Abstract
A laminated structure of Al2O3 and MgO deposited by atomic layer deposition (ALD) is used to realize a thin film encapsulation technology in organic light-emitting diodes (OLEDs). This film was targeted to achieve an excellent barrier performance. As the thickness of MgO layer increased from 0 nm to 20 nm, its physical properties transformed from the amorphous state into a crystalline state. The optimized cyclic ratio of ALD Al2O3 and MgO exhibited much lower water vapor transmission rate (WVTR) of 4.6 × 10−6 gm−2/day evaluated by Calcium (Ca) corrosion at 60 °C&100% RH, owing to the formation of a terrific laminated structure. Top-emitting OLEDs encapsulated with laminated Al2O3/MgO show longer operating lifetime under rigorous environmental conditions. These improvements were attributed to the embedded MgO film that served as a modified layer to establish a laminated structure to obstruct gas permeation, as well as a scavenger to absorb water molecules, thus alleviating the hydrolysis of bulk Al2O3 material.
Export citation and abstract BibTeX RIS
1. Introduction
Organic light-emitting diode devices (OLEDs) have great potential, with features such as vivid full color, perfect video capability, thin and light characteristics [1–3]. A major problem for OLED development in general application is their vulnerability to the permeation of water vapor and oxygen, which can oxidize the low work function alkali metal cathode in the OLEDs [4, 5]. Also OLED materials and polymer substrates in flexible displays should be carried out at lower processing temperature to avoid degrading the electronic performance of OLEDs [6]. The thin film encapsulation (TFE) technique is indispensable for realization of high-performance and flexible OLEDs. Nowadays, atomic layer deposition (ALD)—used to produce gas barriers in food packaging—is considered the most promising method to extend to flexible electronics or OLEDs, because the continuous, pinhole-free nature and excellent uniformity of ALD films leads to high quality gas diffusion barriers [7, 8].
Generally, ALD Al2O3 thin film is proposed for ultralow gas diffusion barrier films application because it has excellent water vapor and oxygen blocking properties. But in the deposition of Al2O3 as gas barrier for thin film encapsulation, the Al2O3 film itself is intended to experience hydrolysis in high temperature and humidity [9]. Hence, a highly dense compound bond is required to postpone this degradation. Despite this, Al2O3 film has been employed as a building block in laminated structures for configuration of highly impermeable gas barriers, such as Al2O3/ZnO, Al2O3/TiO2, and Al2O3/ZrO2 stacked structures [10–12]. But few of these laminated structures solve the hydrolysis problem in bulk Al2O3 film. Magnesium oxide (MgO) is characterized by a wide band gap (7.8 eV), dielectric constant (κ) equal to 9.8, low refractive index of 1.72, and by a prominent chemical stability, making it a potential material in semiconductor and display technologies [13]. In this study, an interlayer of MgO was introduced into the laminated Al2O3/MgO in the field of thin film encapsulation for OLEDs; this embedded MgO film was employed as a scavenger to absorb penetrating water molecules, aiming to relieve degradation by hydrolysis under ambient humidity.
Many reports have shown that the MgO film can be deposited by ALD using a variety of precursors. The earliest attempts at ALD MgO film utilized Mg(C2H5)2 or Mg(Cp)2, both of which reacted with H2O [14, 15]. The growth rate observed for these two kinds of precursors ranged from 1.8 to 3.5 Å/cycle between 600 and 900 °C. Another attempt at MgO film involved -diketonate precursors with O3, H2O, or H2O2 [16–18]. The growth per cycle observed using Mg(thd)2 and H2O was only ∼0.25 Å. But as for the OLED encapsulation, the above precursors with higher deposition temperature are not suitable for practical application. Most recently, the deposition of MgO using ALD sequential exposures of Mg(CpEt)2 (that with a higher volatility) and H2O in the temperature below 90 °C has been studied [19]. At this lower temperature, the growth per cycle was between 0.68 and 1.2 Å. In our study, MgO has been deposited by precursors of Mg(CpMe)2 and H2O at 80 °C, which can significantly lower the cost for production because the price of precursor Mg(CpEt)2 is much more expensive than the Mg(CpMe)2 owing to the material purification process from the methyl to ethyl group.
