Abstract
Heteroatom-doped graphitic frameworks have received great attention in energy research, since doping endows graphitic structures with a wide spectrum of properties, especially critical for electrochemical supercapacitors, which tend to complement or compete with the current lithium-ion battery technology/devices. This article reviews the latest developments in the chemical modification/doping strategies of graphene and highlights the versatility of such heteroatom-doped graphitic structures. Their role as supercapacitor electrodes is discussed in detail. This review is specifically focused on the concept of material synthesis, techniques for electrode fabrication and metrics of performance, predominantly covering the last four years. Challenges and insights into the future research and perspectives on the development of novel electrode architectures for electrochemical supercapacitors based on doped graphene are also discussed.
Export citation and abstract BibTeX RIS
Introduction
Energy, a fundamental demand for humankind, is mainly sourced from fossil fuels. Our future requirements are expected to increase considerably due to population growth and changing lifestyles. The world energy assessment report estimates that global energy consumption is expected to reach about 27.6 terawatts (TW) by 2050 [1]. Currently, there is a scientific consensus about the environmental damage caused by the emission of carbon dioxide and other greenhouse gases, which primarily originate from our dependence on the diminishing fossil fuels. Despite the introduction of several advanced technologies to circumvent these issues, the US Energy Information Administration's annual energy outlook 2015 anticipates that energy-related carbon emissions will increase [2].
Driven by society's needs, the development of energy-efficient devices is being constantly pursued and/or improved. Today, energy is stored and derived from various electrochemical energy devices, such as lithium-ion batteries, solar cells and fuel cells. Although the world has become enamoured with lithium-ion battery technology, which is suitable for portable electronic goods, it is not the best candidate yet for electric vehicles despite the batteries' higher operating voltage and energy density. In addition, the sources available to produce energy are either intermittent, scarce or location-specific, the sun for example. Therefore, to reduce the energy dependence on fossil fuels and to curb the emission problems, low-cost renewable and alternative energy storage systems are highly desirable.
Electrochemical supercapacitors are promising and efficient energy storage devices because they can store and release energy quickly. Unlike batteries, they can be integrated with microelectronic devices and can operate for very long periods of time without losing their energy storage capacity. With their high power density and long cycle life, they can play complementary functions when hybridised with a lithium-ion battery, known for its relatively high energy density, thus bridging the gap between energy and power demand, a key requirement for hybrid electric vehicles and power backup for portable electronics. Categorised by their charge storage mechanism, they are classified into electrochemical double layer capacitors (EDLCs) and pseudocapacitors (PCs). Typically, carbon-based electrode materials with high specific surface area exhibit predominantly static double layer capacitance (DLC), where energy is stored via reversible ion adsorption at the electrode/electrolyte interface. The EDLC-based supercapacitor is one of the most developed forms of electrochemical capacitor so far. On the other hand, PCs store charges via reversible redox reactions occurring on or near the electrode surface. Electrically conducting polymers and transition metal oxides are widely employed for the construction of such pseudocapacitor electrodes. A combination of EDLC and PC can store and release electrical energy by nanoscopic charge separation at the interfaces between the electrode and the electrolyte.
Graphene and graphene-based materials are deemed to be very suitable for electrochemical energy applications, in particular for electrochemical supercapacitors, given their exotic properties, namely electrical conductivity and large surface area. Significant advances have been made in the recent past, in which promising results were demonstrated. Graphene is perhaps currently the most extensively examined and widely utilized electrode material in EDLCs. Chemically inert, however, pristine graphene can benefit from the doping of heteroatoms such as boron (B), nitrogen (N), sulfur (S) and phosphorus (P) into its lattice that could further lead to a change in chemical activity, not only improving wettability, but also inducing favorable pseudocapacities for electrochemical supercapacitors. In addition, graphene materials doped with heteroatoms are more advantageous because of their better conductivity, stability, chemical reactivity and sheet-to-sheet separation. In this article, we mainly focus on the importance of heteroatom-doped graphene, summarize briefly its present status and very recent progress in its synthesis, and review the role of such materials as supercapacitor electrodes in some detail.
Strategies for synthesis and capacitive properties of doped graphene
Doping is a form of replacing a carbon atom in the graphitic lattice with a heteroatom that can be done either by in situ processing during carbon material synthesis or through post-processing of the carbon with a heteroatom-containing precursor. In the recent past, doping carbonaceous materials, especially graphene, with heteroatoms has attracted tremendous attention, because it is an efficient way to tailor and enhance the chemical and structural properties that can broaden their applications in energy conversion and storage. Although structural and electronic distortions are inevitable, the generation of charged or active sites on the surface of graphene, by substitutional doping with heteroatoms such as nitrogen [3], sulfur [4], dually doped nitrogen/sulfur [5], boron [6], dually doped nitrogen/boron [7] as well as doping phosphorus [8] into the graphene lattice, is considered important and offers possibilities that may be beneficial for frontier energy applications. In particular, for electrochemical supercapacitors, which can be charged and discharged in seconds, doping can effectively modify the surface properties of graphene, and thus form one of the most prominent families of carbon materials today. In essence, doping heteroatoms, especially nitrogen, into a graphitic lattice may enhance the energy states or density of states (DOS) at the Fermi level, thus overcoming quantum capacitance limitations of nanocarbons, and eventually increasing net specific capacitance, leading to a high power and a high energy density.
The synthetic pathways to dope a graphitic lattice with heteroatoms are manifold, and are thus not limited to a single standard procedure. Although in situ doping (CVD, arc discharge etc) can incorporate heteroatoms into the graphitic structure homogeneously, post-treatment of graphene (thermal treatment, plasma-assisted doping, etc) with reactive heteroatom-containing sources is an attractive approach to obtain heteroatom-doped graphene. As an explosively growing field, doping strategies are becoming more common and several reports have accounted the synthesis and doping procedures of carbonaceous materials including graphene, especially nitrogen for targeted applications such as electrochemical supercapacitors. Accordant review articles published previously are hence recommended [9–12]. In the following section, a brief introduction to the most commonly used technique to obtain heteroatom-doped graphene is described and its capacitive properties are reviewed.
