Abstract
We report the tuning of the internal Mn photoluminescence (PL) transition of magnetically-ordered Sr-doped lanthanum manganite (LSMO)/Mn-doped zinc sulfide (ZnS:Mn) nanocomposites (NCs) by applying a static magnetic field in the range of 0–1 T below the critical temperature of ∼225 K. To do that, we have systematically fabricated LSMO/ZnS:Mn at different concentrations (1:1, 1:3, 1:5 and 1:10 wt%) via a straightforward solid-state reaction. X-ray diffraction and Raman analyses reveal that both phases coexist with a high degree of crystallinity and purity. Electron microscopy indicates that the NCs are almost spherical with an average crystal size of ∼6 nm, and that their surfaces are clean and smooth. The bifunctional character of LSMO/ZnS:Mn was evidenced by vibrating sample magnetometry and PL spectroscopy analyses, which show a marked ferromagnetic behavior and a broad, intense Mn orange emission band at room temperature. Moreover, the LSMO/ZnS:Mn at 1:3 wt% exhibits magneto-luminescent (ML) coupling below 225 K, and reaches the largest suppression of Mn-band PL intensity (up to ∼10%) at 150 K, when a magnetic field of 1.0 T is applied. The ML effect persists at magnetic fields as low as 0.2 T at 8 K, which can be explained by evoking a magnetic-ordering-induced spin-dependent restriction of the energy transfer to Mn states. No ML effect was observed in bare ZnS:Mn nanoparticles under the same experimental parameters. Our findings suggest that this NC can be considered as a new ML compound, similar to FeCo/InGaN-GaN and LSMO/ZnO NCs, useful as q-bits for quantum computation. The results presented here bring forth new avenues to better understand the interaction between semiconductors and perovskites, and exploit their synergistic effects in magneto-optics, spintronics and nanoelectronics.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
Magnetic-ion-doped semiconductor nanocrystals have attracted a great deal of interest because they enable direct observation of unusual phenomena, such as giant Zeeman splitting, magnetic-field induced metal–insulator transition, large Faraday rotation, metal–transition exciton complexes and zero-field magnetization [1]. When magnetically-active atoms at low concentrations are introduced into the cation sites, no extra charge carriers are injected into the host semiconductors (specifically those of the group II–VI), and this allows a number of diverse transport processes, including the multiplicity of decay times, carriers' relaxation and energy and spin transfer, to be studied [2]. Dilute magnetic semiconductor nanoparticles (NPs) that present quantum confinement not only induce size-dependent optical properties, but also show intriguing magnetic ordering below their Curie temperature (TC) [3]. For instance, 2 nm and 4 nm zinc sulfide (ZnS) NPs with Mn-doping levels of 2.5% and 5%, respectively, exhibit ferromagnetic ordering and giant Zeeman splitting below 30 K [4, 5]. This type of interaction between high-spin Mn2+ ions and quantum-confined carriers in Mn-doped ZnS (ZnS:Mn) NPs has been explained in terms of the hybridization of the impurity d-level in the conduction and valence bands of the host lattice [5]. The origin of observed ferromagnetism was attributed to both the movement of d-level within the host lattice and the degree of disorder due to its low dimensionality (excitons confined in the three spatial dimensions) [4]. It was also observed that such magnetic ordering can tune the optical properties of ZnS:Mn NPs. Specifically, the ferromagnetic-ordering-induced spin-sensitive energy transfer to Mn-states in ZnS:Mn NPs below 30 K can produce suppression of the orange emission band photoluminescence (PL) intensity [6], but this magnetic-field-dependent PL (magneto-luminescent (ML) coupling) was only observed at high magnetic fields (20–50 T). It was also reported that the internal Mn-PL transition in ferromagnetically ordered ZnS:Mn NPs is stable at low magnetic fields (down to 1 T), and that no ML coupling is detectable in its paramagnetic phase [5]. Thus, novel ML nanostructures that make this phenomenon observable at low intensity magnetic fields, and materials with ferromagnetic-paramagnetic transition temperatures near room temperature (RT), are needed.
