Abstract
In response to the recognized fragility of reactor-produced 99Mo supply, direct production of 99mTc via 100Mo(p,2n)99mTc reaction using medical cyclotrons has been investigated. However, due to the existence of other Molybdenum (Mo) isotopes in the target, in parallel with 99mTc, other technetium (Tc) radioactive isotopes (impurities) will be produced. They will be incorporated into the labeled radiopharmaceuticals and result in increased patient dose. The isotopic composition of the target and beam energy are main factors that determine production of impurities, thus also dose increases. Therefore, they both must be considered when selecting targets for clinical 99mTc production.
Although for any given Mo target, the patient dose can be predicted based on complicated calculations of production yields for each Tc radioisotope, it would be very difficult to reverse these calculations to specify target composition based on dosimetry considerations. In this article, a relationship between patient dosimetry and Mo target composition is studied. A simple and easy algorithm for dose estimation, based solely on the knowledge of target composition and beam energy, is described. Using this algorithm, the patient dose increase due to every Mo isotope that could be present in the target is estimated. Most importantly, a technique to determine Mo target composition thresholds that would meet any given dosimetry requirement is proposed.
Export citation and abstract BibTeX RIS
For more information on this article, see medicalphysicsweb.org
1. Introduction
Technetium-99m (99mTc) is the most widely used nuclear medicine radioisotope, with up to 40 million diagnostic procedures performed yearly (Einstein 2009, Bénard et al 2014a). Traditionally, 99mTc is obtained from molybdenum-99 generators (99Mo/99mTc), where 99Mo is produced in a reactor using the 235U(n,fission) channel in enriched uranium. However, due to recognized fragility of many reactors in the aging global reactor base, other production methods are being investigated. In particular, direct cyclotron production of 99mTc, which was pioneered by Beaver and Hupf in the early seventies (Beaver and Hupf 1971), has been suggested as an adjunct to 99Mo/99mTc reactor production (Lagunas-Solar 1999, Takács et al 2003, Guérin et al 2010, Gagnon et al 2011, Pillai and Knapp 2012, Bénard et al 2014a). In this method, 99mTc is obtained via proton irradiation of an enriched molybdenum-100 (100Mo) target through the 100Mo (p,2n) reaction, henceforth referred to as cyclotron-produced 99mTc (CPTc).
However, even when using highly enriched 100Mo targets, a large number of unwanted radioactive and stable isotopes will be produced due to the existence of trace amounts of other stable Mo isotopes (92, 94–98Mo) (Lagunas-Solar 1999, Celler et al 2011). While isotopes other than technetium (Tc) can potentially be removed from CPTc samples using chemical purification, all Tc impurities will remain in the sample and will be incorporated into the technetium labeled radiopharmaceuticals (Morley et al 2012, Bénard et al 2014b) due to their chemical equivalency. Injecting radiopharmaceuticals containing Tc impurities may result in increased patient radiation dose compared to those which were labeled with generator-produced 99mTc (Hou et al 2012, Selivanova et al 2015).
Dose increases (DI) due to the presence of Tc impurities in CPTc-labeled radiopharmaceuticals depend on a non-trivial relationship between target isotopic purity and cyclotron irradiation conditions, including beam energy, irradiation time, and target thickness. Previously, we have shown that proton beam energies ranging from 16 to 19 MeV and targets with thicknesses degrading these beam energies to approximately 10 MeV with 3–6 h irradiation times may correspond to the most advantageous production conditions from a clinical perspective, i.e. maximizing 99mTc yield while minimizing patient dose increase due to impurities in CPTc (Hou et al 2012). In addition, the time-period between the end of beam (EOB) and the time of patient injection has to be considered, as the ratio of any long-lived impurities relative to 99mTc will increase with time.