Nitrogen-doped graphene
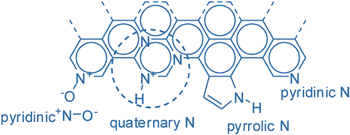
Nitrogen, neighboring carbon in the periodic table, has an atomic size similar to that of carbon but a different electronic configuration. Doping graphene with nitrogen (N-doping) easily manipulates the electronic structure, resulting in three common bonding configurations within the graphitic lattice, namely pyrrolic, pyridinic and graphite-like or quaternary nitrogen (figure 1) [13]. In addition to increasing the charge-carrier density, leading to large interfacial capacitance, the doping configuration, in general, dictates the capacitive property of the N-doped graphene to a large extent, and thus is a key factor in the design of an electrode material for supercapacitor.
Figure 1. Types of nitrogen species that can be incorporated into a graphitic framework, in which the pyridinic N and quaternary N are sp2 hybridized while pyrrolic N is sp3 hybridized. Adapted from [13]. Copyright Springer 2009.
Download figure:
Standard image High-resolution imageNumerous strategies for synthesis including CVD and non-CVD methods (or post-processing techniques) have been employed to fine-tune the physical and chemical properties of these carbonaceous materials to produce N-doped thin films of graphene as well as N-doped graphene powders to suit a variety of applications. In recent years, however, there seems to be a surge in the use of non-CVD methods to dope graphene because the layer distribution of N-doped graphene depends on the synthesis procedure. Methods such as thermal annealing of graphene oxide (GO) [14–17], wet-chemical methods such as hydrothermal [18–23], solvothermal [24, 25], microwave [26–29] and plasma-assisted techniques [3, 30, 31] were commonly used to obtain few-layer N-doped graphene. Interestingly, most of the post-processing techniques reported thus far use GO as the precursor to prepare doped graphene. GO, usually obtained by the corrosive oxidative breaking and/or exfoliation of graphite flakes, is thus perhaps the most studied/used graphene derivative for preparing doped graphene, due to the ease of its mass preparation and its versatile functionality. A series of N-doped graphenes were prepared starting from GO, and the nitrogen content of the resulting N-doped graphene materials ranged from 1% to 16%. As-prepared N-doped graphene materials were widely used as electrodes in supercapacitors with improved device performances and have been reviewed frequently [9, 32–35]. In the following sections we highlight and discuss heteroatom-doped graphene structures that were prepared by post-processing techniques predominantly using GO as a precursor with a doping source, and summarize the most recent advances in the field.
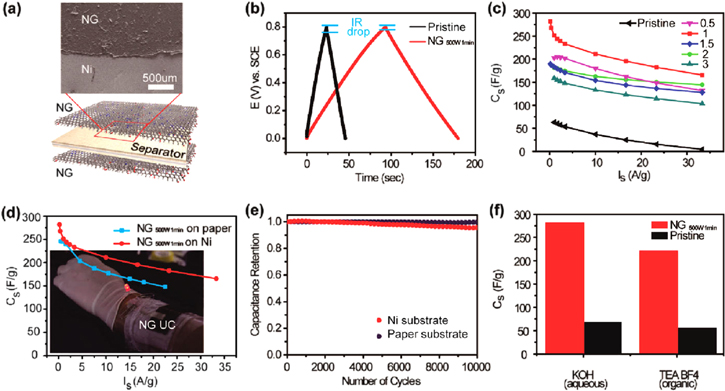
When graphene or reduced graphene oxide (rGO) is exposed to plasma in the presence of ammonia gas or nitrogen, substitutional doping of nitrogen atoms on the carbon lattice occurs, knocking off the oxygen groups [30]. The nitrogen content and configuration can be regulated by the strength of the plasma and exposure time. Thus, appropriate nitrogen configuration can greatly promote the capacitance of N-doped graphene. As a representative example, using such a simple plasma process, Jeong et al developed a wearable N-doped rGO supercapacitor electrode that showed lower resistivity and exhibited a capacitance four times higher than that of pristine graphene, reaching nearly 280 F g−1 at a high current density of 1 A g−1 (figure 2). While maintaining other essential and useful properties for operation of a supercapacitor, the electrode exhibited excellent cycle life with high power capability, and compatibility with flexible substrates (figure 2(d)) [36]. Moreover, the N-doped graphene-based device showed a much higher capacitance than a device based on pristine graphene in both KOH and organic electrolyte (figure 2(f)). The basal-plane pyridinic N on the N-doped graphene was found to exhibit the largest binding energy with K+, which can eventually accommodate a larger number of ions in the electrolyte on the electrode surface. Thus, the basal-plane pyridinic N was claimed to have a dominant role in the enhancement of capacitance.
Figure 2. (a) Schematic illustration of the assembled supercapacitor structure alongside a SEM image showing a top view of the device. (b) Charging and discharging curves measured by galvanostatic characterization. (c) Gravimetric capacitances of supercapacitors based on various N-doped reduced graphene oxide (rGO) and pristine rGO measured at a series of current densities. The numbers in the legend indicate the plasma durations in minutes. (d) Gravimetric capacitances of supercapacitors built on nickel and paper substrates measured at a series of current densities. Inset: a photograph showing a wearable supercapacitor wrapped around a human arm that can store the electrical energy to light up a LED. (e) Cycling tests for the supercapacitors based on Ni and paper substrates up to 10 000 cycles. (f) The specific capacitances measured in aqueous and organic electrolytes. Adapted from [36]. Copyright American Chemical Society 2011.
Download figure:
Standard image High-resolution imageUsing a microwave plasma process, Nolan et al reduced and doped GO simultaneously to prepare N-doped rGO with a total nitrogen content level of ∼5%. While studying the capacitive behavior of the electrode prepared by spray-depositing the aqueous N-doped rGO, it was determined that surface redox events occur at the functional groups of the N-rGO, which contributed to the pseudocapacitive mechanism leading to the charge storage at the electrode [30].
