Abstract
Suspensions of magnetic nanoparticles offer diverse opportunities for technology innovation, spanning a large number of industry sectors from imaging and actuation based applications in biomedicine and biotechnology, through large-scale environmental remediation uses such as water purification, to engineering-based applications such as position-controlled lubricants and soaps. Continuous advances in their manufacture have produced an ever-growing range of products, each with their own unique properties. At the same time, the characterisation of magnetic nanoparticles is often complex, and expert knowledge is needed to correctly interpret the measurement data. In many cases, the stringent requirements of the end-user technologies dictate that magnetic nanoparticle products should be clearly defined, well characterised, consistent and safe; or to put it another way—standardised. The aims of this document are to outline the concepts and terminology necessary for discussion of magnetic nanoparticles, to examine the current state-of-the-art in characterisation methods necessary for the most prominent applications of magnetic nanoparticle suspensions, to suggest a possible structure for the future development of standardisation within the field, and to identify areas and topics which deserve to be the focus of future work items. We discuss potential roadmaps for the future standardisation of this developing industry, and the likely challenges to be encountered along the way.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Magnetic nanoparticles (MNPs) in liquid suspensions are rapidly being implemented in a diverse set of new and emerging technologies [1–6]. Key technology areas include the use of MNPs as biomedical imaging agents; as functionalised particles for in vitro enrichment of cells, viruses, proteins or nucleic acids; as magnetic slurries for the adsorption of hazardous materials or water desalination; and as magnetic oils for use in vacuum seals, lubricants, and loudspeakers. Many of these applications impose stringent requirements on the MNPs to be used, in order to ensure correct functioning and safety [7], but each application requires its own specific characteristics, and the appropriate characterisation parameters vary accordingly. To date, a huge variety of compositions, structures and morphologies of MNPs have been developed to meet established industry needs, while at the same time MNPs continue to be the subject of cutting edge research and development (R&D) aimed at meeting new and emerging industry needs.
Alongside this concerted industry-focused R&D effort, there is clearly a need for the development of appropriate and robust standards relating to the characterisation of MNPs in liquid suspensions. A standard provides an agreement on the current scientific state-of-the-art within a topic, and guidelines on implementing this knowledge for the optimisation of manufacturing, measurement, analysis and safety. Best practice guides provide information and guidance on technical methodologies that have been accepted as being superior to their competing alternatives. Standard operating procedures contain specific step-by-step instructions on how the carry out processes in order to achieve optimal quality, and comply with regulations. Both best practice guides, and standard operating procedures may be incorporated into standards documents. While the creation of new standards is driven by the needs of industry, their development is a task for science. A recently published book discusses the wider topics of metrology and standardisation for the field of nanotechnology as a whole [8] and provides an introduction to the standardisation of MNPs, a subject which we examine in detail here.
It is in this context that we provide here an overview of the current state-of-the-art in the science of magnetic nanoparticles, and the future tasks and challenges we face in order to reach the complete standardisation of MNP products. Throughout the document, we highlight many of the open questions and unsolved problems which currently inhibit progress within the sector. These represent significant opportunities for future research projects, and are intended to provide an overview or roadmap of major topics which should be addressed within the coming years, In part, this article represents some of the conclusions that have been reached over the last four years by a broad consortium of academic laboratories, national metrology institutes and industrial collaborators who have been working together under the auspices of the European Commission's Seventh Framework Programme project 'NanoMag: Nanometrology standardisation methods for magnetic nanoparticles' [9]. The document presented here is intended to act as a precursor to the future development of MNP standards.
The review is structured as follows. Initially, we provide an overview of the potential standardisation routes for MNPs which might be implemented. We then volunteer a possible framework for future standards documents based on both the constraints of the subject matter, and the requirements for document development stipulated by the International Organization for Standardization (ISO). To conclude, we present an overview of the standardisation work which is currently underway in the MNP field, discuss additional tasks which need to be undertaken, and provide a roadmap for the future of the topic.
2. Routes towards standardisation of MNP suspensions
MNP suspensions form a vast and diverse topic which spans a huge variety of industries, each with highly specialised requirements. Specific terminology is required to describe the unique aspects of MNPs, many of which have yet to achieve universal definitions in the literature. In addition, there are a multitude of techniques for both the synthesis and characterisation of MNP suspensions. A particular selection of these techniques and definitions will be required for a specific product; this is dictated by the application for which it is destined.
Furthermore, our understanding of the health and safety implications for nanomaterials, from production and release criteria through to storage requirements and disposal methods, is still developing. This is because there are fundamental physical characteristics and factors applying to nanoparticles, which are distinct from those applicable to the same materials when they are in the bulk form. As such, the very large amount of accumulated knowledge that exists for bulk materials cannot simply be applied to the nanoparticulate state, and new standards are needed.
2.1. Standardisation methods
There are two conventional routes towards the standardisation of materials, which in principle might be applicable to the case of MNPs in liquid suspensions. These are: (1) the adoption of standardised synthesis processes intended to result in particles with predictable and reproducible properties; and (2) the post-synthesis characterisation of MNPs in order to establish the specific characteristics of a batch of particles after manufacture [10]. We conclude that the standardisation of characterisation methods is the approach best suited to the MNP sector. Based on this, we propose a framework for the organisation of future standards documents in order to provide coverage for this varied and complex area. The proposed structure is based on the compilation of portmanteau documentation covering vocabulary/definitions; sampling; measurements; labelling; and applications.
2.1.1. Standardisation of synthesis methods.
It has long been noted that batch-to-batch reproducibility in nanoparticle synthesis is a significant challenge, and a key obstacle to the development of marketable products. Variations in particle output are commonly the result of either the uncontrollable presence (or variation) of contaminants in raw materials, and/or the uncontrollable variation in production process parameters. Even with access to detailed procedural instructions, issues with reproducibility become amplified when trying to reproduce synthesis processes in different laboratories. Furthermore, the problem of reproducibility in synthesis increases with the batch size under production [11]. As a result, at present the standardisation of synthesis methods remains a significant challenge.
A further consideration is the large variety of synthesis routes which are available for producing MNPs. The precise details of individual synthesis techniques are frequently kept secret in order to protect the intellectual property of manufacturers, and prevent competitors from copying their products. Due to the reluctance of the MNP industry to share the precise details of individual manufacturing processes, it is unrealistic to attempt to create global standards for them. This, coupled with the complexities that would be involves in drawing up standardised techniques for every possible MNP synthesis route, mean that we do not regard the standardisation of synthesis methods as the best option.
In this review, we therefore choose to concentrate primarily on the standardisation of characterisation methods, rather than on synthesis procedures. It should be noted, however, that a small number of published standards do exist pertaining to vocabulary and processes relevant to MNP production [12–22], in addition, some standards of relevance are currently under preparation at the International Electrotechnical Commission [23]. The study of the exact parameters which dictate variations in nanoparticle synthesis, and the quest for reproducibility remain ongoing topics in front-line research [24]. The reproducible synthesis of identical MNPs in different labs remains a scientific goal. With greater understanding of the interplay between the important limiting factors, standards for MNP synthesis can be expected to develop in the future.
2.1.2. Standardisation of characterisation techniques.
As the standardisation of MNPs synthesis requires significant R&D advances, post-synthesis characterisation promises a faster and in particular more reliable route towards achieving the harmonisation of MNP products. A vast variety of characterisation techniques exist, probing both magnetic and non-magnetic parameters. In many cases, multiple measurement techniques are capable of probing the same parameter through alternative means [25].
It is notable that while several standards have been published relating to the characterisation of non-magnetic properties of nanoparticles (see section 6.1 for details), to date, no standards for the magnetic characterisation of nanoparticles have been formulated. One stumbling block is that to develop standardised measurements, it is very helpful to be able to work with standard reference materials. This, as we have just discussed, remains a significant challenge for MNPs. At present, no approved reference materials exist for MNP characterisation measurements.
That said, some de facto 'quasi-standard' materials are being used for specific purposes. One example is the use of the commercially available MRI contrast agent Resovist® in the development of magnetic particle imaging, a novel method for mapping MNP distributions [26]. This is a pragmatic approach, which, given the highly-fragmented nature of the MNP market sector, is an informative one. Indeed, it has led us to the conclusion that rather than attempting to establish only a few 'one-size-fits-all' standards, a more application-specific 'mix-and-match' approach should be adopted. The mix-and-match approach combines different, but complementary, items from a wider list in order to form a coordinated set which meets the necessary requirements. We discuss this below, both from a pedagogical perspective, and in the context of recent developments towards a MNP suspension standard that is being undertaken through the International Organization for Standardization (ISO).
2.2. A 'mix-and-match' framework for future MNP standardisation
In our opinion, a rational approach to the standardisation of products based on magnetic nanoparticles in liquid suspensions is to consider which necessary constituent documents should logically be covered in any such standard. In effect, the 'mix-and-match' approach is to compartmentalise the documentation into separate but inter-linked classes. If these individual items are suitably well designed, then they can act as building blocks to be drawn together and referenced, when necessary, as any given body of work develops. Existing ISO standards of relevance relating to non-magnetic characterisation of magnetic nanoparticles (e.g. electron microscopy or dynamic light scattering analysis of particle sizes) are already in a suitable format to be coupled into the proposed standardisation structure under the 'measurement standard' class.
The mix-and-match approach is illustrated in figure 1 as a combinatorial map showing the suggested structure around which standards relating to MNP suspensions may be built. Underlying all of the other document types are the vocabulary standards and materials specifications class, whose content feeds into the development of each of the other document types. The connections between the different classes, and the manner in which they build upon the content of others is depicted by the arrows. Descriptions of the constituent document classes are provided in the following text.
Figure 1. Diagram illustrating the 'mix-and-match' framework for the standardisation of products based on magnetic nanoparticles in liquid suspension. Illustrating the inter-relation between the constituent standards classes, viz. definitions; sampling; measurements; labelling; and applications. The diagrams illustrate how different standards of each class may combine as necessary to provide the coverage required for standardisation of a specific MNP application.
Download figure:
Standard image High-resolution image2.2.1. Definitions standards and material specifications.
