Abstract
A biocompatible polymer–gold nanorod (P-AuNR) conjugate was developed as a thermo-chemotherapeutic nano-sized drug carrier for cancer therapy using near-infrared (NIR) light as an external trigger. The amphiphilic polymer, poly(ethylene glycol)-block-poly(caprolactone) (PEG-b-PCL) bearing a disulfide bond, was prepared using a facile synthetic route via copper(I)-free click chemistry and covalently linked to AuNR. The chemical structures and successful conjugation of PEG-b-PCL were analyzed using 1H NMR and FT-IR. Doxorubicin (DOX), a hydrophobic anticancer drug, was effectively loaded into the hydrophobic PCL domain of P-AuNR through a simple dialysis method. P-AuNR showed longitudinal plasmon resonance absorption at the NIR region, thus generating heat under irradiation at 808 nm. Interestingly, exposure of P-AuNRs to NIR induced a structural change in the PCL block from a crystalline to an amorphous state, leading to the temporally controlled release of DOX. No significant release of DOX was observed from P-AuNRs under physiological conditions (pH 7.4), whereas the release rate of DOX was remarkably enhanced in response to NIR irradiation. In vitro cellular experiments to assess cytotoxicity and intracellular drug release behavior of DOX-P-AuNRs demonstrated that the release of DOX could be selectively regulated by NIR irradiation. Overall, DOX-P-AuNRs might have the potential to overcome the indiscriminate toxicity of free DOX.
Export citation and abstract BibTeX RIS
1. Introduction
Nanoparticle-based drug delivery systems have emerged as a potential solution for cancer therapy to surmount the limitations of conventional chemotherapeutics such as poor water solubility and indiscriminate toxicity to cells in our body [1, 2]. Nanocarriers have been shown to improve the water solubility of hydrophobic drugs and facilitate their delivery to tumor tissue through the enhanced permeability and retention (EPR) effect [3, 4]. However, traditional nanocarriers have exhibited sustained release of anticancer drugs via the passive diffusion mechanism, which may not effectively deliver a cytotoxic level of the drug to tumor tissues. To minimize the leakage of the drug and achieve efficient delivery, novel nanocarriers capable of releasing the drug in response to specific stimuli have emerged as alternatives [5–8]. There are various platforms exploiting endogenous stimuli (e.g., pH gradient [9], enzymes [10, 11], redox potential [12], and reactive oxygen species [13, 14]) and exogenous stimuli (e.g., temperature [15, 16], light [17–19], magnetic fields [20, 21], and ultrasound [22, 23]).
Light-sensitive nanocarriers have drawn increasing attention because their physicochemical properties do not rely on changes in specific chemical properties of the environment and the drug release behaviors can be regulated temporally and remotely. Recently, near-infrared (NIR) light has been an attractive stimulus because it can deeply penetrate tissues owing to a minimum overlap in absorption by biological components compared to ultraviolet or visible light which shows the limited penetration depth and photodamage [24–26]. In particular, NIR can be used to generate heat through a combination of nanoparticles (e.g., gold nanorods (AuNRs) [27, 28], gold nanocages [29], carbon nanotubes [30, 31], and graphene oxides [32]), resulting in hyperthermia and tumor ablation [33, 34]. In addition, NIR laser can be simply modulated by the precise spatiotemporal control for targeted photothermal activation of nanoparticles [35, 36].
AuNRs have shown promising potential as nanoparticles for biological imaging and photothermal therapy due to their surface plasmon resonance and strong absorption in the NIR region [28, 35, 37]. Particularly, AuNR-based nanoplatforms are highly useful for simultaneous delivery of heat and chemotherapeutic agents to the tumor for thermochemotherapy because the combination of NIR-based photothermal therapy and chemotherapy may provide controlled spatial and temporal drug release, allowing for high therapeutic efficacy and less cytotoxicity. In this study, in an attempt to develop the nanocarrier for targeted thermochemotherapy of cancer, we prepared amphiphilic polymer-coated AuNRs (P-AuNRs) which can release the drug in response to NIR irradiation (figure 1). As the amphiphilic polymer, disulfide bond-bearing poly(ethylene glycol)-b-poly(caprolactone) (PEG-b-PCL) was prepared by copper(I)-free click chemistry, which is simple and efficient without any copper-induced toxicity [38, 39]. Both PEG and PCL are highly biocompatible polymers, approved by the US Food and Drug Administration. PEG has been used to prevent non-specific interaction with blood proteins for prolonged circulation of nanoparticles, and PCL has been extensively used as the hydrophobic segment for encapsulation of the anticancer drug. Doxorubicin (DOX), the chosen model anticancer drug, was encapsulated into the P-AuNR, and the potential use of DOX-loaded P-AuNRs as a thermo-chemotherapeutic agent was investigated.