Apart from the plasma approach, GO can also be hydrothermally treated in the presence of a nitrogen precursor—ammonia gas [37] or urea [22, 38] for example—to dope N into the graphitic lattice while reducing the oxygen functionalities simultaneously. Using urea as a dopant and reductant, N-doped graphene nanosheets with a large specific surface area (593 m2 g−1) and nitrogen level as high as 10.13 at.% were synthesized. In 6 M aqueous KOH electrolyte, the synthesized nanosheets achieve a specific capacitance of 326 F g−1 at a current density of 0.2 A g−1 along with excellent cycling stability, maintaining initial capacitance (99.85% coulombic efficiency after 2000 cycles) [22]. Similarly, N-doped graphene hydrogel with a large specific surface area of 1500 m2 g−1 and a nitrogen content of 5.86 at.% N (with dominant pyrrolic N) was synthesized by the hydrothermal method with urea. Specific capacitance as high as 308 F g−1 was achieved at a current density of 3 A g−1 [38]. It was inferred that the capacitive performance of N-doped rGO depends not only on the N content but also on the doping configurations. While graphitic N can facilitate electron transfer and pyridinic N can improve the wettability of doped graphene because of their large dipole moments, another study found that pyrrolic nitrogen formation was predominant when hydrothermally treating GO in the presence of hydrazine and ammonia, leading to enhanced capacitance of 194 F g−1 [39]. It should be noted that both pyridinic and pyrrolic N offer high pseudocapacitance as they are electrochemically active in an alkaline aqueous solution, whereas graphitic nitrogen enhances the conductivity of the graphene material.
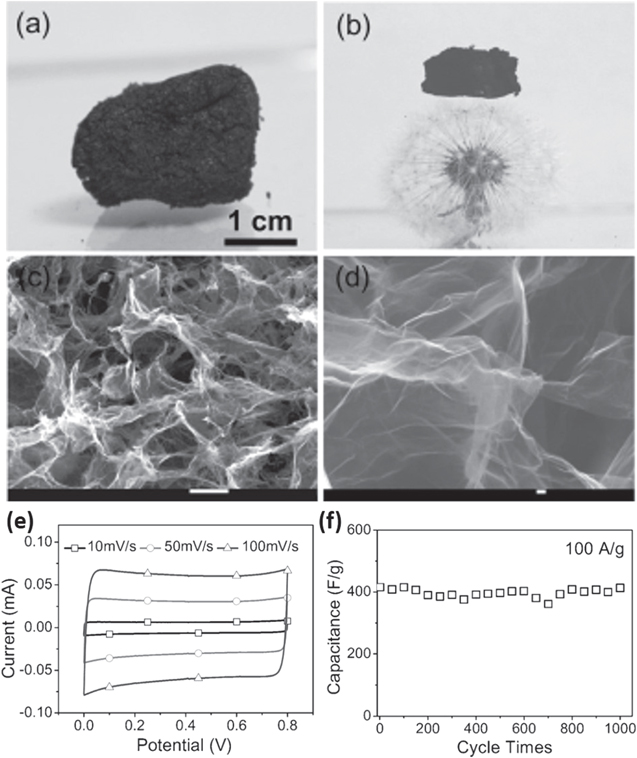
Following this initiation, a macroscopic three-dimensional (3D) N-doped, ultralight graphene framework (GF) with a high conductivity of (1.2 ± 0.2) × 103 S m−1 was prepared by hydrothermal treatment of aqueous GO suspension with 5 vol.% pyrrole in an autoclave at 180 °C for 12 h to form an N-containing hydrogel, which was freeze-dried and subsequently annealed at 1050 °C for 3 h under argon (Ar) atmosphere (figures 3(a)–(d)) [40]. The as-prepared GF with an ultralow density of 2.1 ± 0.3 mg cm−3 had a high thermal stability and was comparable to that of the lightest silica aerogels. The supercapacitors fabricated from the N-doped GF presented high-performance capacitive behaviors in both two- and three-electrode systems due to the combination of heteroatom doping and 3D porous structure. At a current density of 1 A g−1, the GF presents a specific capacitance of 484 F g−1 in 1 M aqueous LiClO4 electrolyte, which nearly approached the theoretical electrochemical double layer capacitance of 550 F g−1 for pure graphene. At the current density of 100 A g−1, the GF still had a capacitance of 417 F g−1. The framework also exhibited long cycle life (figure 3(f)). Although it is strongly believed that the enhanced pseudocapacitance originates from doping, it has recently been suggested that quantum and interfacial capacitance effects, in which the N atom provides additional mobile electrons to the graphitic lattice, are responsible for the increased capacitance of a N-doped graphene supercapacitor [41].
Figure 3. (a), (b) Photographs of an as-prepared superlight graphene framework (GF) and one with a piece of GF size of 1.8 cm × 1.1 cm × 1.2 cm standing on a dandelion. (c), (d) SEM images of the sample in (a). Scale bars: (c) 10 μm, (d) 100 nm. (e) Current–voltage (CV) curves of a GF electrode in 1 M aqueous LiClO4 solution. (f) Cyclic stability of the GF-based capacitor with a current density of 100 A g−1. Adapted from [40]. Copyright Wiley Publishing group (2012).
Download figure:
Standard image High-resolution imageVery recently, it was shown that 3D macroscopic N-doped graphene hydrogels, which were prepared from organic amines and GO as precursors, exhibited excellent supercapacitive performance. The organic amine was used as the nitrogen source and to control the assembly of graphene sheets in the 3D structures. At a charge/discharge rate of 185 A g−1, a specific capacitance of 113.8 F g−1 and a high power density of 205 kW kg−1 were obtained. 92.5% of the capacitance was retained at a current density of 100 A g−1 after 4000 cycles [19]. When hydroxylamine was used as a dopant and reductant, the N-doped rGO (N-rGO) hydrogel exhibited a specific capacitance of 205 F g−1 [42]. A macroscopic N-rGO hydrogel prepared at relatively low temperature exhibited a capacitance of 217.8 F g−1 at a current density of 1 A g−1 [43].
An interconnected network architecture with carbon nanotubes (CNTs) inserted in-between graphene nanosheets (here N-doped graphene or N-rGO) could simultaneously alleviate the agglomeration of N-rGO and promote the diffusion of ions in the electrolyte (enhancing surface wettability). Thus, fully utilizing the carbon framework's specific surface area, the network architecture can enhance the conductivity, and therefore the specific capacitance of the supercapacitor device. On this basis, 3D N-doped graphene–CNT networks (NGCs) were obtained by hydrothermal treatment, freeze-drying and subsequent carbonization of GO-dispersed pristine CNTs in the presence of pyrrole. The resulting NGCs used as a supercapacitor showed a specific capacitance of 180 F g−1 with good rate capability and retained 96% of the initial capacitance after 3000 cycles [44].