These documents fulfil the role of introducing and defining terminology specific to a given topic within the standards structure. Definitions standards are those whose scope is limited to the definition of technical terms. Materials specifications typically contain definitions specific to the material in question, as well as additional information such as lists of appropriate characterisation measurement techniques, safe handling guidelines and labelling requirements.
While some vocabulary pertaining to nanoparticles has already been included within published standards, a number of key definitions, particularly relating to magnetic characteristics, have yet to be accepted universally. It is not, for example, possible to define a measurement technique to establish superparamagnetic behaviour in a MNP sample, until the exact meaning of the term is defined in the literature. Harmonisation of the terminology used to describe MNPs is therefore the first requisite step in the path towards standardisation.
2.2.2. Sampling standards.
The need to obtain a representative usable sample is universal and intrinsic to all techniques for the measurement of MNP suspensions. Sampling documents are reliant on the terminology contained within definitions standards, and are a requisite to feed into documents on best practise for conducting characterisation measurements. Other aspects of sample preparation and handling may also require the compilation of standardised codes of practise. Examples of this include the correct pipetting techniques for handling nanoparticle dispersions, or the correct methods for digestion of samples for measurement of iron concentration.
2.2.3. Measurement standards.
As previously described, a separate standard should be established for each characterisation technique. Measurement standards are reliant upon both definitions and sampling standards. They are in turn a prerequisite in order to formulate more specific standards for particular applications. While a number of characterisation techniques for nanoparticles have already been the subject of ISO standards, at present none exist for characterisation of the magnetic properties of MNPs. This is a key area which requires development.
2.2.4. Labelling and storage standards.
Establishing industry consensus on the requirements for MNP labelling for use in specific applications can greatly aid end-users of the material. Appropriate storage, disposal and health warnings are also of significant importance, particularly given the unique and relatively unknown nature of nanomaterials. Labelling and storage documents are reliant on the previous definition of relevant terminology, and are a necessary reference in the compilation of a comprehensive application standard.
2.2.5. Application standards.
This class of document is the most advanced in terms of the standardisation infrastructure required, as it draws on the content of an established library of definitions, measurement and labelling standards. An application standard should define the important parameters of relevance for MNPs destined for a particular application, and give recommendations for, or define the characterisation techniques suitable to measure them. Information on the appropriate labelling of the characterised particles should also be included.
The proposed structure is intended to allow the development and cross referencing of many interlinked standards with the least possible confusion or amendment. Each application standard can draw upon the specific documents which are relevant to it. Additional measurement and application standards may be developed as and when the need arises.
To give a concrete example (see figure 2), one possible application standard that could be envisioned might be on the requirements for MNPs for use in magnetic hyperthermia therapy. Such an application standard would not stand on its own, but instead would be accompanied by both a definitions standard (defining the terms associated with single-core and multicore MNPs) and a material specification (defining the properties characteristic of MNP suspensions, and how they may be measured). There would also be a sampling standard on how to obtain appropriately representative aliquots from an MNP suspension, plus a series of measurement standards corresponding to each of the parameters needed to fully characterise the MNP suspension with respect to its intended application purpose: in this case, of magnetic heating [27]. The list of measurement standards provided in figure 2—of chemical analysis, hydrodynamic diameter, dynamic magnetic properties and intrinsic loss power—are examples of the sorts of standards that would be required here [28]. (Note this is a hypothetical list only: in practise, it will take a good deal of work to develop the standards before a definitive list may be determined.). Lastly there would be labelling standards (we list here two—concentration and stability of the suspension, and the magnetic heating capacity of the MNPs themselves—but there may be others too), and the application standard itself, which would bring the entire set together and in which the inter-relations between the constituent standards would be presented and explained.
Figure 2. Hypothetical example of a set of standards documents that together might constitute the 'mix-and-match' set of definitions, sampling, measurement and labelling standards to support an application standard covering MNP suspensions for use as magnetic hyperthermia agents.
Download figure:
Standard image High-resolution imageIn sections 6–10 below, we will examine the current state-of-the-art in each of the classifications of standard described here. We shall then identify the gaps in the existing documentation, and future requirements for MNP standards content.
However, before that, it is important to recognise that nanomaterials standardisation work is currently ongoing in a number of organisations around the world, and in particular that there is a programme under way at ISO that is specifically directed towards nanotechnology standards. We review this groundwork within the next section. Details of draft standards relating to MNP suspensions which are currently under development at ISO can be found in section 9 of this review.
3. Steps currently being undertaken by ISO towards MNP suspensions standardisation
This document concentrates primarily upon standardisation within the framework of the International Organisation for standardisation (ISO). This is due to the global reach of their documents, and the large body of relevant work already underway within the organisation. Recent years have seen significant ISO activity towards the standardisation of many aspects of nanotechnology. In 2005 an ISO technical committee (ISO/TC 229) was inaugurated with the specific goal of nanotechnology standards development.
Those developing new standards for MNPs should be aware of the definitions and terminology already provided within the ISO 80004 'Nanotechnologies—vocabulary' series of documents. Furthermore, for MNPs being developed for use in human healthcare, the content of the ISO technical report 'Nanotechnology—vocabulary in human healthcare' [30] may be relevent. ISO has issued its own guidelines regarding the expected content and structure of prospective ISO standards [29]. Two key stipulations are given in these guidelines: (1) that a separate standard is required for any test method which is likely to be referred to in a number of other documents; and (2) that if multiple test methods are capable of probing a particular characteristic, then each method should be the subject of a unique document.
In addition, ISO has published a 'metrological checklist' to aid assessment of the readiness of particular measurement techniques for the development of standards [31]. This checklist can be used by standardisation organisations, metrology institutes, companies, environmental agencies or other stakeholders intending to propose the development of a new ISO standard for measurements on engineered nanomaterials. It formulates the basic requirements for developing a quality management system for measurements on the nanoscale. The content of the metrological checklist is summarised as follows (MNP specific comments are below the italics):
- (1)The material subjected to the measurement procedure should be clearly described.While some terminology is clearly defined, many terms relating to the internal structure of MNPs, their magnetic properties, and magnetic interactions are not yet covered.
- (2)The definition of the material to be measured should not be unnecessarily restrictive.Definitions must be consistent across the full spectrum of MNP types spanning from small (5 nm) single-core to large multicore particles, and the variety of particles in between.
- (3)The measurand should be clearly described.A measurand is a physical quantity measured by a specific instrument. In the study of MNPs measurands may be probed by remote observations of the system, from which we infer MNP properties using models. While some physical properties of MNP suspensions can be unambiguously defined, e.g. the iron concentration, a physically exact definition can be difficult for others, e.g. for saturation magnetisation.
- (4)It should be clearly indicated whether the measurand is defined operationally (by the methods used), or whether the measurand is an intrinsic, structurally defined property.To illustrate this point, dynamic magnetic susceptibility is an example of an operationally defined property. This requires a careful definition of the field amplitude and temporal variation in order to interpret the measurand's definition correctly.
- (5)The measurement unit should be clearly defined, and the metrological traceability of the measurement should be pursued.Measurements should be defined using SI units. Metrological traceability means that the measurement result can be related to an established reference measurement through a documented unbroken chain of calibrations with defined uncertainties [32]. Metrological traceability in the strict sense has not yet been demonstrated for the magnetic characterisation techniques of MNPs.
- (6)The measurement technique should be demonstrated in the literature by at least one, but ideally multiple laboratories. Results should ideally have been published within peer-reviewed journals.Many published studies have attempted to provide comparisons of the non-magnetic properties of particles [33, 34]. For example, thorough interlaboratory comparison of nanoparticle size analysis has already been reported [39]. Comparative studies of magnetic properties measurements are sparsely reported at present [35–38]. Additional studies to provide further coverage is a key task for the future, these could eventually be performed within the organisational framework of VAMAS [40] or using the model offered by other published interlaboratory comparisons [41].
- (7)Quality control tools should be developed in order to validate the proficiency of a laboratory in performing the technique.Proficiency testing involves the use of interlaboratory comparisons for the determination of laboratory performance, the tests are performed by accredited providers [42]. Results of proficiency tests can be useful for customers, regulators, laboratory accreditation bodies and other organizations that specify requirements for laboratories [43]. Currently, there exists no comprehensive methodology to perform proficiency tests for characterization of MNPs. Achieving this would require the development of suitable protocols for sample treatment and measurements, standardized representation of results and data analysis, and the availability of stable MNP reference materials which could be used in such tests.
- (8)The instrumentation required in order to perform the measurement should be widely available.The availability of instruments varies significantly depending upon the measurement technique in question.
- (9)An uncertainty budget for the measurement should be developed, or at least a list of the measurement uncertainty components must be provided.The measurement uncertainty is a non-negative parameter that expresses the level of doubt about the validity of a result given by a measurement [43]. An uncertainty budget is a quantitative statement of the measurement uncertainty, of the constituent components of that measurement uncertainty, and of their calculation and combination. For the magnetic properties of MNP, full uncertainty budgets have been presented in a few isolated cases including Mössbauer spectrometry [44] and magnetic hyperthermia [45]. Generally, it is a task for the future to describe the magnetic properties measurements of MNPs according to the internationally accepted 'Guide to the expression of uncertainty in measurement' [46].
- (10)Characterisation methods must be properly validated in at least one laboratory. This means that the working range, sensitivity, repeatability and other factors must be known.The level of advancement varies dramatically between different techniques. In general, a significant amount of work is still required in order to achieve this for the majority of measurements.
From this, it is clear that the 'mix-and-match' methodology proposed in section 2 is well aligned to meet the requirements placed by the ISO drafting guidelines, and that careful attention needs to be given not only to the measurements standards, but also how they relate and feed into each of the other classes of standards. We therefore now proceed to review the current status and future requirements for the five classes of documents introduced in the mix-and-match structure for MNP standards.
4. Standards for MNP definitions and technical specifications
It is impossible to form a coherent standardisation structure for MNP suspensions without first defining the terminology, concepts and properties which must be considered. In this section, we examine the current coverage of ISO standards in the field and introduce some of the key concepts which must be defined in order to standardise both MNPs and their suspensions.
