Abstract
The gas injection line of the latest spherical aberration-corrected environmental transmission electron microscope has been modified for achieving real-time/atomic-scale observations in moisturised gas atmospheres for the first time. The newly developed Wet-TEM system is applied to platinum carbon electrode catalysts to investigate the effect of water molecules on the platinum/carbon interface during deactivation processes such as sintering and corrosion. Dynamic in situ movies obtained in dry and 24% moisturised nitrogen environments visualize the rapid rotation, migration and agglomeration of platinum nanoparticles due to the physical adsorption of water and the hydroxylation of the carbon surface. The origin of the long-interconnected aggregation of platinum nanoparticles was discovered to be a major deactivation process in addition to conventional carbon corrosion.
Export citation and abstract BibTeX RIS
1. Introduction
In recent years, proton exchange membrane fuel cells (PEMFC) and direct methanol/oxygen fuel cells (DMFC) [1] have shown great promise as new energy technologies because of their low operating temperature, fast start-up and potential for miniaturization. However, major improvements are still needed to enable their widespread use in electric automobiles. PEMFCs and DMFCs typically consist of bipolar plates, electrodes, a membrane separator and an electrode catalyst. One of the biggest challenges is the synthesis of robust electrode catalysts [2]. In order to dissociate H2 and O2, PEMFCs use platinum nanoparticles on conductive carbon black (Pt/carbon) as the electrode catalyst at both the anode and cathode [3].
It is now well established that the degradation of the carbon support at the cathode limits the lifetime of Pt/carbon catalysts and thus the performance of the PEMFC. The main degradation processes of the Pt/carbon electrode catalyst system of platinum particle sintering, platinum dissolution, carbon support corrosion, carbon monoxide corrosion and detachment from the carbon support have been reported previously [4, 5]. These degradation processes were indicated by structural changes shown in electron micrographs and through electrochemical measurements.
Ex situ and in situ microscopy show the dynamic behaviour of the fuel cell catalyst. This is very valuable for improving the understanding of degradation mechanisms and thus contributes to the critical catalyst design to improve robustness. Differentially pumped environmental transmission electron microscopy (ETEM) [6] has proven to be one of the most efficient tools for in situ visualisation of the deactivation of heterogeneous catalysts in a reactive gas atmosphere at the nanometer scale [7–9]. The analytical ETEM was first applied to Pt nanoparticles on carbon in the oxygen environment in 2009 by Kishita et al [10]. Improvements in microscope resolution brought about by spherical aberration correction have recently enabled us to achieve atomic resolution environmental STEM of Pt-Pd model catalysts [11]. Additionally, real-time ETEM movies [12, 13] of Pt/carbon fuel cell catalysts at the atomic level have been recorded in the anode operating environment (H2) in addition to nitrogen (N2), oxygen (O2) and air environments to replicate the cathode operating environment.
However, the effect of water on Pt/carbon catalysts has not been investigated by high-resolution ETEM due to difficulties in achieving a moist environment in a TEM. A basic design of the wet environment TEM (Wet-TEM) using a differential pumping E-cell was reported in 2002 by P L Gai [14]. Recently, the window-type holder was modified in order to investigate the carbon corrosion of Pt surfaces in moisturised air by Yaguchi et al [15]. However, humidity in the specimen chamber could not directly be detected or controlled in these previous wet-ETEM studies.
Here, we report the developments of a progressive wet-ETEM system equipped with a mass spectrometer, a thermostatic chamber and a two-pressure gauge system, which allows the study of the dynamic behavior in heterogeneous catalysts with controlled moisturized environmental conditions. The new wet-ETEM system was applied to the Pt/carbon electrode catalysts of PEMFC, and Pt nanoparticle behavior in this environment is presented at the atomic scale for the first time.
2. Experimental procedure
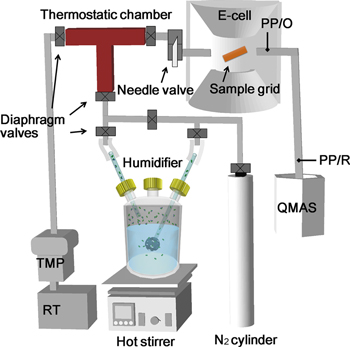
Figure 1 shows a schematic of the present gas injection line modification, which we call the wet-ETEM system.
