Abstract
Stable iron nanoparticles have been synthesized from naturally occurring and abundant Fe-containing bio-precursors, namely hemoglobin and myoglobin. The formation of stable iron nanoparticles was achieved through a one-pot, single-phase chemical reduction approach. The reduction of iron ions present in the bio-precursors was carried out at room temperature and avoids the use of harsh chemical reagents. The size distribution of the product falls into the narrow 2–5 nm range and the particles were found to be crystalline. This method can be a valuable synthetic approach for producing bio-conjugated nanoparticle systems for biological applications.
Export citation and abstract BibTeX RIS
1. Introduction
The past decade has witnessed impressive growth in the synthesis and applications of nanoparticles [1–6]. There has also been a great deal of applications oriented towards utilizing metal nanoparticles for biological systems [4, 5]. Most of the research relied on gold and magnetic materials based on oxides integrated with the biological systems [4–8]. The compatibility of the metal nanoparticles in biological systems depends on factors such as solubility in bio-compatible media and its rapid availability for the intended application within the bio-system of interest [7, 8]. These demands require the genesis of the stable metal nanoparticles from bio-inspired materials and prolonged stability in aqueous media, and there is a large potential to synthesize metal nanoparticles of interest directly from bio-precursors that contain the required metal.
A wide range of synthetic methods have been employed to produce iron nanoparticles (Fe-NP) [9]. Thermal decomposition of Fe precursors (e.g. organometallic compounds) and chemical reduction of iron salts have been widely employed for the synthesis of Fe-NP. However, the majorities of the as synthesized Fe-NP are found to be unstable and are often susceptible to oxidation, leading to the formation of iron oxide nanoparticles [9–11]. This drawback (oxidation) has had serious implications in the development of applications of Fe-NP [9]. Special core–shell structures, either with oxide or other metal surface coatings, have been created to ensure the protection of Fe-NP from oxygen [12, 13]. Though such coatings have resulted in stable Fe-NP the magnetic properties have largely been known to suffer [9]. It is therefore critical to develop newer synthetic methods to realize stable Fe-NP. Stable Fe-NP are expected to find application in catalysis [14], magnetic [9], electrical [9], environment remediation [15, 16] and sensors [9]. Moreover, because of their high magnetic moments they are also expected to have serious implications in biomedical applications such as drug delivery, labeling and MRI contrast agents [17].
The size distribution from the Fe-NP generated by the thermal decomposition of organometallic precursors such as iron pentacarbonyl yields particles in the size range of 6–20 nm [9]. The typical size range for the Fe-NP obtained by various chemical reduction methods are 2–50 nm. While numerous reports for the chemical synthesis of Fe-NP using Fe salts are available only a few reports for the synthesis of Fe-NP using bio-molecules as either precursors or protecting groups are known. Recently, it has been shown by Lacroix et al [18] that it is possible to control the growth of Fe-NP in the organic structures using the organometallic precursor {Fe(N[Si(CH3)3]2)2}2 to obtain anisotropic cubic Fe-NP. Tsang et al [19] has shown that Fe-NP with graphitized carbon coating could be prepared using ferritin, a bio-precursor for iron, by decomposition and subsequent carbonization (by heated to high temperatures ∼900 °C). More recently, Varma et al has demonstrated a green chemical route for the synthesis of Fe-NP in water using iron salts such as FeCl3, and capped the particles with polyphenol groups present in natural materials such as tea [20], where the polyphenols act as both as the reducing and capping agent, allowing the stable synthesis of Fe-NP. Most recently, Njagi et al [21] have extended the approach developed by Verma et al by using Sorghum Bran extracts, rich in polyphenols, for the aqueous phase synthesis of amorphous Fe-NP from iron salts.
Here we show that stable Fe-NP, a key material that can have bio-catalytic applications, can be prepared directly from bio-precursors, such as hemoglobin and myoglobin, which are both abundant and ubiquitous in nature, and a main component of blood using a simple 'one-pot single-phase' synthetic reduction strategy. These bio-precursors contain Fe ions (Fe2+ ions) coordinated with four nitrogens of porphyrin molecules present in large globular proteins. A schematic depiction of the process along with a proposed mechanism is shown in figure 1.