4.1. Coverage of published standards
Two published ISO documents deal specifically with vocabulary relevant to nanoparticles, although they do not consider the particular case of nanoparticles formed from magnetic materials:
In the technical specification document ISO 17200:2015, a number of characteristics relating to dry powders of nanoparticles are defined [47]. While some of this document's content is of relevance for MNP suspensions, in general the scope is not sufficient for those working with either magnetic particles, or for nanoparticles in suspension.
In the ISO 26824:2013 document, a dictionary of agreed definitions with relevance to the characterisation of nanoparticles is set out [48]. While not being comprehensive, its content includes material relevant to the characterisation of particles in both dry powders and in liquid dispersions. Vocabulary of relevance to dynamic light scattering, small angle x-ray scattering, and zeta potential measurements is also presented.
Hence, although some terminology of relevance to MNPs and MNP suspensions is defined within existing documents, there is currently a lack of cohesion, consistency and coverage. Moreover, the existing standard definitions primarily describe the outer contours and surfaces of materials at the nanoscale. There is a lack of definitions, firstly of the MNP itself, and secondly of an ensemble of particles in a suspension medium. With regards to the former, there is almost no discussion at present of either the internal structures and/or the compositions of nanoscale particles or other nanomaterials, both of which are critical in the study of MNPs as it is the physical arrangement that dictates the magnetic properties of the particle. Taking this a step further, nanoparticle suspensions consist of both the particles and the liquid within which they are dispersed and have properties and considerations which are not applicable in the case of dry powders. There is currently a lack of definitions or descriptions of nanoparticle suspensions within the literature. Such properties and definitions are not unique to magnetic NPs, and thus belong within a specific document on the appropriate terminology and concepts for generic nanoparticle dispersions.
Somewhat remarkably, at present there is no description or definition of the magnetic properties of MNPs within published standards. The term 'superparamagnetic' is mentioned within [49], however no description of the meaning is offered beyond it being an effect observed within 'small particles formed of metal oxide'. This is an effect which is well established within the scientific literature [50], and which underpins many applications of MNPs. It will be impossible to develop measurement standards in order to characterise MNPs until their unique magnetic properties have been defined.
In the following subsections, we introduce key concepts in the classification and description of magnetic nanoparticles, which we believe are of vital importance specifically for the characterisation and description of MNPs.
4.2. Standardisation requirements
In the following sections, the major characteristics and properties of MNPs which must be considered for accurate characterisation are outlined. These are the topics which should eventually be covered within definitions standards in order to offer coverage suitable for all application possibilities.
4.2.1. Structure of magnetic nanoparticles.
A vast variety of particle structures have been reported in the literature. However, all of the known variants have included some, or all, of the following components. As such, we consider that in order to aid the harmonisation between MNP manufacturers and users, the following concepts should be incorporated into the standardisation literature.
4.2.1.1. Magnetic cores.
The fundamental building blocks of magnetic nanoparticles are called cores [11]. They are individual nanoscale magnetic objects formed of a magnetic material. Each core may be a single crystallite of magnetic material (meaning that the crystalline lattice is coherent and unbroken throughout the object), or have a polycrystalline structure (meaning that the crystalline lattice within the object has two or more distinguishable orientations).
The simplest MNP is that which contains just one magnetic core – a 'single-core' magnetic nanoparticle. Figure 3 shows a schematic diagram of a single-core MNP, along with other constituent components (matrix, functional shell, hydrodynamic surface layer) that we discuss further below.
Figure 3. Schematic diagram of a single-core magnetic nanoparticle. Note that the magnetic core is a single magnetic object that may be either a monocrystalline or polycrystalline single magnetic domain, which responds to an applied magnetic field in a single, net, coherent manner.
Download figure:
Standard image High-resolution imageThe size and composition of each magnetic core, as well as their spatial distribution relative to other cores (see later with respect to 'multicore' MNPs), affects the magnetic properties of the overall particle system. When reduced below a critical size, it becomes energetically unfavourable for domain walls to form within a magnetic core. As a result, cores smaller than this limit will form single domains, with all of the spins aligned in a common direction.
Magnetic cores can be formed from a range of metals and metal oxides, often with the choice of material dictated by the choice of application. For example, while pure magnetic metals offer high magnetisation (magnetic moment per unit volume) values, they are generally not suitable for biomedical or environmental applications due their toxicity and sensitivity to oxidation. In general, particles formed from metal oxides exhibit a greater long term stability, although pure metal may be rendered stable if protected from oxidation by sufficient layers of oxide or other materials such as gold or carbon.
Magnetic iron oxides, such as magnetite (Fe3O4) and maghemite (γ-Fe2O3) are among the most commonly-used materials in MNP systems for biomedical application, due to their non-toxicity and stability from oxidation [51]. To date iron oxide particles are the only MNP products so far approved for administration to humans [52], these being: the MRI contrast agent Resovist®, the iron replacement agent Feraheme®, the sentinel node detection agent Sienna+®, and the magnetic thermoablation agent NanoTherm™.
Other magnetic metals and oxides (including iron, nickel, cobalt and more complex oxides) have all been studied, with many exhibiting unique and useful properties. One of the key challenges to the introduction of products implementing these particles, however, is the need to conduct thorough studies of the environmental and health impact which such nanomaterials may incur. This is an ongoing topic in the development of MNP standardisation [53].
4.2.1.2. Non-magnetic matrix.
If dispersed in a liquid, uncoated magnetic cores tend to aggregate over time due to the influence of Van der Waals forces and magnetic dipolar interactions. This tendency towards aggregation is detrimental to many applications, where stability of the particle dispersion over time is often a key requirement. To reduce the risk of aggregation or sedimentation, a non-magnetic encapsulating matrix (sometimes referred to as a coating) may be added to the cores, either as an integral part of the original synthesis route to the material, or a part of a post-production treatment phase. Such matrices/coatings stabilise the colloid by providing steric and/or electrostatic repulsion between the particles to prevent aggregation [54, 55].
In addition to stabilising the MNP suspension, matrix coatings may serve a variety of other functions, including aiding the particles' biocompatibility [54, 56], or providing an anchor layer to which functional groups may be attached [57, 58]. The material used for the coating is dictated by the application for which the particles are destined, with organic materials such as sugars or starches being commonly used for coatings, particularly in biomedicine.
It should be noted that it is not always necessary for the cores to be covered with a non-magnetic matrix, but it is also true that a large majority of the MNPs currently being produced, studied and used around the world do have such a matrix coating. It should also be noted that in almost all cases the matrix is produced as part of the synthesis stage rather than afterwards. The matrix material is often a relatively dense polymeric or organic material that is insoluble in the intended suspension fluid for any given system, so that it is the matrix, rather than the magnetic core, that has a surface interfacing with the suspension medium. Furthermore, the matrix is often relatively rigid, and is either intrinsically electron-dense (in the case of silica), or can be made to be electron-dense by staining with heavy metal ions. By doing this the particles exhibit measurable Z-contrast, and are therefore both visible and distinct in transmission electron microscopy.
Given these features, it is not surprising that the term 'particle' has come to be applied to the composite core-plus-matrix system. In other words, the 'particle' in 'magnetic nanoparticles' usually refers to a system comprising a magnetic core encapsulated within a non-magnetic matrix. In systems where there is no matrix—commonly referred to as 'naked' or 'bare' nanoparticles, the 'particle' in question is the magnetic core on its own.
4.2.1.3. Functionalised shell and hydrodynamic layer.
Both biomedical applications and environmental remediation techniques commonly require that functional ligands be attached to the surface of the MNPs. By comparison with the core(s) and matrix of a particle, the ligands have relatively low density and different composition, however, this can be difficult to detect directly. They are closely associated with the surface of the particle, and are bound to that surface either through covalent or ionic force that they can withstand the shearing stresses associated with the boundary interface between the particle and the suspension medium.
The incorporation of a functionalised shell is an important aspect of many applications of MNPs in liquid suspensions. These include: to enable binding to biomolecular targets for immunoassays; to enhance the accumulation of particles within a specific biological organ or tumour; to act as a vector to transport drugs around the body; and to facilitate the isolation of pollutants from water.
Lastly, a hydrodynamic layer—or, more generally, a boundary layer—exists at the interface between the particle (and its functionalised shell, if present) and the suspension medium. (Strictly speaking, the term 'hydrodynamic layer' applies only when the suspension medium is water-based.) The boundary layer forms as a natural consequence of the very different viscosities of the particle and the suspension medium, in that the fluid right next to the particle sticks to the particle, and acts to shear or 'slow down' the fluid next to it. The boundary layer thickness is then defined by the distance from the particle surface at which the influence of the particle on the dispersing medium is no longer apparent. In water-based suspensions, the hydrodynamic layer is typically of the order of 5–10 nm thick. When the MNPs are immersed in biological liquids like blood or serum they are intrinsically surrounded by an additional layer of proteins, the so-called corona, further increasing the effective hydrodynamic diameter [59].
4.2.1.4. Multicore particles.
Multicore magnetic nanoparticles—as illustrated in figure 4—are particles that contain more than one distinguishable magnetic core, where 'distinguishable' means that the cores are embedded within a non-magnetic matrix, and that there is a physical separation between neighbouring cores that is filled with the matrix material. If the cores of a muticore particle are sufficiently tightly bunched, then the term 'nanoflower' is used to describe them. This class of particles has been demonstrated to show great promise for a variety of applications including magnetic hyperthermia therapy [45, 60] and magnetic resonance imaging [61]. Note that if there were no physical separation between adjacent cores, then the assembly would behave as a polycrystalline single-core particle.
Figure 4. Schematic diagram of a multicore magnetic nanoparticle.
