Abstract
Symmetries regarding system-surroundings interchange are used to propose the attribution of different meanings to the terms 'adiabatic' and 'thermally insulated' and address the resulting implications. It is also shown that entropy generation can be interpreted as the ratio of lost work by the temperature at which such loss occurs, and that it occurs always in the system.
Export citation and abstract BibTeX RIS
1. Introduction
Conceptual difficulties in thermodynamics are often enhanced by the fact that some definitions and concepts are not consistently or clearly stated in the literature, their understanding becoming thus conditioned by our intuition and previous knowledge.
One such concept is that of adiabatic process, which in turn is closely related to the concept of adiabatic wall. The former is of utmost importance in thermodynamics because not only does it appear, for instance, in Caratheodory's statement of the second law [1], but it is also studied in its theoretical and practical aspects. Essentially, a process is adiabatic if the system is enclosed by adiabatic walls, i.e. walls that inhibit any flow of energy through them that would result from a temperature difference between system and surroundings. There are however other situations where, although the walls are not adiabatic, the process can still be considered adiabatic provided it is sufficiently quick to inhibit such energy flow [2, 3].
On the other hand, the term thermally insulated is often used as an alternative to adiabatic depending on the author, a situation which is evident from the references cited in [4], which use either the term adiabatic or thermally insulated indifferently. If the two terms mean the same, which is the idea tacitly assumed, there is no problem in opting for one or the other, as long as consistency is maintained when discussing topics where the concept is present.
However, our proposal is different: to continue to use the already existing terms of adiabatic and thermally insulated, but to give them different meanings. As we shall see, although in many cases the advantage in doing so is minimal, there are others where distinguishing between such terms brings added value to their understanding as it helps clarify the underlying concepts. For instance, in the case of the adiabatic piston problem, our proposal enables the adiabatic piston to be described by a process which is adiabatic but not thermally insulated, a distinction which is decisive to overcome important conceptual issues raised in the literature, see e.g. [5], with regard to this problem.
Finally, this study provides a good example of the use of symmetry as a powerful tool to clarify concepts and definitions, thereby being relevant also from a didactical and pedagogical perspective.
2. Process-invariant quantities
For the sake of intuitiveness, consider PVT systems. A system-surroundings interaction constitutes a thermodynamic process, which is assumed here to be quasistatic (reversible or irreversible) [6], meaning that the system develops through a sequence of equilibrium states, such assumption not implying loss of conceptual generality, because if the actual process is non-quasistatic, we use the concept of identical process [7], to consider in its place a quasistatic process identical to it as detailed in [8].
Given that throughout a quasistatic process all thermodynamic variables, of both system and surroundings, are defined [6], we can envisage a system-surroundings interchange and define as process-invariant an equation or quantity that remains unchanged under such interchange. In other words, a process-invariant exhibits full symmetry and does not depend on which is labelled as system or as surroundings. If subscript 'e' (for external) is used for surroundings variables, a system-surroundings interchange is a conceptual operation which corresponds solely to removing subscript 'e' from surroundings variables and assigning it to system variables. If when doing so we obtain a formally identical expression, i.e. indistinguishable from the starting one, we are faced with a process-invariant. Process-invariants are physically very relevant because they express properties of a process, which are independent of what is labelled as system or as surroundings.
Using the fundamental relation [6] and the conservation of energy, a thermodynamic process is described by the process-invariant

where T, S, P and V are, respectively, temperature, entropy, pressure and volume.
Another process-invariant is the entropy generation  which satisfies the relation
which satisfies the relation

constituting a concise statement of the second law of thermodynamics. Unlike energy, which is always conserved, entropy is generated in irreversible processes, which constitutes an additional difficulty in grasping this concept. One possible interpretation of entropy is that it is generated whenever work is lost, i.e. work that is dissipated and can no longer be used as such. More precisely, entropy generation can be defined as the ratio of lost work  by the temperature at which it was dissipated, i.e.
by the temperature at which it was dissipated, i.e.