Figure 1. Schematic depiction of the 'one-pot single-phase' synthetic method adopted for the synthesis of Fe-NP. Both hemoglobin and myoglobin form a stable red color solution in pyridine–water mixture (3:1 v/v). The globular proteins containing the Fe ions bound to porphyrin moieties in both hemoglobin and myoglobin are shown on the left. In the water–pyridine solvent mixture there is dissociation of the Fe ions from the pockets of the porphyrin. Upon addition of the reducing agent sodium borohydride and subsequent vigorous stirring the reduction reaction is initiated and the color of the solution changes from reddish in appearance to brownish (shown on the right). The final Fe-NP formed are capped primarily with pyridine along with capping via the amino acids on the globular protein. The Fe-NP remain soluble and stable in the water–pyridine mixture for extended time periods.
Download figure:
Standard image2. Experimental section
All chemicals were purchased from Sigma-Aldrich and were used as received.
2.1. Synthesis of iron nanoparticles from hemoglobin and myoglobin
50 mg of human hemoglobin (0.735 µmol, 0.164 mg of iron) or 50 mg of horse myoglobin (2.94 µmol, 0.164 mg of iron) was dissolved in 5 ml of water with slight sonication. To this solution 15 ml pyridine was added to form a homogeneous red colored solution. Separately, 30 mg of sodium borohydride (0.793 mmol) was dissolved in 3 ml of water. This solution was then injected into the previously prepared hemoglobin solution with vigorous stirring. The reduction reaction is carried out at room temperature. The vial was capped and allowed to stir for an hour. The reaction was performed without any additional capping agent. Within an hour, the initial red colored solution turned blackish brown indicating the formation of iron nanoparticles (Fe-NP). The color of the solution remains unchanged for one day even after exposure to air. All of the chemical handling and synthesis were performed in a chemical hood with proper ventilation. (Caution: given the natural sources of these bio-precursors we handled them as bio-hazardous materials and used appropriate methods for their handling and disposal.)
2.2. Characterization of Fe-NP
A JEOL 1230 high-contrast transmission electron microscope and a JEOL 2100-F, equipped with EELS and EDX spectrometers, were employed for imaging and detailed analysis of the nanoparticles. The selected-area diffraction patterns and EELS were obtained with the JEOL 2100-F. Image J software was used for obtaining the particle size distribution by measuring the diameters of at least 100 nanoparticles. UV–visible spectra were obtained on a Shimadzu spectrophotometer. TEM samples were prepared by casting a droplet of the blackish brown solution of iron nanoparticles obtained individually from the respective bio-precursors on the carbon grids followed by drying it in air. The diffraction patterns were taken in the selected-area mode, where many crystals contribute to the pattern and allow a structure determination on a larger-scale. EELS spectra were taken with a Gatan imaging filter in the spectroscopy mode. Due to the large amount of residual organic material around the Fe crystals, contamination did not allow spectroscopy with a focused electron beam. Instead, a specimen area somewhat larger than the diameter of one Fe particle was selected with an aperture in the image plane of the spectrometer, and a spectrum from this area was measured. The elemental analysis was performed using proton-induced x-ray emission (PIXE) by Elemental Analysis Inc.
3. Results and discussion
In a typical synthesis, we took a known amount of hemoglobin in a vial and dissolved it completely in a solvent mixture of water–pyridine (1:3 v/v) (more details in section section 2). To this homogeneous solution a large excess of sodium borohydride (dissolved in water) was added and the solution was stirred vigorously. Within minutes the solution changes color from reddish to brown as shown in figure 1. This solution remains stable for an extended period of time (approximately one week). After one week we have observed that some white colored aggregates are formed in the bottom of the vial. However, the solution still contains the Fe-NP (as observed by TEM). The reduction of hemoglobin and myoglobin using sodium borohydride (NaBH4) yields nearly monodisperse, small (2–5 nm) and stable Fe-NP (figure 2). The precipitated aggregates do not show any well-defined morphology and are most likely from the excess porphyrin and globular proteins present in the bio-precursors. The same protocol is followed for myoglobin and similar observations have been made.