Download figure:
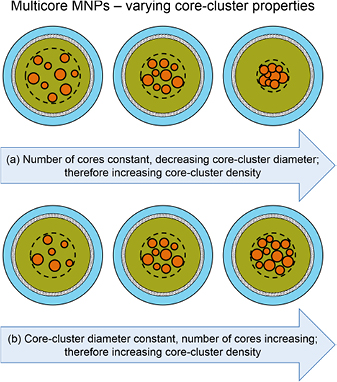
Standard image High-resolution imageIn defining the structure of multicore MNPs, it is convenient to use the term 'core-cluster' to describe the dispersion of the cores within the matrix (see figure 4). This leads to other parametric descriptions that may be applied to multicore MNPs, including the number of cores per particle; the core-cluster diameter; and the density of the core-cluster. Changes in these parameters reflect significant changes in the packing arrangement, meaning that the spacing of the cores within the particle is variable, and can greatly affect its characteristics—as illustrated in figure 5.
Figure 5. Illustration of factors affecting the properties of the core-cluster within a multicore magnetic nanoparticle. The term nanoflower may be applied for the most densely packed core-assemblies.
Download figure:
Standard image High-resolution imageMulticore MNPs are often a natural product of one-pot synthesis routes in which the magnetic cores and the non-magnetic matrix material coexist at the time of formation. The presence of numerous cores may also be advantageous in functional terms, such as by increasing the overall magnetic content of given size particle, while still allowing the formation of stable colloids. Furthermore, magnetic dipole-dipole interactions between the cores in multicore particles have been implicated as being beneficial for applications including magnetic hyperthermia [62] and magnetic particle imaging [63].
4.2.2. Particle size.
Due to their internal structure, and the additional complexity of particles distributed in liquid suspensions, a number of important size quantities are necessary in order to properly describe MNPs.
4.2.2.1. Core, core-cluster, particle, functionalised shell and hydrodynamic sizes.
The core size describes the sizes of the individual magnetic cores, whether the particles are single-core or multicore entities. In multicore MNPs the core-cluster size refers to the size of the region of the non-magnetic matrix within which the cores occur. The particle size describes the size of the entire particle, i.e. the combination of the magnetic core or cores plus the non-magnetic matrix that those cores are embedded within. All of these are sizes that are in principle measurable in both the liquid suspension state and in the dried state.
For particles in suspension, however, two further sizes are significant. The functionalised shell size refers to the size of the physical particle as well as that of the (frequently biomolecular) functionalised surface layer. The hydrodynamic size includes the boundary layer between the particle (and its functionalised shell if present) and the suspension medium.
4.2.2.2. Size distributions.
All size quantities relating to any particulate or nanoparticulate matter have an associated size distribution, that is to say there is a mean value and a range which is quantified by a distribution width. The distribution of particle sizes can vary greatly and has a strong impact on their characteristics and potential uses. The polydispersity index of a particle ensemble is given by the standard deviation of the particle sizes divided by the mean of their sizes. If this value is smaller than 0.25, then the particles are described as being monodisperse or uniform. When the distribution is larger than this, the particles are described as being polydisperse [64, 65]. Standard techniques for characterising particles as mono- or polydisperse are given in the following ISO standards [66, 67]. Particle sizes are typically described by a log-normal distribution, or more complex multi-parameter models may be required [68, 69]. As with all MNP characterisation, appropriate sampling must be achieved in order to give a representative value for the full population of particles.
When contemplating particle/core size distributions, a common issue which must be considered is whether the measurement output is a distribution in terms of volume, number or intensity. Additional information on this subject can be found in [70].
4.2.3. Zeta-potential and stability of suspensions.
The zeta (ζ) potential is a measure of the potential difference between the dispersion medium and the stationary layer of fluid attached to the dispersed particle. The ζ-potential provides a key indicator of the stability of colloidal dispersions, which is critical to many applications.
It follows, then, that this value is an important metric for any nanoparticle solution, but particularly so for MNPs as small attractive forces between magnetic cores may exceed the repulsion, resulting in aggregation of the dispersion. In general, the larger the value of the ζ-potential, the greater the stability of the suspensions against aggregation. Further stability can be conferred to the particle dispersion by the addition of charged functionalised ligands to the particle surface through a process known as 'electrostatic stabilisation'. Finally, the choice of solvent used in the particle suspension will also greatly influence the ζ-potential value obtained as variation in the pH value can greatly affect the stability of the suspension.
Alternatively, MNPs may be stabilised sterically by the addition of a coating layer of large polymer molecules such as polysaccharides. Electrostatic and steric stabilisation methods may be combined to form electro-steric stabilisation. Steric stabilisation methods are less susceptible to changes in the in the ionic strength or pH, and thus steric or electro-steric stabilisation is preferable for some applications [71].
4.2.4. Chemical composition.
The chemical composition of nanoparticle products is of utmost importance, especially when validating them for use in medical, biological, or environmental technologies. Analysis of the chemical composition can also indicate the extent of impurities which are present within particles. For iron oxide MNPs, Mössbauer spectroscopy or x-ray diffraction can further aid in discerning which ferrite phases are present. Thus far magnetite (Fe3O4) and maghemite (γ-Fe2O3) are the only ferrites which have been approved for use in humans [72]. To characterise MNPs for some applications, a complete understanding of the particle composition including the crystal structure, extent of defects and the presence/extent of impurities may be necessary [73].
Magnetism in nanoparticle suspensions
In this section we introduce key magnetic concepts which are necessary considerations when characterising magnetic nanoparticles in liquid suspensions.
4.2.4.1. Superparamagnetism.
Superparamagnetism occurs when a single domain magnetic core is sufficiently small such that the thermal energy is sufficient to overcome the energy barrier to magnetisation reversal [50]. This allows the magnetic moment of the core to flip between orientations in the easy axes, within the timescale of the used measurement technique (measurement time). The temperature above which a particle exhibits superparamagnetic behaviour is the blocking temperature (TB). Below this, the thermal energy is insufficient to flip the magnetic moment within the measurement time [74], and the particle is described as being thermally blocked. For iron oxide MNPs, typically core sizes less than 20 nm are superparamagnetic at room temperature (when measured using common DC magnetometry apparatus with measurement times of several seconds).
Above the blocking temperature, thermally activated switching occurs frequently (see following section on relaxation mechanisms), as a result the average magnetisation of a large ensemble of cores is zero in zero field. An ensemble of superparamagnetic particles exhibits no remanent magnetization in zero applied field and no magnetic coercivity although a magnetisation may be temporarily induced within the ensemble by the application of a magnetic field. If the magnetic field is removed, the ensemble magnetisation quickly decays due to thermal agitation of the particle orientations. The ability to induce a large magnetisation in superparamagnetic particles, without causing aggregation of the MNP suspension (as tends to happen in thermally blocked particles), is of great interest in a large number of applications including sensing and focussing [75, 76].
It should be noted that the observation of superparamagnetism is closely linked to the timescale of the measurement technique used. A particle may appear to be superparamagnetic when probed using one measurement technique, and yet appear to exhibit a remanent magnetisation when measured using a method with a shorter measurement time.
In liquid suspensions of MNPs, superparamagnetic properties may still be observed within thermally blocked particles if they are suitably free to rotate within the suspension liquid and the thermal agitation of the system is sufficient to result in random reorientation of the particle via Brownian reorientation.
4.2.4.2. Relaxation mechanisms.
The orientation of MNPs in suspension can relax via two distinct mechanisms: Néel relaxation or Brownian relaxation. In Néel relaxation, the magnetic moment flips direction within the particle due to thermal agitation, with an average time between these reversal given by (τN) [77]. For a temperature T, a system of non-interacting (in zero field) single-core particles has a characteristic Néel relaxation time τN given by:
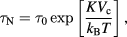
where τ0 is a material dependent time constant (typically in the range of 10–12–10–9 s), KVc is the total anisotropy energy of the particle core, and kB is the Boltzmann constant. The Néel relaxation behaviour of a given type of particle is dictated by the size, shape and distribution of the cores (which may be interacting if the packing density in the core-cluster is high) within the matrix.
For liquid suspensions of MNPs, reorientation of a particle's magnetic moment may also be caused by a physical rotation of the whole particle within the suspension medium and unless they have been specifically immobilised, this provides an additional degree of freedom to the magnetisation alignment of nanoparticles. Magnetisation realignment through physical rotation of the particle is known as Brownian relaxation, which occurs with characteristic time τB which is given by:
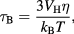
where  is the hydrodynamic volume of the particle, and
is the hydrodynamic volume of the particle, and  is the viscosity of the carrier liquid.
is the viscosity of the carrier liquid.
A liquid suspension of nanoparticles may be dominated by either Néel or Brownian relaxation depending upon a combination of factors including the particle size, structure (single or multicore), the liquid in which they are dispersed and the temperature of the system.
In a multicore particle, individual cores are unable to physically rotate due to the matrix in which they are anchored and whilst this prevents Brownian relaxation of the individual cores from occurring, the overall particle, however, may still rotate within the suspension. In this case, the relaxation behaviour of the particle is determined by a combination of Néel relaxation within the individual cores and the Brownian relaxation of the overall particle. As a result, a multicore particle may appear to exhibit either Néel or Brownian relaxation in the same manner as a single-core particle.
For both single- and multicore particles. The effective relaxation time,  ,of the MNP suspension accounting for both Néel and Brownian contributions can be deduced using:
,of the MNP suspension accounting for both Néel and Brownian contributions can be deduced using:
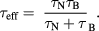
Further discussion of the contributions from both Néel and Brownian relaxation processes is described in [78].
Relaxation behaviours are important in a variety of MNP applications, particularly in biomedicine. These include magnetic biosensor detection systems [79–82], inducing localized magnetic hyperthermia treatment to kill tumour cells [83], as well as providing or enhancing contrast in magnetic particle imaging [84] and magnetic resonance imaging [85]. The type of relaxation exhibited by a MNP suspension therefore has great implications for its applicable uses, and so this is a critical characterisation parameter.
4.2.4.3. Interactions between particles.
Interactions between MNPs within a system can have a strong influence the group behaviour [86], and thus are an important consideration during magnetic characterisation. The magnetic moment of individual MNPs can easily exceed 10 000 μB (Bohr magneton), and the resultant dipole interactions act over long distances to influence the magnetic properties of the system. For a set of randomly distributed particles with average magnetic moment µ and average separation d, an estimate of the dipole interaction energy of a particle using:
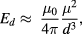
where µ0 is the permeability of free space.