In this experiment, the laminated Al2O3/MgO film can generally improve the reliability of thin film encapsulation. The multilayered Al2O3/MgO with optimized thickness and cyclic ratio can realize a barrier film with high density, which achieves a superior WVTR as low as 4.6 × 10−6 gm–2/day. Top-emitting OLEDs (TE-OLEDs) encapsulated with Al2O3/MgO laminated structure exhibit excellent storability, without any visible black spot growth under challenging conditions (temperature = 60 °C, humidity = 100%) for 600 h.
2. Experiments
For the experiment, a laminated structure of Al2O3/MgO was grown at 80 °C with BENEQ TFS500 system, using TMA, Mg(CpMe)2, and H2O as precursor gases. In this investigation, we fabricated different kinds of laminated Al2O3/MgO thin film as a comparison to identify the role of the MgO film in the multilayered structure. In this study we looked at two groups of laminated Al2O3/MgO. One had a variable content of MgO in the laminated structure, through the control of the ALD deposition cycle with Al2O3/MgO ratios of 50/2, 50/10, and 50/50. The other group had a fixed ratio of Al2O3 to MgO, applied in cycles, with cycle length ratios of 5/1, 25/5, and 50/10. The pulse time for TMA and H2O was fixed at 0.25 s, while the purge time was 10.0 s. The precursor Mg(CpMe)2 was heated at 70 °C and bubbled with nitrogen to be injected into the reaction chamber. The pulse and purge times for Mg(CpMe)2 were 0.4 s and 10 s. In fabrication of the laminated Al2O3/MgO, the respective Al2O3 or MgO thin film was deposited following the completion of line purge with the previous precursors. The schematic illustration of laminated Al2O3/MgO is shown in figure 1.
Figure 1. Schematic diagram of the laminated Al2O3/MgO barrier film.
Download figure:
Standard image High-resolution imageThe film thickness and reflective index of the deposited films were measured by spectroscopy ellipsometry (SE). The microstructure of the films was analyzed by x-ray diffraction (XRD). The chemical bonding states on the bulk Al2O3 and MgO films were observed using x-ray photoelectron spectroscopy (XPS) (Thermo, ESCALAB 250). The film interfacial property of laminated Al2O3/MgO used as barrier film was investigated by high-resolution transmission electron microscopy (HR-TEM) (JEOL, JEM-2100F). In this study, the structure of the green TE-OLED was Glass/Ag(100 nm)/IZO(7.6 nm)/MeO-TPD:F4-TCNQ(156 nm), 4 wt%)/NPB (20 nm)/VOM1511:GD-5 (40 nm)/Bebq2: (25 nm)/LiF(1 nm)/Mg:Ag (9:2, mass ratio, 15 nm)/ZnSe(24 nm). Herein, the ZnSe acts as a capping layer (CPL) in TE-OLEDs, which is a transparent and non-conducting capping layer for refractive index matching to optimize optical transmission. All the above materials information can be found in our previous work [20].
3. Results and discussion
Figure 2 shows the growth characteristics of the ALD Al2O3 and MgO thin films on Si substrates; film thicknesses are plotted versus number of ALD cycles. It can be observed that there was a good linear relationship between the fitting line slope and film saturated growth rate for the film thickness of Al2O3 or MgO. Additionally, the linear extrapolations of the plots passed though the origin for both processes, indicating that both the Al2O3 and MgO deposition lacked a significant nucleation delay and a postponement of film growth. This provides a good guarantee for the realization of the laminated Al2O3/MgO film with the merits of reliable depositional continuity and precise thickness control. The growth rates of Al2O3 and MgO were determined to be 0.82 and 1.01 Å/cycle from the linear fit of the plots. The refractive index of Al2O3 was 1.645, while that of MgO film was 1.721. Both values are quite similar to those of previous reports [21, 22], which indicates that the film deposited by the selected precursors TMA, Mg(CpMe)2, and H2O has formed a relatively dense structure.