Lanthanum strontium manganite, La0.67Sr0.33MnO3 (LSMO), exhibits perovskite-type colossal magnetoresistance and ferromagnetic metal–paramagnetic insulator transition at ∼355 K [7]. LSMO possesses excellent physical properties, including high spin polarization, half-metallic-like conductivity, good thermal stability, and spin charge and orbital ordering, which are suitable for applications in nanospintronics and nanoelectronics [8]. Mixed with ferroelectric materials, LSMO forms a new generation of multiferroic hybrids that present a prominent magnetoelectric (ME) effect, wherein the ferromagnetic state can be switched on by applying an external electric field and vice versa [9]. This represents a significant breakthrough given that the ME coupling measured in single-phase materials is usually weak [10]. Nevertheless, there is a lack of ME nanocomposite (NC) materials that exhibit multifunctional behavior at RT and that are cost-effective. The fabrication process in the past was limited to the primary use of two or more perovskite ceramics, or those integrated with magnetic oxides [11, 12]. There is a lack of reports on NCs that consist of LSMO and semiconductors (except the LSMO/ZnO system) that show a marked ME effect, i.e., their ME voltage coefficient can be directly measured [13–15]. It is believed that the ME effect in nanostructured composites originates from the magnetic–mechanical–electrical interaction between the magnetostrictive and ferroelectric phases mediated by the strain–stress coupling existing at their interface [16]. Thus, an alternative pathway to indirectly measure the ME effect in LSMO/semiconductor NCs would be to change their optical properties by applying an external magnetic field [17]. Specifically, the deformation of the ferromagnetic grains (due to magnetostriction) in the presence of an external magnetic field is transmitted to the piezoelectric grains, which induces electric polarization, which in turn modifies the strain and built-in internal electric field in the semiconductor, and affects the optical response [17]. As a proof of concept, Chen and co-workers showed the feasibility of optically detecting the ME effect at RT in FeCo-coated InGaN/GaN multiple quantum wells subject to external magnetic fields down to 50 mT [17, 18]. The ME coupling was explained in terms of a piezoelectric-induced electric polarization-mediated effect, which results in the screening of the built-in internal electrical field, strain reduction and the subsequent tilting of the band structure of the FeCo-coated InGaN/GaN [17]. Nevertheless, further investigation is required to better understand the ML coupling that correlates the relaxation processes and energy-transfer mechanisms in the ferromagnetic phase. To date, there are no reports on the mixing of nanostructured LSMO in its fully spin-polarized state with luminescent semiconducting sulfide-based matrixes, and the study of their corresponding ML coupling is still a challenge.
We report here the synthesis of LSMO/ZnS:Mn NCs via a straightforward solid-state reaction, the effect of ferromagnetic LSMO on the structural and optical properties of host ZnS:Mn NPs, and the study of ML coupling present in the NCs by monitoring the suppression of the PL emission band intensity when a static magnetic field in the range of 0–1 T is applied.
Experimental techniques
Synthesis of LSMO nanoparticles
LSMO was fabricated via a solid-state reaction route as reported elsewhere [8], using high-purity La2O3, SrO and MnO2 (La:Sr:Mn = 0.67:0.33:1.0) as starting raw chemicals, which were purchased from Sigma Aldrich, USA. Precursors were stoichiometrically mixed in methanol and high-energy ball-milled for 10 h. The soft mixture was then dried and ground many times in order to break up some big agglomerates. The resulting powder was calcined at 670 K for 3 h to remove organic by-products, and then re-calcined at 1470 K for 8 h. The calcined products were ball-milled for 1 h and crushed to get finer powder. The powder was then uniaxially pressed at 3.7 × 109 N m−2 in order to make pellets 25 mm in diameter and 5 mm in thickness. The pellets were sintered at 670 K for 3 h, and then at 1570 K for 4 h in a covered crucible with alumina powder.
