Abstract
The effects of the Cl adatom on Na-decorated graphene are studied using first principles density functional theory under the generalized gradient approximation to calculate the adsorption energy, geometric structure, charge density difference, and density of states. When Na and Cl adatoms are simultaneously absorbed on opposite sides of graphene, the adsorption energy of Na increases by about 1 eV and the adsorption system becomes more stable because graphene can effectively transfer the 3s valence of the Na adatom to the Cl adatom.
Export citation and abstract BibTeX RIS
1. Introduction
Graphene, which is a 2D single layer with an sp2-bonded carbon lattice, has many prominent intrinsic chemical and physical features such as excellent mechanical strength, extraordinarily high electrical and thermal conductivities, good light transmission, etc [1–10]. So it has potential in various applications, including electronics [11], optics [12], catalysis [13, 14], sensors [15, 16], and energy storage [17, 18]. Due to it has exceptionally high specific surface area, which is up to 2675 m2 g−1 [19], graphene can be doped by adsorbing atoms of other elements, and then controlling the self-assembly structure can manufacture new types of graphene-based nanodevices. Because this adsorbate-graphene system may significantly expand graphene applications, it has received much attention in the past few years [20–25].
Alkali atoms have simple electronic structures and relatively high ratios of adsorption energy to the bulk cohesive energy, suggesting that alkalis can form 2D layers on the surface of graphene [26]. Therefore alkali-decorated graphene systems have been investigated in a wide range of studies [27–33]. The adsorption energy of Na adatom on graphene is lower than other alkali adatoms, limiting the applications of Na-decorated graphene [26, 34–37]. However, because Na is abundant and easily extracted, Na-decorated graphene applications are highly anticipated.
On the other hand, Cl atoms have the highest electron affinity and the fourth highest electronegativity of all reactive elements. Because the electronegativity of carbon atoms is in between those of Na and Cl atoms, graphene may transfer the 3s valence of Na to Cl when they are adsorbed simultaneously on opposite sides of graphene. Thus, co-adsorption may improve the adsorption energy of Na on graphene and alter the charge distribution around the Na adatom, enhancing the performance of Na-decorated graphene nanomaterials.
In this work, the co-adsorption of Na and Cl adatoms on graphene is studied using first principles density functional theory (DFT). Specifically, their adsorption energies and electronic structures are reported.
2. Methods
A 5 × 5 supercell (50 carbon atoms) was used as the graphene model to eliminate the coupling interaction of adjacent adatoms. The calculations used fixed supercell dimensions and a lattice constant of 2.47 Å, which is slightly larger than the experimental value of 2.46 Å [26]. The minimum distance perpendicular to the layer was 25 Å, making the interactions among the perpendicular cells negligible.
All the calculations were performed within the first-principles DFT under the generalized gradient approximation (GGA) of perdew, burke, and ernzerh (PBE). The Vienna ab-initio stimulation package (VASP) was used to perform all calculations [38–40]. Ion cores were modeled with the projector augmented wave (PAW) potentials. The semicore 2p63s1 states of Na and the 3s23p7 states of Cl were treated explicitly as valence states. The cutoff for the plane waves' kinetic energy was 500 eV. During the relaxation process and the total energy calculations, the reciprocal space was sampled using the gamma point-centered methodology with a 9 × 9 × 1 k-point grid, whereas a 13 × 13 × 1 mesh was adopted for the DOS calculations. A Gaussian smearing with a width of 0.05 eV was used for the occupation of the electronic levels. All atoms were allowed to relax until the residual forces were less than 0.01 eV Å−1 in the optimization. Spin polarization is not considered in this work.
Figure 1 shows the graphene model with a single Na or Cl adatom adsorbing at three different sites: the T-site (directly above a carbon atom), B-site (above the midpoint of a carbon–carbon bond), and H-site (above the center of a hexagonal ring of graphene).
Figure 1. Atomic structure of 5 × 5 graphene with three different adsorption sites: hollow (H), bridge (B), and top (T).
Download figure:
Standard image High-resolution image3. Results and discussion
Table 1 lists the results for a single Na adatom or Cl adatom adsorbed on graphene. The adsorption energy is defined as
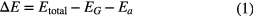
where ΔE is the adsorption energy, Etotal is the total energy of the whole system (including the adsorbates and graphene), EG is the total energy of pure graphene, and Ea is the total energy of the isolated adatom. The adsorption geometry is obtained from the positions of the atoms after relaxation. The adatom height (h) is defined as the difference in the z-coordinates of the adatom and the average of the z-coordinates of the C atoms in the graphene layer. In some cases, the distortion of the graphene layer is significant [26]; it is defined as the difference of the z coordinates between the C atoms which have the maximum and minima value of the z coordinate in graphene layer [41]. The Bader method was used in the charge transfer analysis.