Figure 2. Thickness growth characteristics of Al2O3 and MgO thin films with increasing ALD cycles.
Download figure:
Standard image High-resolution imageIn the issues of thin film encapsulation technology, the penetration, diffusion, or reaction process of water molecular in the internal film structure are important factors in determining the quality of barrier film. Hence, the work is expected to shed light on intriguing studies that suggest how water molecules can have a profound effect on the barrier performance for the single Al2O3 and MgO film. The corrosion test (at 60 °C, 100% RH condition) results, for zero, two and seven days, for single Al2O3 and MgO film with thickness of 100 nm on Si substrate are shown in figure 3. By observing the change of film surface morphology, it can be seen that neither film can effectively prevent the water from causing corrosion with generation of many white dots. The results also confirm that water vapor is easily transmitted through both these bulk materials. In addition, the result for MgO on Si substrate shows more reactive areas than the Al2O3 film, which may be due to the film crystallinity, which will possibly induce more molecular reaction of water with the underlying film.
Figure 3. Microscopic image of Al2O3 and MgO film corroded under harsh environmental conditions.
Download figure:
Standard image High-resolution imageIn order to confirm the phenomenology of the hydrolysis process, the chemical composition and bonding states of Al2O3 and MgO film placed under high temperature and humidity (T = 60 °C, RH = 100%) for seven days were investigated in detail with regard to the O1s, Mg1s and Al2p core level spectra using x-ray photoelectron spectroscopy (XPS). To avoid surface contamination of the samples during the XPS measurements, the films were sputtered off the extreme surface of samples exposed to ambient air.
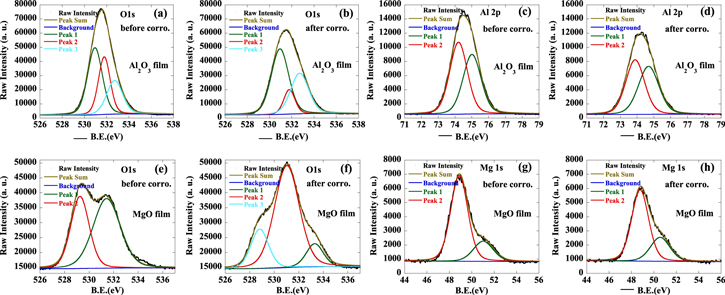
Figures 4(a)–(d) show high-resolution images of O1s and Al2p core level spectra for Al2O3 films before and after corrosion in the specified ambient conditions. Three peaks were used for fitting the O1s spectra as shown in figure 4(a). The main component (O1sA) at the BE of 530.9 ± 0.1 eV is attributed to the oxide ions of the alumina matrix. The O1sB peak observed at 531.8 ± 0.1 eV can be identified as oxygen vacancies or hydroxyl groups bonded with Al atoms. The third peak, O1sC at 532.7 ± 0.1 eV, is associated with the adsorption of water molecules at the surface [23]. As seen from the table 1, the percentage of O1sA, O1sB, and O1sC for the as-fabricated Al2O3 film was 44.76%, 31.01%, and 24.23%, while the value was 53.29%, 12.78%, and 33.94% for the film aging at the condition of high temperature and humidity. The increment of O1sC percentage in internal Al2O3 film indicated that there is a certain proportion of –OH formation when immersed in humid ambient atmosphere, which suggests that the Al2O3 film will experience a hydrolysis process with the assistance of diffusing water molecules. Meanwhile, the proportion of oxide ions in the alumina matrix slightly increased, implying that the as-deposited Al2O3 has a loose network structure that reacts with the absorbed water molecules, thus making more intact Al–O bonds. As shown in figures 4(c), (d), this same phenomenon is reflected in the Al2p core level spectra, which shows that the Al2pB at 74.8 ± 0.2 eV has been scaled up when the water molecular has been diffused, meaning that the partial Al–O bonds have been converted to the AlOOH or Al(OH)3 species. Therefore Al2O3 alone does not ensure the quality of barrier performance once the film experiences extremely hard conditions.
