Abstract
SnO2 is an attractive anode material for lithium-ion batteries (LIBs) due to its high theoretical specific capacity (1491 mAh g−1), low cost, and environmental benignity. The main challenges for SnO2 anodes are their low intrinsic conductivity and poor cycling stability associated with their large volume changes during the charge and discharge process. Here, we present a simple chemical vapor deposition method to fabricate three-dimensional SnO2/carbon on Cu foam electrodes for LIBs. Such a three-dimensional electrode combines multiple advantages, including a continuous electrically conductive network, short pathways for electron transport and ion diffusion, and porous space to allow for the volume expansion of SnO2 nanoparticles. With this anode, superior electrochemical performance is achieved with a high reversible specific capacity of 1171 mAh g−1 at a current density of 100 mA g−1. A stable cycling performance as well as an excellent rate capability is also achieved. These outstanding lithium-storage properties suggest the strategy is a reliable approach for fabricating high-performance LIB electrodes.
Export citation and abstract BibTeX RIS
1. Introduction
As one of the most dominant electrochemical energy storage (EES) system, lithium-ion batteries (LIBs) have been widely utilized in various devices and equipment. Besides the applications in electric vehicles (EVs) and hybrid electric vehicles (HEVs), LIBs are the foremost power source for portable electronics, such as cell phones, laptops, and digital cameras, which accompany us in our daily lives [1–3]. SnO2-based nanomaterials are proposed as promising anode materials for LIBs because of their low cost, environmental friendliness, and high theoretical capacity (1491 mAh g−1), which is much higher than that of currently commercial graphite anodes (370 mAh g−1) [4–7]. However, one big issue for SnO2-based anodes is their huge volume change (∼300%) during lithium insertion and extraction, which leads to cracking and pulverization of the electrode and induces rapid capacity fading. In addition, the low conductivity further hinders their high rate capability [8–11]. Therefore, fabrication of designed nanostructured materials to accommodate huge volume changes and enhance conductivity is indeed necessary for SnO2-based electrodes with excellent cycling stability and rate capability.
To alleviate the above-mentioned challenges, great efforts have been carried out to develop novel nanostructures of SnO2-based anodes, such as nanoparticles [12, 13], nanosheets [14, 15], nanoboxes [16, 17], SnO2/carbon nanohybrids [18–20], and SnO2/graphene nanocomposites [21–23], thus leading to improved lithium-storage properties. Recent works have demonstrated that the chemical vapor deposition (CVD) method is a powerful technology for surface modification, device development, and electrode material fabrication [24, 25]. A series of Si- and Ge-based anodes have been successfully grown on metallic current collector substrates using CVD methods [26–28]. Benefiting from good electrical contact between the current collector and nanomaterial, more active materials can contribute to the capacity. In addition, without any polymer binder and slurry process, the as-prepared electrode was directly used as a LIB anode, ensuring continuous and fast conductive pathways throughout the whole electrode. Nevertheless, fabrication of high-performance SnO2-based anodes is rarely reported because the main challenge in this strategy is the achievement of nanoscale size and uniform distribution of the active material on the metal substrate.
In this paper, we report a simple strategy for fabrication of SnO2/carbon nanohybrids on Cu foam (denoted as SnO2/C-Cu) by the CVD method and subsequent carbon coating by hydrothermal reaction. The Cu foam with a three-dimensional (3D) interconnected architecture, and homogenous and large macro-porous surface effectively confines the uniformly embedded SnO2. Without the addition of any polymer binder, highly conductive Cu foam was directly used as the current substrate to ensure fast electronic transport. The nanostructured void spaces between the adjacent SnO2 nanoparticles can provide ample space to accommodate the substantial volume change, and facilitate electrolyte diffusion and lithium ion migration. Moreover, the carbon coating can further promote electronic transport, prevent structural cracking, and preserve the integrity of the electrode. When evaluated as an anode for LIBs, the SnO2/carbon nanohybrids exhibit a high reversible capacity of 1171 mAh g−1, with excellent rate capability.