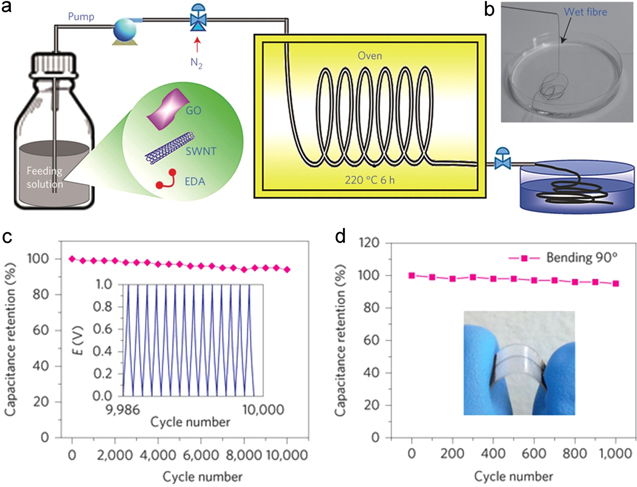
Hierarchically structured carbon composite fibers made of an interconnected network of single-walled carbon nanotubes (SWNTs) with interposed N-rGO sheets were recently prepared by a wet-spinning technique using a silica capillary column as a hydrothermal micro-reactor (figures 4(a) and (b)) [45]. In addition to the high electrical conductivity of 102 S cm−1, the resultant mesoporous fibers with large surface areas of 396 m2 g−1 showed a good tensile strength of 84 MPa to 165 MPa. Consequently, micro-supercapacitors fabricated with these composite fibers featured a specific capacitance up to 305 F cm−3 in H2SO4 electrolyte in a three-electrode set-up and 300 F cm−3 in polyvinyl alcohol (PVA)/H3PO4 electrolyte in a two-electrode cell with negligible loss in the cyclic stability or rate capability. This is one of the highest volumetric capacitance values reported among porous structured carbon composite materials (figure 4(c)). The hybrid fibers were robust enough when they were subjected to mechanical bending tests. They remained flexible and structurally consistent over their length and retained 97% of their initial capacitance after bending 1000 times at 90°, demonstrating the potential for flexible electronics.
Figure 4. (a) The fibre was synthesized by injecting a homogeneous solution containing acid-oxidized SWNTs, GO and EDA through a pump into a flexible silica capillary column, followed by in situ thermal treatment in an oven at 220 °C for 6 h before a continuous fibre was pushed into a water reservoir by a pressurized nitrogen flow. (b) Photograph of the as-prepared fibres collected in water. (c) Cycle life of the micro-supercapacitor. Inset: galvanostatic charge/discharge curve after 10 000 cycles between 0 and 1 V at 250 mA cm−3. (d) Capacitance retention after 1000 cycles up to 90° bending angle. Inset: photograph of a bent micro-supercapacitor. Adapted from [45]. Copyright Nature Publishing Group 2014.
Download figure:
Standard image High-resolution imageSeveral other groups evaluated the capacitive properties and studied the effect of nitrogen doping in a graphene lattice using various methods. The most recent examples over the past two years are summarized in table 1.
Table 1. Summary of performances of different N-doped graphene materials (2014–2015).
| Materials | N content (%) | Sp. Capacitance (F g−1) | Scan rate/Current density | Energy density/Power density | Electrolyte | Cycle life | Reference |
|---|---|---|---|---|---|---|---|
| N-rGO | 4.68 | 261 | 0.5 A g−1 | — | 1 M H2SO4 | 3000 | [46] |
| N-rGO | 9.83 | 364.6 | 10 mV s−1 | — | 1 M H2SO4 | 500 | [47] |
| N-rGO | 4.5 | 459 | 1 mA cm−2 | 25.8–63.5 W h kg−1/0.7–15.5 kW kg−1 | 1 M H2SO4 | — | [48] |
| N-rGO | 0.48 | 280 | 5 mV s−1 | — | 1 M H2SO4 | 40000 @100 mV s−1 | [49] |
| N-rGO | 3.97 | 246.4 | 1 A g−1 | — | 6 M KOH | 2000 | [50] |
| N-rGO | 4.7 | 3.4 F cm−3 (areal) | 20 mA cm−2 | 3.0 × 10-4 W h cm−3 (Volumetric energy density) | PVA-H3PO4 | 2000 | [51] |
| N-rGO | 10.85 | 313 | 0.1 A g−1 | — | 6 M KOH | 3000 @1 A g−1 | [52] |
| N-rGO aerogel | 5.0 | 290 | 1 A g−1 | 149.1 W h kg−1 | EMIMBF4 | 1000 | [53] |
| N-rGO | 6.85 | 242 | 1 A g−1 | 8.4 W h kg−1/250 W kg−1 | 1 M H2SO4 | 5000 | [54] |
| N-rGO microwave | 7.34 | 151 | 5 A g−1 | — | 6 M KOH | 5000 | [28] |
| 3D N-rGO porous | 11.3 | 138 | 1 A g−1 | — | 1 M Et4NBF4/PCa | — | [55] |
| N-rGO crumpled | 5.8 | 270 | 1 A g−1 | 24 W h kg−1/1.5 kW kg−1 | 1 M H2SO4 | 2000 | [56] |
| N-rGO/CNT paper | 8.4 | 294 | 1 A g−1 | — | 6 M KOH | 1000 | [57] |
| N-graphene | 2.64 | 324 | 0.1 A g−1 | — | 6 M KOH | — | [58] |
| N-rGO | — | 187.8 | 5 mV s−1 | 25 W h kg−1/10 kW kg−1 | 1 M TEABF4/PC | 730 | [59] |
| N-rGO | 7.34 | 340 | 0.5 A g−1 | — | 6 M KOH | 2000 | [60] |
| N-rGO silk cocoon | 15.2 | 348 | 5 mV s−1 | 9.8 W h kg−1/9.9 kW kg−1 | 1 M H2SO4 | 1000 @10 A g−1 | [61] |
| N-rGO aerogel | 8.4 | 223 | 0.2 A g−1 | — | 1 M H2SO4 | 2000 @1 A g−1 | [62] |
| N-rGO sol–gel | 9.77 | 245 | 0.25 A g−1 | — | 6 M KOH | 2000 | [63] |
| N-rGO | 7.41 | 170 | 0.2 A g−1 | — | 6 M KOH | 1000 | [64] |
| N-rGO buckygel | 3.8 | 145 | 20 mV s−1 | — | 6 M KOH | 5000 | [65] |
#EMIMBF4—(1-ethyl-3-methylimidazolium tetrafluoroborate). a1 M Et4NBF4/PC; TEABF4/PC—(tetraethylammonium tetrafluoroborate/propylene carbonate).
