Abstract
Polydimethylsiloxane (PDMS) is a commonly used silicone elastomer with broad applications. Particularly for bioengineering use, PDMS is treated with oxygen plasma with which its surface is oxidized to allow positive interaction with water and live cells. In exchange for the acquisition of hydrophilicity, the oxidized PDMS becomes mechanically brittle so that resulting formation of cracks affects the system in various ways. However, tensile strength (TS), which is an inherent capacity of a material to withstand tensile loads before breaking and is thus a key parameter limiting the use of the material, remains unclear regarding oxidized PDMS. Here we determine the TS of oxide layers created on the surface of PDMS based on micro-stretch experiments using a custom-made device. We show that the surface layer displays cracks upon tensile loading of small strains of within 10% to have a TS of ~10–100 kPa, which is approximately two orders of magnitude lower than that of unmodified PDMS. We further show that the TS sharply decreases with oxidation duration to become highly brittle, while the thickness of the resulting oxide layer finally reaches a plateau even with prolonged plasma treatment. Consequently, we suggest that gradual surface modification of PDMS takes place only within a finite region even with prolonged plasma treatment, as distinct from previously held assumptions. These quantitative data provide critical design information for the oxide layer of plasma-hydrophilized PDMS.
Export citation and abstract BibTeX RIS
1. Introduction
Polydimethylsiloxane (PDMS) is one of the most commonly used silicone elastomers. Particularly in bioengineering applications such as microfluidics, the originally hydrophobic PDMS is converted to attain hydrophilic surface properties upon treatment with oxygen plasma, allowing for close interaction with water solutions, proteins, and live cells [1]. Bulk material properties of PDMS such as Young's modulus and viscoelastic properties, which are useful information in designing engineering systems, have been reported in literature (e.g. [1–6]). Previous studies also reported a local increase in Young's modulus at the surface of PDMS after treatment with oxygen plasma that endows hydrophilicity (e.g. [2, 4]).
There is actually another important, but less characterized drastic change that emerges at the surface of PDMS after oxidation, i.e. brittleness [7]. PDMS, a silicone elastomer, is literally elastic in its original state and exhibits high extensibility where a stretch of more than 100% strain is possible (i.e. stretchable without breaking to the double of its original length) [1]. Meanwhile, oxygen plasma-treated PDMS becomes apparently fragile as the surface is converted to a thin silica-like layer [8–10]. The rest of the bulk PDMS other than the surface may be unaffected with plasma treatment and hence keep high extensibility; thus, even subtle deformation can cause cracks only at the fragile surface and deteriorate the system in a variety of ways. For example, optical properties of the PDMS such as transparency will be impaired. In addition, unaltered, originally hydrophobic PDMS may be exposed through a crack, potentially resulting in incompatible chemical interaction with the surrounding water solutions. Moreover, PDMS sheet is often used in biological studies to mechanically stretch cells plated on it as cells are known to regulate their function in a stretch-dependent manner [11, 12]. In this kind of experiments, stretch of oxidized PDMS sheets inevitably yields cracks to potentially induce unexpected cellular response to the newly emerged features [12].
Material properties of oxidized PDMS have been investigated in previous studies (e.g. [2, 4]); however, to our knowledge, quantitative measurement regarding the brittleness—i.e. a practical factor limiting the use of the material—remains to be achieved. Here we characterize the breaking of the thin silica-like layer formed on the surface of PDMS upon oxygen plasma treatment to measure its tensile strength (TS). TS—generally defined as the maximum tensile stress (i.e. an internal tensile force divided by the cross-sectional area of interest) that a material can withstand while being stretched before breaking—is an intrinsic property specific to the material. TS is determined here, based on measurement of tensile strain (i.e. the current length divided by the original length of the material before stretching), as the product of the breaking strain (or fracture strain) and the Young's modulus of the surface layer. We then discuss mechanisms underlying oxidation duration-dependent changes in the material properties based on measurement of accompanied changes in the thickness of the surface layer. We further investigate how the mechanical resistance changes over time.
