Abstract
The review addresses the prospects of global hydrogen energy development. Particular attention is given to the design of materials for sustainable hydrogen energy applications, including hydrogen production, purification, storage, and conversion to energy. The review highlights the key role of oxide-supported metal or alloy nanoparticles as catalysts in the hydrogen production via the conversion of natural gas or alcohols. An alternative approach is the pyrolysis of hydrocarbons giving hydrogen and carbon. The direct production of high-purity hydrogen can be performed using electrolysis or membrane catalysis. Apart from conventional hydrogen storage methods such as the compression and liquefaction, the hydrogen alloy absorption and chemical conversion to liquid carriers (ammonia and toluene cycles) are considered. Fuel cells, containing catalysts and proton-conducting membranes as the key components, are used for hydrogen energy generation. Binary platinum alloys or core – shell structures supported on carbon or oxides can be employed to facilitate the oxygen electroreduction and CO electrooxidation in low-temperature fuel cells. High conductivity and selectivity are provided by perfluorinated sulfonic acid membranes. The high cost of the latter materials dictates the development of alternative membrane materials. A crucial issue in high-temperature fuel cells is the necessity of reducing the operating temperature and ohmic losses. This problem can be solved by designing thin-film materials and replacing oxygen-conducting ceramic membranes by proton-conducting membranes.
The bibliography includes 290 references.
Export citation and abstract BibTeX RIS
| S.P.Filippov. Academician, Doctor of Technical Sciences, Director of the ERI RAS. |
| E-mail: fil_sp@mail.ru |
| Current research interests: energy, system analysis, forecasting, thermodynamics, mathematical modelling. |
| A.B.Yaroslavtsev. Corresponding Member of the RAS, Doctor of Chemical Sciences, Professor, Head of the Laboratory at the IGIC RAS, Head of the Department at the Higher School of Economics University. |
| E-mail: yaroslav@igic.ras.ru |
| Current research interests: ionic conductivity, membranes, materials for lithium- and sodium-ion batteries. |
1. Introduction
Energy is one of the most important inputs for a multitude of industries and operation of various devices, which determine the everyday life and even the lifestyle of a modern person. Until recently, the global energy consumption was doubled, on average, every 30 years. 1 Throughout history, wood was the most important source of energy over a long period of time and was then replaced by coal in the end of the 19th century. Coal was the main energy source until petroleum surpassed it in the early 1950s. All these changes were associated exclusively with economic viability and efficiency of the use of fuel. However, despite all advantages, the dominant role of petroleum is not so unambiguous — it accounts for less than a third of the primary energy production. Switching to alternative sources of energy is currently debated. It should be emphasized that this issue is dictated not by economic reasons but by environmental considerations.
The energy production from carbon-containing sources leads to environmental pollution. Although serious concerns about environmental impacts of carbon dioxide are not univocal, the hazard of accompanying gases such as nitrogen and sulfur oxides is evident. Therefore, the question of which sources of energy will be dominant in the mid-21st century is of great interest. According to guidelines for energy development in advanced countries, these will be renewable energy sources (RESs), in particular solar batteries, wind generators and tidal power. 2–4 However, the application of these sources is limited by the duration and stochastic nature of the natural phenomena. Therefore, energy storage systems are needed to ensure uninterruptible energy supply. Metal-ion batteries, redox batteries and the hydrogen cycle hold the most promise. 4–6 Taking into account self-discharge processes, which occur in batteries, the latter cannot be used for the long-term storage of energy produced from renewable energy sources, for example, to reduce the imbalance between the energy production and consumption in summer and winter, as well as during periods of unfavourable weather.
Fuel cells (FCs), in which the energy is produced by the oxidation of hydrogen-containing fuels by oxygen, can also be considered as renewable energy sources utilizing hydrogen generated by the conversion of biomass 7,8 or alcohols. 9,10 It should be emphasized that, unlike most of energy-generating devices, FCs are environmentally friendly (they produce only water) and highly efficient. Besides, a significant area of the Russian Federation is still not electrified. In line with the trends in decarbonization of the energy industry, the use of hydrogen for energy supply to remote and isolated areas and for field works and vehicles is one of the best solutions.
These trends led to the growing interest in hydrogen energy, 11–13 which is implemented in programmes for hydrogen energy development adopted by a number of developed countries, including Russia. 14 These programmes include the manufacture of FCs and different devices based on them, in particular vehicles. The programmes envisage the development of the hydrogen energy infrastructure, such as systems for hydrogen refueling, storage and production.