Figure 1. Schematic illustration of the polymer–gold nanorod (P-AuNR) conjugate for NIR light triggered thermochemotherapy.
Download figure:
Standard image High-resolution image2. Experimental section
2.1. Materials
Gold(III) chloride trihydrate (HAuCl4 · 3H2O, 99%), hexadecyltrimethylammonium bromide (CTAB, 99%), sodium borohydride (NaBH4, 99%), silver nitrate (AgNO3, 99%), ε-caprolactone (ε-CL, 97%), 2-hydroxyethyl disulfide, tin(II) 2-ethylhexanoate (Sn(Oct)2), triethylamine (TEA), p-toluenesulfonyl chloride (99%), sodium azide, dibenzocyclooctyne-acid (DBCO-acid, 95%), N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), N,N'-dimethylformamide (DMF), tetrahydrofuran (THF), dimethyl sulfoxide, doxorubicin hydrochloride (DOX·HCl), hydrogen chloride-methanol solution (1.25 M HCl), and cell counting kit-8 (CCK-8) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Methoxy(polyethylene glycol) amine (mPEG-amine, MW = 5000) was obtained from Laysan Bio, Inc. (Arab, AL, USA). Cell culture products including fetal bovine serum (FBS), cell culture medium RPMI 1640, Dulbecco's phosphate buffered saline (DPBS), antibiotic–antimycotic solution (AA), and trypsin-EDTA were purchased from WelGENE (Seoul, Korea). Human breast carcinoma cell line MDA-MB-231 was obtained from the American Type Culture Collection (Rockville, MD, USA). All other chemicals were analytical grade and were used without further purification.
2.2. Preparation of CTAB-coated AuNRs (CTAB-AuNRs)
CTAB-AuNRs were prepared based on the seed-mediated growth method according to the literature with slight modification [40]. In brief, a mixture of CTAB (5 ml, 0.10 M) and HAuCl4 (128 μl, 0.01 M) in deionized water was stirred, and then ice-cold NaBH4 (450 μl, 0.01 M) was added to the stirred solution. After 2 min of vigorous stirring, the brownish yellow color of the gold seed solution formed. Next, HAuCl4 (500 μl, 0.01 M) was added to the mixed solution of CTAB (7.5 ml, 0.1 M) and AgNO3 solution (90 μl, 0.01 M) in deionized water, followed by gentle mixing with the addition of L-ascorbic acid (75 μl, 0.1 M). Consequently, the color of the growth solution changed from dark yellow to transparent. Lastly, 24 μl of seed solution was added to the growth solution at 27 °C, resulting in the formation of a dark red solution within 10–20 min. The excess CTAB in the AuNR solutions was removed by centrifugation twice at 12 000 rpm for 10 min.
2.3. Synthesis of azide-functionalized PCL with the disulfide bond (N3-PCL-SS-PCL-N3)
Disulfide group-bearing PCL was synthesized by ring opening polymerization of ε-CL as previously reported [41]. Three grams of ε-CL (26.28 mmol), 46.3 mg of 2-hydroxyethyl disulfide (0.30 mmol), and 10 ml of toluene were added to a Schlenk flask under nitrogen and the mixture was degassed by three freeze-pump-thaw cycles. Then, 33.3 mg of Sn(Oct)2 (0.082 mmol) in 5 ml of toluene was added to the flask through a syringe. The reaction proceeded for 24 h at 100 °C. The resulting polymer (PCL-SS-PCL) was precipitated in cold diethylether, filtered, and dried in a vacuum at room temperature.