2. Materials and methods
2.1. Sample preparation for stretch experiment
PDMS used here (Sylgard-184, Dow Corning Toray; its TS is 6.7 MPa or 3–12 MPa according to the manufacturer's specification or literature [1, 3], respectively) is supplied as two-part liquid component kits with the polymer base and curing agent. First, PDMS prepolymer was mixed at a 20:1 base/curing agent ratio at room temperature, stirred, degassed using a vacuum, and poured into a custom-made mold that consists of several acrylic parts, similar to those described elsewhere [13], designed to form a hollow cavity with a dumbbell-like shape (figure 1(A)). The mold supporting the poured prepolymer was oven cured at 60 °C for 2 h. After ejected from the mold, the dumbbell-shaped PDMS was further oven cured at 60 °C for 20 h and mounted onto specialized jigs for stretch experiments described below. In a separate process, another PDMS prepolymer was mixed, as a test sample for the stretch experiments, at a weight ratio of either 50:1, 60:1, 70:1, 80:1, 90:1, or 100:1 and poured onto the top surface of the dumbbell-shaped base PDMS. The sample PDMS (~500 µm in thickness) is fully flattened on the base PDMS with an aid of application of an air stream using a pipette connected to a compressor and is then exposed to oxygen plasma using a plasma generator (4 mA in current, 600 V in voltage, and 20 Pa in pressure; SEDE-P, Meiwafosis) for either 60, 75, 90, 105, or 120 s. Here the sample PDMS is too soft to be hooked up to a stretch device described in the following section; thus, the relatively stiff base PDMS is necessary to stably sustain the whole structure. For storage of the samples, we put them in a light-blocking desiccator at room temperature for 7 months. Change in hydrophilicity of the surface was evaluated by examining the contact angle between the surface and ultrapure water (4 µl droplet) using a 90°-tilted stereomicroscope (S8APO, Leica) with a camera (EC3, Leica).
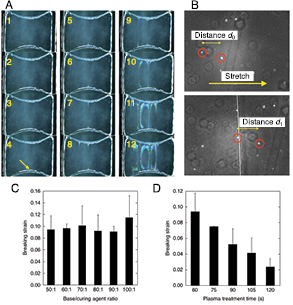
Figure 1. Custom-made device allows stretch experiment for a hydrophilized PDMS sample on an upright/inverted microscope. (A) Top and side schematic views of the base PDMS that supports a sample PDMS on the surface. The numbers shown for the size are in mm. The end parts with a 1.5 mm radius of curvature (R1.5) are for clamping connection to the linear stage-equipped stretcher. (B) Schematic view of the stretcher. The PDMS specimen is not depicted. (C) Photograph showing a PDMS specimen mounted on the stretcher. (D) Side view of an ultrapure water droplet, taken by stereomicroscopy, shows that the contact angle between the water and the surface of a sample PDMS significantly decreases after the exposure to oxygen plasma, suggesting alteration to a hydrophilic state.
Download figure:
Standard image High-resolution image2.2. Stretch experiment
The base PDMS described above, supporting a PDMS sample, was mounted onto the specialized jigs prior to the oxygen plasma treatment and subsequent stretch experiments because PDMS tends to easily rupture at the surface once treated with plasma so that even the act of mounting can break the sample portion if done after plasma treatment. The jigs were made of acrylic parts and Teflon bolts so that the insertion of the PDMS with the non-metallic jigs is supposed not to affect the intensity of direct current-driven plasma. The oxygen plasma-treated sample was then clamped along the both ends of the dumbbell-shaped base to a custom-made micro-stretch device (figures 1(B) and (C)). The device is fabricated on an aluminum alloy plate (A5052) and is equipped with a precision linear stage (TSD-60121SR, Sigma-koki) to uniaxially stretch the sample within the horizontal plane on the stage of a microscope. Images were taken using a camera (EC3) under a stereomicroscope (S8 APO, 10× magnification) to obtain the whole views of the sample PDMS while stretching the sample at a quasi-static strain rate of ~6 × 10−4 s−1 (that corresponds to 20 µm s−1). Alternatively, in separate experiments, stretch-induced cracks were imaged every 2 s at a high magnification (40×) using a camera (ORCA-R2, Hamamatsu) under an inverted phase contrast microscope (IX-73, Olympus) during stretching at the quasi-static strain ratio. In the latter experiments, local strains or changes in the distance between neighboring minute foams, which are inevitably present in the Sylgard-184 PDMS [6] but can be used as a fiducial marker for measuring strains of the material, were analyzed using image analysis software ImageJ (NIH). Breaking strain ε is quantified as (d1 − d0)/d0 where d0 and d1 represent the distances between two fiducial markers before and after a new crack appears due to stretch in the middle of them, respectively.