Table 1. Energetic and structural properties of a single Na adatom or Cl adatom adsorbed at a hollow (H), bridge (B), or top (T) sites of graphene. Properties listed include the adsorbed energy (ΔE), adatom height (h), graphene distortion (Δhc), and charge transfer (Δq).
| Adatom | Site | ΔE(eV) | h(Å) | Δhc(Å) | Δq(e) |
|---|---|---|---|---|---|
| Na | H | − 0.837 | 2.205 | 0.009 | − 0.905 |
| B | − 0.708 | 2.348 | 0.063 | − 0.846 | |
| T | − 0.698 | 2.361 | 0.074 | − 0.864 | |
| Cl | T | − 1.115 | 3.077 | 0.012 | 0.514 |
| B | − 1.112 | 3.102 | 0.016 | 0.525 | |
| H | − 1.094 | 3.157 | 0.018 | 0.512 |
Of the three adsorption sites are considered, the site with the largest adsorption energy is referred to as the favored site [26]. For the Na adatom, the most favored site is the H-site, while the T-site, which has the minimum adsorption energy, is the least favored. The energy difference between these two sites is 0.139 eV, but the difference between the T- and B-sites is small (~0.01 eV). When the Na adatom adsorbs on the favored site, the small graphene distortion of 0.009 Å is almost negligible.
Although the Cl adatom binds most strongly to the T-site, the difference in adsorption energies of the three sites is very small (~0.02 eV). That is, the migration energy barrier for a Cl adatom is very small [42]. Similarly, the difference in graphene distortion at the three sites is small. When a Cl adatom adsorbs on the T-site, the stable height is about 3.077 Å, which is larger than the bond length of CCl4 (1.77 Å), indicating that the character of this bond differs from a C–Cl covalent bond [42, 43].
Table 2 shows the result when a single Na adatom adsorbs above the graphene and a single Cl adatom adsorbs below the graphene. We calculated nine combinations of Na and Cl adatoms when they adsorbed on the same hexagonal. The adsorption energy is defined as
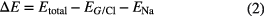
where ΔE is the adsorption energy, Etotal is the total energy of the whole system, EG/Cl is the total energy of the system when a single Cl adatom adsorbs on graphene, and ENa is the total energy of an isolated Na atom.
Table 2. Energetic and structural properties of Na and Cl adatoms adsorbed simultaneously above and below graphene. Properties listed are the sites of adatoms, adsorbed energy (ΔE), adatom height (h), graphene distortion (Δhc), charge transfer (Δq), and the distance of the Na and Cl adatoms along zaxe (d(Cl–Na)z).
| Adatom | Site | ΔE(eV) | h(Å) | Δhc(Å) | Δq(e) | d(Cl_Na)z(Å) |
|---|---|---|---|---|---|---|
| Na/Cl | H/T | − 1.758 | 2.239/2.815 | 0.084 | − 0.887/0.680 | 5.054 |
| H/B | − 1.746 | 2.246/2.851 | 0.045 | − 0.882/0.689 | 5.097 | |
| H/H | − 1.727 | 2.266/2.920 | 0.014 | − 0.881/0.682 | 5.186 | |
| B/T | − 1.685 | 2.332/2.827 | 0.137 | − 0.878/0.683 | 5.159 | |
| B/B | − 1.681 | 2.364/2.839 | 0.068 | − 0.875/0.682 | 5.203 | |
| B/H | − 1.626 | 2.366/2.929 | 0.075 | − 0.873/0.679 | 5.295 | |
| T/T | − 1.709 | 2.311/2.799 | 0.176 | − 0.872/0.685 | 5.110 | |
| T/B | − 1.665 | 2.350/2.851 | 0.131 | − 0.865/0.673 | 5.201 | |
| T/H | − 1.610 | 2.365/2.936 | 0.096 | − 0.871/0.675 | 5.301 |
The adsorption energy of the Na adatom increases about 1 eV and the maximum energy difference is 0.15 eV among the nine combinations, indicating that the migration energy barrier is the same as a single Na adatom adsorbed on graphene. The adsorption energy of the Na adatom is the highest when Na adatom adsorbs above graphene at H-site and Cl adatom adsorbs below graphene at T-site. Thus, the H/T-site combination is the most suitable when Na and Cl adatoms adsorb simultaneously on different sides of graphene. If the Na adatom is fixed at a specific site, varying the location of Cl adatom adsorption has a negligible effect on the adsorption energy of the Na adatom. However if the site of the Cl adatom is fixed, the adsorption energy of the Na adatom is the highest when Na adsorbs at the H-site, while the B- and T-sites are about the same.
The charge loss and adsorption height are similar for the Na adatom adsorbed on graphene and on the Cl-graphene system (tables 1 and 2). However, the Cl adatom receives more charge from the simultaneous adsorption system; it is about 0.18 e and its adsorption height decreases nearly 0.26 Å.