Boron-doped graphene
Apart from nitrogen, doping graphene with other heteroatoms such as boron (B) to improve supercapacitor performance is relatively less explored. Boron, having one electron less than carbon, has unique properties and thus doping B into a graphene lattice is highly amenable. Early work on doping B into pyrolytic graphite was shown to enhance space charge capacitance [66]. Later on doping B into other forms of carbon including carbon nanotubes and mesoporous carbon was found to improve properties with a significant boost in specific capacitance [67]. When doped into graphene, B acts as a p-type dopant and the B atom forms sp2 hybridizations in the carbon lattice. In-plane substitutional doping is more stable than out-of-plane bonding, and therefore the methodology of incorporation influences the electrochemical properties of the resultant B-doped graphene. Consequently, several strategies for synthesis were carried out to dope B into the carbon lattice. An accordant review by Rao et al detailing many synthesis procedures is recommended [9].
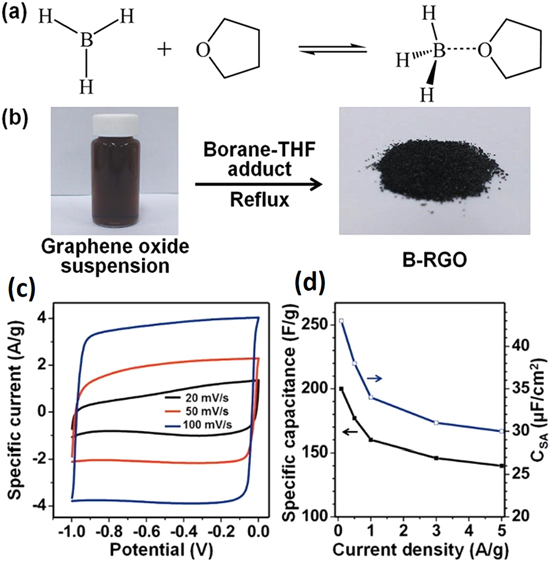
Recently, Han and co-workers were among the first to report the energy storage properties of B-doped graphene with high surface area produced through a scalable one-pot solution method for high-performance supercapacitors [6]. GO was reduced and doped using a borane (BH3)–tetrahydrofuran (THF) adduct under reflux, subsequently drying the sample in a vacuum under gentle conditions to yield 1.1 at.% of B incorporated into graphene platelets (figure 5). The resultant compound with a high specific surface area of 466 m2 g−1 showed excellent supercapacitor behavior including high specific capacitance values of 200 F g−1 (figure 5(d)) in a two-electrode configuration and 193 F g−1 in a three-electrode configuration with a good rate performance using an aqueous electrolyte. In addition, more than 95% of the original capacitance was retained after 4500 electrochemical cycles, and no distortion of the CV curves was observed after the cycle test, indicating a stable electrode material.
Figure 5. Boron doping of graphene prepared by hydroboration. (a) BH3–THF adduct and (b) reaction scheme for the reduction of GO and preparation of boron-doped graphene (B-rGO). The electrochemical performance of a B-rGO electrode in 6 M KOH solution using a two-electrode configuration, showing (c) the CV curves at various scan rates (two-electrode configuration) and (d) gravimetric capacitance and surface-area-normalized capacitance at various scan rates. Adapted from [6]. Copyright American Chemical Society 2012.
Download figure:
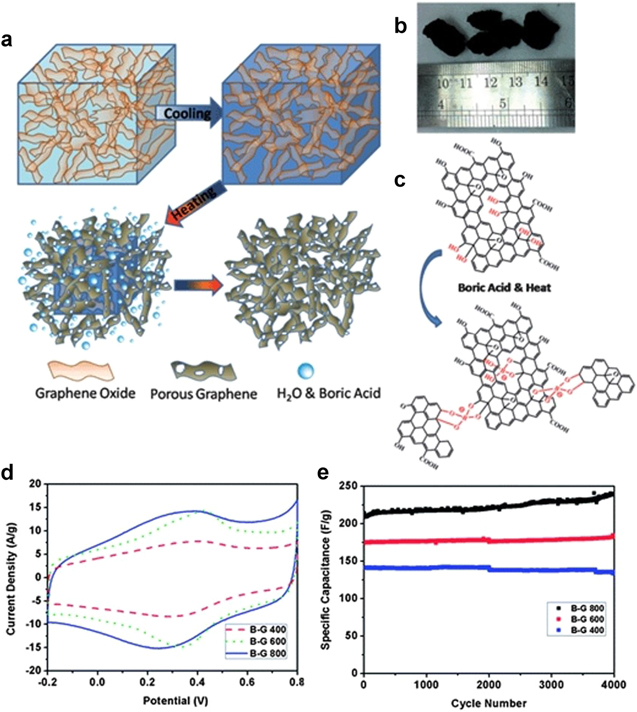
Standard image High-resolution imageIn an another interesting approach, 3D B-doped graphene was made by the so-called 'fried-ice' concept with porous morphologies. A GO gel containing boric acid was immersed in a dry ice–acetone bath to solidify the GO gel. Further, the frozen solid was exposed to thermal treatment. It was found that the morphology, boron content and specific surface area of these porous graphene materials could be tuned by controlling the reaction temperature. A specific surface area of 622 m2 g−1 was achieved due to the steaming effect (figure 6). The porous B-doped graphene electrodes show promising performance in supercapacitors with a gravimetric capacitance of up to 281 F g−1 in 2 M aqueous H2SO4 electrolyte [68]. Pseudocapacitance contributed to the doped B atoms and the remaining oxygen-containing functionalities were found to co-exist with EDLC (figure 6(d)). In addition, the long-term cycling stability demonstrates the robust electrochemical retention of the B-doped porous graphene (figure 6(e)).