2.3. Evaluation of tensile strength
TS is determined as the product of the breaking strain ε and the Young's modulus E, the latter of which was previously obtained regarding the same type of Sylgard-184 PDMS [4]. Briefly, regarding the measurement of E, contact-mode atomic force microscopy (AFM, NanoWizard II, JPK Instrument) was performed using a silicon nitride cantilever (OMCL-RC800PB-1, Olympus) on the PDMS treated with oxygen plasma for either 0, 50, 300, or 500 s. E is determined from the acquired force curves (i.e. the relationship between the force and cantilever displacement) with the Hertz model, together with the information on the opening angle of the pyramidal tip of the cantilever that the manufacturer provided and the Poisson's ratio of the PDMS, 0.5. Here, a linear regression function was obtained, based on the least-squares method, regarding the relationship between the average value of E (MPa) and corresponding plasma treatment time t (s). Thus, E for arbitrary values of t is determined from the regression function to evaluate the TS at different plasma treatment times of 60, 75, 90, 105, and 120 s that we applied for the measurement of ε.
2.4. Atomic force microscopy of stretched samples
PDMS sheets (Sylgard-184, 10:1 base/curing agent weight ratio), initially treated with oxygen plasma (2.4 W, 20 Pa) for either 1, 30, or 60 min, were stretched and then fixed with scotch tape at the ends on a slide glass. We found that cleavages were consequently produced in a direction perpendicular to the externally applied stretch. The height of the cleavages was analyzed from the profile of the 3D geometry obtained by contact-mode AFM (NanoWizard II). To validate our assumption that the height of the cleavages corresponds to the thickness of the oxide layers, micropatterning experiments were performed as follows. A copper electron microscopy (EM) grid (Athene Old 400 G204, EM Japan) was put on the surface of PDMS, followed by oxygen plasma treatment (2.4 W, 20 Pa) for either 1 or 30 min. The EM grid has square holes (30 × 30 µm2) and thus locally blocks the exposure to the plasma at the portions holding the bars, thus allowing for creation of spatially distinct (i.e. plasma-treated or non-treated) regions or micropatterning [14]. After the EM grid was removed using forceps, the PDMS surface was subjected to contact-mode AFM to obtain force curves and adhesive forces, which were compared to those obtained from PDMS sheet samples possessing stretch-induced cleavages.
3. Results and discussion
3.1. Oxygen plasma-treated PDMS breaks upon less than 10% strain at the surface layer
Sylgard-184 PDMS, which is one of the most used silicone elastomers, is known to be highly extensible, allowing for more than 100% tensile strain [1]; but, upon oxygen plasma treatment followed by the formation of a thin silica-like layer, it becomes brittle at the surface in exchange of the acquisition of hydrophilicity [7]. Here we attempted to quantify the critical strain for rupture using a custom-made stretch device, which enables stretching of a sample PDMS coated on a base PDMS (figure 1). The jigs holding the sample were all comprised of non-metal materials to allow the exposure to spatially uniform oxygen plasma. Moreover, the device was designed to minimize application of unexpected stretch and resulting rupture of the sample throughout the process of the setup. We confirmed that the initially hydrophobic sample PDMS mounted on the jigs was altered to a hydrophilic state after the oxygen plasma treatment (figure 1(D)). This is quantitatively consistent with our previous measurements in which water contact angle decreased on the modified PDMS surface from originally ~90° to much less than 90° afterward [10].
To figure out where rupture is initiated upon stretch, we initially observed the whole part of samples. We found that the first rupture always appeared at the central constricted parts as expected (figure 2(A), arrow). The rupture ran in a direction perpendicular to the stretch and then extended as stretch proceeded, thereby producing a straight cleavage. With further stretch, several additional cleavages were created in parallel to each other. Even with the emergence of cleavages under extensive strains loaded up to several tens of percent, the PDMS specimens did not break as a whole, suggesting that the rupture occurs only at limited portions or within the surficial oxide layer, and the rest of the bulk material or unmodified interior sample PDMS still remains high extensibility.