It is worth noting that attempts were made to accelerate the widespread use of hydrogen energy. Since the 19th century, hydrogen and hydrogen-containing gases, produced primarily by the gasification of charcoal and coal, were utilized in rather large volumes for gas lighting of cities, in internal combustion engines for vehicles and in industry. In the 1980s, the fear of depletion of world global oil reserves spurred renewed interest in hydrogen. It was proposed to replace petroleum products by hydrogen, produced from coal or using nuclear fuels, resources of which are an order of magnitude greater than those of petroleum. 15 However, more recently it was shown that the growth rate in global vehicle fuel demand will slow down, while petroleum resources are sufficient to meet the global energy demand for the foreseeable future. 16
The following main geopolitical factors have renewed the interest in hydrogen energy:
- —the Paris Agreement on climate change on 12 December 2015;
- —efforts of hydrocarbon-importing countries to greatly reduce their dependence on foreign exporters;
- —formation of the global market of devices ensuring the use of renewable energy sources.
According to the Paris Agreement on climate change, the goal is to limit global warming to well below 2 °C above pre-industrial levels. This agreement requires the reduction of energy consumption (energy saving obligation) and decarbonization of the energy sector and economy. The latter implies the replacement of fossil fuels by renewable energy sources and the use of carbon-free energy supply, primarily hydrogen.
According to the estimates, 12 a twofold reduction of CO2 emission by 2040 and, for the first time in the history of mankind, the reversion to a downward trajectory of energy consumption are required under the Paris Agreement. These challenges necessitate the development of new technologies. New materials, both functional and structural, in particular hydrogen energy materials, will play the key role in the solution of these issues. Such materials should help in increasing the performance of devices, elongating their service life and decreasing the cost. This problem is addressed in several reviews published in the past half-and-one year. 17–20 However, definite materials are required for the design of each hydrogen energy device, the efficiency of implementation of a particular technology being dependent on their quality. Hence, the hydrogen energy development requires the exploration of materials science issues related to the construction of new materials and optimization of their properties. Advances in this challenging research area are not covered in literature reviews.
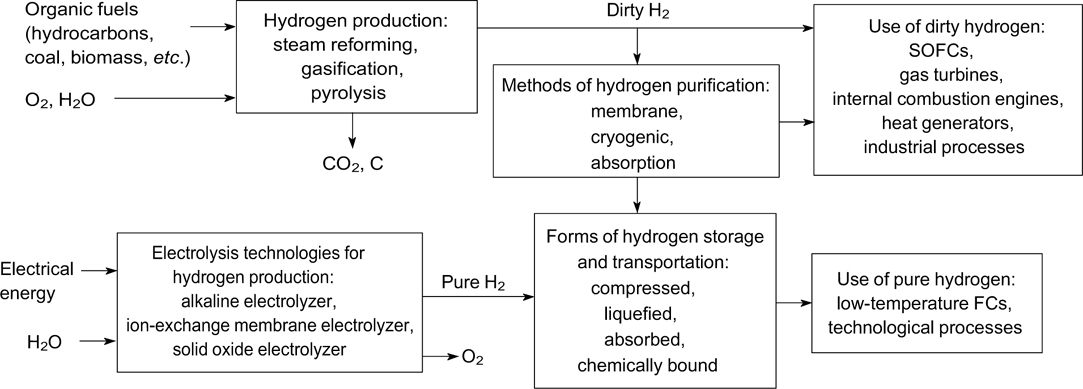
This review is devoted to the prospects of hydrogen energy development and the creation of main types of materials suitable for hydrogen energy, including the production, purification and storage of hydrogen and its conversion to energy (Fig. 1). Evidently, it is impossible to consider all publications in this rapidly growing research area. Hence, selected important advances are discussed in each section of the review, and related publications are briefly summarized, which, in our opinion, can provide insight into the state-of-the-art of hydrogen energy.
Figure 1. Main directions of hydrogen energy. SOFCs are solid oxide fuel cells.
Download figure:
Standard image2. Prospects of hydrogen energy development
Currently, the global demand for hydrogen is ∼115 million tons per annum (mtpa) 21 (Table 1). Seventy mtpa of hydrogen are used in individual form, mainly for the ammonia production and oil refining; ∼45 mtpa are used as mixtures with different gases for methanol production, direct metal reduction, etc. The share of hydrogen in the final energy consumption is ∼3%. Hydrogen is not currently utilized in the electric power generation for economic reasons.