With regards to the influence of isotopic composition on patient dose (Hou et al 2012), our investigations show that there exists the possibility that the same irradiation conditions will generate similar amounts of 99mTc, but differing amounts of other radioisotopes (see for example, table 5 in Celler et al 2011), which will result in highly variable DI (see table 4 in Hou et al 2012)). Our previous dosimetry analysis indicated that for beam energies below approximately 20 MeV, the most favorable target for 99mTc production should not only contain a large percentage of 100Mo, but it also needs to have a relatively small amount of 94–97Mo which will result in production of significant amounts of radioactive Tc isotopes other than 99mTc. For higher beam energies (e.g. 24 MeV), additional constraints on the amount of 98Mo may be required (Hou et al 2012). Therefore, it is crucial that before any production run, the targets which will result in DI above acceptable limits are identified and omitted (defining these DI limits is beyond the scope of this publication as they have to be carefully determined based on patient safety considerations).
Specific requirements for target composition leading to production of CPTc samples corresponding to acceptable DI are very difficult to define. Although, for any given target, the patient DI can be predicted based on the theoretical or experimental yield estimations for each Tc product (Celler et al 2011, Hou et al 2012, Selivanova et al 2015), it is impossible to reverse these calculations, i.e. specify target composition that will result in production of CPTc samples leading to DI below the predefined limit, because there are potentially an infinite number of targets that will lead to the same DI level.
The objective of this work is to address this problem and to determine the relationship between Mo target specification (i.e. threshold amounts of stable Mo isotopes which can be present in the target) and the resulting DI. Questions, which are investigated in this work, are as follows:
- (a)What is the relationship between patient dose and DI, and the Mo target isotopic composition?
- (b)How does each of Mo isotopes present in the target influence patient dose?
- (c)How do we identify Mo targets that will lead to acceptable DI levels and distinguish them from those that will not?
To answer these questions, we have developed an algorithm which allows us to calculate patient dose based solely on the knowledge of target composition. As will be shown, application of this algorithm does not require complicated yield and dosimetry estimations for each produced Tc isotope. Instead, it uses tabulated values of patient doses that would result from injection of CPTc produced by irradiation of targets composed of 100% of each of the seven stable Mo isotopes. We have created such a table and used it to identify the 'worst offender' of the molybdenum isotopes, i.e. the one which affect(s) the patient dose the most. Additionally, examples of how this algorithm can be used to set target composition thresholds for any given DI limit are presented.
2. Dosimetry estimation based on isotopic compositions of Mo target
2.1. Algorithm
The absorbed patient dose in a target organ T originating from a mixture of technetium radioisotopes that are present in a source organ S is equal to the sum of activity-weighted dose corresponding to each technetium radioisotope present in the injected radiopharmaceutical (Loevinger et al 1988, Hou et al 2012):

where ![${{\left[{{D}_{~{{r}_{T}}\leftarrow {{r}_{S}}}}\right]}_{CPTc}}$](https://content.cld.iop.org/journals/0031-9155/61/2/542/revision1/pmbaa0aabieqn001.gif) represents the absorbed dose in organ T from CPTc activity present in source organ S (expressed in Sv),
represents the absorbed dose in organ T from CPTc activity present in source organ S (expressed in Sv), represents the target region and
represents the target region and  represents the source region,
represents the source region,  (expressed in Bq) represents the activity of Tc isotope
(expressed in Bq) represents the activity of Tc isotope  in the CPTc sample, and
in the CPTc sample, and ![${{\left[{{d}_{{{r}_{T}}\leftarrow {{r}_{S}}}}\right]}_{i}}$](https://content.cld.iop.org/journals/0031-9155/61/2/542/revision1/pmbaa0aabieqn006.gif) represents the dose coefficient (in units of Sv Bq−1) corresponding to the Tc isotope
represents the dose coefficient (in units of Sv Bq−1) corresponding to the Tc isotope  .
.