Figure 2. Stretch experiment reveals the breaking strains of the oxide layer as a function of the PDMS base/curing agent ratio and plasma treatment time. (A) Sequential stereomicroscopic images of the whole views of a PDMS specimen subjected to quasi-static unidirectional stretch. Time proceeds in the order of the numbers described in each panel. The yellow arrow represents the primary crack, which extends in a straight line as time passes. (B) Phase-contrast micrographs before (upper panel) and after (lower panel) the primary crack appears as recognized as a white straight line. Breaking strains were quantified based on the distances between fiducial markers marked by red circles. (C) Breaking strain as a function of the base/curing agent ratio at a constant plasma treatment time of 60 s. Data are expressed as mean ± standard deviation. (D) Breaking strain as a function of the plasma treatment time at a constant base/curing agent ratio of 50:1. Data are expressed as mean ± standard deviation.
Download figure:
Standard image High-resolution imageNext, focusing on the targeted constriction where prone to rupture, the stretch-induced rupture formation was observed at a higher magnification with phase-contrast microscopy (figure 2(B)). Here, local breaking strains were examined at different base/curing agent weight ratios between 50:1 and 100:1 for a fixed plasma treatment time of 60 s. The result showed that the oxidized PDMS exhibits rupture at a local strain of on average ~9%, thus losing extensibility by at least one order of magnitude compared to unmodified PDMS (figure 2(C)). There was no significant difference in the strains regarding the change in the base/curing agent weight ratio. We then investigated the effect of changing plasma treatment time from 60 s to 120 s at an interval of 15 s while keeping the base/curing agent weight ratio to a fixed value of 50:1. We found a significant decrease in the breaking strains from 9.4% to 2.3% on average with extending the duration of plasma treatment (figure 2(D)). In a separate preliminary experiment, we observed that plasma treatment for more than 30 min resulted in formation of so fragile oxide layers on the PDMS surface that their breaking strains were difficult to be precisely determined for such long treatment durations. Thus, we performed the experiment for determining the breaking strains up to a treatment time of 120 s as our measurement limitation.
The breaking strain was thus highly sensitive to the plasma treatment time but not to the amount of the curing agent, suggesting that the resistance to molecular rupture of oxidized PDMS is predominantly affected by plasma-driven changes in the 'intramolecular' structure of the base material rather than in the 'intermolecular' one between the base material and curing agent; more specifically, oxygen plasma treatment has been suggested in previous studies to modify a part of the major repeated architectures of PDMS, [Si(CH3)2–O]n, to those include Si2–O in which two silicon atoms are captured in motion by the presence of a single oxygen atom [8–10]; therefore, the restricted molecular motion would result in elevated elastic modulus at a macroscopic scale [4] as well as in lowered resistance to rupture (figure 2(D)). In contrast to this significant contribution of the intramolecular structural changes to the rupture, the loss in the amount of the curing agent within the whole PDMS or intermolecular changes between the base material and curing agent had little apparent effect on the magnitude of the resistance to rupture.
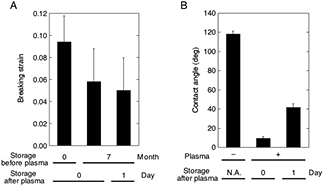
We also investigated the effect of sample storage on the breaking strain (figure 3). With or without the storage in a light-blocking condition for 7 months after curing, the PDMS samples were treated with oxygen plasma for 1 min and then tested for measuring the breaking strain. The breaking strain of the sample with the long-term storage (5.8%) was significantly lower than that without the storage (9.4%). This deterioration in mechanical strength may be caused due to the long-term exposure to the dry air and resulting surface oxidation. We further measured the effect of sample storage after the plasma treatment. Here we first stored PDMS samples for 7 months and then treated them with oxygen plasma for 1 min. After a lapse of 1 d during which the PDMS samples were stored in a light-blocking condition, they were subjected to mechanical stretch until rupture occurred. We detected no significant change in the breaking strains between the samples with (5.0%) or without (5.8%) the 1 d storage after the plasma treatment although the average value decreased with the storage. In contrast, the water contact angle for PDMS samples stored for 7 months after curing was, on average, 118°, 9.4°, and 41.7° for control (i.e. measured without plasma treatment), day 0 (i.e. measured just after the plasma treatment for 1 min), and day 1 (i.e. measured 24 h after the plasma treatment for 1 min), respectively. This is consistent with the well-known hydrophobic recovery [15], which has been interpreted to happen because of the diffusion of a low-molecular weight siloxane (incomplete reticulated fragment) into the surface oxide layer. Thus, these results suggest that chemical properties and macroscopic mechanical properties are not always correlated; specifically, hydrophobic surface properties are recovered to some extent after storage, but the mechanical resistance is rather increasingly lost over time.