Azide-functionalized PCL was prepared, according to the procedure as reported with slight modification [42]. In brief, the hydroxyl group of PCL-SS-PCL was tosylated, followed by conversion to the azide group. First, PCL-SS-PCL (2 g, 0.20 mmol) was dissolved in dry methylene chloride and p-toluenesulfonyl chloride (389.1 mg, 2.04 mmol) was added under a nitrogen atmosphere. The mixture was cooled in an ice water bath, and TEA (206.4 mg, 2.04 mmol) was added dropwise using a syringe. After removing the ice water bath, the reaction was allowed to proceed for 24 h at room temperature. The solvent was evaporated using rotary evaporation and dissolved in THF, followed by filtration of insoluble products. Thereafter, tosylated PCL (Ts-PCL-SS-PCL-Ts) was isolated by precipitation in cold methanol, filtered, and dried in a vacuum at room temperature. Second, the end groups of Ts-PCL-SS-PCL-Ts were displaced with azide groups in the presence of sodium azide. In brief, the excess amount of sodium azide (132.7 mg, 2.04 mmol) was added to Ts-PCL-SS-PCL-Ts (1 g, 0.10 mmol) in dry DMF under nitrogen atmosphere. The reaction was stirred for 24 h at room temperature. Then, the solution was concentrated using a rotary evaporator, diluted with methylene chloride, and purified through basic alumina column to remove salt. Finally, azide-functionalized PCL (N3-PCL-SS-PCL-N3) was obtained by precipitation in cold methanol, filtered, and dried in a vacuum at room temperature. To prepare N3-PCL-SH, N3-PCL-SS-PCL-N3 was dissolved in THF containing D,L-dithiothreitol (DTT) with catalytic amount of sodium methoxide and stirred for one day, followed by precipitation in excess amount of diethyl ether. The resulting N3-PCL-SH was obtained after being dried in a vacuum at room temperature.
2.4. Synthesis of DBCO-functionalized poly(ethylene glycol) (PEG-DBCO)
To activate the carboxyl group of DBCO-acid, EDC (17.3 mg, 0.09 mmol), NHS (10.4 mg, 0.12 mmol), and TEA (18.2 mg, 0.18 mmol) were added to the DBCO-acid solution (20 mg, 0.06 mmol) in 2 ml THF. The mixture was stirred for 3 h and directly added to the mPEG-amine solution (150 mg, 0.03 mmol) in 10 ml THF. The reaction mixture was stirred under nitrogen atmosphere for 24 h, evaporated using a rotary evaporator, and dissolved in deionized water. The resulting mixture was dialyzed (MWCO = 3.5 kDa) against dimethyl sulfoxide for 1 day and distilled water for 2 days, followed by lyophilization.
2.5. Preparation of P-AuNR
PEG-DBCO and N3-PCL-SS-PCL-N3 in a molar ratio of 1:1 were dissolved in DMF (2 ml, 10 mg ml−1), stirred for 1 h at 37 °C, and added dropwise to the AuNR solution (5.0 ml, 0.2 mg ml−1) under vigorous stirring. The reaction was carried out overnight at room temperature. The obtained polymer-coated AuNR was then centrifuged at 12 000 rpm for 10 min, decanted, and then resuspended in 1 ml deionized water three times to get rid of unreacted N3-PCL-SS-PCL-N3 or PEG-DBCO.
2.6. Characterization
1H-NMR spectra were obtained using a Varian Unity 500 MHz spectrometer (Palo Alto, CA, USA). CDCl3 was used as the solvent. The morphology of the particles was observed using transmission electron microscopy (TEM, Philips CM30) at an accelerating voltage of 200 keV. For TEM images, samples were dropped on a 200 mesh copper grid without staining. Absorption spectra of CTAB-AuNR and P-AuNR were measured using a UV–vis spectrophotometer (Optizen 3220UV, Mecasys Inc., Korea). Samples were placed in a quartz cuvette for measurements and distilled water was used as a reference.
The hydrodynamic sizes and zeta potentials of nanoparticles were measured by dynamic light scattering (DLS) using a Zetasizer Nano ZS90 (Malvern Instruments, Worcestershire, UK) with a He-Ne laser (633 nm) at 90° collecting optics. The samples for size measurement and zeta potential were prepared in aqueous solutions at concentrations of 0.1 and 0.01 mg ml−1 of AuNR, respectively. The concentration of AuNRs was measured using an inductively coupled plasma mass spectrometry (ICP-MS Agilent 7500, Agilent Technology Inc., USA). The synthesized polymers were further characterized by FT-IR (Bruker IFS-66v/S, Bruker corp., USA) spectroscopy. The molecular weights and polydispersity indexes (PDI) were measured using gel permeation chromatography (GPC, Shodex-KF 802.5, KF 808L, USA), with chloroform as the eluent at a flow rate of 1 ml min−1.