If the concentration of a suspension of superparamagnetic MNPs is sufficiently high then the magnetic dipole interaction can result in ordering of the particles at temperatures below a critical value T0 [87], as given by:
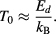
Dipolar interactions can significantly affect the results obtained from static and dynamic magnetization as well as other characterization measurements. They are also a relevant factor when synthesising MNPs for optimal magnetic hyperthermia performance [88–91]. If particles are packed very closely within a core-cluster, then exchange interactions between the cores may start to influence the overall behaviour of the particle.
5. MNP sampling standards
Obtaining accurate representative samples for analysis from bulk synthesis products is a critical requirement for all characterisation measurements, as well as for quality control in production. The sampling is typically based on volumetric (e.g. pipetting) and/or gravimetric (weighing) methods. Minimal uncertainty in sample volume or weight can be achieved by choosing a standardised calibration and measurement technique. For MNP suspensions, volumetric sampling is more straightforward, and traditionally used by the industry. However in most cases it is less accurate than weighing. For example, the accuracy of the volume obtained by pipetting depends on physical factors such as liquid density, viscosity, vapour pressure and evaporation, surface tension, air pressure, temperature of the ambient and the liquid, as well as on calibration accuracy, pipetting technique and operator skills. The uncertainty of the calibrated laboratory micropipettes is in the order of 10−2–10−1 µl [92].
The gravimetric method such as weighing by laboratory balance is more precise and in fact is typically used to calibrate the pipettes or laboratory glassware. Still, the measurement is sensitive to the characteristics of the balance, such as readability, repeatability, nonlinearity, sensitivity accuracy and temperature coefficient, to the influences that caused by environment such as air humidity, temperature, pressure and influences that affect the weighing object such as air buoyancy, way of loading the sample, etc. The uncertainty of the calibrated laboratory balance is in the range of 10−4–10−3 mg [93].
As a general rule, prior to sampling, the MNP suspension needs to be homogenized (e.g. shacked, vortexed, sonicated) to diminish effects of segregation or sedimentation during the storage, and if bulk volume is large, the sampling needs to be done from different locations of the container. Sampling size and number has to be correctly selected based on the following analysis method and sample properties.
When sampling MNP suspensions, precautions should be taken to avoid unintentional exposure to the external magnetic fields or magnetic materials in sampling devices as they can affect composition and properties of the sample.
Accurate and appropriate sampling is a key requirement when compiling an uncertainty budget for a specific measurement. Uncertainty budgets are specified within the metrological checklist (summarised in section 3) as being a necessary precursor to the development of ISO standards for measurement techniques.
5.1. Coverage of published standards
Here, we briefly discuss the existing standards covering the topic of obtaining MNP samples in liquid or powder forms.
Acceptable methods for obtaining particulate samples which are representative of the size, size distribution and surface areas are described in ISO 14488:2007 [94]. This ISO document deals mainly with sampling from dry powders of particles. It briefly covers the sampling of particles in pastes and suspensions. Many of the recommendations which it contains are not suitable for sampling MNPs in liquid suspensions. For example, the standard recommends the 'rinsing residual particles from equipment to ensure the inclusion of all particulate matter in the sample', which is not appropriate for the study of particle suspensions as it would alter their concentration, which could in turn affect their stability.
Best-practise guidelines for the sampling of particles are also found in British standard BS 3406-1 [95], although it is worth noting that this document does not specifically pertain to nanoparticles. Rather, the document primarily deals with particles of much larger dimensions than the nanoscale and whilst there are some sections which discuss the sampling of liquid suspensions and of obtaining samples of particles with sizes of less than 20 µm, this is still 3 orders of magnitude larger than typical particle sizes in MNP suspensions
Techniques for sampling from large volumes of colloidal suspensions such as paints and varnishes are specified in ISO 15528:2013 [96]. While the document deals with the sampling from liquid suspensions, its applicability to the specifics of MNP analysis requires further examination. Indeed, additional information with relevance to the sampling of nanoparticles may be found within ISO/TS 12025 [97], which deals with the quantification of nano-objects released by the generation of aerosols. Even though the techniques listed within the existing documents listed above may provide appropriate samples for some measurement techniques, their validity, however, remains to be established.
Besides the need for standard techniques for acquiring representative samples from bulk material, additional sample processing is sometimes required in order to optimise some measurement techniques. For example, samples containing very polydisperse MNP suspensions may require fractionation of a suspension so as to sort the sample particles into size categories before characterisation with DLS [98].
5.2. Standardisation requirements
Precise sample preparation is of the utmost importance in the analysis of MNP suspensions. As it stands, there are currently no documents within the published literature that specifically address the topic of sampling nanoparticles in suspension. Instead, existing standards have largely been written without consideration of the specific requirements of MNP characterisation methods. It is our recommendation that an evaluation of existing standards should be undertaken, with the sampling requirements of each MNP characterisation technique individually surveyed, as each technique is subject to its own set of unique prerequisites.
Specific considerations that should be made when sampling from liquid suspensions of MNPs regard the residual environmental magnetic field in the preparation space and the magnetisation of any tools or material used in handling the liquid.
6. MNP measurement standards
A rigorous characterisation of MNPs in liquid suspension requires a large number of chemical, structural, and magnetic properties to be considered. However, as it is often the particular usage that determines the necessary parameters for characterisation (illustrated in figures 1 and 2), our 'mix-and-match' approach can simplify the task of amalgamating the necessary information for characterising MNPs for a specific application. Here, we turn our attention to the existing standards that are specific to the characterisation of MNPs and MNP suspensions. We then examine a selection of the most commonly used techniques that are currently used to characterise MNPs and MNP suspensions which, due to their level of applicability, we believe deserve to be standardised.
6.1. Coverage of published standards
There are a number of national and international standards which provide best practice guidelines that should be followed when characterising nanoparticle samples. The level of detail included varies from document to document, with all of them being subject to continuous review and improvement. Of the existing nanoparticle particle standards, the following are applicable to the characterisation of MNPs and MNP suspensions: electron microscopy (ISO 13322-1, [99]) and x-ray diffraction (BS-EN 13925-1:2003, [100]), both of which are limited to dry material only; small angle x-ray scattering (ISO 17867:2015, [101], which, by virtue of analogous principles, can also be applied to small angle neutron scattering; photon correlation spectroscopy (ISO 13321:1996, [66]); dynamic light scattering (ISO 22412:2008, [67]) and determination of the zeta potential (ISO 13099, [102])—all of which use dilute MNP suspensions. Finally, the analysis of particulate material within water using inductively coupled plasma mass spectroscopy is covered in [103–106] (the individual documents all work together to form the full body of information necessary for ICP measurements). In addition there are a number of non-technique-specific documents relating to analysis methods for particle size and colloidal stability [107, 108].
6.2. Standardisation requirements for magnetic measurements on MNPs
Somewhat remarkably, at present there are no standards at either national or international level for any characterisation methods of the magnetic properties of MNPs or MNP suspensions. Accurate and precise determination of the magnetic properties is necessary in order to assess not only the functionality, but also the stability and safety of MNP suspensions. With this in mind, the development of a standardised description would not only benefit research science, by facilitating interlaboratory comparison of results, but also clinical and technical applications of MNPs. Here, we describe the most common measurement techniques that are currently used to characterise the magnetic properties of MNPs. We discuss the state-of-the-art of magnetic characterization and outline obstacles in the interpretation of measurement results that must be overcome for standardization. It is not our intention to provide solutions for each issue, this section rather provides suggestions of important work areas and issues to be addressed in the future.
6.2.1. DC magnetometry.
DC magnetometry describes a variety of measurements in which the magnetic moment of an MNP sample (either as an MNP suspension, an immobilised sample or as a powder) is measured as a function of either an applied static magnetic field H, temperature T or measurement time t. DC magnetometry represents one of the most common measurement techniques reported within the literature, and yet there no standardised methodologies suitable for the application of the technique to MNP characterisation. The unique properties of MNPs mean that the existing document standards, which relate to macroscopic magnetometry, are not transferable to this particular class of measurements [109] and so necessitates a need to develop additional standards that reflect the unique properties and requirements of MNPs.
Through this technique, one can measure the total magnetic moment of an MNP ensemble in saturation and, assuming the volume of the magnetic material is known, the saturation magnetization MS can be deduced. In case of immobilised and/or thermally blocked MNPs, the magnetic remanence and the coercive field can also be measured. The models used to analyse the measurements vary in complexity and number of system parameters. In the case of an ensemble of non-interacting identical particles at T  TB (i.e. superparamagnetic particles) neglecting magnetic anisotropy effects, the ensemble magnetisation can be described by the Langevin function:
TB (i.e. superparamagnetic particles) neglecting magnetic anisotropy effects, the ensemble magnetisation can be described by the Langevin function:

where µpµ0H is the magnetic Zeeman energy and kBT the thermal energy of an MNP with the magnetic moment µp.
In the case of T  TB (i.e. thermally blocked particles), magnetic anisotropy negates the use of the Langevin expression and instead there are a number of models in the literature that may be applied to describe the particles' behaviour, as discussed in [110–112]. Dipolar interactions can occur in MNP systems with complex core-cluster arrangements, in which case the modelling complexity often needs to be increased. In this case, Monte Carlo simulations have been demonstrated as a feasible method to model such systems [113, 114], or a numerical inversion process may be implemented [38]. The thermal response of the MNP sample can also be determined from DC magnetometry measurements. Such measurements may be conducted either in large (saturating or near saturating fields) (S/NS), or under small fields (zero field cooled/field cooled loops, ZFC/FC magnetisation). In S/NS measurements, the sample is initially cooled to cryogenic temperatures under a large applied field after which the sample magnetisation is recorded over the desired temperature range. The temperature dependence of the saturation magnetisation is typically fitted using Bloch-type law:
TB (i.e. thermally blocked particles), magnetic anisotropy negates the use of the Langevin expression and instead there are a number of models in the literature that may be applied to describe the particles' behaviour, as discussed in [110–112]. Dipolar interactions can occur in MNP systems with complex core-cluster arrangements, in which case the modelling complexity often needs to be increased. In this case, Monte Carlo simulations have been demonstrated as a feasible method to model such systems [113, 114], or a numerical inversion process may be implemented [38]. The thermal response of the MNP sample can also be determined from DC magnetometry measurements. Such measurements may be conducted either in large (saturating or near saturating fields) (S/NS), or under small fields (zero field cooled/field cooled loops, ZFC/FC magnetisation). In S/NS measurements, the sample is initially cooled to cryogenic temperatures under a large applied field after which the sample magnetisation is recorded over the desired temperature range. The temperature dependence of the saturation magnetisation is typically fitted using Bloch-type law:
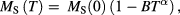
where B is the Bloch constant. In bulk materials, α has a value of 3/2, although deviations from this value have been reported for nanoparticulate samples [113] with suggested mechanisms including the low-temperature freezing of surface spins [115] and or the quantum confinement of spin-waves [116].