Figure 6. (a) Possible formation process of the 3D B-doped porous graphene framework; (b) photo of the as-prepared 3D graphene monoliths; (c) illustration of the interaction between GO and boric acid molecules during the thermal treatment; (d) cyclic voltammograms of the 3D porous graphene framework in a three-electrode system in 2 M H2SO4 solution at a scan rate of 50 mV s−1; and (e) capacitance retention of the porous graphene performed at a current density of 10 A g−1. Adapted from [68]. Copyright The Royal Society of Chemistry 2013.
Download figure:
Standard image High-resolution imageSimilarly, B-doped graphene prepared by a simple pyrolysis process using GO and boric acid (dopant) in an argon atmosphere at 900 °C introduced 4.7% of boron content when pyrolyzed for 3 h. A specific capacitance of 172.5 F g−1 at 0.5 A g−1 was obtained in an alkaline medium and 96.5% of the initial capacitance was maintained after cycling continuously for 5000 times. Notably, the boron doping increased the capacitance by about 80% when compared to pristine graphene. The enhanced electrochemical properties of B-doped graphene were attributed to the incorporation of B atoms and the high percentage of oxygen-containing functional groups in the form of BC2O/BCO2 bonding configurations formed during boron doping [69]. The symmetric supercapacitor could deliver a high energy density of 3.86 W h kg−1 at a power density of 125 W kg−1, and retained 2.92 W h kg−1 at a higher power density of 5006 kW kg−1. In another study, B-doped graphene samples were prepared by thermally treating GO in a boron-rich environment at 1200 and 1500 °C and by subjecting GO to nitrogen plasma. A large increase in the specific capacitance was found in both acidic and alkaline media [70]. In another study, B-doped graphene sheets are used as carbon substrates instead of graphene for loading polyaniline by in situ polymerization. The sandwich-like polyaniline/B-doped graphene exhibited remarkably enhanced electrochemical specific capacitance in both acid and alkaline electrolytes. A specific capacitance about 406 F g−1 in 1 M H2SO4 and 318 F g−1 in 6 M KOH at 1 mV s−1 was observed in a three-electrode configuration [71].
Although recent works highlight the capacitance enhancement from B-doping, it is still debatable and there is no clear answer as to why an increase in capacitance is seen after doping a heteroatom into the graphitic lattice. Recently, Pumera et al demonstrated that the doping process does not lead to enhancement of capacitive behavior and concluded that the main characteristic influencing capacitance is in fact the presence of structural defects within the graphitic structure, independent of doping level [72]. Whether doping heteroatoms into graphene really influences the capacitance of graphene-based supercapacitors is a question that needs to be answered after more in-depth studies. Nevertheless, doping heteroatoms into a graphene lattice is still technologically important and relevant as it is seen as the main means to open and tailor the band-gap of the resulting material.
Boron and nitrogen co-doped graphene structures (BCN)
Boron and nitrogen atoms are adjacent to carbon in the periodic table. As B-doping helps to bring other atoms into the graphitic structure [73], doping graphene with B and N atoms can open the band-gap of graphene, leading to new structures and properties. In addition, the preferential B–N bond formation is in the stable energy state, so that could have a positive effect on the pseudocapacitive performance through the redox reaction.
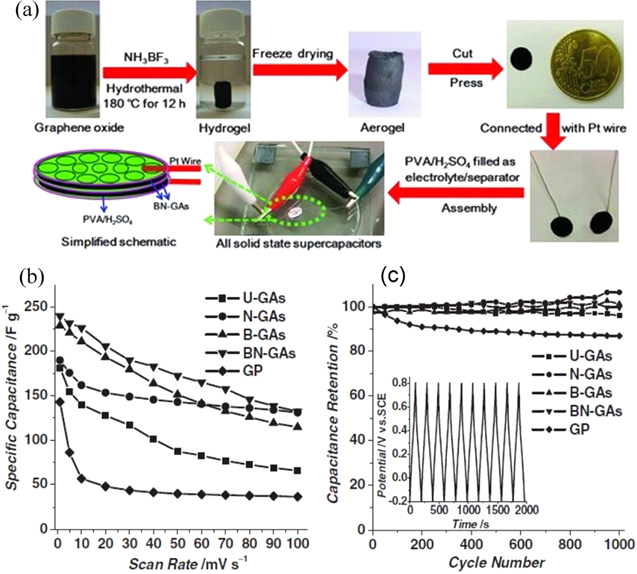
A 3D boron and nitrogen co-doped graphene aerogel (GA) was synthesized by hydrothermally treating GO with ammonia boron trifluoride and subsequent freeze-drying. The interconnected doped graphene sheets with ∼3% nitrogen content and 0.6% boron content facilitate charge transfer between the neighboring carbon atoms. Monolithic BN structures obtained by such a simple process were used as an additive-free composite to fabricate an all-solid-state supercapacitor electrode (figure 7) [74]. Significant synergistic effects from BN-co-doped graphene (BN-graphene) to improve electrocapacitive performance were reported when it was compared to N-doped, B-doped and pristine derivatives of the graphene materials. The highest specific capacitance of BN-graphene was ∼62 F g−1. The monolith also tested in 1 M aqueous H2SO4 electrolyte exhibited a capacitance as high as 239 F g−1 at a scan rate of 1 mV s−1 with high capacitance retention after 1000 cycles (figures 7(b) and (c)). The solid-state supercapacitor also achieves a high energy density of 8.7 W h kg−1 and power density of 1600 W kg−1 with respect to undoped (U-GAs), nitrogen-doped (N-GAs), boron-doped (B-GAs) GAs or layer-structured graphene paper. Interestingly, however, similar values were achieved even with pure B-doping. Later on, a layer-by-layer strategy was also used by the same group to produce B and N co-doped graphene films featuring high volumetric capacitance [75].
Figure 7. (a) Illustration of asymmetric solid-state supercapacitors (ASCs) based on BN-doped graphene aerogels (GAs) formed by a combined hydrothermal process and freeze-drying process. The as-fabricated supercapacitors with a diameter of 7 mm indicated by the dotted green ring and a simplified schematic of ASCs based on aerogels are shown (below left). A possible formation process of the 3D B-doped porous graphene framework is shown. (b) Specific capacitance of undoped aerogels (U-GAs), N-GAs, B-GAs, BN-GAs and graphene paper (GP) electrodes as a function of scan rate. (d) Comparison of the cycling stability of U-GAs, N-GAs, B-GAs, BN-GAs and GP electrodes. Inset is the charge–discharge profile of BN-GAs at 2 A g−1. Adapted from [74]. Copyright Wiley Publishing group 2012.