Figure 3. Hydrophobic recovery occurs after sample storage, but still deterioration in mechanical strength rather continuously proceeds over time. (A) Breaking strain measured with or without two types of storage (i.e. one is long-term storage for seven months after curing but before 1 min plasma treatment; the other is 1 d storage after 1 min plasma treatment on samples that experienced seven months pre-storage). (B) Contact angles between the PDMS surface and water, measured with or without 1 d storage after plasma treatment. Data are expressed as mean ± standard deviation.
Download figure:
Standard image High-resolution image3.2. Tensile strength of PDMS decreases sharply with increasing plasma treatment duration
While failure-bearing capability has been characterized regarding a thin film seeded on a PDMS [16], our results obtained above may be the first, to our knowledge, that quantified the oxidation time-dependent breaking strains of the silica-like layer formed on the surface of PDMS; from which we found that the surface layer withstands only less than 10% strains even with such moderate power of the plasma (2.4 W) for 1 min (figures 2(C) and (D)). The PDMS we used here is highly elastic before reaching fracture (with a low 'loss tangent-delta', an index characterizing viscosity, of <0.07 [6]), and the material is under uniaxial tension in the present experiment. Consequently, the stress and strain are in one- to-one relationship through the Hook's law. Thus, the breaking strains directly measured here would well characterize the breaking criteria. Meanwhile, it was difficult with the current experimental setup to directly measure the breaking stress.
We further evaluated the TS of the surface layer based on the breaking strains ε obtained above and the Young's modulus E obtained previously in a separate AFM but using the oxidized PDMS prepared with the same protocol to the present one [4]. A linear regression curve of the positive relationship between E (MPa) and plasma treatment time t (s) was determined as E = 0.86 + 0.0013t (correlation coefficient R, 0.98) from the published data based on the least-squares method (figure 4(A)). Thus, values of E at arbitrary t including the time we investigated for the present study, i.e. 60, 75, 90, 105, and 120 s, can be determined, yielding the TS at the same plasma treatment times by multiplying ε and E (figure 4(A)). The results show that the TS at the oxide layer is 88 kPa at t = 60 s and sharply decreases with further plasma treatment to 24 kPa at t = 120 s. Thus, the oxide layer is considerably brittle compared to unmodified PDMS in terms of TS by approximately two orders of magnitude, as the latter has a TS of 3–12 MPa according to the manufacturer's specification and literature [1, 3].
Figure 4. The TS of the surface layer sharply decreases with oxidation time because of a significant reduction in the breaking strain regardless of a relatively moderate increase in the Young's modulus. (A) Mean Young's modulus and TS versus plasma treatment time. (B) Normalized changes in the Young's modulus, TS, and breaking strain over time relative to those obtained at 60 s.
Download figure:
Standard image High-resolution imageTo compare relative changes among the three parameters, i.e. TS, E, and ε, those values were normalized by the values at 60 s (figure 4(B)). The results show that, although E was elevated relatively moderately with oxygen plasma treatment (~108% at t = 120 compared to t = 60), the degree of a decrease in ε was larger (~25.2%), thereby playing a predominant role in causing a similar decrease in TS (~27.3%). Thus, the TS of PDMS sharply decreases with plasma treatment time because of the marked reduction in ε, which is more profound in magnitude than an opposite increase in E.