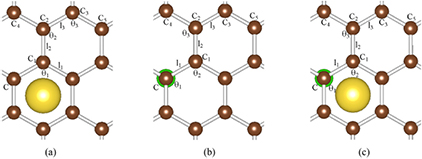
Figure 2 depicts the top views of the adatoms at stable site on graphene where C, l, and θ represent the C atoms, bond lengths, and bond angles, respectively. Yellow and green atoms denote Na adatom and Cl adatom, respectively. Table 3 lists the bond lengths and bond angles of graphene. l0 and θ0 represent bond length and bong angle of pure graphene, while the others stand for bond lengths and bond angles of graphene after adatoms are adsorbed on graphene.
Table 3. Bond lengths and bond angles of graphene. Symbols are marked in figure 2; l0 and θ0 represent the bond length and bong angle of pure graphene.
| Adatom | Site | l0 (Å) | l1 (Å) | l2 (Å) | l3 (Å) | θ0(°) | θ1 (°) | θ2 (°) | θ3 (°) |
|---|---|---|---|---|---|---|---|---|---|
| Na | H | 1.4261 | 1.4328 | 1.4265 | 1.4256 | 120.00 | 120.00 | 119.83 | 120.17 |
| Cl | T | 1.4261 | 1.4262 | 1.4245 | 1.4257 | 120.00 | 120.00 | 119.99 | 120.03 |
| Na/Cl | H/T | 1.4261 | 1.4334 | 1.4251 | 1.4248 | 120.00 | 119.81 | 119.9 | 119.91 |
Figure 2. Top views of adatoms adsorbed at the stable sites on graphene. (C, l, and θ stand for the C atoms, bond lengths, and bond angles, respectively. Yellow and green atoms denote the Na and Cl adatoms, respectively.)
Download figure:
Standard image High-resolution imageThe change in the maximum value of the bond length (bond angle) of graphene before and after adsorption is 0.007 Å (0.19°), adsorption has a negligible effect on the geometric structure of graphene.
Figure 3 shows the charge density difference of the adsorption system, where blue and yellow indicate charge depletion and accumulation, respectively. The charge accumulation on the entire graphene surface suggests that the Na adatom transfers its 3 s valance to the big π bond of graphene (figure 3(a)). The difference in the adsorption energy of the Na adatom when it adsorbs at the B- or T-site is small for the big π bond of graphene. The Cl adatom collects the charge from graphene without obvious charge sharing (figure 3(b)) (i.e. a strong covalent bond does not form between the two). Although the charge density of the H-site is less than that of the T- or B-site, the migration energy of the Cl adatom between sites is very small, which may be due to the relatively long distance (about 3 Å) between the Cl adatom and graphene as well as the larger radius of the Cl adatom after adsorption. It also indicates that there has interaction among Cl adatom and several carbon atoms as figure 3(b) shows.
Figure 3. Charge density difference of the adsorbed system. (a) Na adsorbed at the H-site, (b) Cl adsorbed at the T-site, and (c) Na adsorbed at the H-site above the graphene while Cl is adsorbed at the T-site below the graphene. Yellow and blue indicate charge accumulation and depletion, respectively. Isosurface boundary is set from −0.008 to 0.008.
Download figure:
Standard image High-resolution imageFigure 3(c) shows the charge density difference when the Na adatom is adsorbed at H-site and the Cl-adatom is simultaneously adsorbed at T-site on the opposite side of graphene. The charge density of the C atoms which are located on the edge of graphene is mostly unaffected. Charge accumulation and depletion are mainly concentrated on the Na and Cl adatoms and a few surrounding C atoms. All these indicate that graphene effectively transfers the 3s valence of the Na adatom to the Cl adatom. Although the adsorption distance and charge loss of the Na adatom in the simultaneous adsorbed system is almost the same as the adsorption of the Na adatom alone (figure 3(a)), the additional adsorption of the Cl adatom concentrates the charge, which enhances the interaction between the Na adatom and the graphene. Consequently, the adsorption energy of the Na adatom increases nearly 1 eV. Compared to Cl adatom adsorption on graphene alone (figure 3(b)), the interaction between Cl adatom and graphene (figure 3(c)) is strengthened as well, indicated by the facts that Cl adatom's adsorption height decreased about 0.26 Å and it gained an extra charge of 0.18 e from graphene (tables 1 and 2). For the Na adatom, the decreased charge accumulation on graphene will undoubtedly improve the cation ambient. The significant increase in the adsorption energy and the improved cation ambient may increase the applications of Na-decorated graphene nanomaterials.