Figure 2. TEM images of the Fe-NP obtained from hemoglobin ((a), (b)) and myoglobin ((c), (d)). The mean diameter of iron nanoparticles from hemoglobin and myoglobin is 3.2 nm and 3.6 nm, respectively.
Download figure:
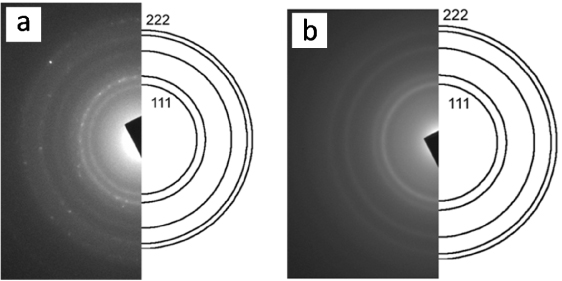
Standard imageThese bio-precursors, hemoglobin and myoglobin, contain varying numbers of iron ions bound to the porphyrin and globular proteins: hemoglobin contains four iron ions per molecule whereas myoglobin has only one. Despite this difference the resulting Fe-NP show a very similar size distribution of 2–5 nm, as measured by transmission electron microscopy (TEM). The TEM images are shown in figure 2 and figure S1 (supplementary information available at stacks.iop.org/Nano/23/055602/mmedia). The average size of these Fe-NP, obtained by measuring over 100 particles for samples made of each bio-precursor (figure S2, supplementary information available at stacks.iop.org/Nano/23/055602/mmedia), are 3.2 nm and 3.6 nm for hemoglobin and myoglobin, respectively. The selected-area electron diffraction (SAED) obtained from the Fe-NP are shown in figure 3. From the diameter and distribution of the rings in the SAED, a lattice constant of 3.6 Å, consistent with the γ-Fe (fcc) crystal, could be determined [22]. γ-Fe is one well-known crystal structure for bulk iron, though in nanoscale it is observed to a lesser extent in comparison to the thermodynamically favored bcc at room temperature. However, γ-Fe has been observed previously in carbon-coated Fe-NP, for example, Fe-NP in CNT [23], Fe-NP with graphitized carbon [19] at high temperatures and Fe-NP with nonionic surfactant micelles [24] at room temperature. The presence of oxides and the α-Fe (bcc) in the nanoparticles can be excluded because these materials would have a different crystal structure and give different diffraction rings as well as different spacings in lattice images. In addition, electron energy-loss spectra (EELS) have been measured that show the presence of a very small amount of oxygen in the organic material surrounding the Fe-NP but no oxygen in the particles themselves. The detection of the core-loss edge from the metal is difficult, however, due to the high energy of the edge and the small size of the metal particles.
Figure 3. The selected-area electron diffraction (SAED) of the Fe-NP obtained from hemoglobin (a) and myoglobin (b). The indexing of the innermost five rings (111, 002, 022, 113, 222) for a γ-Fe (fcc) is illustrated.
Download figure:
Standard imageIn order to understand the formation mechanism of the Fe-NP from the bio-precursors we carried out a series of qualitative UV–visible measurements from the solutions before and after reduction (figure S3, supplementary information available at stacks.iop.org/Nano/23/055602/mmedia). The initial measurements of the precursor yields typical spectra expected for those of the Fe–porphyrin moieties. However, upon reduction there is complete loss of the Fe–porphyrin spectral features, indicating a loss of structural features within the bio-precursor usually after 1 h, and the appearance of a peak at 359 nm indicates the formation of Fe-NP [11]. Elemental analysis of the reduced solutions (containing Fe-NP) and of the original bio-precursors was performed to confirm the presence of Fe in the samples using proton-induced x-ray emission (PIXE, Elemental Analysis Inc.). Given the high sensitivity of the PIXE methods, routinely used for the analysis of trace amounts of metals, it is evident from the measurements that only metallic Fe along with the capped bio-precursors are present in the reduced solutions. Though there is almost a 100% yield of the reduction of the Fe ions producing Fe-NP, the amount of nanomaterial obtained after the reduction is quite low because the original Fe content in the bio-precursors is only 0.28% (supplementary information available at stacks.iop.org/Nano/23/055602/mmedia).