Figure 1. Wet-ETEM system for the differentially pumped E-cell with a quadrupole mass spectrometer.
Download figure:
Standard image High-resolution imageThe wet-ETEM system was tuned to fit with a third-generation differential pumping system of the latest spherical aberration-corrected high-resolution transmission electron microscope (Titan ETEM, FEI Company) based on the original atomic resolution-ETEM design and development [6, 14]. The TitanETEM was operated with an acceleration voltage of 300 kV. In the present Wet-ETEM system, a humidifier (H2O of 400 ml in a capacity of 800 ml) was heated up on a hot stirrer (HS-5BSSD, AS ONE). The Boiling condition of the humidifier was maintained between 368.0 to 373.0 K with 0.1 K degree step accuracy. N2 gas (N2, 99.9998%, Taiyo Nippon Sanso) was allowed to flow once through the humidifier, and the resulting gas was accumulated into an in-house developed thermostatic chamber at 423 ± 2 K to minimize the devolatilization of the boiled H2O. The partial pressure in the thermostatic chamber was maintained at a constant order between 10−1 and 103 Pa. The gas inlet needle valve positions and the pumping rate of the differential pumping ETEM were set to known settings to achieve the equilibrium partial pressures of 10−3 to 10 Pa in the sample area [16]. The humidity and partial pressure in the E-cell were accurately measured using the quadrupole mass spectrometer (QMAS: PrismaPlus™, Pfeiffer Vacuum), and the pressure gauges were mounted at different two positions (PP/O and PP/R in figure 1).
Pt nanoparticles were deposited by sputtering (Ar Ion Sputter E-1030, Hitachi) on a 5 nm thick amorphous carbon film prepared by chemical vapour deposition (CVD coater NC5 Turbo, Enomoto AV) using methane (CH4) and ethylene (C2H4) as precursor gases. Mica sheets (G250-2, Agar Scientific) were used as substrates for the growth of the amorphous carbon film. CVD and ion sputter deposition were carried out at room temperature (288–300 K). Conventional copper micro-grids (Lacey Carbon Film 300 Mesh Cu, Agar Scientific) were used as the support of the prepared Pt/carbon film sample. The Pt/carbon sample was characterised by dynamic aberration-corrected ETEM in a dry N2 or moisturised N2 atmosphere of 10−2 Pa.
To record the dynamic movie files, a high-speed CCD camera (Orius™ CCD, Gatan Inc.) with a fast readout mode of 512 pixel × 512 pixel and video capture software (VisualDub, licensed under the GNU General Public License) were set up with an image size of 620 pixel × 480 pixel and a time resolution of 0.05 s (20 frames s−1). The pixel size was 0.035 nm, and the local current density was estimated as 1.8 × 103 A cm−2. This corresponds to 8.3 × 106 electrons nm−2 (for comparison; in Batson's in situ STEM work, a 100 pA probe that was used to scan over a 4 nm × 4 nm area for a 0.2 s exposure produced a dose of 7.8 × 106 electrons nm−2) [17, 18].
3. Results and discussion
3.1. Control and calibration of humidity and partial pressure in an E-cell
The detection of H2O molecules using mass spectrometry, which had not been achieved in previous studies, was the first challenge of the present wet-ETEM system. The introduction of H2O molecules into the vacuum pumping system of the ETEM is generally more dangerous for the vacuum pumping system of the ETEM in comparison with alcohols (methanol and ethanol, etc.), which have lower melting points such as 176 and 159 K [19, 20]. Because the vaporization in a low-pressure environment (a high vacuum) conducts heat away from liquid H2O; small ice balls are produced in the vacuum. The ice balls can impact the rapidly moving vanes of the turbo molecular pump, which may cause the vacuum condition to be threatened. The gate and needle valves cannot react quickly enough to maintain the vacuum in the TEM column if ice balls are in the pumping system.
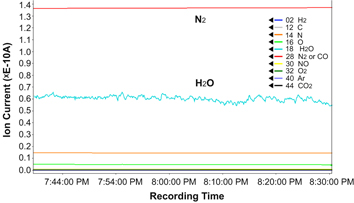
Partial pressure control in a thermostatic chamber at 423 K, which is a new design reported for the first time in this paper, successfully solved the problems occurring from the ice balls. Figure 2 shows a mass spectrometry graph obtained in situ during the present wet-ETEM observation of the Pt/carbon sample in a moisturised N2 environment.