Synthesis of ZnS:Mn nanoparticles
ZnS:Mn nanoparticles were fabricated via a one-pot inorganic chemical route as reported elsewhere [5]. Briefly, 3.227 g of ZnSO4 · H2O (≥99.0%, Sigma Aldrich, USA) and 0.162 g of MnSO4 · H2O (≥99.0%, Sigma Aldrich, USA) were dissolved into 90 ml of high-purity deionized water (HPD), resulting in a 5 wt% Mn doping. An aqueous solution of high-purity Na2S (1.404 g, 90 ml, Sigma Aldrich, USA) was separately prepared and gradually dripped into the first aqueous solution, and then vigorous magnetic stirring was maintained at RT with a controlled reflux system. The reagent mixture rapidly produced a deep orange solution when exposed to UV light, indicative of the compound formation. The flocculate was then removed from the supernatant by ultracentrifugation, copiously rinsed with HPD, and dried at 330 K for 24 h in order to eliminate any remaining by-product. It resulted in fine powders consisting of quasi-spherical ZnS:Mn nanoparticles (75% thereof being of ∼4 nm [5]) that exhibit quantum effects. Cylindrical pellets of the as-synthesized powder were made to conduct PL and ML measurements at low temperatures. The pellets were made using a hydraulic press under an isostatic uniaxial pressure of 3.7 × 109 N m−2. The 25 mm diameter pellets were then heated up at 370 K to remove any surface contamination.
Synthesis of LSMO/ZnS:Mn NCs
LSMO/ZnS:Mn NCs were fabricated via a solid-state reaction route. The LSMO and ZnS:Mn powders, the solid starting materials, were carefully mixed at different weight percentages, LSMO:ZnS:Mn = 1:10, 1:5, 1:3 and 1:1, Keeping a net weight of 500 mg and without using any binder. Individual powders were loaded onto alumina crucible boats, which were then placed into a fused silica tube in a hot-wall horizontal furnace [19]. In separate experiments, the powders were positioned in the middle of the furnace (hot zone), where the temperature was 670 ± 10 K (much lower than the melting points of LSMO and ZnS:Mn and does not induce structural changes). See [19] for a full temperature profile and a description of the apparatus. The reaction tube was first evacuated for several hours down to 465 mPa to purge oxygen, heated to 670 K, and filled to 40–250 Pa with a constant Ar flow at 35 sccm for 12–48 h. Afterward, the tube was left to cool spontaneously to RT. The final products were crushed to get finer powder and then were used to make 25 mm diameter pellets, which were further characterized and analyzed.
Structural, optical and magnetic characterization of LSMO/ZnS:Mn NCs
The phase and structure of the products were studied using an x-ray diffractometer (XRD), Model Siemens D500 with Cu Kα radiation. Raman spectra were collected using a Jobin-Yvon T64000 spectrometer with Ar-ion laser excitation (514.5 nm) and equipped with an optical microscope with 80X resolution. The surface morphology, elemental composition and topography of the products were analyzed using a JEOL JEM-2200FS Cs-corrected high-resolution TEM (HRTEM). All the images were taken in scanning TEM (STEM) mode. To collect temperature-dependent PL spectra, the products were first mounted on the cold-finger of a closed-cycle helium-cryostat operating from 10 to 320 K under high vacuum condition (>0.1 mPa). The products were then excited with a 325 nm He–Cd laser (Model IK 3202R-D) with a maximum power of 3.2 mW mm−2. The PL signal was dispersed by a 0.3 m Acton spectrometer with changeable holographic gratings and analyzed by a Princeton Instrument PI-MAX CCD camera equipped with UV intensifier, operating in the spectral region of 200–950 nm. The magnetic properties of the products were studied using a vibrating sample magnetometer (VSM, Lakeshore 7400).
ML measurements
To record the ML spectra, the products were placed on the cold stage of a closed cycled cryostat (Model 202-E) coupled to an electromagnet (Lake Shore, Model EM4-HVA). The magnetic field strength was monitored with a Gaussmeter probe (Lake Shore, Model 475-DSP). The long-term magnetic field stability was 0.001 T, and no magnetic field strength drift was observed during data collection. The temperature in ML measurements was monitored using a temperature controller (Lake Shore 340) with a stability of ±0.5 K. The products were excited with a 325 nm He–Cd laser (Model IK 3202R-D) with a power of 2.4 mW mm−2. The ML signal was detected by using a fiber optic spectrometer (Ocean Optics, Model USB2000). Different optical filters were used along the optical detection path to selectively record luminescence of interest from studied specimens.