2.7. Temperature changes under NIR irradiation as a function of time
To investigate the photothermal effects of the AuNRs under laser irradiation as a function of time, a near infrared (NIR) laser with irradiation wavelength of 808 nm (Fiber coupled laser system FC-W-808, Changchun new industries optoelectronics technology Co., Ltd, China) and an infrared camera (FLIR T430sc, FLIR Systems, Inc., USA) were utilized. One milliliter of deionized water and an aqueous solution of CTAB-AuNR (0.2 mg AuNR ml−1) and P-AuNR (0.2 mg AuNR ml−1) were irradiated at a fluence of 2 W cm−2 with a beam area of 1 cm2. Each sample was irradiated for 10 min and the temperature was recorded every 2 min using a digital thermometer (TES-1300, TES electrical electronic corp., Taiwan). Additionally, NIR thermographic images were taken before and after 10 min irradiation with the laser.
2.8. Preparation of DOX-loaded P-AuNR (DOX-P-AuNR)
DOX·HCl (0.12 mg) was dissolved in DMSO (5 mg ml−1) containing three equimolar amount of TEA. The solution was added dropwise to the P-AuNR solution (3 ml, 0.2 mg ml−1) and stirred for one day. The resulting solution was dialyzed against an excess amount of distilled water for 24 h to remove unloaded DOX. DOX-P-AuNR was stored at 4 °C under dark conditions. To estimate the loading efficiency (LE) and loading content (LC) of DOX, the DOX-P-AuNR sample was dissolved in hydrogen chloride–methanol solution. Extracted DOX from DOX-P-AuNR was separated by centrifugation (12 000 rpm, 10 min), and the amount of DOX was determined using a fluorospectrometer (FluoroMatye FS-2, SCINCO Co., Ltd, Seoul, Korea). The fluorescence of DOX was measured (excitation = 480 nm, emission = 500–650 nm) based on a calibration curve that was obtained from the same condition at various concentrations of DOX in hydrogen chloride-methanol solution (0.5–50 μg ml−1). LE and LC were calculated as follows
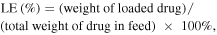
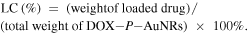
2.9. In vitro drug release
The NIR-dependent drug release profile was analyzed by measuring DOX fluorescence with a fluorospectrometer. Free DOX and DOX-P-AuNRs (DOX concentration = 10 μg ml−1) were dispersed in deionized water, and DOX-P-AuNRs were irradiated with the laser at 2 W cm−2 for 10 min. The solutions were centrifuged at 12 000 rpm for 10 min, and the supernatant was used for the measurement of released DOX. Also, we evaluated the effect of temperature and NIR irradiation on the release behavior of DOX. In brief, DOX-P-AuNRs were dispersed in PBS (1 ml, pH 7.4) and shaken at 100 rpm for 24 h under different temperature (37 °C and 50 °C). The NIR-triggered drug release was carried out by irradiation with an 808 nm laser at 2 W cm−2 for 5 min at designated time points (1, 3 and 5 h). At predetermined time intervals, solutions were centrifuged at 12 000 rpm for 5 min and the supernatants were collected to measure the absorbance of DOX using UV–vis spectrophotometer at 480 nm. The medium was refreshed with equal volume of PBS.
2.10. In vitro cytotoxicity assays
To evaluate the cytotoxicity of CTAB-AuNR and P-AuNR, CCK-8 assay was carried out. The MDA-MB-231 human breast carcinoma cell line was cultured in RPMI 1640 medium supplemented with 10% FBS and 1% AA at 37 °C in an incubator under a humidified atmosphere with 5% CO2. MDA-MB-231 cells (1 × 104 cells well−1) were seeded in 96-well plates, which were incubated for 24 h in 100 μl RPMI 1640 medium containing 10% FBS and 1% AA. After washing with DPBS, the cells were incubated for 24 h with various concentrations of the AuNR solutions. Then, the medium in each well of plate was replaced with 200 μl of CCK-8 solution (10 v/v% in RPMI 1640), and the cells were incubated for an additional 2 h at 37 °C. Cell viability was evaluated by measuring the absorbance at 450 nm of each well with a microplate reader (Synergy HT Multi-Mode microplate reader, Biotek, USA). To assess cell cytotoxicity of DOX-P-AuNR in both presence and absence of NIR irradiation, MDA-MB-231 cells (1 × 104 cells well−1) were cultured and seeded in 96-well plates and then incubated for 24 h as described above. Cells of each plate were treated with free DOX and DOX-P-AuNR with various concentrations of DOX and incubated for 3 h and the media was replaced with fresh media. Plates were then divided into two parts. One plate was irradiated using an 808 nm laser at a fluence of 2 W cm−2 for 5 min and the other plate was kept without laser irradiation. After 24 h of incubation, cell viability was determined by comparing the absorbance at 450 nm with that of cells grown in cell culture medium only.