A system-surroundings interchange fully preserves the boundary properties, in particular whether or not heat can flow through it. If not, the wall is called adiabatic, and therefore the property of a wall being adiabatic is process-invariant. For a non-adiabatic wall, heat crossing the wall due to the difference between the system and surroundings temperatures is anti-symmetric under a system-surroundings interchange, meaning that heat changes its sign under such an interchange (i.e. it is a flux). As this heat is closely related to differences in temperature we qualify it as calorimetric and denote it by  In the light of lost work, how can
In the light of lost work, how can  generate entropy?
generate entropy?
To clarify this issue, let us compare the process shown in figure 1(a) to the one in figure 1(b). In figure 1(a), the system at temperature T is put in thermal contact with surroundings at temperature  and calorimetric heat
and calorimetric heat  flows from surroundings to system, being
flows from surroundings to system, being  since we take energy (i.e. heat and/or work) entering the system as positive (and negative otherwise). It is well-known (see e.g. [6]) that the entropy generated by such process is
since we take energy (i.e. heat and/or work) entering the system as positive (and negative otherwise). It is well-known (see e.g. [6]) that the entropy generated by such process is

but it being a pure heat interaction, how was there lost work and where was it dissipated?
Figure 1. Calorimetric heat interaction between surroundings (at temperature  ) and system (at lower temperature T) either by direct contact (a) or using a Carnot engine CE (b, c), with (c) resulting from (b) through a system-surroundings interchange. Calorimetric heat
) and system (at lower temperature T) either by direct contact (a) or using a Carnot engine CE (b, c), with (c) resulting from (b) through a system-surroundings interchange. Calorimetric heat 

 and
and  lost work
lost work  and
and  and
and 
Download figure:
Standard image High-resolution imageTo answer this, imagine a Carnot engine (CE) working between surroundings and system, see figure 1(b). With  we can adjust CE to remove from surroundings the same heat
we can adjust CE to remove from surroundings the same heat  that was exchanged by direct contact, producing work which is given by the well-known expression (e.g. [6])
that was exchanged by direct contact, producing work which is given by the well-known expression (e.g. [6])

and rejecting to the system the heat  Clearly, the CE being reversible and cyclic, neither
Clearly, the CE being reversible and cyclic, neither  nor
nor  contribute here to entropy generation. Therefore, in figure 1(b), only
contribute here to entropy generation. Therefore, in figure 1(b), only  is eligible to generate entropy, from (3) such generation occurring only if there is some dissipation of
is eligible to generate entropy, from (3) such generation occurring only if there is some dissipation of  If
If  is completely dissipated in the system, which is at temperature T, then the entropy generated is, according to (3),
is completely dissipated in the system, which is at temperature T, then the entropy generated is, according to (3),  which combined with (5) gives an entropy generation expression identical to (4), thereby showing that the process that includes the system, surroundings and the CE in figure 1(b) is indistinguishable from the direct heat exchange process in figure 1(a). Such indistinguishability means that entropy generation in the direct heat exchange process can be interpreted as resulting from the work
which combined with (5) gives an entropy generation expression identical to (4), thereby showing that the process that includes the system, surroundings and the CE in figure 1(b) is indistinguishable from the direct heat exchange process in figure 1(a). Such indistinguishability means that entropy generation in the direct heat exchange process can be interpreted as resulting from the work  this work being designated here as lost work because it is entirely dissipated in the system.
this work being designated here as lost work because it is entirely dissipated in the system.
Figure 1(c) illustrates a system-surroundings interchange where the new calorimetric heat of figure 1(a) is denoted with a prime. Lost work is now denoted by  but bearing in mind the anti-symmetry of calorimetric heat, i.e.
but bearing in mind the anti-symmetry of calorimetric heat, i.e.  it is readily shown to be formally given also by (5), i.e.
it is readily shown to be formally given also by (5), i.e.