Results and discussion
The XRD patterns of the products at 1:1, 1:3, 1:5 and 1:10 wt% are depicted in figure 1(a). The XRD patterns of ZnS:Mn and LSMO are also included for comparison. The diffraction peaks observed were indexed to the diffraction planes of cubic sphalerite ZnS phase [(111), (220), (311), (400) and (331), JCPDS card, no. 77–2100], and of orthor-hombic perovskite LSMO phase [(200), (220), (202), (321), (400) and (323)] consistent with the standard values [5, 7]. The observed well-defined peaks, the absence of secondary phases (including hexagonal ZnO and MnS) in the XRD patterns, and the co-existence of both phases are clear indicators of the successful formation of the composite material with high crystalline quality and purity. The broadening of the diffraction peaks is ascribed to the nanocrystalline nature of the composites. Their calculated average crystal size was ascertained by means of Scherrer's formula, yielding 4–7 nm. It was also observed that the diffraction peaks of LSMO/ZnS:Mn become gradually broader and less intense as the amount of LSMO increases from 1:10 to 1:1 wt%, as expected. Moreover, they were relatively shifted (and consequently overlapped) when compared to bare LSMO and ZnS:Mn. This is probably due to the internal strain between both phases developed during the thermal treatment, and to the lattice mismatch [7].
Figure 1. (a) XRD patterns of LSMO, ZnS:Mn and LSMO/ZnS:Mn at 1:1, 1:3, 1:5 and 1:10 wt%. (b) Micro-Raman spectra of LSMO, ZnS:Mn and LSMO/ZnS:Mn at 1:3 wt%. Solid lines in (b) correspond to the DHOPM fit.
Download figure:
Standard image High-resolution imageThe microstructure quality of LSMO/ZnS:Mn at 1:3 wt% was studied by Raman spectroscopy. Figure 1(b) shows the Raman spectrum of LSMO/ZnS:Mn NCs. The Raman spectra of ZnS:Mn and LSMO are also included for comparison. A broad Raman band peaking at 345 cm−1 and a well-pronounced shoulder at lower frequencies can be observed. To discriminate between the contribution of LSMO and ZnS:Mn, the deconvolution of the band at 345 cm−1 was carried out. Two components were identified, one minor band at 332 cm−1 and one major band at 345 cm−1. When compared to the characteristic transverse and longitudinal optical modes of cubic ZnS (centered at 261 and 342 cm−1), it was found that the minor band is red-shifted by ∼10 cm−1, which indicates that the LSMO/ZnS:Mn is under tensile stress as a result of the synthesis process [20]. The Raman modes observed at 298 and 345 cm−1 and attributed to LSMO (with a slight blue-shift as compared to the matrix of LaMnO3 due to the increased Sr content) do not experience any shift upon NC formation. Similar observations were obtained for the NCs at 1:1, 1:5 and 1:10 wt%. Taken altogether, both XRD and Raman analyses furnish evidence of the formation of pure, highly-crystalline LSMO/ZnS:Mn.
The morphology, size, composition and crystallinity of LSMO/ZnS:Mn were studied by electron microscopy. HRTEM images and crystal size distribution of LSMO/ZnS:Mn at 1:3 wt% are depicted in figure 2. It was observed that the NCs form compact thin clusters of 200–350 nm in diameter with minor void volume (see figure 2(a)). From the gray scale contrasts, they appear to be composed of many near-spherical NPs. A closer look at the clusters reveals the presence of two different constituent NPs with high definition (see figure 2(b)). The lattice-resolved images depicted in figures 2(b) and (d) show that the surface of individual NPs is clean and smooth, and that certain ends are atomically rough. Hence, this method provides an efficient route to develop particulate NCs without any sheathed amorphous phase, having low degree of interdiffusion between the constituents and the surrounding medium. Interplanar spacings of 0.27 and 0.31 nm corresponding to the (200) and (111) planes of LSMO and ZnS:Mn, respectively, were identified. The crystal size distribution obtained by statistical image analysis indicates that the crystal diameters are in the 4–12 nm range, with an average size of ∼6 nm, consistent with that estimated by the XRD analysis (see figure 2(c)). The illumination of the samples with 200 KV electrons did not cause any increase of planar induced defects, damage, phase transformation or amorphous carbon deposition.