2.11. In vitro cellular uptake
To investigate cellular uptake and the intracellular drug release behavior of DOX-P-AuNR, MDA-MB-231 cells were seeded onto gelatin-coated cover slips in 6-well plates at a density of 1 × 105 cells well−1 and incubated for 24 h in 2 ml RPMI 1640 medium with 10% FBS and 1% AA. Media were replaced with media containing free DOX and DOX-P-AuNR (5 μg ml−1 concentration of DOX), and incubated for 3 h at 37 °C. In a similar way as described above, one plate was exposed to an 808 nm laser (2 W cm−2, 5 min) and other plates were kept in the dark without irradiation. Cells were washed twice with DPBS, fixed with 4% paraformaldehyde solution, and the cell nuclei were stained using 4, 6-diamidino-2-phenylindole. Fixed cells were observed under a confocal laser microscopy (LSM 510 META NLO, Carl Zeiss GmbH, Heidelberg, Germany).
2.12. Flow cytometric analysis
To study intracellular release of DOX from DOX-P-AuNR after NIR irradiation, MDA-MB-231 cells were seeded in 6-well plates at a density of 1 × 105 cells and incubated for 24 h at 37 °C in a 5% CO2 humidified atmosphere. The media was replaced with fresh media containing DOX-P-AuNR (2 μg ml−1 concentration of DOX), which was incubated at 37 °C for 3 h. After incubation for 3 h, plates were then kept under irradiation at a fluence of 2 W cm−2 for 5 min or without irradiation. Then, cells were incubated for another 30 min, washed with cold DPBS, and harvested by trypsin treatment. Cell pellets were washed twice with DPBS, and resuspended in 200 μl DPBS to detect DOX fluorescence using flow cytometry (guava easyCyte, EMD Millipore, USA) with laser excitation at 488 nm.
3. Results and discussion
3.1. Preparation and characterization of P-AuNRs
P-AuNRs were prepared by chemical conjugation of disulfide-bearing PEG-b-PCL copolymer onto the surface of AuNRs (figure 2). PEG-b-PCL copolymer was obtained by reacting N3-PCL-SS-PCL-N3 and PEG-DBCO via click chemistry. N3-PCL-SS-PCL-N3 was synthesized through three steps and the chemical structure of polymer, obtained from each step, was analyzed using 1H NMR (figure 3). The characteristic peaks of PCL-SS-PCL appeared at 3.7 ppm (–OH), 2.9 ppm (–S–CH2−), and 1.4 ppm (−CH2−). For N3-PCL-SS-PCL-N3, the characteristic peak of the hydroxyl end group (at 3.7 ppm) of PCL-SS-PCL disappeared and new peaks for benzene ring protons (at 7.8 ppm) and methylene protons neighboring to the azide group (at 3.3 ppm) were detected. As shown in figure S1, a unimodal distribution of N3-PCL-SS-PCL-N3 with a Mn of 7280 and polydispersity index (PDI) of 1.26 was confirmed using gel permeation chromatography. By treating N3-PCL-SS-PCL-N3 with D, L-dithiothreitol, the reduced form (N3-PCL-SH) was also obtained since it directly reacts with the surface of AuNRs through the formation of Au–S bond. N3-PCL-SH had a unimodal distribution with a Mn of 4750 and PDI of 1.26. The chemical structure of PEG-DBCO, synthesized via formation of the amide bond in the presence of EDC and NHS as a catalyst, was confirmed by its characteristic peaks at 3.3 ppm (–CH3 in PEG), 3.5–3.7 ppm (–CH2−CH2−O– in PEG), 5.1 ppm (–NCH2− in DBCO group), and 7.3–7.7 ppm (–aromatic H in DBCO group). From the FT-IR spectrum in figure S2, the peak correlating with an azide stretching frequency of N3-PCL-SS-PCL-N3 appeared at 2100 cm−1, and it disappeared after reaction with PEG-DBCO, demonstrating the success of the click reaction. To confirm the conjugation of polymers onto CTAB-AuNRs via the ligand exchange reaction, we observed the phase transfer behavior after conjugation in the water/oil phase [43]. When the CTAB-AuNRs were conjugated with N3-PCL-SS-PCL-N3 only, the AuNR solution was soluble in chloroform, whereas P-AuNR solution was dispersed in water phase (figure S3).