2. Experimental section
2.1. Materials synthesis
The SnO2 nanoparticles were grown on Cu foam by a CVD method. In detail, a gold catalyst film with a thickness of 3 nm was first deposited on Cu foam by thermal evaporation. Then, commercial SnO2 and graphite powder was mixed with a molar ratio of 1:3. The resulting mixture was placed in a quartz boat and loaded at the center of a 1-inch diameter quartz tube furnace. The gold-coated Cu foam substrate was then placed downstream at different distances from the center of the furnace. The furnace was heated up to 900 °C in 15 min under a high purity argon gas (50 sccm). The growth process was maintained for 15 min at about 6.5 Torr.
Afterwards, glucose-derived carbon-rich polysaccharide was coated on SnO2/Cu foam by a simple hydrothermal reaction. In a typical synthesis, the as-prepared SnO2/Cu foam and 20 ml of 0.1 M aqueous glucose solution were added into Teflon-lined autoclaves for hydrothermal treatment at 180 °C for 3 h. Finally, the obtained materials were treated at 500 °C in argon gas for 2 h to obtain the SnO2/C-Cu electrode.
2.2. Materials characterization
X-ray diffraction (XRD) measurements were performed on a Philips Xpert x-ray diffractometer using Cu Kα (λ = 1.54 Å) radiation. The morphology and structure were observed by scanning electron microscopy (SEM; FEI Quanta 450FEG) and transmission electron microscopy (TEM; JEOL 2100 microscope). X-ray photoelectron spectroscopy (XPS) spectra were obtained using a Kratos Axis Ultra DLD x-ray Photoelectron Spectrometer with Al Kα source (1486 eV) running at 150 W.
2.3. Electrochemical measurements
The electrochemical performance was measured using CR2032-type coin cells. The SnO2/C-Cu anode was directly used as an electrode without any added conductive agent and polymer binder. The electrolyte was 1 mol l−1 LiPF6 in a mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) (1:1 by volume). Charge/discharge tests were recorded on an Arbin BT2043 system between 0.01 ∼ 1.5 V. Impedance and cyclic voltammetry measurements were carried out on an electrochemical analyzer (Ivium Compact State10800).
3. Results and discussion
The uniform SnO2 nanoparticles were first deposited on Cu foam using a simple CVD method. In a low-pressure environment at 900 °C, Sn vapor reacted with graphite to form SnO2. Meanwhile, graphite was oxidized to generate a CO or CO/CO2 vapor mixture. Then, in the low temperature region, Sn vapor reacted with the CO or CO/CO2 vapor mixture to form SnO2 nanoparticles on the Cu foam [29]. The oxidation-reduction reaction can be described as follows:
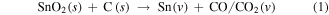
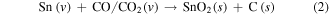
Figure 1 shows SEM images of the as-prepared SnO2 nanoparticles on Cu foam, positioned at a distance of 14.5 cm downstream from the center of the furnace. The low-magnification SEM image (figure 1(a)) indicates that the SnO2 layer fully and uniformly covers the 3D Cu foam. The magnified SEM image (figure 1(b)) shows the SnO2 layer is composed mainly of randomly oriented nanoparticles that form a porous structure, which is beneficial for ion diffusion. In agreement with the SEM observations, the uniform architecture can be illustrated in the energy dispersive x-ray (EDX) (figure 1(c)) and elemental mapping images (figure S1, see online supplementary material available at stacks.iop.org/NANO/27/415401/mmedia). The tin and oxygen elemental mappings reveal a homogeneous distribution of SnO2 on the Cu foam. Online supplementary figure S2 exhibits the SEM images of SnO2 nanoparticles on Cu foam obtained at 13.5 cm and 12.5 cm from the center of the furnace. Comparing figures 1 and S2, the SnO2 layer can have smaller SnO2 nanocrystals and more porous structure between the adjacent SnO2 nanoparticles by increasing the distance to the center of the furnace. These results suggest that the process of heat treatment at a lower temperature can reduce the size of the resulting nanoparticles. Unless otherwise mentioned, electrodes obtained at a distance of 14.5 cm downstream from the center of the furnace are used to exhibit the physicochemical properties of this CVD strategy. The SnO2 nanoparticles are coated with glucose-derived carbon-rich polysaccharide (GCP) by a simple hydrothermal process [30]. Figures 1(d) and (e) reveal typical SEM images of the SnO2/C–Cu hybrid material. After carbon coating on the SnO2 nanoparticles, the hybrid material exhibits no obvious changes in macroscopic morphology. The thickness of SnO2/C on Cu foam is estimated to be about 2 μm (figure S3). The XRD result in online supplementary figure S4 demonstrates that the SnO2 nanoparticles are crystalline and the peaks match well with tetragonal rutile structure of SnO2 (JCPDS No. 41-1445) [31, 32]. The EDX spectrum (figure 1(f)) and elemental mapping images (figures 1(g)–(i) and online supplementary S5) of tin, oxygen, carbon, and Cu further demonstrate the homogeneous distribution of SnO2 and carbon on Cu foam.