Figure 2. (a), (b), (d) HRTEM images of LSMO/ZnS:Mn nanocomposites at 1:3 wt% taken at different magnifications. (c) Particle size distribution of LSMO/ZnS:Mn nanocomposites at 1:3 wt%.
Download figure:
Standard image High-resolution imageIn order to determine the polycrystalline nature and elemental composition of the NCs, we have conducted selected area electron diffraction (SAED, operated in TEM mode) and electron energy loss spectroscopy (EELS, operated in STEM mode) measurements. The SAED rings, EELS spectra and the corresponding scanned regions of thin clusters of LSMO/ZnS:Mn at 1:3 wt% are depicted in figure 3. The SAED rings (carried out on the region shown in figure 3(a)) were indexed to the cubic sphalerite phase of polycrystalline ZnS [(200) and (202)] and to the orthorhombic perovskite phase of polycrystalline LSMO [(220) and (331)] in agreement with bulk XRD results. The line-scanned EELS spectrum (carried out on the region shown in figure 3(c)) shows a low-loss band peaking at ∼165 eV and attributed to the S-L2,3 edge transition (see figure 3(e)) [21]. To discriminate among the contributions of La, Sr, Mn and O, we carried out the deconvolution of the high-loss bands. Two featured regions were identified, one in the range of 500−590 eV containing three bands: a major band centered at ∼531 eV ascribed to the O-2p transition, and two minor bands ascribed to the influence of higher energy states, such as La-5d and Mn-4sp. This feature is characteristic of LSMO, i.e., a pronounced overlapping of the bands O-2p and Mn-3d has been reported [22]. The second region (590–700 eV) consists of a major band centered at ∼641 eV associated to the excitation of electrons from 2p to 3d states (Mn-2p edge). This band undergoes a slight downshift of ∼1 eV (compared to its theoretical value), which is ascribed to the Sr enrichment in La1−xSrxMnO3 systems as a consequence of changes in the electrostatic energy at the Mn site [22]. The origin of the minor band centered at ∼607 eV may be attributed to the interaction between Mn2+ ions in host ZnS and LaMnO3 matrixes. In general, the broadening of these bands is an intrinsic effect in this type of manganite. The EELS probe used in this study was not adequate enough to resolve heavy Zn atoms. Similar observations were obtained for the NCs at 1:1, 1:5 and 1:10 wt%.
Figure 3. (a) Bright-field HRTEM image and its corresponding (b) SAED pattern of LSMO/ZnS:Mn nanocomposites 1:3 wt%. (c) Dark-field HRTEM image and its corresponding (d), (e) line-scanned EELS spectra of LSMO/ZnS:Mn nanocomposite.
Download figure:
Standard image High-resolution imageTo further evaluate the mixing of LSMO NPs with the host semiconducting ZnS:Mn, we conducted vibrating sample magnetometry. The magnetic hysteresis loops of bare LSMO NPs and LSMO/ZnS:Mn NCs at 1:1, 1:3, 1:5 and 1:10 wt% collected at RT are depicted in figure 4. The well-defined M–H curves show that the NCs exhibit ferromagnetism (inherited from LSMO) with saturation magnetizations (MS) of 24.8, 14.4, 8.6 and 4.7 emu g−1 for the samples 1:1, 1:3, 1:5 and 1:10 wt%, respectively. The drop of MS observed (above 30% compared to LSMO, MS = 81.1 emu g−1) is due mainly to the charge transfer change caused by the interaction of ZnS:Mn and LSMO [7, 23, 24]. The fact that MS is more pronounced as the LSMO content increases is a clear indicator that the dielectric ordering coupled to the ferromagnetic phase acts as voids or pores in the presence of an external magnetic field, which disrupts the magnetic circuits with the increased dielectric content in the NC, thus resulting in a significant drop of MS [7, 25]. The values of the magnetic coercivities are small for all the NCs, which indicates the presence of certain short-range ordered regions or magnetic monodomains, which is typical in LSMO [7]. Despite the relative decrease of magnetization, the NCs were attracted in solid form at the container's sidewall when subjected to the influence of a permanent magnet of 6 kOe strength, with a MS of 24.3 emu g−1 for LSMO/ZnS:Mn (1:3 wt%) and 79.9 emu g−1 for LSMO (see insets of figure 4).