Download figure:
Standard image High-resolution imageBorocarbonitrides of high surface area prepared by the urea route have been examined for supercapacitors [76]. It was found that a specific composition (BC4.5N) shows a specific capacitance of 169 F g−1 at a scan rate of 10 mV s−1 in an aqueous electrolyte medium. In an ionic liquid medium, the specific capacitance value of the borocarbonitrides was similar to that of N-doped graphene with capacitance as high as 240 F g−1. In an attempt to reduce GO, ammonia borane was used and it could concomitantly dope B and N into the graphene lattice, yielding BN-doped rGO. Ammonia borane reduction of GO in tetrahydrofuran produced higher nitrogen (1.57 at.%) and boron (1.81 at.%) content and the co-doped graphene exhibited high supercapacitor performance. A specific capacitance of 130 F g−1 at a current density of 1 A g−1 was calculated for the material in an organic electrolyte (1 M TEABF4) [77].
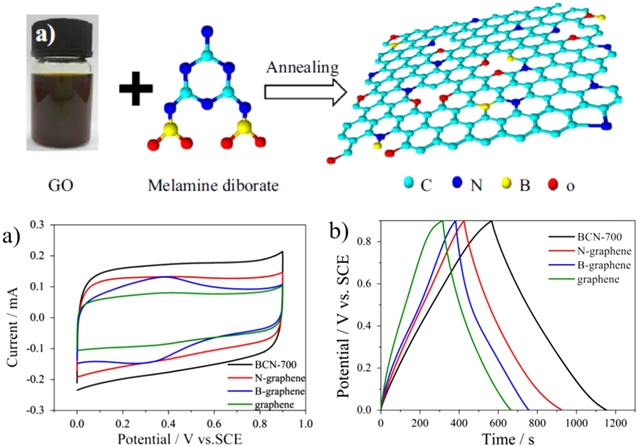
Very recently, boron and nitrogen co-doped graphene or BCN graphene structures were prepared by a simple thermal annealing approach in the presence of melamine diborate as the B and N source (figure 8). The capacitance behavior of the as-prepared co-doped graphene was investigated by cyclic voltammetry and charge/discharge techniques. The specific capacitance of BCN graphene was found to be (700) 130.7 F g−1 at 0.2 A g−1, which was much higher than N-graphene (111.3 F g−1), B-graphene (82.9 F g−1) and undoped graphene (77.4 F g−1). The highest capacitance was attributed to the efficient heteroatom doping (figure 8(b)) [7].
Figure 8. (Top) (a) Schematic of preparation BCN graphene. (Bottom) (a) CV curves of BCN graphene annealed at 700 °C, B-graphene, N-graphene and undoped graphene in 1 M H2SO4 electrolyte solution at a scan rate of 50 mV s−1. (b) Charge/discharge curves of the samples in 1 M H2SO4 electrolyte solution at a current density of 0.2 A g−1. Adapted from [7]. Copyright Institute of Physics Publishing 2015.
Download figure:
Standard image High-resolution imagePhosphorus-doped graphene
With the same number of valence electrons as nitrogen, phosphorus (P) shares similar properties, but it has a larger atomic size and lower electronegativity. Thus, P-doping can cause more structural distortion by transforming the sp2-hybridized carbon to the sp3 state and can also introduce a defect-induced active surface. Given that P-doping introduces more oxygen-containing functional groups to the carbon skeleton (typically over 15%), stable performance of supercapacitors from phosphorus-enriched porous carbons has been reported previously [78]. Apart from carbon motifs, in general, doping a graphene lattice with P is relatively rare. One study recently prepared P-doped graphene by annealing rGO with phosphoric acid at 220 °C [79]. Phosphoric acid activation was found to introduce micropores and phosphorus-containing functional groups on rGO. The supercapacitor electrode prepared offered a capacitance of 367 F g−1 at a scan rate of 5 mV s−1 and an energy density of 59 W h kg−1 in 1 M aqueous H2SO4 electrolyte. Phosphorus doping and increased conducting network due to reduction of GO were attributed to the increased pseudocapacitance. No degradation in specific capacitance was observed after 5000 charge–discharge cycles.
As there was no clear information on the level of P-doping in the graphene lattice, very recently Hulicova-Jurcakova and co-workers synthesized P-doped graphene by annealing a mixture of graphene and phosphoric acid to yield 1.3 at.% of P in the graphene lattice. When tested as a supercapacitor electrode, the P-doped graphene featured a capacitance of 115 F g−1 at a current density of 0.05 A g−1 in 1 M aqueous H2SO4 electrolyte. P-doped graphene can operate stably in the aqueous electrolyte at 1.7 V with excellent cycling performance (3% performance degradation after 5000 cycles at a current density of 5 A g−1). A high energy density of 11.64 W h kg−1 and a high power density of 831 W kg−1 were achieved [8].
N/P co-doped rGO electrodes with 2.9 at.% of N and 4.3 at.% of P showed a capacitance of 165 F g−1 with a retention ratio of over 80% [80]. Similarly, P/N-doped graphene synthesized by microwave-assisted carbonization of tannin cross-linked to melamine in the presence of polyphosphoric acid was evaluated electrochemically for a supercapacitor. In 1 M aqueous H2SO4 and 6 M aqueous KOH, the P/N-doped graphene featured a high specific capacitance of 271 F g−1 and 236 F g−1, respectively [81]. In another instance, N/P co-doped graphene materials of high specific surface area with 1.57 at.% of P and 4.81% of N were also synthesized in an attempt to increase the specific capacitance. An enhanced specific capacitance of 244 F g−1 with stability over 10 000 cycles was achieved in 6 M aqueous KOH electrolyte [82].