Table 1. Projected global demand for hydrogen by 2050, million metric tons of hydrogen per year.
| Usage | Current demand
| Forecasted demand
| Theoretical maximum
|
|---|---|---|---|
| Industry | 115 | 123 | 301 |
| Transport sector | 0 | 301 | 524 |
| Electric power | 0 | 219 | 439 |
| Building heating | 0 | 53 | 106 |
| Total demand | 115 | 696 | 1370 |
| Share of hydrogen in final energy consumption (%) | 3 | 24 | 49 |
The global hydrogen production from natural gas and coal is 76 and 23%, respectively. Approximately 1% of hydrogen comes as a by-product from alkaline electrolysis used to produce chlorine and sodium hydroxide (caustic soda), and only 0.1% of hydrogen are produced by water electrolysis. 21
According to the estimates, 22 the global demand for hydrogen may increase almost six-fold, up to 700 mtpa, by 2050 in alignment with net-zero climate targets to limit global warming. In this case, the share of hydrogen in the final energy consumption will reach 24% (see Table 1). As expected, an increase in the demand for hydrogen will be mainly due to consumption in the transport sector and for the electric power generation, hydrogen being planned to be used for the generation of peak-power traffic, which is important in the case of a high proportion of renewable energy sources in power-generating energy structures. In this case, hydrogen is produced by water electrolysis based on electrical energy generated from RESs and serves as intermediate energy storage systems followed by its use in FCs. According to the estimates, the hydrogen infrastructure, including hydrogen production, transportation and storage, will have $11 trillion in investments. The estimated maximum global demand for hydrogen by 2050 is 1370 mtpa or 49% of global final energy demand. 22 It is expected that organic fuels will be completely replaced in the transport sector, industry, electric power generation and housing and communal services, where it is technically possible.
According to published data, 23 the global demand for pure hydrogen is estimated at a lower value of ∼280 mtpa by 2050 and at 520 mtpa by 2070. Approximately 30% of hydrogen will be consumed by ground transport; 20%, by the jet fuel production along with CO2; 10%, for the synthesis of ammonia followed by its use as a marine fuel; 15%, in chemical industry and metallurgy; 15%, for the generation of peak-power traffic; 5%, for building heating and hot water supply; the remaining 5%, for other needs. It is predicted that ∼40% of hydrogen will be produced from organic fuels with CO2 capture and 60% by water electrolysis.
The main components of hydrogen energy are presented in Fig. 1. To meet net-zero climate targets, hydrogen energy will be based to a large extent on carbon-free primary energy sources, such as RESs, nuclear power and hydrogen production by water electrolysis. The use of organic fuels will require an adequate utilization of large volumes of CO2 — its useful application or disposal, which are associated with significant economic and technological challenges.
The feasibility and scope of the development of hydrogen energy in Russia are important issues. The Unified Energy System (UES) of Russia has a large excess of generating capacities of about 60 GW over needed 155 – 160 GW for the annual maximum power consumption and of 30 GW for the spare capacity. 24 The programme was adopted for the modernization of obsolete and worn-out equipment of the UES. However, in most cases the modernization is reduced to the replacement of worn-out equipment by that of the same kind. The transition to the hydrogen energy may by an alternative. However, Russia is not yet ready for this transition because of the lack of appropriate domestic technologies. The large-scale use of foreign equipment contradicts the requirement of scientific and technological independence of Russia.
The development of hydrogen energy may be stimulated by economic factors. Currently, changes in the structure of energy consumption are observed due to the technological revolution and the impact of economic and social factors. The concentration of population and business in large cities and urban agglomerations leads to a rapid increase in the pressure on the electric power and transport sectors. The energy demand density in metropolitan cities amounts up to (5 − 10) × 103 MW-h km−2 per year or even more. 25 This gives rise to serious environmental problems and difficulties with the introduction of large electric power flows.