Figure 4. XPS spectra of O1s and Al2p in Al2O3 film (a)–(d), O1s and Mg1s in MgO film (e)–(h), before and after corrosion under high ambient temperature and humidity. Peak informations: O1sA (530.9 ± 0.1 eV), O1sB (531.8 ± 0.1 eV), O1sC (532.7 ± 0.1 eV); Mg1sA (48.8 ± 0.1 eV), Mg1sB (50.8 ± 0.2 eV); Al2pA (74.0 ± 0.2 eV), Al2pB (74.8 ± 0.2 eV).
Download figure:
Standard image High-resolution imageTable 1. Summary of the O1s, Al2p, and Mg1s peak properties for Al2O3 and MgO film.
| Al2O3 film | MgO film | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Al2pA | Al2pB | O1sA | O1sB | O1sC | Mg1sA | Mg1sB | O1sA | O1sB | O1sC |
| Before | 74.21 | 75.01 | 530.94 | 531.78 | 532.71 | 48.88 | 51.02 | 529.26 | 531.43 | |
| Corrde | 54.10% | 45.90% | 44.76% | 31.01% | 24.23% | 78.65% | 21.35% | 41.11% | 58.89% | |
| After | 73.88 | 74.68 | 530.89 | 531.79 | 532.69 | 48.77 | 50.56 | 528.82 | 531.04 | 533.76 |
| Corrode | 51.04% | 48.96% | 53.29% | 12.78% | 33.94% | 72.22% | 27.78% | 19.16% | 70.04% | 10.85% |
The signs of water absorption in MgO film become even more apparent as manifested in the O1s and Mg1s XPS core level spectra from figures 4(e)–(h). The as-deposited MgO film did not show any evidence of the related –OH bond that correlated with water molecule absorption. But as long as the film is immersed in the ever deteriorating situation, a peak at the location of 532.7 ± 0.1 eV (O1sC, water molecules in the surface adsorption) has newly emerged, indicating that more and more water molecules from the thin film surface would diffuse into the bulk material and then down to the MgO network. For the Mg1s XPS spectra, this degradation involves obvious increment of the peak at 50.8 ± 0.2 eV (Mg1sB, assigned to the Mg ions of an hydroxide matrix) once the stoichiometric Mg1sA (at the peak of 48.8 ± 0.1 eV) of Mg–O encounter the invading water molecules: thus they will be destroyed once the film network has acquired adequate capacity of absorbed moisture. As a result of all the XPS measurements, we can get the following facts: (1) the Al2O3 film will inevitably go through the hydrolysis process that weakens the barrier performance; (2) the MgO film possesses a strong water absorption capacity. Further down, we will look at the laminated structure of Al2O3/MgO that could change the properties of barrier performance even more radically.
Water vapor transmission rate (WVTR) measurements were carried out to evaluate the gas permeability of the laminated Al2O3/MgO film by using the traditional calcium (Ca) corrosion method, which involves a Ca sensor at 60 °C and 100% RH. The 200 nm-thick Ca film with length/width (L/W) as 10/20 mm was deposited on the patterned titanium electrodes (100 nm) with shape of two narrow bars. The WVTR value was determined using the following equation [24]:

where d(G)/d(t) is the change of Ca conductance as a function of time, and the n is the molar equivalent of the degradation reaction. In this equation, δCa and ρCa are the resistivity (3.4 × 10−8 Ω m) and density (1.55 g cm−3) of Ca; M(H2O) and M(Ca) are the molar masses of water and Ca. According to the geometry of the experimental setup, the ratio of the area of the Ca sensor to the area of the window for water permeation is consistent.