Sulphur-doped graphene
Sulphur doping to the carbon lattice plays a key role in the improvement of catalytic activity and quite a number of works have reported superior activity and performance. Sulphur changes the charge on the neighboring carbon to positive when it is doped with carbon. Consequently, doping graphene with S has been widely reported for oxygen reduction reaction (ORR) activity as well as for electrodes in lithium–sulphur-based batteries. However, in comparison with B and N, S-doped graphene for supercapacitor electrodes is still quite rare and represents an emerging field within carbon nanomaterials research. Earlier, S-doped carbon-based materials were prepared and used as electrodes for electric double layer capacitors, in which S-doping was proposed to enhance the capacitive performances [4, 83]. It was also suggested that the capacitive behavior of such systems is linked to the presence of oxidized sulphur species (sulfones and sulfoxides) [84].
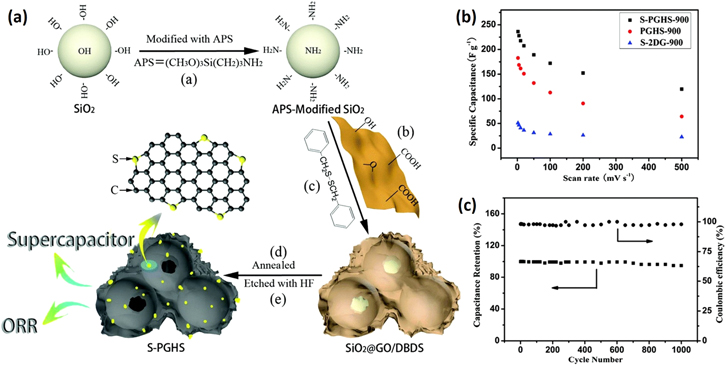
In general, S-doped graphene can be prepared by directly annealing GO and benzyl disulphide in an argon atmosphere or by thermally exfoliating GO in a sulphur-containing gas such as H2S, CS2 or SO2, or ball-milling graphite with sulphur powders. The amount of sulphur incorporated into the graphene lattice strongly depends on the method and dopant source as well as the temperature involved. From this basis, Chen et al prepared 3D S-doped porous rGO hollow nanosphere (S-PGHS) frameworks by directly annealing GO-encapsulated amino-modified SiO2 nanoparticles with dibenzyl disulphide, followed by HF etching (figure 9(a)). With ∼2% of elemental S in the lattice, the 3D porous graphitic framework displayed a very high specific surface area of 496 m2 g−1. The multifunctional material, as-prepared S-PGHS composite, when evaluated as a supercapacitor electrode, displayed high specific capacitance of 343 F g−1 at a current density of 0.2 A g−1. Along with good rate capability and excellent cycling stability, the composite S-PGHS-900 electrode demonstrated a 100% coulombic efficiency for each cycle of charge and discharge processes (figure 9(c)) [85]. It was inferred that the 3D porous graphitic structure with S-doping aided the fast ion diffusion and charge transfer at high current density, leading to enhanced capacitive performance.
Figure 9. (a) Schematic illustration of the preparation of S-doped porous rGO hollow frameworks (S-PGHS); (b) specific capacitance of the samples S-PGHS-900, PGHS-900 (no dopant) and S-2DG-900 (GO mixed with the dibenzyl disulphide alcohol solution directly without using SiO2–NH2 template) as a function of the scan rate; and (c) cycling stability and coulombic efficiency of S-PGHS-900 at a current density of 5 A g−1. Adapted from [85]. Copyright Royal Society of Chemistry 2014.
Download figure:
Standard image High-resolution imageSimilarly, composites of polymer (styrene-sulfonate sodium salt)-derived carbon with highly oxidized GO were synthesized and tested as supercapacitors. Sulphur species located in small pores were found to increase the EDLCs, while the sulfones and sulfoxides located in the larger pores were found to contribute to the pseudocapacitive effect. Increased gravimetric capacitance (∼109 F g−1 at 50 mA g−1) of sulphur-doped graphene materials compared to undoped ones was reported [86]. Very recently, another study demonstrated the synthesis of 3D S-doped rGO aerogels with a specific capacitance of 445.6 F g−1 at a scan rate of 5 mV s−1 [87]. The authors also demonstrated that the operating voltage window could be enlarged up to 3.5 V using an ionic liquid electrolyte.
Binary co-doping of heteroatoms into graphene such as N/S has also attracted considerable attention due to the strengthened or synergistic effect that is thought to contribute to the capacitive performance. Therefore, N and S dual-doped mesoporous carbon-based materials as well as 3D N and S co-doped graphene hydrogels with high specific capacitances have also been reported recently [88, 89].
Summary and future perspectives
Developing new electrocatalysts to address the energy crisis is the most demanding task today. Supercapacitor technology complementing lithium-ion batteries offers a viable alternative to address the crisis. Heteroatom-doped graphitic structures prepared via different approaches have been shown to possess unique and exclusive properties that aid the designing of nanostructured electrode materials for supercapacitor devices. The preceding sections provide an up-to-date survey of the capacitive performances of such heteroatom-doped graphene structures. Despite the many advances made, more attention should be paid to controlling doping levels, which is critical for capacitance. For example, the effect of dopant aggregations on the electrochemical properties can be examined. As it turns out to be difficult to reproduce the doping levels, new methods to improve the reproducibility of heteroatom-doped graphene must be explored. It is clearly evident that many works reported in the literature have focused on N-doped graphene for supercapacitors, and only a handful of reports have studied the capacitive behaviors of other heteroatom-doped or co-doped graphene structures. Besides, a vivid and fundamental understanding of energy storage mechanisms deserves more attention. Although considerable advances have been made with electrode architectures, most studies reported to date use either aqueous alkaline or acidic electrolytes, which are not commercially feasible. Organic, gel and ionic liquid electrolytes to enlarge the voltage window—a key component in building and improving overall supercapacitor performance—should be scrutinized. One way to improve the energy density of the materials based on doped graphene is to incorporate pseudocapacitive materials in the heteroatom-doped graphene lattices. Cost-effective, intelligent and straightforward engineering of electrodes for industry-level applications must be considered in future rather than the design of complicated nanostructured materials. Efforts should focus on more controllable and cost-effective methods for the fabrication of 3D architectures and transform research and development of heteroatom-doped graphene into practical applications.
Acknowledgments
The authors acknowledge the support of Creative Research Initiative (CRI), BK21 Plus, Mid-Career Researcher and Basic Research Laboratory (BRL) programs through the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science and Technology (MEST) and the US Air Force Office of Scientific Research through the Asian Office of Aerospace R&D (AFOSR-AOARD). NAK thanks the University of Queensland for a fellowship.