3.3. The oxide layer thickens with plasma treatment time but reaches a plateau
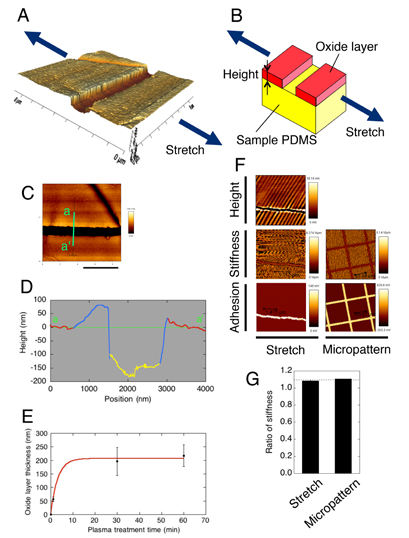
To investigate mechanisms underlying the change in the TS as a function of plasma treatment time, we attempted to determine the accompanied changes in the thickness of the oxide layer. We thought the thickness is measurable by AFM because the sample PDMS would be ruptured upon stretch only within the oxide surface layer but not in the underlying base PDMS that still keeps high extensibility. We thus performed AFM regarding the PDMS samples that were initially treated with oxygen plasma for either 1, 30, or 60 min and then kept stretched with scotch tape to expose resulting cleavages to the AFM probe cantilever. The profile of the 3D geometry showed sharply discontinuous points at which regions are likely to change from the original surface to a newly emerged bottom surface via a transitional region or vice versa (figures 5(A)–(D)). Thus, the height of cleavages was determined as the distance between the original and emerged bottom surfaces and was plotted as a function of the plasma treatment time (figure 5(E)). The height increased significantly between 1 and 30 min, but there was no significant difference between 30 and 60 min, suggesting that the increase finally reaches a plateau.
Figure 5. Atomic force microscopy reveals a non-linear increase in the thickness of the oxide layer with plasma treatment. (A) 3D geometry of a cleavage, created on a surface of plasma-treated PDMS, running in a direction perpendicular to stretch. (B) Model of the stretch-induced formation of a cleavage, in which the oxide surficial layer (show in red) is brittle while the deeper, unaltered PDMS layer (yellow) remains highly extensible so that tensile loading results in formation of a cleavage only within the upper layer. In this model, the height of the cleavage is equal to the thickness of the oxide layer. (C) Height map of the image shown in (A). Scale bar, 4 µm. (D) Profile along the green line a–a' shown in (C). Red, blue, and yellow regions represent planar, transitional, and bottom portions, respectively. (E) Analyzed oxide layer thickness as a function of plasma treatment time. Data are expressed as mean ± standard deviation, with a least-squares regression curve that finally reaches a plateau (thickness = −207e−0.320t + 207; correlation coefficient R, 0.99), shown in red. (F) Validation of the model shown in (B) using stretched and micropatterned samples (oxidation time, 1 min). For stretched sample, the bottom of a cleavage was probed by AFM to obtain spatial distributions of the height (Height) and those of stiffness (Stiffness) and adhesion strength (Adhesion) of force curves. For comparison regarding material properties, a micropatterned area was probed as well (Micropattern). (G) Comparison of stiffness regarding stretched (ratio of original planar region to bottom region) and micropatterned (ratio of plasma-treated region to non-treated region) samples. Data are expressed as mean ± standard deviation. The dashed line represents an estimate for the stiffness ratio between plasma-treated and non-treated regions, made based on the Young's modulus curve of figure 4(A).