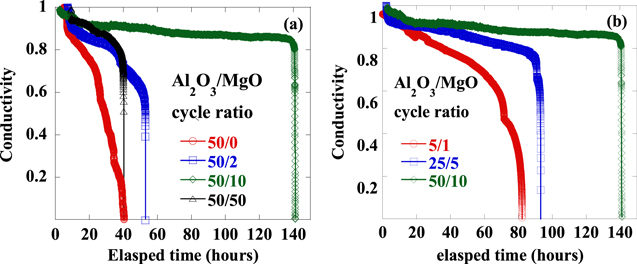
As shown for the Ca test in figure 5(a), the WVTR value for single Al2O3 film is only 8.7 × 10−4 gm−2/day, while it is slightly improved to be 1.6 × 10−4 gm−2/day once MgO is introduced into the laminated Al2O3/MgO film over 50/2 cycles. As the content of MgO laminated into Al2O3/MgO increases with deposition for 50/10 cycles, this laminated film exhibits an excellent barrier performance with WVTR as low as 4.7 × 10−6 gm−2/day. It seems that the introduced MgO is very effective to enhance the blocking effect of the Al2O3 bulk film; meanwhile, the bulk thickness of Al2O3 being equal, the more content of MgO available to the laminated Al2O3/MgO the better. When the MgO content rises up to 50 cycles in the laminated Al2O3/MgO, the barrier property is quite different with degraded WVTR value as 5.9 × 10−4 gm−2/day.
Figure 5. The conductivity of Ca with respect to corrosion time under high ambient temperature and humidity.
Download figure:
Standard image High-resolution imageIn order to confirm the role of MgO content in the laminated Al2O3/MgO film, high-resolution transmission electron microscopy (HR-TEM) was carried out. The laminated Al2O3/MgO films with 50/2, 50/10, and 50/50 ALD cyclic deposition were fabricated on the Si substrate. Figure 6 shows the TEM image and fast Fourier transform (FFT) diffraction pattern of the MgO film with ALD deposition of these three cycles in the laminated Al2O3/MgO. From the diagram it can be seen that the 2-cycle and 10-cycle MgO has a good amorphous state. But apparently, 50-cycle MgO film tended to be crystalline, with consequences that degrade the barrier performance as the grain boundaries provide a channel for water molecule diffusion; this conforms to the result of the WVTR measurement of laminated Al2O3/MgO at 50/50 cycles.
Figure 6. TEM image and FFT of the laminated Al2O3/MgO with different cycles of MgO ALD deposition.
Download figure:
Standard image High-resolution imageThe HR-TEM images and FFT patterns also clearly show that the MgO film with 20 nm (with 200 ALD cycles) is severely crystalline, as shown in figure 7(a), and the crystalline interplanar spacing d is measured to be 0.21 nm. The crystallinity of the MgO ALD films was further investigated using XRD. A 2θ scan from 10° to 90° of MgO film showed diffraction peaks indicative of a crystalline material. Previous reports have proven that the diffraction peaks located at 36.8°, 42.8°, and 62.2° represent the MgO(111), MgO(200), and MgO(220) planes respectively [25]. As shown in figure 7(b), only the (200) diffraction peak can be found in the scanning angle, which was consistent with cubic MgO, as confirmed using the PowderDiffraction Release-2 from the International Centre for Diffraction Data (Newtown Square, PA), while the Al2O3 film does not show any diffraction peak. Therefore, the MgO film has a critical thickness for crystallization, and this in turn will affect the performance of the barrier film. It is encouraging that the MgO film below 1 nm (ALD 10 cycles) has shown a perfect amorphous structure, even during the fabrication of the laminated structure, which marks a solid advance in the process of eliminating the penetration and diffusion of water molecules. What is more, the MgO film in the laminated structure is likely acting as a scavenger, which can effectively absorb the invasive water molecules. That is the reason why the thicker MgO film (under the critical thickness for crystallization) has potential to ensure better barrier performance, owing to its greater water absorption capacity.
Figure 7. (a) TEM image and FFT of the 20 nm-thickness Al2O3 film. (b) XRD spectra of ALD deposited film.