and, if such work is completely dissipated in the system, the entropy generation is  which with (6) again results in an entropy generation expression identical to (4).
which with (6) again results in an entropy generation expression identical to (4).
Even though the above conclusion was obtained assuming  , the same result for
, the same result for  would have been obtained, as required by symmetry, if the other remaining possibility had been considered, i.e. keeping the choice of the system and surroundings but choosing
would have been obtained, as required by symmetry, if the other remaining possibility had been considered, i.e. keeping the choice of the system and surroundings but choosing  Since calorimetric heat is anti-symmetric, i.e.
Since calorimetric heat is anti-symmetric, i.e.  the generation of entropy given by (4) is also process-invariant.
the generation of entropy given by (4) is also process-invariant.
Carefully note, however, that calorimetric heat  is not the only way to generate entropy, since work can also be lost by direct mechanical dissipation. In thermodynamics, work
is not the only way to generate entropy, since work can also be lost by direct mechanical dissipation. In thermodynamics, work  is defined in terms of surroundings variables only [6] by
is defined in terms of surroundings variables only [6] by

unlike configuration work  which is defined in terms of system variables only by
which is defined in terms of system variables only by

While  is the work that surroundings exchange with the system,
is the work that surroundings exchange with the system,  is the part of it that is used to configure the system. Therefore, the difference between
is the part of it that is used to configure the system. Therefore, the difference between  and
and  is a positive (or zero) quantity that can be appropriately taken as the dissipative work
is a positive (or zero) quantity that can be appropriately taken as the dissipative work  i.e. [9]
i.e. [9]

which, from (1), can also be written as

Dissipative work  given above, is thus always positive or zero and is work that was not spent for system configuration but converted into internal energy of system and/or surroundings and, therefore, can no longer be recovered as work. In other words,
given above, is thus always positive or zero and is work that was not spent for system configuration but converted into internal energy of system and/or surroundings and, therefore, can no longer be recovered as work. In other words,  is also lost work that, in line with (3), generates entropy. A direct inspection of (9) shows that dissipative work is a process-invariant. Of course, since
is also lost work that, in line with (3), generates entropy. A direct inspection of (9) shows that dissipative work is a process-invariant. Of course, since  first law
first law  with (1) requires that heat
with (1) requires that heat  be given by
be given by

From the above analysis, if  by (7), (9) and (10),
by (7), (9) and (10),  and
and  i.e. work and heat change sign under a system-surroundings interchange and are therefore anti-symmetric quantities (i.e. are fluxes). Moreover, since in this case
i.e. work and heat change sign under a system-surroundings interchange and are therefore anti-symmetric quantities (i.e. are fluxes). Moreover, since in this case  and
and  the total generated entropy (2) becomes
the total generated entropy (2) becomes

which has to be equal to (4) because no entropy is generated from dissipative work if  Therefore, if
Therefore, if  we get that
we get that 
However, if  the above relations
the above relations  and
and  are no longer valid and, therefore, the anti-symmetry of the terms in (1) is broken, resulting in some conceptual difficulties, namely the somewhat counterintuitive fact that work and heat are no longer fluxes. In other words, for
are no longer valid and, therefore, the anti-symmetry of the terms in (1) is broken, resulting in some conceptual difficulties, namely the somewhat counterintuitive fact that work and heat are no longer fluxes. In other words, for  under a system-surroundings interchange not only the signs of heat and work change but also their absolute values. Evidently, the calorimetric heat
under a system-surroundings interchange not only the signs of heat and work change but also their absolute values. Evidently, the calorimetric heat  should remain a flux and can no longer be identified with
should remain a flux and can no longer be identified with  Thus, the heat
Thus, the heat  must now contain a non anti-symmetric component, which is denoted here by
must now contain a non anti-symmetric component, which is denoted here by  as it is a consequence of the existence of
as it is a consequence of the existence of  i.e.
i.e.