The same Tc isotope can be produced from more than one Mo isotope (Celler et al 2011, Gagnon et al 2011), and the reader is directed to these publications for the detailed description of specific yield calculations. The total activity of each produced Tc isotope is a linear combination of the individual activities resulting from the presence of each Mo isotope in the target, as shown below:

where  represents the activity of Tc isotope
represents the activity of Tc isotope  calculated assuming that the target is composed in 100% of Mo isotope
calculated assuming that the target is composed in 100% of Mo isotope  (
( can be 92, 94–98, 100);
can be 92, 94–98, 100);  represents the relative amount of Mo isotope
represents the relative amount of Mo isotope  in the real molybdenum target used in irradiations. Therefore, the total patient dose estimated using equation (1) can be rewritten as:
in the real molybdenum target used in irradiations. Therefore, the total patient dose estimated using equation (1) can be rewritten as:
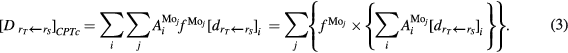
Equation (3) can be substantially simplified:
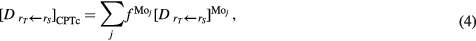
where ![${{\left[{{D}_{~{{r}_{T}}\leftarrow {{r}_{S}}}}\right]}^{\text{M}{{\text{o}}_{j}}}}=\sum_{i}A_{i}^{\text{M}{{\text{o}}_{j}}}{{\left[{{d}_{{{r}_{T}}\leftarrow {{r}_{S}}}}\right]}_{i}}$](https://content.cld.iop.org/journals/0031-9155/61/2/542/revision1/pmbaa0aabieqn014.gif) is equal to the total patient dose from all Tc isotopes produced by reactions on a target which is composed of pure Mo isotope
is equal to the total patient dose from all Tc isotopes produced by reactions on a target which is composed of pure Mo isotope  . Thus, the dose from all Tc impurities relative to that from pure 99mTc, i.e. the dose increase (DI), is given by:
. Thus, the dose from all Tc impurities relative to that from pure 99mTc, i.e. the dose increase (DI), is given by:
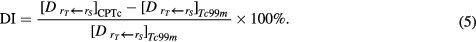
Please note that 99mTc can be produced from both 100Mo and 98Mo. However, the cross section for the 98Mo(p,γ) reaction is approximately thousand times smaller than that for the 100Mo (p,2n) reaction (Celler et al 2011, Qaim et al 2014). Therefore, we can assume that all the 99mTc is produced from 100Mo. Then, equation (5) becomes:
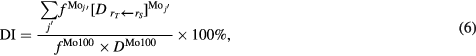
where  identifies the Mo isotope other than 100Mo, i.e.
identifies the Mo isotope other than 100Mo, i.e.  can be 92, 94–98.
can be 92, 94–98.
In summary, equations (4) and (6) can easily be used to estimate the absorbed patient dose and dose increase corresponding to a given target composition, assuming that values of ![${{\left[{{D}_{~{{r}_{T}}\leftarrow {{r}_{S}}}}\right]}^{\text{M}{{\text{o}}_{j}}}}$](https://content.cld.iop.org/journals/0031-9155/61/2/542/revision1/pmbaa0aabieqn018.gif) are known.
are known.
2.2. Patient effective doses corresponding to different Mo isotopes ![${{\left[{{D}_{\text{eff}}}\right]}^{\text{M}{{\text{o}}_{j}}}}$](data:image/png;base64,iVBORw0KGgoAAAANSUhEUgAAAAEAAAABCAQAAAC1HAwCAAAAC0lEQVR42mNkYAAAAAYAAjCB0C8AAAAASUVORK5CYII=)
To better observe the consequences, instead of discussing doses from/to various organs, in this study patient effective doses corresponding to different Mo isotopes ![${{\left[{{D}_{\text{eff}}}\right]}^{\text{M}{{\text{o}}_{j}}}}$](https://content.cld.iop.org/journals/0031-9155/61/2/542/revision1/pmbaa0aabieqn020.gif) are considered. Application of the formalism presented in the previous section requires knowledge of
are considered. Application of the formalism presented in the previous section requires knowledge of ![${{\left[{{D}_{\text{eff}}}\right]}^{\text{M}{{\text{o}}_{j}}}}$](https://content.cld.iop.org/journals/0031-9155/61/2/542/revision1/pmbaa0aabieqn021.gif) values, which depend on the activities of all Tc isotopes produced on the target component
values, which depend on the activities of all Tc isotopes produced on the target component  and on the dose coefficients for each of these Tc isotopes. These dose coefficients have fixed values for any given radioisotope and radiopharmaceutical. However, the produced Tc activities depend on irradiation conditions and, since different isotopes decay with different rates, on the time period between the EOB and the injection. Therefore,
and on the dose coefficients for each of these Tc isotopes. These dose coefficients have fixed values for any given radioisotope and radiopharmaceutical. However, the produced Tc activities depend on irradiation conditions and, since different isotopes decay with different rates, on the time period between the EOB and the injection. Therefore, ![${{\left[{{D}_{\text{eff}}}\right]}^{\text{M}{{\text{o}}_{j}}}}$](https://content.cld.iop.org/journals/0031-9155/61/2/542/revision1/pmbaa0aabieqn024.gif) also depends on the irradiation conditions, i.e. proton beam energy, irradiation time, thickness of the target and patient injection time (after EOB).