Download figure:
Standard image High-resolution imageIn order to further confirm the role of MgO film in laminated Al2O3/MgO, laminated Al2O3/MgO with fixed ratio of Al2O3 versus MgO ALD cycles was fabricated. In this section, we put emphasis on the discussion of the laminated Al2O3/MgO with fixed ratio of ALD deposition cycle as 5/1, 25/5, and 50/10, and these laminated structures were fabricated as presented in figure 8(a) for characterization. Looking back to figure 5(b), the WVTR value for laminated Al2O3/MgO with 5/1 and 25/5 ALD cycle deposition were 2.6 × 10−5 gm−2/day and 7.8 × 10−6 gm−2/day, which were inferior to the laminated Al2O3/MgO with 50/10 cycles, in other words an appropriate content of MgO and appropriate Al2O3/MgO cyclic ratio are guarantees for better WVTR performance. The performance difference caused by the crystalline state can be ignored, because all of these laminated films have an intact amorphous structure, as shown in figure 7(b). However, the TEM images in figures 8(b)–(d) have shown that there is a formation of blend membranes for the Al2O3/MgO with 5/1 and 25/5 ALD cycle, so these laminated structures are comparatively vague. In the deposition of the respective Al2O3 or MgO film, the film-forming process will prompt a number of internal defects (most likely dangling bonds) embedded in the bulk material or interface, thus resulting into a poor barrier performance for TFE. But the Al2O3/MgO with 50/10 ALD cycle shows a clean interface with obvious laminated structure, which will help to improve the quality of WVTR performance.
Figure 8. TEM image and FFT of the laminated Al2O3/MgO with fixed ratio of Al2O3 versus MgO ALD cycles.
Download figure:
Standard image High-resolution imageBased on the above discussion and assumptions, in the binary structure of the laminated Al2O3/MgO, the network of Al2O3 has played the main role in barricading against the penetration and diffusion of gas molecules, while the MgO as a scavenger can alleviate the hydrolysis process of Al2O3 bulk material. It is worth noting that the water absorbing capacity of MgO is proportional to the film content (herein meaning the film thickness), but cannot exceed the critical thickness for crystallization. Additionally, the packed alternatively-grown Al2O3 and MgO for the laminated configuration (5/1 and 25/5 cycles) tend to generate defects in the internal or interfacial film; meanwhile the lower temperature of Al2O3 or MgO film deposition with fewer cycles is more conducive to the formation of compound layers owing to the poor coverage of the multilayered structure. Furthermore, the mismatch of lattice parameters for Al and Mg atoms will expedite the formation of structural imperfections when the Al2O3 or MgO is deposited continuously [26], which will aggravate the penetration of gas species in the internal film. Therefore, a laminated structure is apparently effective to ensure the quality of barrier performance, which is exactly the reason why the laminated Al2O3/MgO with 50/10 ALD cycles has found a wonderful WVTR value, to elongate the lifetimes of film packaging.