Figure 2. QMAS spectra of 24% moisturised N2 gas.
Download figure:
Standard image High-resolution imageThe temperatures of the humidifier, the thermostatic chamber and the back-side pumping line were set at 370.2, 423 and 453 K, respectively. Partial pressure in the thermostatic chamber was maintained at 1 Pa using a manually controlled diaphragm valve and a turbo molecular pump on the back-side pumping line. The needle valve between the E-cell and thermostatic chamber was gently opened until the former pressure gauge showed 10−2 Pa for the partial pressure in the E-cell. As shown in figure 2, we could identify four gas masses of N ((14), O (16) and H2O (18)) and N2 (28) by detecting their corresponding ion currents above the background level (5.0 × 10−14 A) of the quadrupole mass spectrometer. The average ion currents that correspond to the N2 (28) and H2O (18) were 1.37 × 10−9 and 5.95 × 10−10 A during the in situ experiment for 1 h. The current associated with the H2O showed larger fluctuations compared with other gasses due to bubbling in the humidifier. This result proves that the H2O molecules had successfully left the humidifier and passed through the E-cell, where they finally reached the mass spectrometer. The humidity and partial pressure in the E-cell were estimated using two different kinds of calibration charts, presented in figures 3 and 4.
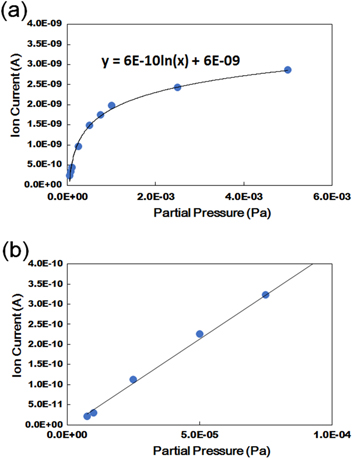
Figure 3. Calibrated pressure estimation charts of N2 gas at the entrance of QMAS at (a) high pressure and (b) pressure lower than 10−4 Pa.
Download figure:
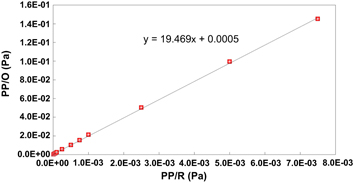
Standard image High-resolution imageFigure 4. Calibration between partial pressures at the first vacuum gauge PP/O and the latter vacuum gauge PP/R on the pumping line.
Download figure:
Standard image High-resolution imageThe calibration curve and line shown in figure 3 enable us to know the partial pressure of each gas at the front of the mass spectrometer. The calibration line in figure 4 gives a more effective measurement of the partial pressure in the E-cell, which is expected to be nearly equal with the values measured by the pressure gauge PP/O.
These calibration curves were prepared using dry N2 gas (99.9998%) before the wet-ETEM experiment. The same settings of the gas cylinder, vacuum pumps, pressure gauges and mass spectrometer were used in both the dry/wet N2 conditions. In the case of the wet-ETEM conditions that correspond to the mass spectrometer graph of figure 2, a humidity of 24% in the E-cell was calculated as the partial pressure ratio between H2O (0.0029 Pa) and the other components, including the N2 (0.0092 Pa).
We report that our progressive wet-ETEM system reproduced the humidity and partial pressure in the E-cell in the ranges of 0 to 30% and 10−3 to 10 Pa. Higher partial pressures of more than 10 Pa in the E-cell showed less reproducibility because the driving current is rapidly increased to exceed the threshold when the inflow of H2O in the turbo molecular pump is started. Concentrations of water greater than 40% could not be achieved in the present wet-ETEM even when the humidifier was heated above 373 K.
3.2. Dynamic wet-ETEM observation of the Pt/carbon in a moisturized cathode atmosphere
The wet-ETEM (moisturised gas environmental transmission electron microscopy) was applied to investigate the origin of the short lifetimes of the Pt/carbon electrode catalysts. The structural weaknesses of the Pt/carbon in the cathode atmosphere (mixture of H2O, N2 and O2) have been a big obstacle to overcome in the improvement of PEMFC for a long time. Our earlier study had clarified one main deactivation mechanism of Pt nanoparticle sintering due to carbon support shrinkage in an oxygen atmosphere [12]. Pt nanoparticle sintering in pure nitrogen (N2, 99.9998%) and in 24% moisturized nitrogen gas atmospheres were compared to study the influences of H2O synthesis at the PEMFC cathode.