also depends on the irradiation conditions, i.e. proton beam energy, irradiation time, thickness of the target and patient injection time (after EOB).
While it is possible to calculate ![${{\left[{{D}_{\text{eff}}}\right]}^{\text{M}{{\text{o}}_{j}}}}$](https://content.cld.iop.org/journals/0031-9155/61/2/542/revision1/pmbaa0aabieqn025.gif) for a broad range of irradiation-injection conditions, according to Hou et al (2012), patient dose would increase when using long irradiation time and late injection time after EOB. Thus, we performed the calculation assuming the 'worst-case irradiation-injection scenario', i.e. the doses were estimated for samples produced using 12 h irradiations and injected at 24 h after EOB, assuming targets thick enough to completely stop the proton beam. Therefore, for shorter irradiation times and/or earlier injection times, patient doses based on our results would be overestimated (Hou et al 2012).
for a broad range of irradiation-injection conditions, according to Hou et al (2012), patient dose would increase when using long irradiation time and late injection time after EOB. Thus, we performed the calculation assuming the 'worst-case irradiation-injection scenario', i.e. the doses were estimated for samples produced using 12 h irradiations and injected at 24 h after EOB, assuming targets thick enough to completely stop the proton beam. Therefore, for shorter irradiation times and/or earlier injection times, patient doses based on our results would be overestimated (Hou et al 2012).
Table 1 summarizes patient effective doses ![${{\left[{{D}_{\text{eff}}}\right]}^{\text{M}{{\text{o}}_{j}}}}$](https://content.cld.iop.org/journals/0031-9155/61/2/542/revision1/pmbaa0aabieqn026.gif) per unit beam current (mSv μA−1) due to all technetium isotopes that would be created assuming a target that is 100% pure Mo isotope j. These values have been calculated using our CYD software (Hou et al 2014) for five different irradiation energies ranging from 16 MeV to 24 MeV under the 'worst-case irradiation-injection scenario' conditions defined above. For consistency with our CPTc dosimetry publication (Hou et al 2012), we employed the same three commonly used Tc radiopharmaceuticals, namely sestamibi™, phosphonate, and pertechnetate.
per unit beam current (mSv μA−1) due to all technetium isotopes that would be created assuming a target that is 100% pure Mo isotope j. These values have been calculated using our CYD software (Hou et al 2014) for five different irradiation energies ranging from 16 MeV to 24 MeV under the 'worst-case irradiation-injection scenario' conditions defined above. For consistency with our CPTc dosimetry publication (Hou et al 2012), we employed the same three commonly used Tc radiopharmaceuticals, namely sestamibi™, phosphonate, and pertechnetate.
Table 1. The effective dose per unit beam current ![${{\left[{{D}_{\text{eff}}}\right]}^{\text{M}{{\text{o}}_{j}}}}$](https://content.cld.iop.org/journals/0031-9155/61/2/542/revision1/pmbaa0aabieqn027.gif) (mSv μA−1) due to technetium impurities which would be produced if a target composed of 100% of each of the stable Mo isotopes was irradiated by a 16, 18, 20, 22, and 24 MeV proton beam. The data was generated using the CYD software under the 'worst-case irradiation-injection scenario', i.e. thick target, 12 h irradiation time and 24 h after EOB injection time.