ZFC/FC magnetisation measurements are more commonly found in the literature and tend to form typical part of the characterisation of almost all MNP samples. To measure ZFC magnetisation curves, one begins by initially cooling a demagnetised sample in the absence of a magnetic field. Once the minimum desired temperature is reached (T < TB, preferably < 10 K), a small magnetic field (ca. 1–20 mT) is applied and the sample magnetisation is recorded as a function of increasing temperature until T > TB (typically room temperature). Following this, the FC magnetisation curve—the cooling curve is then recorded as the temperature is decreased to T < TB, under the same applied field. From such measurements it is possible to determine the blocking temperature and, provided that the core is size is well characterised, the magnetic anisotropy constant can be estimated [117].
The presence of dipolar interactions within an MNP sample—either within the core-cluster or between particles, will shift the blocking temperature peaks in the ZFC/FC magnetisation curve [86]. These are highly sensitive to sample preparation and may be minimised by using sufficiently dilute MNP suspensions, although such effects may still occur in systems where core-clusters are interacting amongst themselves. Further complications may also arise when studying very small particles (<10 nm). This is because for such ultrasmall particles, their anisotropy energy is comparable to the thermal energy of the system.
6.2.2. Dynamic magnetic analysis.
AC susceptibility (ACS) measurements probe the magnetisation of a sample under the application of a sinusoidal AC magnetic field. The field amplitude is selected to be small enough to probe only the linear dynamic magnetisation response of the MNP ensemble. The dynamic magnetic response is recorded as a function of the excitation frequency. It is described by two components, one that is in-phase with the excitation field (real part), and one which is out-of-phase (90 degree phase shift, the imaginary part). In relation to the applied field, the recorded signal is interpreted as a complex magnetic susceptibility (with the above mentioned real and imaginary parts). The frequencies typically employed are in the range of 1 Hz–10 MHz. The response of the sample at varying temperatures may also be measured.
From ACS spectra a number of parameters can be extracted, these include the median hydrodynamic size distribution of particles [118], the core size distribution (if additional parameters are known), whether Néel or Brownian relaxation dominates and the characteristic relaxation times of the system [78]. In some cases, an estimate of the specific absorption rate of particles (see section 6.2.3) may be made from the imaginary part of the AC susceptibility [122].
Temperature dependent AC susceptibility measurements (ACS versus temperature at different excitation frequencies) can provide information about the core volume distribution and allow the magnetic anisotropy constant to be obtained if the MNP system is non-interacting. Analysis is somewhat complicated if there is a distribution of particle shapes within the sample with differing anisotropies.
At present analysis of measurement data is currently conducted using the techniques based on those described in [119–121]. The development of AC susceptibility is a valuable tool for MNP analysis, with relevance to a wide variety of applications [80, 122]. A standardised approach to both measurement and analysis will provide great value to manufacturers.
A similar method to ACS is magnetorelaxometry (MRX), where the magnetic response versus time of an ensemble of MNP is observed after switching off an external pre-magnetizing field (typically in the mT range). Devices have been demonstrated using both SQUID magnetometry [123–125], as well as fluxgate magnetometers [126, 127] which have the advantage of not requiring cryogenic cooling, and other techniques [128]. Applications of MRX are rapidly developing at present. The major topics at present can be divided into those which aim at (1) quantification and localisation of MNPs administered to organisms, and (2) measurements that probe the interactions of magnetic nanoparticles with their environments. An excellent overview of these applications is given in [129].
Rotating magnetic field (RMF) is a characterisation method with similarity to ACS, although the technique is still under development. It measures the viscous drag forces between the particles in a liquid dispersion and the carrier fluid in which they are dispersed [130, 131]. A constantly rotating magnetic field is applied to the sample at a range of frequencies. The system measures the phase lag between the field vector and the rotating moment of the particle ensemble via monitoring the real and imaginary components of the complex magnetisation. RMF allows the Brownian time constant of particles in suspension to be measured and it can also be used to probe the colloidal stability and binding state of the particles [132–134]. It is also possible to find the magnetic moment of the particles, and if the saturation magnetisation is known, then the core size can also be determined.
6.2.3. Intrinsic loss power for magnetic heating.
When an alternating magnetic field is applied to MNP suspensions, it is possible to observe heating within MNP suspensions. There are number of mechanisms that underpin this effect, including the particle system used and the magnitude and frequency of the applied field. Magnetic hyperthermia therapy is based upon utilising the heat to cause damage to tumour cells that have been loaded with MNPs. A more complete discussion of the application can be found within section 8.1.2.1.
The effectiveness of a given particle type for magnetic hyperthermia therapy is characterised by the specific loss power (SLP), which is a measure of the heat which it dissipates. The SLP of an MNP suspension describes the heating power P generated per unit mass of magnetic material m within it [135], and can be expressed as:
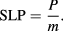
While the heating power produced by a specific nanoparticle type is dictated by its physical and magnetic properties, the power produced also scales linearly with the frequency f and quadratically with the strength of the alternating magnetic field H applied (at low fields). The intrinsic loss power (ILP) of an MNP material attempts to remove these factors, and is given by:
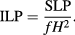
A quantitative and comparable measurement of these values is deceptively difficult to achieve, as indicated by the vast scatter of results published in the literature. One major hurdle is that the majority of measurement setups are non-adiabatic, meaning that heat is continuously lost from the MNP suspension during measurement. A number of techniques to compensate for these losses are currently under investigation [136]. More rarely, attempts are made to conduct measurements under adiabatic conditions [45, 137, 138], which requires a significant investment of both time and resources.
At present there is no standard methodology for measuring the SLP of MNP suspensions and the current state-of-the-art allows only for the direct comparison of particle efficiencies using identical equipment. The realisation of a safe, efficient and reliable cancer therapy based on magnetic hyperthermia will be significantly furthered by the creation of a standardized methodology for characterizing the ILP of MNP systems [27, 135, 139–141].
6.2.4. NMR relaxivity measurements.
The effectiveness of MNP suspensions as contrast agents is characterised using the NMR T1 and T2 parameters [142]. These describe the relaxation of hydrogen nuclei (i.e. protons) via the spin-lattice (longitudinal) relaxation and the spin-spin (transversal) relaxation, respectively, following the application a radio frequency pulse. These values are modified in the vicinity of MNPs due to magnetic field arising from the magnetic core [143, 144], although the spatial configuration of the core/core-cluster as well as and surface modifications of the particle is known to affect the relaxivity of protons [145, 146].
The relaxivity Ri of MNP suspensions is given by:
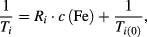
where Ti represents either the longitudinal (T1) or transversal (T2) relaxation time in at a concentration c(Fe) in (mmol l−1) and the relaxation time Ti(0) of the pure solvent. A common testing method is to conduct T1 and T2 measurements for a series of concentrations of the same particle type, and compare the results with the above relationship [147].
MNP suspensions are regularly used to modify the contrast in magnetic resonance imaging (MRI), a non-invasive in vivo imaging technique with a broad range of diagnostic applications. For a longer discussion of this technique and the key literature of relevance, please see section 8.1.1.1. It is worth noting that the technique by which magnetic resonance relaxivity is assessed is not yet universal, and a truly harmonised system for calibration and measurement protocols is needed.
6.2.5. Magnetic particle spectroscopy.
In magnetic particle spectroscopy (MPS), a MNP sample is exposed to a sinusoidally varying magnetic field at a specific excitation frequency (typically ca. 25 kHz). The excitation field has sufficient amplitude so as to exploit the nonlinearity of the magnetisation curve. The magnetic response is monitored using a single or gradiometric induction coil, while the measured signal is a convolution of the excitation frequency with higher odd harmonics that are dictated by the nonlinear magnetisation curve of the MNP sample. It follows, then, that a Fourier transformation of the signal separates the harmonics of the MNPs and the signal originating from the excitation field. The observed spectrum depends on a variety of characteristic parameters of the MNPs, including the core and particle size distributions, the magnetic anisotropy, temperature as well as the frequency of the excitation field.
As a specific quantitative method, MPS is suited to characterize the MNP content in a larger sample of biological material. Standardization of this method has not been performed so far.
MPS is closely related to the recent innovation of magnetic particle imaging (MPI) [84], with both techniques proportional to the non-linear magnetisation response of MNP suspensions [26]. Indeed, whilst MPS has great potential for the optimization of tracers for MPI, the results obtained by this technique are also influenced by properties of the suspension, such as the hydrodynamic size and the viscosity of the liquid in which they are suspended (if Brownian relaxation dominates) [148–150].
6.2.6. Magnetic separation (as a test of colloidal stability).
Magnetic separation is a technique by which the stability of an MNP suspension in the presence of varying magnetic field gradients can be probed. The application of a magnetic field gradient to the MNP suspension results in a magnetic force, causing the MNPs to move towards high field regions. The velocity of the MNP is determined by a combination of the magnetic properties of the MNP and the hydrodynamic properties of the whole suspension, described by the viscosity of the solvent, the hydrodynamic MNP diameter and the temperature, also taking stochastic Brownian motion of the particles into account [151, 152].