which, from (9b) and (10), can also be written as

showing that, since  we get that
we get that  a well-known [10] thermodynamics expression where the equality sign corresponds to processes for which
a well-known [10] thermodynamics expression where the equality sign corresponds to processes for which  and the inequality one to those for which
and the inequality one to those for which  by contrast to the Clausius relation
by contrast to the Clausius relation  where the former sign corresponds to reversible processes and the latter to irreversible ones [10].
where the former sign corresponds to reversible processes and the latter to irreversible ones [10].
Figure 2 illustrates the work interaction, and the fact that work is defined by (7) as  means that the sum of all work-related arrow-lines connecting to surroundings (or to system) should be
means that the sum of all work-related arrow-lines connecting to surroundings (or to system) should be  implying that there can be no other work-related arrow-line connected to surroundings. So, the reading of the figure 2 is that surroundings interact with the system through work
implying that there can be no other work-related arrow-line connected to surroundings. So, the reading of the figure 2 is that surroundings interact with the system through work  part of it,
part of it,  being used for system configuration and the other part,
being used for system configuration and the other part,  being entirely lost by dissipation into the system. However, this conclusion is paradoxical: since
being entirely lost by dissipation into the system. However, this conclusion is paradoxical: since  is process-invariant, i.e. it exhibits total symmetry, the fact that it can only influence the system breaks this symmetry, resulting in a contradiction. Such contradiction is all the more evident if we consider a system-surroundings interchange, because the dissipation of work now occurs where, before the interchange, was surroundings, and physics cannot depend on which is chosen as system or as surroundings.
is process-invariant, i.e. it exhibits total symmetry, the fact that it can only influence the system breaks this symmetry, resulting in a contradiction. Such contradiction is all the more evident if we consider a system-surroundings interchange, because the dissipation of work now occurs where, before the interchange, was surroundings, and physics cannot depend on which is chosen as system or as surroundings.
Figure 2. Work interaction  between surroundings (at temperature
between surroundings (at temperature  ) and the system (at temperature T), showing the two components in which
) and the system (at temperature T), showing the two components in which  is divided: configuration work
is divided: configuration work  and dissipative work
and dissipative work  why cannot
why cannot  have direct effects in surroundings? (see text).
have direct effects in surroundings? (see text).
Download figure:
Standard image High-resolution imageAn attempt to solve this contradiction is to understand the effect of  on surroundings as heat, the natural choice being the heat component
on surroundings as heat, the natural choice being the heat component  that appears in (12) as a consequence of the existence of
that appears in (12) as a consequence of the existence of  But how can heat be involved in a pure work interaction such as that shown in figure 2? And even if it were, if
But how can heat be involved in a pure work interaction such as that shown in figure 2? And even if it were, if  how can heat flow from system to surroundings?
how can heat flow from system to surroundings?
If  we may consider that instead of all
we may consider that instead of all  being dissipated in the system, part of it, say
being dissipated in the system, part of it, say  feeds a Carnot refrigerator (CR) that removes the heat
feeds a Carnot refrigerator (CR) that removes the heat  from the system and supplies the heat
from the system and supplies the heat  to surroundings, as shown in figure 3(a). On the other hand, if
to surroundings, as shown in figure 3(a). On the other hand, if  the heat removed from the system and the one supplied to surroundings are accomplished through a CE, producing the work
the heat removed from the system and the one supplied to surroundings are accomplished through a CE, producing the work  which we choose to be dissipated in the system, so that the net work dissipated in the system is
which we choose to be dissipated in the system, so that the net work dissipated in the system is  as shown in figure 3(b). Since
as shown in figure 3(b). Since  is an attempt to explain the effect of
is an attempt to explain the effect of  in surroundings, it must be negative and satisfy
in surroundings, it must be negative and satisfy