(mSv μA−1) due to technetium impurities which would be produced if a target composed of 100% of each of the stable Mo isotopes was irradiated by a 16, 18, 20, 22, and 24 MeV proton beam. The data was generated using the CYD software under the 'worst-case irradiation-injection scenario', i.e. thick target, 12 h irradiation time and 24 h after EOB injection time.
| Irradiation Energy | Mo isotope | The effective dose ![${{\left[{{D}_{\text{eff}}}\right]}^{\text{M}{{\text{o}}_{j}}}}$](https://content.cld.iop.org/journals/0031-9155/61/2/542/revision1/pmbaa0aabieqn028.gif) (mSv μA−1) (mSv μA−1) |
||
|---|---|---|---|---|
| Sestamibi™ | Phosphonate | Pertechnetate | ||
| 16 MeV | 92 | 8.23E − 03 | 7.10E − 03 | 2.21E − 03 |
| 94 | 2.03E + 01 | 1.40E + 01 | 2.29E + 01 | |
| 95 | 1.03E + 02 | 5.06E + 01 | 1.11E + 02 | |
| 96 | 3.21E + 02 | 1.61E + 02 | 3.58E + 02 | |
| 97 | 1.72E + 02 | 8.71E + 01 | 1.94E + 02 | |
| 98 | 6.82E − 01 | 1.40E − 01 | 8.91E − 01 | |
| 100 | 1.04E + 00 | 7.06E − 01 | 1.29E + 00 | |
| 18 MeV | 92 | 8.57E − 03 | 7.40E − 03 | 2.30E − 03 |
| 94 | 2.30E + 01 | 1.59E + 01 | 2.58E + 01 | |
| 95 | 1.19E + 02 | 5.99E + 01 | 1.29E + 02 | |
| 96 | 3.80E + 02 | 1.90E + 02 | 4.23E + 02 | |
| 97 | 2.95E + 02 | 1.49E + 02 | 3.32E + 02 | |
| 98 | 9.86E − 01 | 2.03E − 01 | 1.29E + 00 | |
| 100 | 1.38E + 00 | 9.39E − 01 | 1.71E + 00 | |
| 20 MeV | 92 | 8.82E − 03 | 7.61E − 03 | 2.37E − 03 |
| 94 | 2.47E + 01 | 1.72E + 01 | 2.75E + 01 | |
| 95 | 1.33E + 02 | 6.83E + 01 | 1.45E + 02 | |
| 96 | 4.37E + 02 | 2.18E + 02 | 4.85E + 02 | |
| 97 | 4.33E + 02 | 2.19E + 02 | 4.86E + 02 | |
| 98 | 1.37E + 00 | 3.06E − 01 | 1.78E + 00 | |
| 100 | 1.69E + 00 | 1.14E + 00 | 2.08E + 00 | |
| 22 MeV | 92 | 8.97E − 03 | 7.74E − 03 | 2.41E − 03 |
| 94 | 2.59E + 01 | 1.80E + 01 | 2.86E + 01 | |
| 95 | 1.46E + 02 | 7.63E + 01 | 1.59E + 02 | |
| 96 | 4.95E + 02 | 2.46E + 02 | 5.48E + 02 | |
| 97 | 5.70E + 02 | 2.88E + 02 | 6.40E + 02 | |
| 98 | 1.29E + 01 | 6.04E + 00 | 1.47E + 01 | |
| 100 | 1.92E + 00 | 1.30E + 00 | 2.37E + 00 | |
| 24 MeV | 92 | 9.04E − 03 | 7.80E − 03 | 2.43E − 03 |
| 94 | 2.67E + 01 | 1.86E + 01 | 2.93E + 01 | |
| 95 | 1.57E + 02 | 8.35E + 01 | 1.71E + 02 | |
| 96 | 5.51E + 02 | 2.73E + 02 | 6.09E + 02 | |
| 97 | 6.92E + 02 | 3.50E + 02 | 7.77E + 02 | |
| 98 | 5.57E + 01 | 2.77E + 01 | 6.29E + 01 | |
| 100 | 2.08E + 00 | 1.41E + 00 | 2.57E + 00 | |
3. Target specification for CPTc
Using the proposed algorithm and table 1, it is possible not only to estimate DI for any particular target enrichment but also to set acceptance criteria for Mo targets based on any given DI levels. Such dose-based target composition specifications will allow the user or 100Mo target manufacturer to disqualify targets that will lead to an unacceptably high DI level before cyclotron runs are performed.