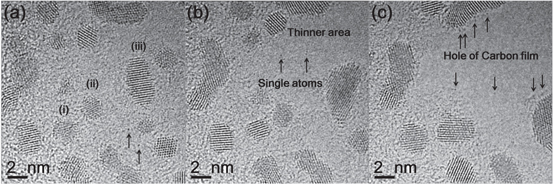
A higher spatial resolution and better time resolution enabled the study of the Pt atom's behaviour. Real-time movies of a Pt/carbon sample in dry N2 and in 24% moisturised environments with an exposure time of 0.05 s are provided in the supporting material. Figures 5(a)–(c) are the selected-area captured (SAC) images at 0, 20 and 40 s after the initiation of the observation. Black dots on the surface of the carbon film during exposure to N2 indicated the presence of Pt atoms. In previous reports, the surface diffusivity of the Pt atoms on graphite and on Pt(110) surfaces are 0.01–0.1 × nm2 s−1 [21] and more than 0.1 × nm2 s−1 [22], respectively, at ambient temperature. The shrinkage of smaller nanoparticles and the selective growth of bigger nanoparticles known to result from Ostwald ripening [16, 18] were not observed until the structure of the carbon film appeared, as shown in figure 5(b). The volume and surface area of each Pt nanoparticle, indicated as (i)–(iii) in figure 5(a), were stable under electron irradiation of 8.3 × 106 electrons nm−2 for initial exposures of several seconds. The present result supports the constant exchange of single atoms between nanoparticles of different sizes, which had been suggested in previous in situ environmental STEM work using the same type of Pt/carbon sample [11, 23]. We believe that nanoparticles maintain their volume, surface area and density without the structural change of the carbon support film. In addition, by using single atoms as markers, the thinning of the carbon film and the formation of a small hole in the carbon film was clearly observed in 0.01 Pa of a pure N2 environment by EB irradiation.
Figure 5. SAC images of Pt/carbon in 0.01 Pa of a pure N2 environment. Parts (a), (b) and (c) correspond to the atomic structures with electron beam (EB) heating for 0, 20 and 40 s, respectively.
Download figure:
Standard image High-resolution imageWe found that Pt aggregates (agglomerates) were observed mainly around such holes in the carbon film. For example, particle (ii) and particle (i) migrated toward each other when the edge of the carbon hole moved to the outside surface, resulting in the formation of a larger single crystal. Particle (iii) moved symmetrically to the opposite side from the edge of the carbon hole. We believe that under a gaseous environment with EB radiation, there is a large driving force for the surface diffusion of Pt atoms and for the growth the nanoparticles.
In contrast, in the 24% moisterised nitrogen environment, the coalescence [12, 23] of the nanoparticles was far more rapid. In figures 6(a), (b), the Pt nanoparticles started to rotate and migrate; they eventually made one large connected aggregation, as shown in the Selected Area Captured (SAC) images of figure 6(c). Nanoparticles (i) and (ii), which are approximately 2 nm in size, had already begun to migrate and rotate after just 5.5 s.
Figure 6. SAC images of Pt/carbon in a 24% moisturised N2 environment. (a), (b) and (c) correspond to the atomic structures with electron beam (EB) heating for 0, 5.5 and 14 s, respectively.
Download figure:
Standard image High-resolution imageThrough such coalescence processes, no holes in the carbon film were created in the 24% moisturised N2 environment. The granular pattern of the carbon film in figure 6(c) was also seen to survive for 14 s. Electron energy loss spectroscopy showed that the thicknesses of the carbon films at two regions from one microgrid sample were similar. This means that the damage and shrinkage of the carbon support is not the reason for the long connected aggregation observed in the moisturised N2 environment.
Pt single atoms could not be observed between the nanoparticles because their diffusion was too fast for the 0.05 s exposure time. In the present wet-ETEM study, the same conditions of partial pressure, electron density, magnification and conversion of an electron beam were used in both of the dry/wet N2 conditions. We discuss the origins of such rapid mobility and long interconnected structured discovered in the 24% moisturised N2 atmosphere by comparing with the ones observed in a dry N2 atmosphere.