Figure 2. Synthetic routes for the P-AuNR conjugate.
Download figure:
Standard image High-resolution imageFigure 3. 1H NMR spectra of (a) PCL-SS-PCL, (b) Ts-PCL-SS-PCL-Ts, (c) N3-PCL-SS-PCL-N3, and (d) PEG-DBCO.
Download figure:
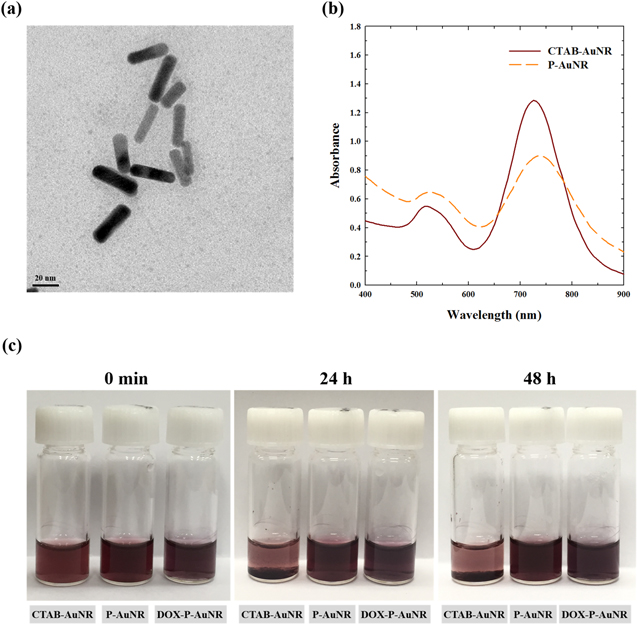
Standard image High-resolution imageP-AuNRs was readily obtained by reacting CTAB-AuNRs with PEG-b-PCL through formation of the Au–S bond. The TEM image demonstrated that the size of P-AuNR was 32.6 ± 4.1 nm and 9.7 ± 1.4 nm for average length and width (aspect ratio = 3.36), respectively (figure 4(a)). The particle size and zeta potential of P-AuNR, measured by DLS, were 74.9 ± 5.79 nm and −10.9 ± 0.38 mV, respectively (table S1). The negative surface of P-AuNR might be due to the presence of PEG on the surface [44, 45]. It is interesting to note that the average size of P-AuNRs, measured by DLS, was higher than that observed using TEM, which might be due to the differences in the experimental condition: i.e., DLS measures the hydrodynamic size in an aqueous environment, whereas TEM observes the sample in its dried condition. As shown in figure 4(b), P-AuNRs had the longitudinal plasmon resonance absorption of AuNRs at 740 nm, slightly red-shifted from CTAB-AuNRs (730 nm). This implies that P-AuNRs have potential to generate heat by absorbing the energy via irradiation at the NIR region. To investigate the colloidal stability of nanoparticles, CTAB-AuNRs, P-AuNRs, and DOX-P-AuNRs were incubated in serum-containing buffer solution, mimicking the biological condition (figure 4(c)). P-AuNRs and DOX-P-AuNRs demonstrated excellent stability for 48 h without any visible aggregation, whereas CTAB-AuNRs as the control were aggregated and precipitated after 24 h.
Figure 4. TEM image of (a) P-AuNR. (b) UV/Vis spectrophotometer spectra of CTAB-AuNR and P-AuNR. (c) Photographs of CTAB-AuNR, P-AuNR, and DOX-P-AuNR suspended in PBS (pH 7.4) containing 10% fetal bovine serum.