3.1. DI estimates for targets of 100Mo + xxMo
Before specifying target composition criteria, it is useful to investigate how patient dose would increase if only 100Mo and a single other Mo isotope (marked as xxMo) are present in the target. Although no real-life target will have only one Mo impurity, this kind of a study can provide an 'extreme' upper limit for the amount of each Mo isotope in the target and indicate the 'worst Mo offender'. This analysis is presented in figure 1. Each figure section corresponds to one beam energy (16–24 MeV) and contains a set of curves, each of which corresponds to one 100Mo + xxMo target. The curves indicate DI levels based on the different percentage amounts of each xxMo isotope in the target (as shown on the x-axis). Pertechnetate and the 'worst-case irradiation-injection scenario' were used in the calculations. Once a clinically-acceptable level of patient DI is defined, the target with isotopic composition leading to larger DI than this level can be eliminated from further processing. Please note that the DI is not linearly proportional to the Mo isotopic composition and the curves of 92Mo are not equal to 100% but increasingly close to 100%.
Figure 1. Different DI levels that are due to the percentage amounts of each xxMo isotope in the 100Mo + xxMo target for irradiation energies from 16 to 24 MeV. The targets were assumed thick enough to stop the proton beam; 12 h irradiation and injection at 24 h after EOB were considered. Pertechnetate was used in these calculations.
Download figure:
Standard image High-resolution image3.2. Molybdenum target specifications based on the corresponding DI levels
Considering all seven stable molybdenum isotopes (92, 94, 95, 96, 97, 98, 100Mo) that are potentially present in the target, and the complexity of the relationship between irradiation conditions and the production yields for various technetium isotopes, determination of a single threshold for the amount of each Mo isotope so that their combined contributions do not exceed the predetermined DI limit represents a very complicated problem. Therefore, to simplify the problem in our analysis we adopted an approach based on the following considerations:
- (a)It is generally easier to separate isotopes when their isotopic masses are farther apart. Thus, for example, it is easier to separate 92Mo from 100Mo than it is to separate 98Mo from 100Mo.
- (b)Not only 92Mo is the easiest to separate from 100Mo, but also, as shown in figure 1, 92Mo content is not projected to significantly affect DI.
- (c)Reactions on 94–97Mo isotopes, leading to production of 94–96Tc technetium impurities, are the main contributors to DI.
- (d)98Mo content will not significantly affect DI at energies lower than 20 MeV. However, its importance becomes obvious when the proton beam energy is greater than 22 MeV, as shown in figure 1.
- (e)For the targets which were obtained by our group (shown in table 2), the amounts of 92–97Mo were at similar levels, while the amount of 98Mo varied and was always higher than the others. This suggests that such targets may be relatively easy to produce.
Table 2. Examples of patient dose increase for five targets with the corresponding true calculated patient dose increase. Compositions of five enriched 100Mo targets, which were previously obtained by our group, are listed in the table. The percent abundance of each isotope is as reported on the Certificate of Analysis supplied by the vendors Trace (2011) and Isoflex (2011, 2013).