Figure 4. M–H hysteresis curves of LSMO and LSMO/ZnS:Mn nanocomposites at 1:1, 1:3, 1:5 and 1:10 wt%. Insets show the optical images of the LSMO/ZnS:Mn at 1:3 wt% (upper) and LSMO (lower) when exposed to a permanent magnet (magnetic field strength of ∼6 kOe).
Download figure:
Standard image High-resolution imageIn order to shed light upon the effect of the LSMO on the optical properties of ZnS:Mn, we carried out PL spectroscopy. The RT PL spectra of LSMO/ZnS:Mn NCs at 1:1, 1:3, 1:5 and 1:10 wt% are depicted in figure 5(a). The PL spectrum of bare ZnS:Mn is also included for comparison. It was observed that all the NCs exhibit a broad orange emission band centered at ∼598 nm, which is ascribed to the internal Mn2+ ion transition between the 4T1 first excited state (spin 3/2) and the 6A1 ground state (spin 5/2) [5]. This band, characteristic of ZnS:Mn NPs, is responsible to provide a pathway for the energy transfer from s-p electron–hole pair band states (host ZnS) to the Mn2+ ion d-electron states. A marked suppression of the Mn PL intensity was seen as the content of LSMO increases, and was attributed to the interaction between the self-trapped excitons (located on MnO6 octahedra) and the surface defect (vacancy) states of LSMO with the d-electron states of ZnS:Mn [26]. A moderate suppression (∼20%) along with a weak red-shift (∼10 nm) of the Mn PL intensity of LSMO/ZnS:Mn at 1:3 wt% was also found when the temperature increased from 8 to 300 K (see figure 5(b)). This decrease in PL intensity can be described in terms of the carrier transfer process from donor states to Mn3+ ions in LSMO. Given that the conduction electrons can freely move in the NCs, some of them can be captured by the Mn3+ ions and then increase the 4T1-6A1 transition probability as the temperature decreases. It is worth noting that the orange emission band of bare ZnS:Mn typically experiences a PL intensity increase when the temperature is increased from 8 to 300 K [5], which contrasts with that observed for LSMO/ZnS:Mn. This effect can be attributed to the carrier transfer from donor states to Mn2+ ions in host ZnS. The upshift observed as the temperature decreases correlates well with the temperature-dependent band-gap shrinkage previously reported for ZnS:Mn systems [5, 27]. The suppression in PL intensity may also result from the electrons trapped in the donor states that are thermally activated towards the conduction band [5]. Figure 5(b) also shows that the interaction of LSMO with ZnS:Mn induces the partial depletion of the surface states of ZnS:Mn when the temperature decreases from 300 to 8 K, thus enabling the visualization of the blue emission band of ZnS:Mn (ascribed to the donor–acceptor pair transitions) centered at 452 nm. Similar observations were obtained for the NCs at 1:1, 1:5 and 1:10 wt%. Despite the substantial decrease in PL intensity of the orange band in LSMO/ZnS:Mn when compared to bare ZnS:Mn, the luminescent signal coming out from the NC when excited with UV light remains strong even at 1:1 wt% (see insets of figure 5), and its suppression is most likely related to thermal effects.
Figure 5. (a) PL spectra of ZnS:Mn and LSMO/ZnS:Mn nanocomposites at 1:1, 1:3, 1:5 and 1:10 wt% and at 300 K. (b) PL spectra of LSMO/ZnS:Mn at 1:3 wt% at 8 and 300 K. Insets show the optical images of the ZnS:Mn (left) and LSMO/ZnS:Mn (right) when exposed to 325 nm light.