or, equivalently,

Figure 3. System-surroundings work interaction  which decomposes into configuration work
which decomposes into configuration work  and dissipative work
and dissipative work  (a) if
(a) if  part of
part of  is supplied to a Carnot refrigerator which delivers
is supplied to a Carnot refrigerator which delivers  to surroundings, the remaining
to surroundings, the remaining  being dissipated in the system; (b) if
being dissipated in the system; (b) if  a Carnot engine delivers
a Carnot engine delivers  to surroundings, the produced work is combined with
to surroundings, the produced work is combined with  being both dissipated in the system.
being both dissipated in the system.
Download figure:
Standard image High-resolution imageAdopting the sign convention that  for the CR and
for the CR and  for the CE, as shown in figure 3, in both cases the lost work by dissipation in the system is given by
for the CE, as shown in figure 3, in both cases the lost work by dissipation in the system is given by  which, using (3), gives
which, using (3), gives

The work supplied to CR or produced by CE is given by (5) with  instead of
instead of  i.e.
i.e.

So, inserting (17) into (16) and using (15), we get

where  and
and  are related by
are related by

Performing a system-surroundings interchange and remembering that after the interchange the new quantities are denoted with a prime,  being process-invariant, (15) becomes
being process-invariant, (15) becomes

which with (15) gives

showing that under a system-surroundings interchange, dissipative heat  is not anti-symmetric, because
is not anti-symmetric, because  unlike calorimetric heat
unlike calorimetric heat  which satisfies
which satisfies