The MNP concentration is monitored by observing the intensity  of light transmitted through the suspension. Concentration c follows then the Beer–Lambert-Bougers relationship:
of light transmitted through the suspension. Concentration c follows then the Beer–Lambert-Bougers relationship:
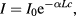
where I0 is the light intensity transmitted in the absence of any particles, L is the length of the optical light path, and α is the absorption coefficient of the particle suspension.
Provided that the transmission intensity is appropriately calibrated using suspensions of known concentration, the evolution of particle concentration under applied field gradients may be measured. A variety of field gradients can be applied in a typical system, with gradients up to 60 T m−1 commonly used [153].
This technique provides a suitable method to test MNP suspensions which would otherwise be stable over the measurement time scales as well as assessing the stability against applied fields of a given particle system. Practically, magnetic separation utilizes typical sample volumes in the range of 40–100 µl, and can probe MNP suspensions with concentrations ranging from 0.05 mgFe ml−1 up to 0.3 mgFe ml−1. Separation times are in the range of hours for most field gradients used.
Magnetic separation is also critical to a number of applications where MNPs have functional groups added to the surface in order to bind to specific target molecules [6]. The particles and attached material are then removed from the fluid by the application of a field gradient. Examples of applications include the isolation and measurement of specific biomolecules, and the removal of pollutants from water (see section 8.1.3 for details).
Colloidal stability of a nanoparticle suspension is a key factor in determining its viability for a multitude of applications. As a result, the standardisation of this technique can be expected to have a strong positive impact on the industry. The implementation of round-robin measurements of known nanoparticle compositions may be used to compare the accuracy and reproducibility of measurements using different equipment.
7. MNP labelling standards
Appropriate labelling is of great importance for all products. The specific information included should be informative, appropriate and unambiguous for the customer. While the labelling requirements of a customer will be dictated by the application for which the particles are destined, it is necessary to discern the characteristics which are scientifically appropriate for inclusion.
This requires commonly accepted definition of terms, specification of measurement procedures and expression of results. Especially for magnetic properties of MNP suspensions, none of these conditions are readily available. Consequently, labels for marketed MNP-based products often contain only the chemical composition and a geometrical size parameter, under the title 'magnetic' or 'superparamagnetic nanoparticles'. Quantitative magnetic properties are rarely indicated, for the above-mentioned reasons.
7.1. Coverage of published standards
Generalised information regarding the labelling of nanomaterials (not specifically particles) may be found in [154]. The document is short and non-specific, and thus it is of little relevance for the particular requirements of MNP based industries. The content is intended to provide optional guidelines to be used at the user's discretion. The overarching message is that, for the time being at least, any decision on the appropriate labelling content for nanomaterials should be agreed between the manufacturer and customer on a case-by-case basis. Work is currently being undertaken within ISO to provide a new version of the document with more information and coverage.
There are however more strict regulations for labelling chemical [155, 156] and medical products [157, 158]; with the main focus on occupational, environmental and end-user safety. Even these regulations are relevant to the major application areas of MNPs, such as medical diagnostics and treatment, none of them are directly connected to the magnetic properties of the products and therefore they are not scrutinized in this review.
7.2. Standardisation requirements
A key topic in developing future standardisation on MNPs is to establish the terms and definitions that could potentially be used on labels for MNP suspensions. MNPs possess many physical parameters that are relevant for certain application, but it would not make sense to list them all on each label. Thus, the precise requirements for labelling should be dictated by the needs of the end-user, in a mix-and-match approach. Some of this information may be deduced from currently available knowledge, while in some cases additional studies may be required in order to ascertain the necessary understanding. Nonetheless, an exhaustive list of all of the potentially valid parameters should be established. This list should enter the content of a labelling standard for MNP. The parameters on this list should be metrologically assessed measurands, with published standards for best practice in conducting their measurement. With a framework available to indicate the valid parameters which are scientifically valid for use in labelling, the manufacturer and user can then agree on those parameters from the list which are necessary when supplying a particle suspension for a specific purpose.
8. MNP applications standards
At this point we turn our attention to an examination of the current state of applications of MNPs and MNP suspensions. There are currently no standards (at either national or international level) that stipulate the requirements necessary for the characterisation of MNPs for any specific end-user technologies, which is hardly surprising given the lack of standardisation in vocabulary, methods, and measurement techniques. Moreover, many of the technologies are themselves still developing, which in itself also contributes to lack of established documentation. However, the development and adoption of standards to stipulate the language, characterisation methods and appropriate labelling of MNPs for specific purposes will only serve to accelerate the development and approval of new MNP-based products intended for clinical and technological purposes.
MNP suspensions designed for use in medical applications are subject to particularly stringent testing by regulatory bodies (such as the Food and Drug Administration, or the European Medicines Agency) before they can be administered for any market by regulatory bodies. Regulatory approval may be eased if the product proposed for approval has been manufactured and characterised according to established standards. Therefore, the development of a body of work using the 'mix-and-match' approach proposed in section 2 is anticipated to greatly facilitate the approval and uptake of MNP based medicine and technologies across the world.
In the following, we will give a brief description of some of the key innovative technologies which are in the process of implementing MNPs along with some discussion of their major requirements in terms of characterisation and future standardisation.
8.1. Emerging applications and their standardisation requirements
8.1.1. Medical imaging techniques.
Magnetic nanoparticles find uses in medical imaging as both contrast agents in MRI, and as tracers in MPI. Imaging technologies are not limited to magnetic nanoparticles; gold nanoparticles and nanorods are used in photoacoustic imaging, whilst radioactive tracers are used to generate contrast in positron emission tomography. However, MNPs offer high spatial resolution and so make a good contrast agent for use in MRI. The contrast arises from the very nature of the magnetism within the nanomaterial. In the case of MPI imaging, the imaging signal arises from the induced magnetisation measured directly from the MNP tracer within the imaging medium. The minimally invasive nature of both of these techniques, combined with the insights which they provide, make them powerful tools in current and future diagnostics. We discuss the two key applications, and refer to the key measurements outlined in sections 6.2.2, 6.2.4 and 6.2.5.
8.1.1.1. Magnetic resonance imaging (MRI).
Magnetic resonance imaging is non-invasive and non-ionising imaging technique with the potential to provide high contrast images of living tissue (and also blood flow) with accessible spatial resolutions of 50 µm or smaller, providing high-quality in vivo imaging of both healthy and diseased soft tissue and organs. These advantages have resulted in the technique becoming an invaluable tool to medical professionals.
MRI is based upon the NMR principle as discussed previously (section 6.2.4). The imaging contrast arises from the protons in water molecules. Thus, due to differences in the water to fat ratio, organs and tissue within the body provide a natural contrast within the body, from which images can be reconstructed based upon the different relaxivities. MRI systems commonly implement large magnetic fields (3 T or more).
Nanoparticles are regularly employed as contrast agents, either by increasing the T1 signal (commonly known as positive contrast agents, such as paramagnetic material like Gd) or by reducing the T2 signal (often called a negative contrast agent, such as superparamagnetic nanoparticles, like iron oxide, [159–161]). The negative contrast offered by FeO particles often limits their use in tissues which have low intrinsic T2 signals (e.g. lung tissue). To circumnavigate this issue, innovative techniques based on either pulse sequences [162, 163], or the engineering of ultrasmall MNPs with positive T1 contrast [164] are being developed. Further advances in resolution and sensitivity of T2 agents may be offered by the use of more complex metallic ferrites including CoFe2O4 and NiFe2O4. However, there are issues regarding toxicity and the long term effects of introducing cobalt and nickel based nanoparticulate materials into the body, and these materials are not yet approved for use in humans. Widespread adoption of these new materials will depend upon the evaluation of their long-term health impact and elimination from the body.
While a significant number of standards exist which cover the safety of the MRI [165–171]; none have yet been published to address the use of magnetic nanoparticle based contrast agents [172].
8.1.1.2. Magnetic particle imaging.
Magnetic particle imaging has great potential as a technique for minimally invasive in vivo imaging. Briefly, the principle of the technique is based upon the reconstruction of the spatial distribution (concentration) of a MNP tracer within the medium to be imaged [84].
To achieve this, a sinusoidal AC magnetic field (drive-field) typically around 25 kHz frequency is used to excite the MNPs with the sample, and the induced magnetisation is measured using pick-up coils. Due to the nonlinear magnetisation curve of the MNPs, the Fourier transform of the induced magnetisation signal contains additional higher-harmonics of the drive-field waveform. Analysis of these higher harmonics provides a MNP-specific imaging signal free from any background (tissue) contributions.
To provide spatial encoding of the MNP signal, a (quasi-) static magnetic field gradient is applied to the sample in order to saturate the MNPs across the majority of the volume to be imaged. This saturating field drops to zero within a small region known as the field free point (FFP). The FFP is scanned across the imaging volume and the magnetic response from each region (voxel) is recorded.
The spatially dependent harmonic data can be reconstructed into an image of the MNP density distribution via one of two methods. In the system function (SF) based technique [4], the scanner response to a small sample of tracer is mapped for all possible locations within the imaging volume. Reconstruction of imaging data is conducted by an inversion process; the spectra measured during the imaging measurement, and the accompanying system function, are used to find the concentration of MNPs within each region during the MPI measurement. Alternatively, X-space reconstruction requires no SF measurement [173, 174], and avoids the noise which forms an intrinsic part of a SF measurement; however, additional details about the passage of the FFP through the sample space are required in order to implement it.
The underlying physics of MPI is very similar to that of MPS [175], a technique which is discussed in section 6.2.5 of this document. The potential of this imaging modality for use in medical and biological studies was established in 2009 by the real-time in vivo imaging of a mouse's heart [176].
A key task in the development of MPI for clinical use is the development of safe, optimised tracer materials based on MNPs in liquid dispersion. Key factors of consideration in assessing a potential tracer are the general magnetic properties [177–179], the stability of the particles in dispersion, especially under applied fields, and finally the proving the performance by imaging objects or organs in animals.