| Mo isotope | Target composition (%) | ||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| 92Mo | <0.003 | 0.006 | 0.06 | 0.09 | 0.005 |
| 94Mo | <0.003 | 0.0051 | 0.03 | 0.06 | 0.005 |
| 95Mo | <0.003 | 0.0076 | 0.04 | 0.1 | 0.005 |
| 96Mo | <0.003 | 0.0012 | 0.05 | 0.11 | 0.005 |
| 97Mo | <0.003 | 0.0016 | 0.08 | 0.08 | 0.01 |
| 98Mo | 0.17 | 0.41 | 0.47 | 0.55 | 2.58 |
| 100Mo | 99.815 | 99.54 | 99.27 | 99.01 | 97.39 |
| 92–97Mo used in the estimations | 0.003 | 0.0076 | 0.08 | 0.11 | 0.01 |
| 18–0 MeV, 24 h after EOB | |||||
| True DI | 1.73% | 1.57% | 31.94% | 52.12% | 5.72% |
| Estimated DI | <10% | <10% | <50% | >50% | <10% |
| 24–0 MeV, 24 h after EOB | |||||
| True DI | 6.02% | 11.41% | 50.84% | 71.66% | 69.49% |
| Estimated DI | <10% | <20% | >50% | >50% | >50% |
| 18–11 ± 1 MeV, 24 h after EOB (experimental data) | |||||
| Irradiation time (h) | 2.8 | 0.25 | 1.3 | 6.4 | |
| True DI | 1.59% | — | 12.96% | 16.52% | 9.25% |
| Estimated DI | <10% | <10% | <50% | >50% | <10% |
Based on the above factors, in our target specification study, the same threshold for the percentage amounts of 92–97Mo in the target is considered, while 98Mo content is defined separately. Combining these assumptions with equation (6), the relationships between the amounts of 92–97Mo and 98Mo in the target and the resulting DI can be quantified. The results are shown in figure 2. The calculations were performed for the same 'worst-case irradiation-injection scenario' as was used to obtain data displayed in figure 1. Only situations leading to patient DI up to 50% were considered. Additionally, in each sub-figure doses corresponding to five targets, which were obtained at our sites, are displayed (for target compositions see table 2). Due to the different abundances of 92–97Mo in each of the experimental targets, the maximum abundance of 92–97Mo in each target was considered when testing target specifications (refers to the '92–97Mo used in the estimations' in table 2). Using this figure, the patient DI corresponding to a given target can be estimated. We estimated theoretical DI for each target for 18 MeV and 24 MeV beam energies using figure 2 as an example (shown in table 2 as 'Estimated DI'). In parallel, DI levels corresponding to actual target compositions were calculated (shown as 'True DI') using our CYD software. Additionally, in the last part of this table DI corresponding to Tc impurities determined in our experimental studies are listed (target II was not used in the experiments). The experiments were performed at the BC Cancer Agency using a 19 MeV cyclotron. Details about target preparation, irradiation and purification have been described elsewhere (Morley et al 2012, Bénard et al 2014a, Hanemaayer et al 2014).
Figure 2. The relationships between a single fraction of 92–97Mo and 98Mo in the target and patient dose increases for irradiation energies ranging from 16 to 24 MeV. The 'worst-case irradiation-injection scenario' was assumed in dose calculations (see text). The positions of five targets listed in table 2 are also shown in each sub-figure as examples indicating the patient DI corresponding to each of these target compositions for different irradiation energies.
Download figure:
Standard image High-resolution imageRelationships presented in figure 2 not only can be used for a rough estimation of patient DI, they can also be considered for the Mo targets corresponding to acceptable DI. For such targets the abundances of 92–97Mo and 98Mo must be below the DI curve defined as the acceptable limit, while targets with abundances above this curve must be rejected. For instance, shown in figure 2, if 10% DI is set as the acceptable DI level, only target I, II and V would be considered for 18 MeV and only target I for 24 MeV proton beam energy.
4. Discussion
In this work, we investigated the relationship between patient radiation dose from CPTc-based radiopharmaceuticals and the compositions of the enriched 100Mo targets used to produce CPTc samples. Our aim was to design a simple and fast method that would allow us to define target isotopic composition thresholds leading to clinically-acceptable dose increases for injections involving CPTc-labeled radiopharmaceuticals. The main component of such target-based dosimetry is the estimation of patient dose corresponding to each of the Mo isotopes that are present in the target (table 1). Combining the data presented in table 1 with equations (4) and (6) provides a straightforward method for estimating patient dose and dose increase for any given Mo target. This method can be used instead of performing complicated yield estimations for each of the Tc products.
Both table 1 and figure 1 show that reactions on 95–97Mo, leading to 94–96Tc production, contribute the most to patient radiation dose. Moreover, doses from technetium isotopes produced on 92Mo–97Mo remain approximately constant for proton beam energies ranging from 16 MeV to 24 MeV, while doses from reactions on 98Mo vary with proton energy. Above 20 MeV, high levels of 98Mo result in substantial dose increases because the (p,3n) reaction channel opens. The (p,3n) reaction with 98Mo results in production of 96gTc, which influences patient dose the most. In addition, figure 1 shows the 'extreme' upper limit for each Mo isotope providing information about patient dose increases from 10% to 100%, under the assumption that the target contains only two isotopes (100Mo + xxMo). Targets with each of Mo isotopes larger than this limit cannot be used for CPTc production for clinical use.