Figure 4(a) is a unified version of figure 3, showing quantities that model the work interaction. Figure 4(b) shows how quantities in figure 4(a) change under a system-surroundings interchange.
Figure 4. System-surroundings work interaction; system temperature T and surroundings temperature  (a) shows dissipative heat
(a) shows dissipative heat  and the two
and the two  components (configuration work
components (configuration work  and dissipative work
and dissipative work  ); (b) shows how quantities in (a) change under a system-surroundings interchange. A set of numerical values is given for merely illustrative purposes.
); (b) shows how quantities in (a) change under a system-surroundings interchange. A set of numerical values is given for merely illustrative purposes.
Download figure:
Standard image High-resolution imageAdding (4) with (18), the total generated entropy is given by
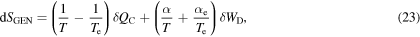
an equation that, as it should, shows that the total generated entropy  is a process-invariant, i.e. applying the transformation
is a process-invariant, i.e. applying the transformation  (23) remains unchanged. It is important to note that to fully characterize the process it is necessary to introduce the new parameters
(23) remains unchanged. It is important to note that to fully characterize the process it is necessary to introduce the new parameters  and
and  which satisfy
which satisfy  and whose meanings were already explained. Finally, being the entropy generation always determined by (3) and since the lost work, given by
and whose meanings were already explained. Finally, being the entropy generation always determined by (3) and since the lost work, given by  or
or  for the heat interaction in figure 1 and by
for the heat interaction in figure 1 and by  for the work interaction in figure 3, is always dissipated in the system, the answer to the question of where entropy is generated is that it always is in the system, in line with Prigogine (see p 16 of [11]). Consequently, no entropy generation occurs in surroundings, being this the fundamental characteristic underlying the reservoir concept.
for the work interaction in figure 3, is always dissipated in the system, the answer to the question of where entropy is generated is that it always is in the system, in line with Prigogine (see p 16 of [11]). Consequently, no entropy generation occurs in surroundings, being this the fundamental characteristic underlying the reservoir concept.
3. Adiabatic versus thermally insulated
As far as known to us, a distinction between  and
and  is virtually absent from textbooks, which propitiates confusion between them, or the assumption of their equivalence. Moreover, such distinction also suggests a distinction between the concepts of adiabatic and thermally insulated, which is the main purpose of this study. Given that before a system-surroundings interchange, see (12),
is virtually absent from textbooks, which propitiates confusion between them, or the assumption of their equivalence. Moreover, such distinction also suggests a distinction between the concepts of adiabatic and thermally insulated, which is the main purpose of this study. Given that before a system-surroundings interchange, see (12),  and after such interchange
and after such interchange  from (21) and (22), we get
from (21) and (22), we get  meaning that, if
meaning that, if 
 is not necessarily zero which contradicts the definition of adiabatic process as one for which
is not necessarily zero which contradicts the definition of adiabatic process as one for which  since such definition does not exhibit invariance under a system-surroundings interchange, i.e. the adiabatic concept should not depend on which is labelled as system or surroundings. Therefore, we propose defining adiabatic process as one for which
since such definition does not exhibit invariance under a system-surroundings interchange, i.e. the adiabatic concept should not depend on which is labelled as system or surroundings. Therefore, we propose defining adiabatic process as one for which  and not one for which
and not one for which  Moreover, as mentioned earlier, since wall properties exhibit the same symmetry as
Moreover, as mentioned earlier, since wall properties exhibit the same symmetry as  under a system-surroundings interchange, and the adiabatic wall definition has to be compatible with that of adiabatic process, an adiabatic wall should be defined as one that inhibits
under a system-surroundings interchange, and the adiabatic wall definition has to be compatible with that of adiabatic process, an adiabatic wall should be defined as one that inhibits  and not one that inhibits
and not one that inhibits  On the other hand, we propose that
On the other hand, we propose that  be the defining condition for a thermally insulated process. Except when
be the defining condition for a thermally insulated process. Except when  (i.e.
(i.e.  which happens, from (15) with (19), when
which happens, from (15) with (19), when  or
or  ), with these new definitions, the terms 'adiabatic' and 'thermally insulated' take on different meanings, even though they have up to now been used indifferently in the literature (see [4] and references therein).
), with these new definitions, the terms 'adiabatic' and 'thermally insulated' take on different meanings, even though they have up to now been used indifferently in the literature (see [4] and references therein).
Before concluding it is worth underlining some implications of the definitions proposed in this study. One of them is that defining calorimetric heat in terms of its symmetry property using (22) dispels apparent difficulties in reconciling the concept of calorimetric heat with that of temperature difference in the case of a reversible process, and with wall properties in the case of the adiabatic piston's second process [5]. Firstly, consider the reversible process. If the definition of  is made to depend on temperature difference between the system and surroundings, in reversible processes
is made to depend on temperature difference between the system and surroundings, in reversible processes  has to be zero because for such ideal processes
has to be zero because for such ideal processes  an apparent contradiction which is usually addressed by considering a reversible process as the limit of a succession of irreversible processes with a constant
an apparent contradiction which is usually addressed by considering a reversible process as the limit of a succession of irreversible processes with a constant  for which
for which  of each succession term becomes arbitrarily small. However, by using the symmetry of (22),
of each succession term becomes arbitrarily small. However, by using the symmetry of (22),  is defined even though
is defined even though  Secondly, consider the case of the so-called adiabatic piston, which is another example where
Secondly, consider the case of the so-called adiabatic piston, which is another example where  is better defined using (22) rather than resorting to wall thermal properties. In fact, after mechanical equilibrium is reached (
is better defined using (22) rather than resorting to wall thermal properties. In fact, after mechanical equilibrium is reached ( but
but  ), the piston undergoes a second process, which lasts until thermalization is reached (
), the piston undergoes a second process, which lasts until thermalization is reached ( ) [5]. Therefore, even though the piston has zero thermal conductivity, the piston has to be considered non-adiabatic, because thermalization requires heat flow through it [5]. As dissipative work is absent during this process such heat has to be calorimetric, i.e. it satisfies (22), and thus there is no conflict with the zero thermal conductivity of the piston, since (22) applies regardless of wall thermal properties.
) [5]. Therefore, even though the piston has zero thermal conductivity, the piston has to be considered non-adiabatic, because thermalization requires heat flow through it [5]. As dissipative work is absent during this process such heat has to be calorimetric, i.e. it satisfies (22), and thus there is no conflict with the zero thermal conductivity of the piston, since (22) applies regardless of wall thermal properties.
Furthermore, using (13), system entropy variation dS can be expressed as