Figure 1. (a), (b) SEM images and (c) EDX spectrum of the pristine SnO2 nanoparticles on Cu foam at a distance of 14.5 cm downstream from the center of the furnace. (d), (e) SEM images, (f) EDX spectrum and (g)–(i) EDX elemental mapping images of the SnO2/C-Cu hybrid material.
Download figure:
Standard image High-resolution imageTEM images (figures 2(a) and (b)) illustrate that the SnO2/carbon nanohybrids consist of some small and quasi-continuous nanoparticles. The enlarged TEM image reveals that the surface of the nanoparticles is covered with an amorphous carbon layer with a thickness of several nanometers, as indicated by arrows in figure 2(b). Obviously, due to significant shrinkage of GCP during carbonization, the carbon layer is attached tightly to the nanoparticles, which is beneficial for maintaining the integrity and enhancing the conductivity of the hybrid nanostructure [30, 33]. The high-resolution TEM (HRTEM) image in figure 2(c) shows two interplanar spacings of 0.33 nm and 0.21 nm, corresponding to lattice spacings of the (110) and (210) crystal planes of SnO2. The nanohybrids were also investigated by XPS, as shown in figures 2(d), (f) and online supplementary S6. The binding energy of Sn 3d5/2 of the sample is 486.0 eV, which is higher than that of Sn0 (484.7 eV) but lower than that of Sn4+ (487.2 eV), indicating there may be a nonstoichiometric oxygen deficiency (SnO2−x) or a small amount of Sn2+ in the SnO2 nanoparticles [14, 34]. But according to the XRD results, the main phase of the as-prepared nanoparticles is SnO2. The C 1s XPS spectrum shows two peaks located at 284.6 eV attributed to the C=C of sp2 hybridized carbon atoms and a small peak at 287.8 eV due to residual traces of C–OH and/or C=O organic group from the precursors [35].
Figure 2. (a), (b) TEM images and (c) HRTEM image of the SnO2/carbon composite. (d) Sn 3d and (f) C 1s XPS spectra of the SnO2/carbon composite.
Download figure:
Standard image High-resolution imageA series of electrochemical tests were carried out to investigate the properties of the SnO2/C–Cu electrodes. Figure 3(a) shows the first five cyclic voltammetry (CV) curves at a scan rate of 0.1 mV s−1 in the voltage window 0.01−2 V. In the first cycle, the two large cathodic peaks at 1.1 V and 0.8 V can be assigned to the reduction of SnO2 to Sn and Li2O as well as the decomposition of electrolyte on the surface of the nanoparticles and the formation of solid-electrolyte interphase (SEI) film. In addition, one broad peak at about 0.28 V can be ascribed to the alloying process of Sn to LixSn [14, 36]. In reverse scanning, two broad peaks at 0.47 V and 1.35 V are seen, which correspond to the phase transition from LixSn alloy and Li2O to SnO2, respectively [4, 37]. From the second cycle onwards, the well-overlapped peaks imply excellent cyclability of the SnO2/C-Cu electrode. The reversible reaction of SnO2 can be described as follows:
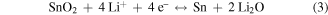
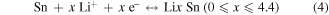
Figure 3. Electrochemical performance of the SnO2/C–Cu electrode: (a) CV curves of the first five cycles between 0.01 and 2.0 V at a scan rate of 0.1 mV s−1. (b) Galvanostatic charge/discharge profiles at 0.1 A g−1 between 0.01 and 1.5 V. (c) Cycling performance at 0.2 A g−1 between 0.01 and 1.5 V. (d) Rate capability between 0.01 and 1.5 V.