Download figure:
Standard image High-resolution image3.2. Photothermal effect under NIR irradiation
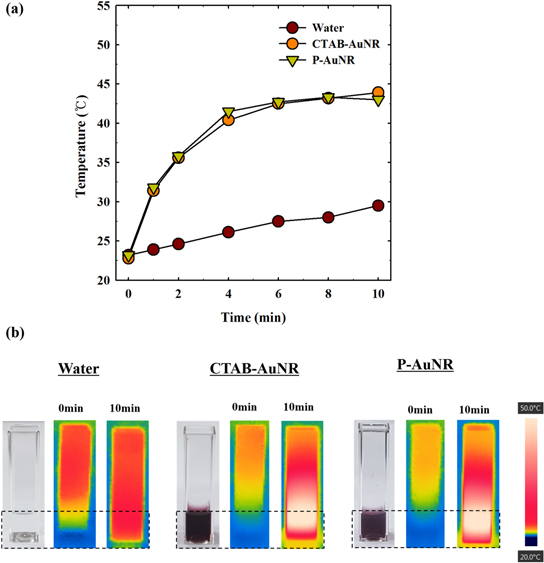
To evaluate the photothermal effect of AuNRs under NIR irradiation, we measured changes in temperature of the AuNR solution at the irradiation fluence of 2 W cm−2 using the 808 nm laser (figure 5(a)). CTAB-AuNRs and P-AuNRs demonstrated a similar profile with rapid temperature increase (>10 °C) within 2 min at the same concentration of AuNRs (0.2 mg ml−1), while water showed little change. Thermographic images in figure 5(b) also indicated that both CTAB-AuNRs and P-AuNRs can efficiently induce thermal conversion when irradiated with NIR light. These results imply that the photothermal conversion efficiency of AuNRs was not significantly affected by the chemical modification with PEG-b-PCL copolymer.
Figure 5. (a) Temperature changes under NIR irradiation (2 W cm−2) as a function of time. (b) NIR thermographic images after 10 min irradiation. Concentration of AuNR: 0.2 mg ml−1.
Download figure:
Standard image High-resolution image3.3. In vitro drug release
In order to investigate NIR light-triggered drug release, we physically encapsulated DOX into the P-AuNRs through the hydrophobic interaction between DOX and the PCL block. The LE of DOX was 69% (table S1), and DOX release amount for 3 h at 37 °C was determined by using a fluorospectrometer (figure 6(a)). The fluorescence emission of DOX from DOX-P-AuNRs was significantly quenched in the absence of laser irradiation, implying no significant release of DOX. In comparison, the fluorescence of DOX was recovered (59.4%) when DOX-P-AuNRs were irradiated for 10 min. These results imply that DOX-P-AuNRs can encapsulate DOX with a slow drug diffusion rate without laser and rapidly release the drug upon exposure to NIR light. Next, we investigated drug release profile as a function of time in temperature-dependent manner (37 °C, NIR-induced heating and 50 °C) to confirm that DOX is released owing to the phase transition of PCL block by melting. As demonstrated in figure S4, the melting temperature of PCL was 50 °C–52 °C, which was verified using differential scanning calorimetry. Without laser irradiation, the amount of released DOX for 24 h was low (31.0% ± 0.64%) at 37 °C; however, DOX was rapidly released under both NIR laser-induced heating and 50 °C, resulting in the burst release of 70.6% ± 0.64% and 62.6% ± 0.97% of DOX, respectively (figure 6(b)). This temperature-dependent release of DOX might be due to thermal transition of PCL regime to the loosened amorphous phase at 50 °C [46, 47]. This result indicated that NIR-induced heating affected the release of DOX in the same manner as thermal heating did.
Figure 6. (a) Emission spectra of DOX under NIR irradiation (2 W cm−2). (b) In vitro drug release of DOX. The error bars represent mean ± SD (n = 3).
Download figure:
Standard image High-resolution image3.4. In vitro cytotoxicity evaluation
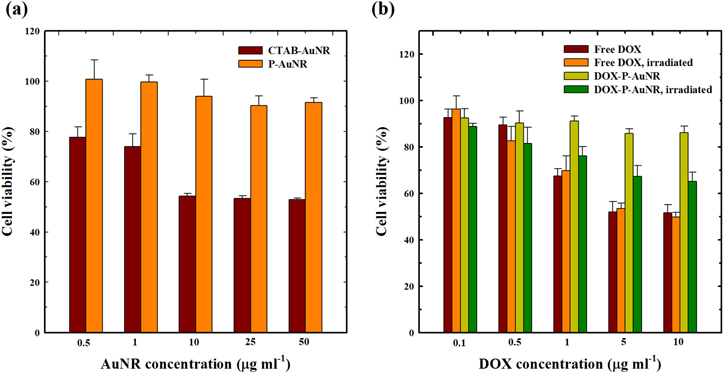
The in vitro cellular cytotoxicity was evaluated using CCK-8 assays in the human breast cancer cell line MDA-MB-231 (figure 7(a)). First, we compared the cytotoxicity of CTAB-AuNRs and P-AuNRs since CTAB is limited to biomedical applications owing to its toxicity. P-AuNRs did not exhibit significant cytotoxicity (>90%), whereas CTAB-AuNRs showed 50% cell viability when they were treated with 50 μg ml−1 of AuNRs. We also conducted the in vitro antitumor efficacy study of DOX-P-AuNRs in presence or absence of NIR light (figure 7(b)). The cell viability of free DOX-treated group was not particularly dependent on NIR irradiation. At 10 μg ml−1 of free DOX, the cell viabilities with and without NIR irradiation were 49.8% ± 1.88% and 51.6% ± 3.45%, respectively. At the same concentration of DOX, DOX-P-AuNRs showed 86.1% ± 2.87% of cell viability in the absence of NIR light, whereas the NIR irradiation led to notably decreased cell viability (65.1% ± 4.10%), indicating that release of DOX from DOX-P-AuNRs was controlled by NIR irradiation. These results were in accordance with in vitro drug release behavior and suggested that P-AuNR could reduce the indiscriminate toxicity of DOX by utilizing the external stimuli, NIR light.
