016a). The process led to the production of yttrium oxide with a final purity of 99.8%, while europium oxide had a purity of 91%. Technology to recover all REEs involving terbium has been proposed using alkaline fusion before leaching with sulphuric acid (Ippolito et al 2017). Leaching was followed by precipitation with oxalic acid and calcination. Approximately 99% of Y and Eu, 80% of Tb, 65% of La, 63% of Ga and 60% of Ce was recovered. Another improvement of the hydrometallurgical processing was proposed using an intense frictional action (Van Loy et al 2017). The activation energy change increased the leaching efficiency of REEs from 0.9% to 81% at the ambient temperature. Also, novel green extractants such as ionic liquids have also been applied to recover REEs from fluorescent lamps (Yang et al 2013). N, N-dioctyldiglycol amic acid (DODGAA) was used to recover REEs from sulphuric and nitric media. The system showed selective separation of REEs from the impurities (Zn, Al, etc) present in the waste, giving the advantage to commercially available extractants such as PC-88A. Even though several processes were tested and developed, the processing of the fluorescent lamps is currently not feasible from an economic point of view due to REE prices.
3.5.5.3. Permanent magnets.
The majority of REE-based permanent magnets are made of neodymium-iron-boron (NdFeB) material, due to its high magnetic strength, inexpensive matrix material (iron) and suitable stability compared to available alternatives. The NdFeB magnet contains approximately 31 wt%–32 wt% REEs, predominantly Nd, Pr and a minor amount of HREEs such as Dy, Tb and Gd, but their content depends on the applications (Yang et al 2016). Dy is used as an additive and its concentration in NdFeB magnets varies widely depending on the application (Binnemans et al 2013). There have been many studies concerning the recovery of Nd and other associated REEs (Pr, Dy, Tb) through different types of metallurgical processes: hydrometallurgical, pyrometallurgical, electrochemical or combinations of these techniques. Pre-dismantling and up-concentration through physical processing are critical for viable chemical or metallurgical recovery. Recycling of the minor amounts of REEs from the magnet scrap is technically and economically challenging (Yang et al 2016). In pyrometallurgical processing, recycling can be applied using the following approaches: (1) material recovery, in which waste materials are treated in the smelting process as a secondary source; (2) alloy re-use, in which the metals are regenerated and used again as alloys for the production of magnets; and (3) recycling, where alloys are utilized without prior treatment (Firdaus et al 2016). To recover and separate valuable components, several methods such as oxidation (Nakamoto et al 2012), chlorination (Murase et al 1995), liquid molten extraction (Chae et al 2014), hydrogenation (Zakotnik et al 2009) and electrolysis (Gutfleisch et al 2013) were tested. Even though several processes have been developed for the recovery of REEs from spent magnets using the pyrometallurgical approach, they are still not fully optimized, and thus are not utilized on an the industrial scale (Firdaus et al 2016).
In hydrometallurgical treatment, the dissolution of magnet scrap can be performed in three different ways (Yang et al 2016): (1) complete dissolution of the NdFeB magnet (with or without a prior roasting step using H2SO4 or HCl (Abrahami et al 2015, Bandara et al 2016), (2) roasting followed by selective leaching of the REEs (Vander Hoogerstraete et al 2014), and (3) selective conversion of REEs in the solid magnet or magnet scrap directly to a new solid phase (Itakura et al 2006). Alternatively, the transformation of REEs in the magnet scrap into REE compound precipitates could be realized, based on the solubility of REE salt at different temperatures or under hydrothermal conditions (Yang et al 2016). Different extractants, naphthenic acid, D2EHPA, PC 88A, Ionquest 801, versatic acid, trialkyl-methyl ammonium chloride (Aliquat 336), Cyanex 272, TBP, and others are used for REE extraction in various industries (Jha et al 2016, Tunsu et al) and were tested for the separation of REEs from the magnets.
Even though several recycling approaches for the recovery of REEs from permanent magnets have been tested and designed, it was concluded that the direct re-use of the magnets or alloys is impossible, and the technological challenges for REE recovery are substantial (Binnemans et al 2013).
3.5.6. Résumé.
REEs have strategic importance in the production of advanced materials utilized in modern and 'green' applications. Since REEs possess specific properties, there exist only limited or no possibilities for their substitution (ERECON 2015). Moreover, due to preferably controlled exploration, which occurs only in a few countries, the accessibility of REEs contributes to their criticality for the EU, USA, Japan and other industrialised economies. The export quotas, duties and licenses introduced by the Chinese government played a role in the record peak in the prices of REEs in 2011 and 2012 (ERECON 2015, USGS 2017d). Subsequently, significant effort has been made in the EU and worldwide to determine and implement alternative sources to REEs. Spent portable and car NiMH batteries, obsolete fluorescent lamps and magnets are considered as promising and feasible secondary sources of REEs. Several technologies and recycling processes have been developed and designed to recover valuable REEs using pyrometallurgical or hydrometallurgical approaches. However, due the recent decline in the price of REEs, the majority of these technologies are currently not commercially applied. Nevertheless, their existence is a strategic solution in the case of future limited accessibility of REEs.