It is a major task for the future to define a standardized way of describing the imaging performance of MPI tracers. There exists a variety of MPI scanner principles, readout methodologies and image reconstruction approaches [180–184]. In order to obtain a standardized assessment of the imaging performance, it will be necessary to consider the actual concentration of the MPI tracers and the signal to noise level as well as other parameters of the MPI scanner [185]. Furthermore, a clear physical description of the relation between magnetic properties of MPI tracers and the imaging resolution is needed [186].
8.1.2. Biological and therapeutic applications.
Therapeutic applications of MNP suspensions span a broad market and includes magnetic hyperthermia therapy [194], targeted drug delivery [187, 188], gene delivery [189], and the stimulation/suppression of the human immune system [190–192]. Despite the differences in the physical principles of therapy, standardisation of technical terms as well as the synthesis and characterisation methods would provide a reliable understanding of the MNP properties [27, 193] which benefit them all.
8.1.2.1. Magnetic hyperthermia therapy.
Magnetic hyperthermia therapy has the potential to impact the methodology used to treat a variety of cancers within humans. This is achieved by targeting functionalised MNP solutions, as well as high frequency AC fields, at specific cancerous organs in order to create local heating to damage the tumour tissue. Successful magnetic hyperthermia trials have been reported in animal models [194], and more recently, successful clinical trials are under way conducted using NanoTherm™ particles at the Charité Hosptial (Berlin, Germany) [195, 196]. As the number of companies entering this market continues to increase, we expect competition to drive the development of both laboratory based and clinical equipment.
The ability of MNP solutions to dissipate heat per unit mass of magnetic material (SLP, ILP) are key requirement in crafting safe efficient treatments; however, a number of other particle parameters must also be properly determined before the particles can be administered and the procedure conducted in vivo. Examples of these include the verification of the particle sizes, establishing superparamagnetic behaviour and chemical composition (impurities increase toxicity).
Also, the metrology in the determination of heat generation is currently purpose of intensive research activities [139, 197].
8.1.2.2. Diagnostic applications/molecular sensing.
By far the most established industry at present is the isolation, detection and/or removal of specific biomolecules using functionalised MNP suspensions. For detailed discussion of the techniques, we refer the reader to a number of excellent reviews already published on the subject [198–202]. Briefly, the technique relies upon the tethering of MNP labels to target molecules via the use of suitable functionalised MNP coatings. Once the particle label is linked to the target, a number of options are possible. The MNPs and their attached cargo may then be extracted into a smaller volume using magnetic field gradients, to either concentrate the target analyte for detection of low concentrations, or else to clean the wider sample solution of the target molecule.
A large number of diagnostic systems currently available on the market are based on this type of magnetic immunoassay technology, with some large corporations (e.g. Roche Diagnostics, Abbott) already producing well established diagnostic products. These products commonly implement well established micrometer sized magnetic beads which are large multicore MNPs, two major manufacturers of these beads are Thermo Fisher Scientific and Merck. It follows, then, that any move towards standardisation should be conducted in close liaison with the major companies involved.
Magnetic particle spectroscopy has been demonstrated as a potential method for probing the binding state of nanoparticles within biological samples, by monitoring the reduction of rotational freedom of functionalised MNPs as a way to establish whether or not they have bound to target biomolecules [203, 204]. This technique has been shown to be sensitive to the detection of blood clots, by monitoring the presence of thrombin, a biomolecule released during the clotting process [205]. Whilst further work is necessary to establish the detection limits of this application, it has great potential to revolutionise the detection and treatment of deep vein thrombosis, a potentially life-threatening medical condition. Though the MPS technique is an active topic in scientific research, no standardisation has been developed so far.
8.1.3. Environmental remediation and engineering based applications.
Magnetic nanoparticle suspensions form the cornerstone of a number of innovative techniques to aid in the desalination of seawater and the removal of heavy metal pollutants. With increasing global attention on sustainability and environmental protection this field can be expected to grow dramatically in the coming years. The requirements for MNPs for use in environmental application, as catalysts or in seals, lubricants and soaps are not, in general, as demanding as for other applications such as biomedical use. However, standards may help to improve and push the development of new products using the unique properties of MNPs, to make the application more reliable and to gain their acceptance in society. A summary of some of the most promising methods currently under development can be found below.
8.1.3.1. Forward osmosis and seawater desalination.
The desalination of sea water is a key opportunity for relieving strain on resources in water-stressed regions across the globe [206]. Realisation of this technology requires the minimisation of energy consumption in the desalination process. Forward osmosis is capable of producing an easily-cleaned liquid with a higher osmotic pressure (draw solution) than the seawater. Briefly, the two solutions are separated by a thin semi-permeable membrane across which the water molecules can osmose. Water molecules diffuse from the sea water into the more concentrated draw solution, leaving behind the salts and impurities which are too large to traverse the membrane. After the osmosis process has ended, the draw solution is cleaned and the resulting fresh water is ready for use.
MNP suspensions offer unique opportunities for manufacturing draw solutions since the particles can be quickly and easily separated from the osmosed fluid via the application of external magnetic fields [207]. Surface functionalisation and particle sizes have both been demonstrated to influence their efficiency as a draw solutes [208, 209]. Additional efficiency has recently been demonstrated by implementing magnetic nanoparticles with thermosensitive coatings [210, 211], thus allowing the same solute to be reused multiple times in the preparation of fresh draw solutions.
8.1.3.2. Heavy metal ion removal.
MNP suspensions have also been applied to the removal of toxic heavy metal pollutants from water. Both bare and functionalised composite nanoparticles have been shown to completely remove aqueous chromium ions and arsenic, [212–214], with the potential to benefit the clean-up of industrial pollution arising from mining and manufacturing industries. However, in these applications it is more the large surface and specific chemical activity of MNPs that are the most important parameters, rather than their magnetic behaviour, and other non-magnetic nanoparticle solutions may be equally as viable. It remains to be seen whether a standardization of magnetic properties of MNP and MNP solutions will directly benefit such applications.
8.1.3.3. Catalysts for industrial reactions.
The surface functionalisation of MNP solutions has led to their use as catalysts in reactions, such as in synthesis of biodiesel fuel [215]. Specific functionalisation of the particle surface provides a huge active surface area, with colloidal dispersion of MNP providing a homogeneous distribution of the catalyst throughout the reactant liquid, and finally the magnetic core meaning the material can be easily separated of the material following completion of the reaction.
8.1.4. Seals, lubricants and soaps.
Magnetic nanoparticles in liquid suspension have been demonstrated for use in vacuum seals with products currently available from companies including Ferrotec and Roseal. The products are commonly described as ferrofluids or magnetic liquids. They are suitable for use in high-vacuum environments, in cases where it is necessary to facilitate rotation and movement of components [216–218].
The incorporation of MNP suspensions into lubricants allows precision targeting of the location of these materials [219, 220], which is of particular interest for the development of highly resilient and long-running precision mechanisms. In a similar way, the incorporation of MNP and MNP suspensions into soaps has the potential to aid a variety of applications including the clean-up of oil spills [221].
9. Standardisation work underway and future needs of industry
Although individual measurements of the characteristics of MNPs and MNP suspension are well established in the scientific literature, there is a lack of cross-laboratory comparisons of MNP metrology. The circulation of standard reference samples for 'round-robin' style interlaboratory comparison measurements, and comparisons of multiple measurement techniques which probe the same characteristic are a necessary ongoing task. This type of comparative work between different measurement laboratories forms a key aspect of pre-normative work in the development of new standards (as specified by the metrological check-list). It is a task for the physics and metrological communities to conduct these consistency checks within the coming years. Two major research collaborations are currently being undertaken to further these goals. The NanoMag project aims to standardize, improve and redefine analysis methods for magnetic nanoparticles [9], while the RADIOMAG project works towards uniting experts and harmonising technologies in the fields of magnetic hyperthermia and indirect radiation therapy [139].
In addition to the numerous published standards listed in the previous sections, a number of standardization bodies are currently assessing the need for, and feasibility of, new standards in the field of nanotechnology. Indeed, ISO is currently developing two work items relating to MNP suspensions: ISO 19807 [222] and ISO TC/229 N 1421 [223]. The first of these is a draft material specification with the aim of providing coverage relevant to all types of MNP suspensions, and aims to introduce some of the unique terminology required by the field, whilst collate existing ISO standards of relevance and specifying the characterisation measurements which may be appropriate for use. The latter is an example of an early stage application standard which has recently been introduced to the ISO/TC 229 work list, and aims is to provide guidelines for MNP and MNP suspensions that are destined for the separation of circulating tumour DNA from biological samples and which is a subsection of the wider group of methods for magnetic separation of target molecules using functionalised MNPs. Such draft documents represent the first steps of standardisation into the field of MNP suspensions and we expect the list of documents under development can be expected to expand significantly in the coming years.
10. Conclusion
Significant advances have recently been made in the development of MNPs tailored to meet the needs of emerging end-user technologies. While progress has been made in our understanding of the underlying physics of these systems, much work is still required. Manufacturing techniques continue to improve in their reproducibility and yields; however, results still fall short of industry requirements. Similarly, great advances have been made in a variety of characterisation measurements and accompanying particle models, however there is currently a lack of consistency checking between different laboratories and measurement techniques. While a number of studies are currently in progress to investigate specific aspects of MNP characterisation [152, 153], a formidable task remains in order to demonstrate agreement between different types of MNP characterisation measurements. These tasks include proving the invariance of results obtained using the same measurement technique in different laboratories, and also the consistency of using different characterisation techniques to probe the same parameter of a MNP sample. The article is intended to serve as a precursor to future standards developments relating to MNPs in liquid suspension. It outlines the major topics in contemporary nanoparticle development which deserve to receive attention in the development of harmonised terminology, measurement techniques and health and safety for MNP products. By presenting an overview of these unsolved questions and obstacles to progress, we intend to provide a resource for identifying future topics for research, and a roadmap for future development within this field.
Acknowledgment
Financial support from the European Commission Framework Programme 7 under grant agreement No. 604448 'NanoMag', and the DFG project 'QuantMPI: Establishment of quantitative Magnetic Particle Imaging (MPI) application orientated phantoms for preclinical investigations' (grant TR 408/9-1) are gratefully acknowledged.