Figure 7. In vitro cytotoxicity of (a) AuNRs and (b) DOX-loaded P-AuNRs. The error bars represent mean ± SD (n = 5).
Download figure:
Standard image High-resolution image3.5. In vitro intracellular drug release behavior
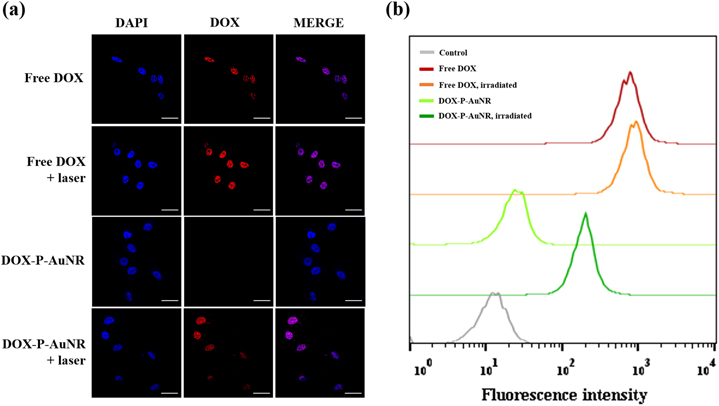
Finally, we assessed NIR-triggered intracellular drug release behavior by using the MDA-MB-231 cell line (figure 8(a)). As expected, free DOX showed laser-independent cellular uptake and accumulated into the cell nuclei. For DOX-P-AuNRs, no significant fluorescent signals were detected in the absence of NIR irradiation at the intracellular compartments, which might be due to the negligible drug release. Interestingly, under NIR irradiation, the nuclei of cells exhibited the strong fluorescence, indicating rapid release of DOX. The intracellular drug release was also confirmed using flow cytometric analysis. As illustrated in figure 8(b), fluorescence intensity of DOX from DOX-P-AuNRs was remarkably enhanced when exposed to NIR laser compared to the laser-untreated condition, which was consistent with the confocal laser microscopy images. These results indicate that DOX-P-AuNRs could enter tumor cells and release DOX by responding to the external light source.
Figure 8. (a) Confocal microscopic images of DOX 3 h post-incubation. The nuclei of cells are stained with DAPI (blue) and the fluorescence from DOX is red. (b) Flow cytometric analysis (concentration of DOX: 2 μg ml−1). The scale bar is 20 μm.
Download figure:
Standard image High-resolution image4. Conclusion
In summary, AuNR-based drug carriers were designed for thermochemotherapy using NIR light as the external stimuli. The polymer, PEG-b-PCL, was prepared via biorthogonal click chemistry and can be used to form AuNR-polymer conjugates with good colloidal stability and low cytotoxicity, substituting the toxic stabilizer of AuNRs with an Au–S covalent bond. Owing to the hydrophobic PCL segment, DOX was effectively loaded into P-AuNRs. The NIR-triggered increase in temperature and drug release profile were verified by detection of the fluorescence signal of DOX. DOX-P-AuNRs effectively released DOX when they were irradiated with the 808 nm NIR laser source, which might be due to the thermal transition of PCL block from crystalline to amorphous form. Overall, AuNR-based polymer conjugates had the potential to be developed as drug carriers for DOX.
Acknowledgments
This work was financially supported by the National R&D Program for Cancer Control (1420040) of MW, the Basic Science Research Programs (20100027955 & 2015R1A2A2A0500N1390) of NRF, and the National Research Council of Science and Technology (NST) through the Degree and Research Center (DRC) Program (2014), Republic of Korea.