or equivalently, inserting (12) and (21) in (24a), and using (20),

meaning that both for  and
and  we have
we have  i.e. the principle of entropy increase, which was commented in [4], can now be formulated as follows: both for thermally insulated (
i.e. the principle of entropy increase, which was commented in [4], can now be formulated as follows: both for thermally insulated ( ) and for adiabatic (
) and for adiabatic ( ) processes the system entropy cannot decrease. However, an important difference exists between the two: while for a thermally insulated process system entropy increases or remains unchanged depending on whether the process is irreversible or reversible, respectively, for an adiabatic process system entropy remains unchanged, not only for reversible processes, but also for those irreversible processes where
) processes the system entropy cannot decrease. However, an important difference exists between the two: while for a thermally insulated process system entropy increases or remains unchanged depending on whether the process is irreversible or reversible, respectively, for an adiabatic process system entropy remains unchanged, not only for reversible processes, but also for those irreversible processes where  i.e. those for which
i.e. those for which  see (15) with (19). Moreover, since the second rhs term in (24b) is always positive or zero, the decrease in entropy of a system can only be achieved by means of calorimetric heat, that is, the process cannot be adiabatic.
see (15) with (19). Moreover, since the second rhs term in (24b) is always positive or zero, the decrease in entropy of a system can only be achieved by means of calorimetric heat, that is, the process cannot be adiabatic.
Finally, let us note that our proposal is compatible with the Caratheodory statement of the second law, which states [1]: in the neighbourhood (however close) of any equilibrium state of a system of any number of independent coordinates, there exist other equilibrium states which are inaccessible by means of reversible adiabatic processes. In the case of the above statement, the process being reversible, one has  and the concepts of adiabatic and thermally insulated coincide, i.e.
and the concepts of adiabatic and thermally insulated coincide, i.e.  and thus our proposal is peaceful.
and thus our proposal is peaceful.
4. Conclusion
The concepts of process-invariant and of lost work, defined, respectively, as an equation or quantity that remains invariant under a system-surroundings interchange and work that becomes unusable as such, were applied in the context of entropy generation being interpreted as the ratio of lost work by the temperature at which it is dissipated. It was found that entropy generation always occurs in the system, i.e. no entropy is generated in surroundings, the latter being the fundamental characteristic underlying the reservoir concept. Is was also shown that the effect dissipative work has on surroundings is equivalent to a heat interaction, leading to distinguish between calorimetric heat  and heat
and heat  which in turn propitiated a distinction between adiabatic and thermally insulated processes, the former being now defined as one for which
which in turn propitiated a distinction between adiabatic and thermally insulated processes, the former being now defined as one for which  and the latter as one for which
and the latter as one for which  The proposed distinction between adiabatic and thermally insulated dispelled apparent difficulties in reconciling the concept of calorimetric heat with that of temperature difference in the case of a reversible process, and with wall properties in the case of the adiabatic piston's second process. Lastly, although for both thermally insulated
The proposed distinction between adiabatic and thermally insulated dispelled apparent difficulties in reconciling the concept of calorimetric heat with that of temperature difference in the case of a reversible process, and with wall properties in the case of the adiabatic piston's second process. Lastly, although for both thermally insulated  and adiabatic
and adiabatic  processes system entropy cannot decrease, an important difference exists between the two: while for thermally insulated process the system entropy increases or remains unchanged depending, respectively, on whether the process is irreversible or reversible, for an adiabatic process system entropy remains unchanged not only for reversible processes, but also for those irreversible processes where dissipative work is entirely converted into dissipative heat
processes system entropy cannot decrease, an important difference exists between the two: while for thermally insulated process the system entropy increases or remains unchanged depending, respectively, on whether the process is irreversible or reversible, for an adiabatic process system entropy remains unchanged not only for reversible processes, but also for those irreversible processes where dissipative work is entirely converted into dissipative heat  the latter being defined as
the latter being defined as 