Download figure:
Standard image High-resolution imageA correlative plateau can be observed in the charge/discharge profiles at 0.1 A g−1 between 0.01 and 1.5 V (figure 3(b)). The first charge and discharge capacities are 1171 and 3234 mAh g−1, respectively. Capacity loss can be mainly ascribed to the formation of the SEI film and the irreversible insertion of Li+, which helps accomplish the reduction of SnO2 to Sn [22, 30]. For a practical high-performance anode, low initial Coulombic efficiency cannot be accepted. However, the initial Coulombic efficiency can be greatly improved by pre-inserting lithium into the anode to compensate the consumption of lithium due to SEI formation [38, 39]. Figure 3(c) shows the cycling performance at a current density of 0.2 A g−1. As expected, the hybrid electrode shows very slow fading. A high capacity of 846 mAh g−1 is delivered after 100 cycles, which retains 77% of its second discharge capacity. Such excellent cycling stability is superior to that of many other SnO2-based anode materials [31, 32]. Besides the excellent cycling performance, the SnO2/C–Cu anode also exhibits convincing rate capabilities. Figure 3(d) shows the charge/discharge capacities of the anode at various current densities from 0.1 to 3 A g−1 between 0.01 and 1.5 V. As the current increases, the anode only displays a small decrease in capacity. Remarkably, even at a high current density of 3 A g−1, a reversible capacity of 328 mAh g−1 can still be obtained for the SnO2/C-Cu anode. When the current density is reduced back to 0.1 A g−1, a high capacity of about 1086 mAh g−1 can be recovered and kept stable in the following 10 cycles. As a comparison, we tested the rate capabilities of SnO2/C–Cu electrodes obtained at a distance of 13.5 cm and 12.5 cm downstream from the center of the furnace (online supplementary figure S7). Both of them exhibit lower charge/discharge capacities at each current density than that of the SnO2/C-Cu electrode at 14.5 cm. This is because the SnO2/carbon nanoparticles at 14.5 cm are much smaller than those at 13.5 and 12.5 cm, and consequently have shorter lengths for electron transport and ion diffusion, which is beneficial for fast charge and discharge processes. At a current density of 2 A g−1, the SnO2/C–Cu electrodes at 13.5 cm and 12.5 cm can only deliver 270 and 143 mAh g−1, respectively, while the SnO2/C–Cu electrodes at 14.5 cm exhibit a capacity of 443 mAh g−1 (figure 3(d)), indicating that the porous and small SnO2 nanoparticles on the Cu foam are indeed beneficial for the improvement of lithium-storage performance. The performance of the SnO2/C–Cu electrode has also been compared with those of recently published, SnO2-based LIB anodes, especially SnO2 on metal substrate anodes, and the comparison result is presented in online supplementary table S1. It can be seen that the SnO2/C–Cu electrode studied in this work exhibits great advantages in terms of reversible capacity and rate capability.