Figure 4 shows the DOS (density of states) of graphene which belong to the system of pure graphene, a single Na adatom adsorbed at the H-site on graphene, a single Cl adatom adsorbed at the T-site on graphene, and simultaneous adsorption of a Na adatom at the H-site above the graphene and a Cl adatom at the T-site below the graphene. ED denotes the energy of the Dirac point. For Na adsorbed alone on graphene, the Fermi level shifts to a higher energy relative to ED, reflecting a greater occupation of the graphene states because graphene collects the charge. In contrast, for Cl adsorbed on graphene, the Fermi level shifts to a lower energy relative to ED, indicating that graphene transfers its charge to the Cl adatom. However, when Na and Cl adatoms are adsorbed simultaneously on opposite sides of graphene, the Fermi level is close to ED, demonstrating that graphene transfers most of the obtained charge from the Na adatom to the Cl adatom. Under the three adsorption circumstances, the orbit where the Fermi level is occupied indicates that graphene is metalized. Compared to the pure graphene, there is a peak at the Fermi level in the DOS when a Cl adatom is adsorbed on graphene, suggesting hybridization between the Cl adatom and graphene. The hybridization can be revealed from the PDOS of the C atoms and the Cl adatom (figure 5).
Figure 4. Graphene DOS of the adsorbed system from the top: pure graphene, Na adsorbed at the H-site, Cl adsorbed at the T-site, simultaneous adsorption of Na at the H-site and Cl at the T-site on opposite sides of graphene. Fermi energy is set to zero.
Download figure:
Standard image High-resolution imageFigure 5. PDOS of C atoms in the adsorption systems. Numbering system of the model is described in figure 5.
Download figure:
Standard image High-resolution imageFigure 5 shows the PDOS of several C atoms near the adatoms. The first column (from left to right) shows top views of the adsorption system with the numbering sequence of the C atoms, the C atom PDOS of pure graphene, and three C atoms' PDOS of Na-graphene adsorption system. The second and the third column show the PDOS of six C atoms in the Cl adsorbed on graphene, and Na/Cl co-adsorbed on graphene, respectively. For Na adsorbed on graphene, the Fermi level of C atoms shift to the right of the Dirac point, indicating that the C atom receives charge from the Na adatom. Peaks appear on the left-shoulder of the s-orbital and pz-orbital due to the action of the Na adatom, but the peak height decreases with the distance between the Na and C atoms, it indicate that the charge which Na adatom transfers to graphene occupies the lower energy orbital of the C atoms. For Cl adsorbed on graphene, the Fermi level of C atoms shift to the left of the Dirac point, indicating that Cl adatom receives charge from graphene. Hybridization between the Cl adatom and the C atoms occurs near the Fermi level; the shorter the distance between the Cl and C atoms, the stronger the hybridization. The degree of the hybridization is not only related to the distance between the Cl and C atoms but also with the distribution of carbon atoms. Although the 5th carbon atom is farther from the Cl adatom than the 3rd carbon atom, hybridization of the 5th carbon atom is stronger. These observations indicate that hybridization occurs between the Cl adatom and the big π bond but not between the Cl adatom and a single C atom.
The height of the left shoulder of the s-orbital and the pz-orbital of the C atoms are drastically decreased for Na/Cl co-adsorption system and the Fermi level of C atoms seems to move back the Dirac point vicinity, which strongly suggest that graphene transfers the most charge that collect from Na adatom to the Cl adatom. The interaction with the Na adatom weakens the hybridization between the 1st C atom and the Cl adatom. However, hybridization of the other C atoms is unaffected, suggesting that the hybridization between the Cl adatom and the big π bond differs from general oriented hybridization. The migration energy and adsorption energy of the Cl adatom, changes in the bond length and bond angles of graphene which caused by Cl adatom indicate that the interaction between Cl adatom and grphene is mainly form ionic bonding [42–46]; but due to the hybridization between the Cl adatom and the big π bond of graphene, weaker covalent bonds also form between the Cl adatom and a plurality of C atoms.
4. Conclusion
In this paper, the adsorption energy, geometric structure, charge density difference, and density of states are calculated using first principles density functional theory under the generalized gradient approximation when the Na adatom alone, the Cl adatom alone, and Na/Cl simultaneous adsorption on a 5 × 5 graphene supercell. The Na adatom and graphene form an ionic bond. Although the Cl adatom and graphene mainly form an ionic bond, weaker covalent bond also form between the Cl adatom and the big π bond of graphene. For simultaneous adsorption of Na and Cl on opposite sides of graphene, graphene effectively transfers the 3s valence of the Na adatom to the Cl adatom, significantly increasing the adsorption energy of the Na adatom, stabilizing the adsorption system, and improving the cation ambient of the Na adatom.
Acknowledgments
This work was supported by the Natural Science Foundation of China under Grant No. 41076057, No. 41476082 and the 48th Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, No. 82214226.