Download figure:
Standard image High-resolution imageIn the previous sections, we demonstrated the bifunctional (magnetic and luminescent) character of high-quality LSMO/ZnS:Mn NCs. In this section, we evaluate the possibility of tuning their optical properties by applying a static magnetic field (in contrast with the thermal effects) above and below their ferromagnetic transition temperature. In doing so, we performed ML measurements by orienting the magnetic field parallel to the pellet surface. Thorough precautions were taken to eliminate ambient temperature fluctuations (±1 K), laser beam intensity drift (±0.001 mW) and magnetic field instability (±10 Oe) over extended periods of time (>60 min) during the collection of ML spectra. All the NCs were tested, but only the 1:3 wt% NCs showed a detectable ML effect. The temperature-dependent PL spectra in the absence (zero field) and in the presence of an applied magnetic field of 1.0 T (∼10 kOe) are depicted in figure 6. As for the orange emission band, no ML coupling was observed in the range of 310–225 K, since LSMO is in its ferromagnetic state (TC ∼345–355 K) and ZnS:Mn is in its paramagnetic phase (TC ∼30 K). A prominent and reproducible ML coupling was observed below 225 K. The calculated percentage of the drop of PL intensity at 200 K was ∼3%, and reaches a peak value of ∼10% at 150 K, indicating a strong correlation between the ferromagnetic ordering of LSMO and the internal Mn–PL transition. An almost constant value of 4% was observed between 100 and 35 K. A slightly increased value of ∼5% was obtained when ZnS:Mn underwent a phase change (ferromagnetic ordering below 30 K) until 8 K. It is noteworthy that such ML coupling was not observed in ferromagnetically-ordered ZnS:Mn, i.e., in the absence of LSMO at low temperatures and under the influence of an applied magnetic field of 1.0 T [5]. The significance of our findings lies in the fact that the ML coupling in ZnS:Mn NPs was previously reported at much larger magnetic fields (50 T) and below 30 K [6], while we have found a more intense ML effect at low magnetic fields (below 1 T) near RT by mixing LSMO with host ZnS:Mn.
Figure 6. (a)–(c) PL spectra of LSMO/ZnS:Mn nanocomposites at 1:3 wt% and at 300, 200 and 8 K in the absence and presence of an external magnetic field of 1.0 T. (d) Maximum PL intensity (band at 598 nm) as a function of the temperature of LMSO/ZnS:Mn at 1:3 wt% in the absence and presence of an external magnetic field of 1.0 T. Inset shows the optical image of the LSMO/ZnS:Mn at 1:3 wt% when exposed to both 325 nm light and a permanent magnet (magnetic field strength of ∼6 kOe).
Download figure:
Standard image High-resolution imageThe observed ML coupling can be explained in terms of the spin-selective energy transfer from the photo-excited excitonic states to the Mn3+ and Mn2+ ion d-electron states induced by the ferromagnetic ordering in LSMO and ZnS:Mn, respectively. No significant drop in PL intensity was observed for the blue emission band in the entire range of temperatures (310–8 K) when subjected to an applied magnetic field (0.2–1.0 T), as expected. Further observations also indicate that the internal Mn–PL transition is sensitive to the presence of magnetic fields as low as 0.2 T, when both LSMO and ZnS:Mn are magnetically-ordered (see figure 7(a)). The percentage drop of PL intensity at 8 K and subject to 0.0, 0.2, 0.4, 0.6, 0.8 and 1.0 T fell in the range of 1%–4.5%. The linear dependence of the Mn-PL intensity with respect to the magnetic field (shown in inset of figure 7(a)) is substantially different to the modified exponential-decay suppression, I ∼f[H/T]exp[αH/T], observed in Mn2+ internal transitions of magnetic semiconductor quantum dot systems at much larger magnetic fields (up to 50 T), and is presumably a direct consequence of the spin-selective energy transfer from excitons to Mn states that is caused by the magnetic ordering. The intensity of the internal Mn-PL transition (assumed to be proportional to the transition probability for energy transfer when the field polarizes the Mn spins) can be fitted using a model based on the dynamics of excitonic complexes, which can be expressed as a first approximation for low magnetic fields (H < 1.0 T), as follows [6, 28]:
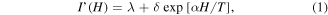
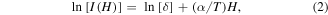