When control reduction experiments were carried out in the absence of pyridine no Fe-NP could be observed, most likely because pyridine makes possible the dissociation of the iron ions from the pockets of porphyrin in both of the bio-precursors. Additionally, without pyridine the globular proteins do not denaturize in water, leaving the bio-precursors stable. While several solvent combinations were tested, only the pyridine–water mixture results in the formation of stable Fe-NP. Other water-miscible solvents (such as alcohols, DMF, THF) did not allow for a uniform solution to be formed by dissolution of the bio-precursors, thus impeding the single-phase synthetic route. Moreover, large excess of the reducing agent (NaBH4) and pyridine in the water–pyridine solvent mixture allows for complete reduction and maintaining the stability of the Fe-NP, respectively. These experimental observations are in good agreement with the proposed mechanism (figure 1) that there is significant separation of the different moieties present in the bio-precursor under the reaction conditions specified.
The reduction reaction of the bio-precursors in a pyridine–water mixture has been carried out without applying inert conditions or the use of high temperature and corrosive reagents. Despite avoiding the precautionary note (inert conditions) often required for synthesis of stable Fe-NP, the reactions were carried out in an ambient lab atmosphere and it was found that the Fe-NP synthesized from the bio-precursors remain stable in solution over an extended time period even when exposed to air (opening and closing of the vials). It is well known that both pyridine [11] and amino acid groups [18, 25] are capable of capping the Fe-NP. It has been previously observed that Fe-NP capped with pyridine tends to oxidize within minutes of exposure to air [11]. While the role of the globular proteins containing amino acids is difficult to delineate we hypothesize that they provide protection to the Fe-NP in addition to the pyridine molecules because of the affinity of the amino acid groups for the surface of Fe-NP. It is noteworthy that the Fe-NP does not separate out from solution even when centrifuged at extremely high speeds (50 000 rpm) and also do not separate out when placed in magnetic fields with strengths of the order of 0.4 T. This can be attributed to the fact that a large amount of pyridine is present in the final solution, the nature of the bulky capping agents such as globular proteins and the smaller size distribution observed for the Fe-NP (2–5 nm). Though the Fe-NP are stable in the solvent mixture despite containing a reasonable amount of water; for realistic use in biological applications appropriate solvent conditions in aqueous conditions must be found, perhaps by solvent phase-transfer methods to render these Fe-NP more usable, therefore allowing for direct use from solution when required.
4. Conclusions
In summary, the synthesis of extremely stable Fe-NP in solution has been achieved by chemical reduction of iron ions in the bio-precursors, such as hemoglobin and myoglobin, in a significantly shorter period of time (∼1 h) by a one-pot single-phase approach carried out at room temperature. The synthetic protocols developed here avoid the use of harsh reaction conditions such as high temperature, corrosive reagents, etc, usually employed for the synthesis of Fe-NP. The Fe-NP was found to be highly crystalline (γ-Fe) with 2–5 nm in diameter. This general method can further be advanced to other bio-precursors for creating usable nanoparticles.
Acknowledgments
This work was supported through the NSF Materials World Network program for funding through grant DMR-0801012. We thank Drs Julio Rodriguez-Manzo and Florian Banhart, Institut de Physique et Chimie des Matériaux, Université de Strasbourg, and Dr Ashavani Kumar, MEMS, Rice University for helpful discussions during different stages of this project.