To gain insight into the mechanism of the excellent electrochemical performance and the structural changes of the SnO2/C–Cu electrode, the cycled nanohybrids were further investigated using SEM, XPS, TEM, HRTEM, XRD and EDX elemental mapping images. The SEM images of the cycled electrode show the formation of an SEI layer on the surface of the SnO2/carbon nanohybrids (figures 4(a) and (b)). No obvious exfoliation of SnO2 from the Cu foam was observed, suggesting that there is a strong interaction between the in situ deposited SnO2 and Cu foam, which keep the SnO2 nanoparticles tightly adhering to the Cu substrate. Figure 4(c) shows the XPS spectra for Sn 3d of the SnO2/C–Cu electrode after cycling. It can be seen that two peaks assigned to Sn 3d5/2 and 3d3/2 (at 486.4 and 494.7 eV) appear, indicating the formed metallic Sn can be oxidized to generate SnO2 (online supplementary figure S8). The crystal structure of SnO2 can be further confirmed by HRTEM analysis. Figure 4(e) shows obscure lattice fringes with the distance of 0.21 nm, which can match with the (210) facet of SnO2. The fast Fourier transform (FFT) image further confirms the crystalline structure of SnO2. The observation of SnO2 in the cycled electrodes demonstrates that Sn and Li2O can reversibly react to form SnO2 during charge/discharge cycling. Moreover, the elemental mappings of tin, oxygen, and carbon still show a homogeneous and continuous dispersion throughout the Cu foam network (figure 4(f)). This reflects that the carbon coating can effectively suppress the aggregation of Sn nanoparticles during lithiation and delithiation reactions.
Figure 4. (a), (b) SEM images, (c) XPS spectra for the Sn 3d before and after cycling, (d) TEM, (e) HRTEM and FFT images, and (f) STEM and EDX elemental mapping images of the SnO2/C–Cu electrodes after 100 cycles at 0.2 A g−1.
Download figure:
Standard image High-resolution imageElectrochemical impedance spectroscopy (EIS) was also carried out for the SnO2/C–Cu electrode before and after the cycling test (online supplementary figure S9). A decrease in the total impedance was observed after cycling, which is mainly due to slow infiltration of the electrolyte into the porous nanohybrids and the conductivity increase of SnO2/carbon after the electrochemical activation process during charge/discharge cycling [40, 41]. Due to the excellent integrity and reduced impedance of electrodes, the SnO2/C–Cu anodes exhibit both superior capacity and rate performance.
The outstanding electrochemical performance of the SnO2/C–Cu electrodes can be possibly attributed to the following reasons. First, the macroporous Cu substrate and abundant void space between neighboring SnO2 nanoparticles not only help maintain efficient electron and ion transport, but also mechanically accommodate the vast volume changes of the SnO2 nanoparticles during lithiation and delithiation. Second, in situ deposited SnO2 on Cu foam can effectively enhance the physical connections and electrical contact of SnO2 nanoparticles with the 3D conductive substrate. Moreover, the absence of an electrochemical inactive polymer binder can further facilitate high electron transport with continuous and fast conductive pathways throughout the electrode, thereby maximizing the effective utilization of the active materials and ensuring a reversible lithiation/delithiation process even at high current rates. Last, the carbon coating layer not only counteracts the pulverization of the electrode material, but also maintains a stable SEI layer and prevents the electrolyte from further decomposition, which leads to a good cycling stability of the SnO2/C–Cu electrodes.
4. Conclusion
In conclusion, we present a reliable strategy to fabricate 3D porous SnO2/C–Cu electrodes as high-performance LIB anodes using a CVD method combined with subsequent hydrothermal reaction. By taking advantage of the porous structure, which was intrinsically integrated with the Cu substrate, nanosized SnO2 particles, and conductive carbon coating, the SnO2/C–Cu electrode achieved high reversible capacity, stable electrochemical cycling, and excellent rate capability. Moreover, this facile method can be further extended to build other 3D hybrid electrodes, such as GeO2/carbon on Cu foam (denoted as GeO2/C–Cu). We will report the research development of the GeO2/C–Cu anodes in a further paper. We believe that the rational material design and the obtained promising electrochemical results can encourage more research into other high-performance anodes for next-generation LIBs.
Acknowledgments
W W acknowledges financial support from the University of Wyoming's School of Energy Resources and support from US Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Award DE–FG02–10ER46728.