4. Brief summary and conclusions
Criticality has become an important issue today as technologically-advanced economies such as the EU, USA and Japan are strongly dependent on the supply of certain raw materials. This paper has reviewed the current situation of Co, Nb, W and REEs. These elements are extremely critical from a European perspective as they are currently of high economic importance and present a high supply risk and are expected to be crucial for emerging technologies.
One possible solution to tackle the problem of CRMs is a substitution by other, less critical ones. For the selected elements, different possibilities are suggested in the literature (ERECON 2015, MSP-REFRAM 2016, Shedd 2017a, Papp 2017a), but potential disadvantages have to be taken into account, including the possibility of significantly degraded material properties or higher prices compared to the original element (Grilli et al 2017). It has furthermore to be considered that some of the suggested substitutes might not work in practice. For example, the substitutability index of Co is 1 (EC 2017a), which means that it is de facto indispensable for certain applications. As criticality is mercurial, any significant increase in demand for substitutes will influence their economic importance and supply risk, and, as a consequence, the material might be shifted into criticality.
Considering the complications associated with the substitution of different elements, one logical step for industrial economies is to promote substitution with secondary resources, which essentially amounts to the promotion of recycling. Recycling is an effective tool to decrease the demand for primary CRM resources. Unlike polymers, the elements investigated in this paper do not undergo a degrading process. However, it is clear that a recycling rate of 100% is not possible due to several reasons.
During the use phase of products, material is lost by dissipation. In particular, CRMs used under extreme conditions are subjected to abrasion (e.g. W used in cemented carbides for tooling) and corrosion (e.g. Nb in alloys), which means that a certain fraction of the CRMs is spread into the environment and heavily diluted. Gutowski (2008) has outlined that the work to extract a material from a mixture is monotonically increasing when the concentration gets lower and thus dissipated metals are lost for recycling.
Another important issue is the complexity of products. In order to take into account the number and concentrations of materials used for an item, Dahmus and Gutowski (2007) introduced a factor of material mixing. The authors showed that products only get recycled if they are rather simple (i.e. they contain a low number of materials) and/or the value of the extractable materials is high. Otherwise, when products are complex and their material value is low, recycling is not economic and simply will not take place. Dahmus and Gutowski (2007) further outlined that products are becoming increasingly more complex over time. Any partial substitution of critical materials such as Co, Nb, W and REEs will make the products more complex and reduce recyclability. Therefore, one has to realise that replacing a CRM with other elements might cause the opposite effect to that intended. Namely, an increased demand for the CRM as the recycling rate is declining.
Finally, recycling demands a separate collection of end-of-life items containing a certain metal. End-of-life products that end up in incorrect waste streams mean that recycling will not happen.
In view of these circumstances, it is surprising that the recycling rates of the important materials Co, Nb, W and REE are extremely low. The European Commission (EC 2017c) reports an end-of-life recycling input rate of 0.0% for Co and 0.3% for Nb only. The situation is slightly better for REEs (LREEs: 3.0%/HREEs: 8.0%), but far from being sufficient. Only W exhibits an end-of-life recycling input rate of 42%. In view of the above-mentioned findings, this W success rate seems excellent. It has to be concluded that the EC has in theory recognized the problem, but obviously the policy has not improved the situation to date.
Acknowledgments
The authors are members of the WG4 working group entitled 'value chain impact' within the European COST action CA15102 'Solutions for Critical Raw Materials Under Extreme Conditions' (CRM-EXTREME), which was initiated in 2015 (COST 2015). The action addresses possibilities for substitution of CRMs in high-value alloys and metal-matrix composites. CRM-EXTREME considers the substitution of CRMs including Co, Nb, W and REE in the energy, transportation and machinery manufacturing industry applications under extreme conditions of temperature, loading, friction, wear and corrosion.
Within CRM-EXTREME, the WG4 working group focused on 'value chain impact' has been established. WG4 addresses the CRM substitution issue via a holistic approach, recognizing that all the implications of the product life cycle (production, usage, recycling, etc.) and the accompanying value chain are interconnected, and that positive and negative effects in one part of the chain can reverberate in the other parts. Specifically in the CRM-EXTREME WG4 activity, it is foreseen that a lasting contribution in the area of life cycle assessment and/or value chain analysis will be based on the analysis of material and energy flows, including dedicated emphasis on material losses such as improved recycling technologies.
This publication is based upon work from COST Action CA15102 supported by COST (European Cooperation in Science and Technology): 'Solutions for Critical Raw Materials Under Extreme Conditions (CRM-EXTREME)', www.crm-extreme.eu. Furthermore, the authors would like to acknowledge networking support from COST CA15102.




