In a pure N2 atmosphere, Pt aggregations were produced around the holes in the carbon film due to a higher density of the Pt atoms, as shown in figures 5(c) and 7(a). The nanoparticles were pretty stable on the amorphous carbon surface, which is similar to our observations of the nanoparticles in a high vacuum. The studies are consistent with our previous findings, which elucidate the stability of Pt nanoparticles on carbon substrates [11, 12 and 16].
Figure 7. Reaction scheme depiction of the deactivation process of the Pt electrode catalysts at (a) pure N2, (b) water vapor in plane view and (c) water vapor in a cross-sectional view on the cathode in a PEMFC.
Download figure:
Standard image High-resolution imageWe believe that thermal heating and sputtering by electron and gas molecules are the driving force in vacuums and in lower-pressure nitrogen atmospheres. On the other hand, the Pt nanoparticles showed higher mobility and formed a linked aggregation in a moisturised nitrogen atmosphere as an additional deactivation was enhanced by H2O vapor (figures 6(c) and 7(b)). In comparison with a pure nitrogen atmosphere, it is reasonable to assume that the physical adsorption of water and hydroxylation of the carbon surface contribute to the mobility and agglomeration of the nanoparticles (figure 7(c)). Ex situ heating experiments in 24% moisturised N2 and biasing experiments also indicated the formation of such an agglomeration.
The present in situ wet-ETEM experiments suggest that stronger trapping sites on the carbon supports can be induced for use in an H2O environment on the PEMFC cathode. Conventional amorphous carbon and carbon black (including graphite) supports have already been substituted by single-walled nanotubes [24], multi-walled nanotubes [25–27], stacked nanocups [28] and graphitic nanofibres [29] as the electrocatalyst supports in fuel cells in laboratories. The oxygen reduction reaction activities of these nanostructured carbon supports are higher than that of conventional amorphous carbon. From the view point of robustness in H2O vapour, our results, which have less dangling bonds on the surface, stand by such supporting materials. Pt nanoparticles supported on oxide [30] and oxide-carbon compounds [31, 32] are also expected to improve the lifetime of electrode catalysts used in present PEMFC systems.
Finally, the presented wet-ETEM system can be mounted on every type of differential pumping E-cell. The swapping of a turbo molecular pump and the tuning of the zero-level pumping line of the E-cell can improve the maximum partial pressure and humidity of the present wet-ETEM system [13]. Although our wet-ETEM system, which includes a glass humidifier and Teflon tubing, uses preferred safe driving gases, such as N2, O2 and air, a stainless steel cylinder and sealing segments enables a moisturised H2, CO and CO2 environment for wider applications.
4. Conclusions
To visualise the influence of moisture on the deactivation of the Pt/C catalysts in PEMFC in real time at the atomic scale, a wet-ETEM system was developed for spherical aberration-corrected transmission electron microscopy. The introduction of a thermostatic chamber and mass spectrometer achieved a stable moisturised N2 environment in the E-cell and allowed the direct detection of H2O for the first time. The movements of the platinum nanoparticles were successfully investigated in 24% moisturized N2 and pure N2 atmospheres.
The Pt/carbon sample showed considerable structural weakness in a moisturized N2 atmosphere. We discovered the rapid migration and long interconnected structured produced from well-dispersed Pt nanoparticles in a moisturised environment. The deactivation process, which is due to moisture (hydroxylation) of the carbon supports, is discussed; during this deactivation process, we used the movement of platinum nanoparticles measured in moisturized nitrogen and pure nitrogen atmospheres for comparison.
Acknowledgements
This work was partly supported by a Grant-in-Aid for Young Scientific Researchers (B) (No. 24710110) from the Japan Society for the Promotion of Science (JSPS), Japan. The ETEM facility was partly supported by a grant from the Chubu Economic Federation and Aichi Prefecture. We thank Dr Y Sasaki of JFCC for allowing our usage of the ETEM. We also thank the Young Leaders Cultivation Program of Nagoya University for their financial assistance for KY. The work at York (PLG, EB, LL, MW and KY), which we gratefully acknowledge, was supported under EPSRC (UK) critical mass grant EP/J018058/1.