where λ is a constant that represents all the contribution from field independent processes (including direct excitation of Mn2+ ions by the laser and Auger-type transfer via Mn2+ ion pairs), δ is the transition intensity at a certain magnetic field (H), and α = −gMnμB/kB is a constant at a given temperature (T) that denotes the strength of Mn–Mn interaction, with gMn being the Lande g-factor for Mn2+, including direct excitation, μB the Bohr magneton, and kB the Boltzmann constant [6, 28]. By fitting the experimental data (shown in the inset of figure 7(a)) to this model and introducing the value of temperature (8 K), one obtains α = 1.272 ± 0.215 signifying that at low-field measurements the direct Mn–Mn antiferromagnetic interactions are enhanced (when compared to those reported at high-field measurements) [7], probably due to the single Mn ion excitations and to the weak contribution from Mn–Mn clusters [6]. Through this model, it is noted that fields as low as 0.2 T are sufficient to saturate the Mn spins within the ferromagnetic LSMO domains. Thus, the suppression of the Mn–PL transition due to the presence of LSMO can be explained by evoking a magnetic-ordering-induced spin-dependent restriction of the energy transfer to Mn states. It is believed that the magnetic nature of stoichiometric LSMO originates from the ferromagnetic ordering of Mn3+ ions along the Mn–O planes, and that such ordering is initiated by the Mn3+–O–Mn3+ anisotropic superexchange interaction, which is stabilized at RT by the Jahn–Teller effect [29]. A change in temperature favors the suppression of this distortion modifying the structural, magnetic and transport properties of LSMO. It has been also reported that the ground (6A1) and first excited (4T1) levels of the five d-electron states of Mn split into six and four non-degenerate states, respectively, under the influence of an applied magnetic field [6]. To make the Mn-PL transition dipole-allowed, the conservation of Mn2+ spin state (ΔSz = 0) must be met. At RT the interaction between the d-electron states (in their first excited state) and the Mn3+ ions is overpowered by thermal effects. At the critical temperature around 225 K (presumed to be a phase transition temperature), the two effects are essentially balanced. As the temperature is further decreased below 225 K, the distortion in the MnO6 octahedra caused by the Jahn–Teller effect induces a strong interaction resulting in a blocking from the 4T1 level to the ±5/2 states of 6A1 as the field increases (LSMO being fully spin-polarized), thus reducing the PL intensity. This blocking leads to a linear decay behavior of the internal Mn–PL intensity, consistent with the magnetic field dependence shown in the inset of figure 7(a). The schematic of the proposed mechanism is depicted in figure 7(b). This magnetic-ordering-induced spin-dependent model can explain the results presented here. Further studies are needed to better understand the dynamics of excitonic complexes below TC in LSMO/ZnS:Mn NCs, and to confirm that T = 225 K is its phase transition temperature.
Figure 7. (a) PL spectra of LSMO/ZnS:Mn nanocomposites at 1:3 wt% at 8 K when exposed to a variable magnetic field. Inset shows the corresponding maximum PL intensity (band at 598 nm) as a function of the magnetic field (strengths of 0.0–1.0 T). (b) Schematics of the magnetic-ordering-induced spin-dependent restriction mechanism for the internal Mn–PL transition of LSMO/ZnS:Mn.
Download figure:
Standard image High-resolution imageConclusions
We have synthesized 6 nm LSMO/ZnS:Mn NCs with a strong bifunctional character via a straightforward solid-state reaction. The highly crystalline nature, absence of secondary phases, well-defined ferromagnetic behavior and prominent PL emission profile of the hybrid material indicate that they are of high quality and purity. The strong correlation between the ferromagnetic ordering of LSMO and ZnS:Mn and the internal Mn–PL transition observed for LSMO/ZnS:Mn at 1:3 wt% indicates that LSMO is disrupting the spin-selective energy transfer from excitons to Mn states in ZnS:Mn. This research represents a step ahead to improve luminous efficiency in magneto-optical devices by optimizing the magnetic field effect, and advances the effective integration of semiconductor nanostructures and perovskites.
Acknowledgments
This project was partially supported by the Institute for Functional Nanomaterials (NSF Grant 1002410) and PR NASA EPSCoR (NASA Cooperative Agreement NNX15AK43A). The authors thank the valuable assistance of Mr Oscar Resto for providing the HRTEM images, Mr Jabril Vilmaney for helping acquire the VSM curves, and Dr Ram S Katiyar for his research facilities. WMJ acknowledges the support from the NSF CAREER Award No. DMR-1056493.