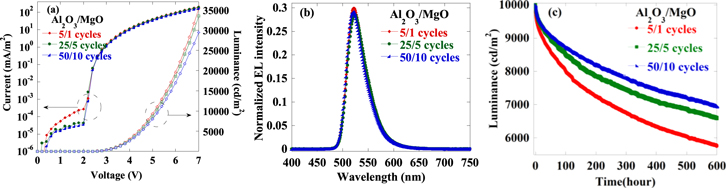
The WVTR results have heralded that encapsulated thin film based on laminated Al2O3/MgO film may have preferable barrier performance when applied in TE-OLEDs. Studies on I–V–L characteristics were undertaken to compare the electrical behaviors of the TE-OLEDs before and after fabrication of different laminated Al2O3/MgO film. Herein we will put emphasis on OLED devices with and without laminated Al2O3/MgO encapsulation. As seen in figure 9(a), only slight differences are apparent in I–V–L property between TE-OLEDs devices with and without encapsulation, indicating that the performance of the TE-OLEDs did not change distinctly in the process of thin film encapsulation. What is more, the influence of differently laminated Al2O3/MgO in the encapsulation of TE-OLEDs is negligible. Likewise, the electroluminescent (EL) spectra show no difference between the bare and encapsulated devices, and also depend hardly at all on cyclic deposition of laminated Al2O3/MgO film, as shown in figure 9(b). Based on the above experimental data, the light out-coupling effect is not obvious when using laminated Al2O3/MgO for encapsulation. Furthermore, lifetime characterization was performed to further verify the quality of the encapsulating Al2O3/MgO film. Figure 9(c) shows the typical plots of normalized luminance versus operating time for TE-OLEDs in a nonstop constant-voltage mode with a starting luminance of 10 000 cd m−2, which was measured in constant temperature and humidity using a luminance meter (KONICA MINOLTA CS-2000). For this study, the lifetime is defined as the decay time that the luminance decreases to 5 000 cd m−2. Herein the luminance of the OLED device measured is taken to be merely an indicator of Al2O3/MgO barrier performance against external permeated water/oxygen deterioration—being based on the assumption that the internal mechanism of the OLED itself is the same. As shown in figure 9(c), the luminance of the TE-OLEDs encapsulated with laminated Al2O3/MgO (50/10 cycles) barrier film measured in an oven deteriorated slower (with lifetime exceeding 600 hours) than the luminance of the devices with 5/1 or 25/5 Al2O3/MgO film capping, suggesting that the degradation induced by the H2O or O2 gas permeation into the TE-OLEDs was blocked effectively by the laminated film with appropriate deposition cycles, which can be attributed to its better performance in WVTR.
Figure 9. (a)The I–V–L behavior (b) EL spectra, and (c) TE-OLEDs luminescence versus continuous operation times for laminated Al2O3/MgO with different cyclic deposition.
Download figure:
Standard image High-resolution imageThe photography images of encapsulated TE-OLEDs stored in oven (60 °C, 100%) for 600 h were recorded to verify the effect of barrier film. The black spots are usually caused by cathode delamination due to reactions with ambient H2O or O2 gas. As shown in figure 10, the TE-OLEDs encapsulated with laminated Al2O3/MgO of 50/10 cyclic deposition display a good image without obvious black spots. For further observation, the TE-OLEDs encapsulated with 5/1 or 25/5 cycles laminated Al2O3/MgO have been most weakened with generation of many large-sized black spots, and the proportion of the these black spots is thriving, having created wider permeation paths to cause further damage. After being encapsulated with a densely laminated Al2O3/MgO barrier film, the initial growth of black spots alleviates, with a very slow rate—manifesting an excellent barrier performance for this kind of laminated structure.
Figure 10. Photography of three comparative TE-OLEDs before and after aging in oven.
Download figure:
Standard image High-resolution image4. Conclusions
In summary, physical properties of MgO embedded in laminated Al2O3/MgO were investigated for thin film encapsulation. The TEM results demonstrated that 5 nm MgO film tended to be crystalline, which is not preferred for barricading the gas permeation. But thinner MgO film presented a good amorphous state, meaning it can act as a water scavenger that can delay hydrolysis of the bulk Al2O3 film. Specific cyclic ratio deposition of the respective Al2O3 and MgO thin film is required to form an appropriate laminated structure, preventing production of blended membranes with undesirable internal defects or dangling bonds. The shelf-time of TE-OLEDs encapsulated with laminated Al2O3/MgO (50/10 ALD cycles) was significantly enhanced. Our work has expounded that MgO interlayers in laminated Al2O3/MgO could provide a facile route to prepare high-performance films with superior barrier properties, which is of great potential in flexible displays.
Acknowledgments
The authors are grateful to the National Basic Research Program of China (Grant No. 2015CB655000) the National Natural Science Foundation of China (Grant No.51502093), Science and Technology Program of Guangdong Province (2015B090915001, 2016B090906002), Pear River S&T Nova Program of Guangzhou (No. 201610010052) and Tip-top Scientific and Technical Innovative Youth Talents of Guangdong Special Support Program (No. 2015TQ01C777).