Figure 2 presents the relationship between the amounts of 92–97Mo and 98Mo in the target and the corresponding DI levels for five beam energies. This figure has two different functions. First, using this figure, the upper limit of the patient DI corresponding to a given target can be estimated, as shown in table 2. Although the amounts of Mo isotopes in the five targets considered in this study varied, we used the assumptions discussed in section 3.2 and assigned a single value for the amounts of 92–97Mo, i.e. maximum amounts among 92Mo to 97Mo. Table 2 confirms that DI values estimated from figure 2 consistently exceed the calculated DI, but also are relatively close to them. While the experimental DI and values determined from figure 2 for Target I and V show relatively good agreements; large differences can be found for Target III and IV. These differences are mainly due to shorter irradiation times of experiments using target III and IV resulting in lower amounts of impurities (Celler et al 2011, Hou et al 2012).
Second, figure 2 can be used when defining target accept/reject criteria for target specifications. Once an acceptable level of patient DI is defined (for example it can be set to be equal to or less than 10%), the threshold amount for each Mo isotope in the target can be determined. This threshold will depend on proton beam energy and its value can be obtained from figure 2. Table 3 shows two examples of target specification for proton beam energies ranging from 16–24 MeV assuming a dose increase limit equals to 10% at 24 h after EOB. Based on our discussion in section 3.2, the same amounts of 92–97Mo for all the energy levels were used while amounts of 98Mo decreased with increasing proton energy. Similar target specifications can be set by users based on figure 2 and their own requirements.
Table 3. Two examples of 100Mo target composition specifications, expressed as percentage of total molybdenum content, based on 10% dose increase at 24 h after EOB.
| Mo isotope | Target composition (%) | ||
|---|---|---|---|
| 16 MeV ⩽ E ⩽ 20 MeV | 20 MeV < E ⩽ 22 MeV | 22 MeV < E ⩽ 24 MeV | |
| Example I | |||
| 92Mo | 0.013 | 0.013 | 0.013 |
| 94Mo | 0.013 | 0.013 | 0.013 |
| 95Mo | 0.013 | 0.013 | 0.013 |
| 96Mo | 0.013 | 0.013 | 0.013 |
| 97Mo | 0.013 | 0.013 | 0.013 |
| 98Mo | 3 | 0.4 | 0.08 |
| 100Mo | 96.94 | 99.54 | 99.86 |
| Example II | |||
| 92Mo | 0.008 | 0.008 | 0.008 |
| 94Mo | 0.008 | 0.008 | 0.008 |
| 95Mo | 0.008 | 0.008 | 0.008 |
| 96Mo | 0.008 | 0.008 | 0.008 |
| 97Mo | 0.008 | 0.008 | 0.008 |
| 98Mo | 6 | 0.85 | 0.2 |
| 100Mo | 93.96 | 99.11 | 99.76 |
5. Conclusions
In this study, we investigated the effect of the isotopic composition of the Mo target used for cyclotron production of 99mTc on patient dose increase due to injection of a radiopharmaceutical labeled with cyclotron-produced 99mTc relative to that labeled with pure 99mTc. A simple and quick dose estimation method was proposed. A table of effective patient doses corresponding to technetium produced on each of the stable Mo isotopes has been provided. This table, together with the proposed formalism can be used to define a threshold of each Mo isotopic impurity present in the target for CPTc production. Thresholds for the amounts of stable Mo isotopes allowed in the target for different levels of patient dose increase have been provided. As an example, target compositions corresponding to 10% patient dose increase at 24 h after EOB were considered for five different proton beam energies. Additionally, Mo target accept/reject criteria have been discussed for different ratios of 92–97Mo and 98Mo in the target. Such criteria can be used to verify if any given Mo target is acceptable for clinical CPTc production.
Acknowledgments
This work was supported by Natural Resources Canada through the Isotope Technology Acceleration Program (ITAP) and TRIUMF core funding.



![${{\left[{{D}_{\text{eff}}}\right]}^{\text{M}{{\text{o}}_{j}}}}$](https://content.cld.iop.org/journals/0031-9155/61/2/542/revision1/pmbaa0aabieqn019.gif)