Download figure:
Standard image High-resolution imageTo validate that the height of the cleavages corresponds to the thickness of the oxide layer (figure 5(B)), micropatterning experiments were performed (figure 5(F)). Here, using a plasma lithography-based micropatterning technique [14], plasma-treated (2.4 W, 20 Pa, 1 min) and non-treated regions were spatially separated on identical PDMS samples and were probed by AFM to obtain force curves and adhesive forces (figure 5(F), Micropattern). Data were also obtained regarding other oxidized PDMS samples (2.4 W, 20 Pa, 1 min) containing stretch-caused cracks (figure 5(F), Stretch). Extensive stretch sometimes caused wrinkling of the oxide layer in an almost parallel direction to the stretch due to Poisson's effect-driven compressive loading [4, 17], but here we focused only on the depth of cleavages appeared. The comparison of the data shows similar material characteristics between such cleavage-containing samples and micropatterned samples. Specifically, the slope of the force curve (i.e. stiffness), which is now difficult to be converted to the absolute Young's modulus because of the current boundary conditions incompatible with the Hertz model but should at least correlate with the local stiffness, is larger at original planar regions than at emerged bottom regions within cleavages. For micropatterned samples, plasma-treated regions were larger in the stiffness than non-treated regions. Because cracks are newly emanated upon stretch applied after plasma treatment, the emerged bottom regions would be less affected by the plasma. Thus, regions that are closer to native conditions of bulk PDMS were consistently softer in stiffness. Relative comparison of spatially averaged data showed that the original planar regions in the stretched samples were larger in stiffness than the bottom regions by 1.084-fold (figure 5(G)); in addition, the plasma-treated regions in the micropatterned samples were larger than the non-treated regions by 1.107-fold. These relative increases are in quantitatively good agreement with an estimate, made for the increase in the Young's modulus based on the regression in figure 4(A), of 1.091-fold (=E(t = 60 s)/E(t = 0 s) = 0.938/0.86) (figure 5(G), dashed line). Likewise, qualitatively similar tendency was observed regarding the adhesive force; larger adhesive forces were detected at the bottom of cleavages and non-treated regions in micropattened samples. Thus, the results of the micropatterning experiments support the working model of figure 5(B).
3.4. Model of time evolution of PDMS properties under plasma treatment
TS in general is a material property intrinsic to the material itself but independent of the size/geometry of the samples investigated; therefore, the above result demonstrating the time-dependent decrease in the TS indicates that gradual compositional changes occur at the PDMS surface through the exposure to oxygen plasma; in other words, the material itself changes. Here we also found that the thickness of the oxide layer, responsible for the TS, is increased through plasma treatment in a limited manner with a finite plateau value. On the other hand, earlier studies often made an unverified assumption that is inconsistent with our experimental result [18, 19]. Specifically, the Young's modulus—which is another inherent material property and is indeed increased, by a first plasma treatment, at the surface layer compared to that of native silicone elastomers—was assumed to be unchanged thereafter even with further exposure to plasma; consequently, the thickness of the oxide layer must also be assumed to increase unlimitedly to be compatible with the former assumption of the unchanged inherent material properties after a first plasma treatment [18, 19]. Our results, however, draw a conclusion that is different from these previous assumptions: even prolonged exposure to plasma of a constant power eventually results in formation of an oxide layer with a finite thickness. We thus submit an alternative model that the inherent mechanical properties gradually change under exposure to oxygen plasma because of graded substitution of PDMS into oxygen atom-containing molecular structures [8–10], but such changes take place within limited surficial layers as long as the power of input plasma is unchanged (figure 6). This view is consistent with our previous results that peeling off the oxide layer results in exposure of deep layers of the bulk PDMS, which exhibits chemically similar properties to those of unmodified PDMS [10].
Figure 6. Model of surface modification of PDMS by oxygen plasma treatment. Transition occurs gradually within a limited surface region from native (shown in yellow) to oxidized (red) PDMS.
Download figure:
Standard image High-resolution image4. Conclusion
We quantitatively characterized stretch-induced breaking of the oxide layer formed on the surface of PDMS upon oxygen plasma treatment. In contrast to the high extensibility of unmodified PDMS, here we demonstrated that a minute strain of less than 10% as well as a TS smaller by two orders of magnitude than that of bulk PDMS are enough for inducing cracks at the oxide layer. The TS decreases with oxidation down even to our measurement limitation, while the thickness of the resulting oxide layer increases to finally reach a plateau even with prolonged plasma treatment. Consequently, we suggest that gradual surface modification of PDMS occurs upon oxygen plasma treatment only within a finite region, as distinct from the manner assumed in previous studies. These quantitative and qualitative findings will be useful as basic design information regarding how much mechanical stresses and strains are permitted before rupture within the oxide layer of plasma-hydrophilized PDMS.
Acknowledgments
This study was partly supported by JSPS KAKENHI Grant Numbers 15H03004, 26560208, and 25242039.