Additional problems arise with the large-scale adoption of electric cars. These issues are the multiplied demand for electric power, the fluctuation of electricity demand throughout the day and the necessity of the installation of extensive networks of charging stations, which may include high-power electrochemical batteries and hydrogen FCs. Electrochemical batteries can displace hydrocarbons in passenger vehicles; hydrogen FCs, in heavy-duty and passenger vehicles and in rail, river and maritime transport. In particular, Daimler Trucks is developing a hydrogen FC 40-ton Mercedes-Benz GenH2 truck with a 1000 km range (80 kg of liquid hydrogen). 26 Hydrogen passenger vehicles are also under development. For instance, Toyota introduced a second-generation Mirai hydrogen fuel cell electric vehicle with an 850 km range (5.6 kg of hydrogen). 27 A hydrogen infrastructure is being constructed. Toshiba proposed a technology for a multi station (H2OneTM Multi Station) 28 to supply both hydrogen fuel to fuel cell vehicles and electric power; in this multi station, hydrogen is produced by water electrolysis using electric power generated by RES.
Two thirds of the Russian territory have no centralized electric power supply, and three-fourth no centralized gas supply. This is because of economic inefficiency of the installation of such systems due to a low energy demand density (<10 MW-h km−2 per year for electricity). 25 These territories are suitable for the creation of a RES-based distributed generation infrastructure. 29 In such systems, electrochemical batteries can cover the daily electric load demand, and hydrogen technologies (FCs, electrolyzers, storage systems) can provide long-term (weekly, seasonal) energy storage, thereby ensuring the efficient and reliable electric power supply for consumers.
Fuel cells are a key technology making the transition to hydrogen energy energetically and economically feasible. In many countries, the development of FCs is supported at the government level. For instance, the major component of the National Hydrogen Strategy of Germany is the multi-billion euro National Innovation Programme for Hydrogen and Fuel Cell Technology. 30 Fuel cells are extensively developed in the United States, Japan, South Korea and many other countries. National programmes were adopted in many countries. 31 Governments provide support to the creation of demonstration areas for testing FCs of different types under actual operating conditions and demonstrating the technological potential for accelerating the formation of the relevant market. Germany follows suit and starts the development of hydrogen regions (HyLands) with the aim to elaborate new technologies and accelerate the market introduction of FCs and other hydrogen technologies.
The main competitive advantages of FC power-generating systems are high energy efficiency, reliability, low pollutant emissions, quietness and long-term autonomous off-grid operation with remote control of operating parameters. Hence, there are no significant limitations regarding the direct installation of FCs at consumer sites, which make them promising technologies for the distributed generation. A comparison of the available literature data 32 shows that FC power-generation systems with an electric power of up to 1 MW are unrivaled in efficiency (Fig. 2). 11 Similar efficiency parameters (∼60%) can be reached only by the best combined-cycle plants with an electric power of 1 GW or higher.
Figure 2. Efficiency ranges for different technologies at a rated load versus the capacity of the plant: FCs, gas-piston engines (GPEs), microturbines (MTs), gas-turbine plants (GTPs), steam-turbine plants (STPs), combined-cycle plants (CCPs). The Figure was drawn by the authors of the review using data from Ref. 32.
Download figure:
Standard imageA combination of solid oxide fuel cells (SOFCs) and gas microturbines also holds promise. Such hybrid plants are developed in Japan by the Mitsubishi Heavy Industries (MHI) with the financial support of the government. 33 Several demonstration plants with an electric power of 250 and 1000 kW were created. The goal is to raise the efficiency of hybrid plants to 70%. 34
Fuel cell power systems have a great potential for the improvement. 31 Unlike conventional heat generation systems, FCs are not subjected of limitations of the Carnot cycle because there are other physical limits on the maximum power efficiency (thermodynamic efficiency). These limitations are much less stringent in the low-temperature range, which is of critical importance for promising power systems.
Fuel cells are characterized by a radically different dependence of the thermodynamic efficiency on the temperature of the process, as follows from thermodynamic calculations using the data obtained in Ref. 35 (Fig. 3). The efficiency increases with decreasing temperature, which is beneficial for the practical application of FCs in the power production systems. Thus, the higher the potential electrical efficiency, the lower the requirements for structural materials, the longer the lifetime and the higher the reliability. The temperature of about 120 – 150 °C is sufficient for the efficient utilization of the heat of exhaust gases for heat recovery purposes. At this temperature, the thermodynamic efficiency of FCs is higher than 90%. Under these conditions, the efficiency of heat generation systems does not reach even 30%. Consequently, the central pillar in the development of FCs for the stationary power production is the decrease in the operating temperature with retention of performance of the power generation system.
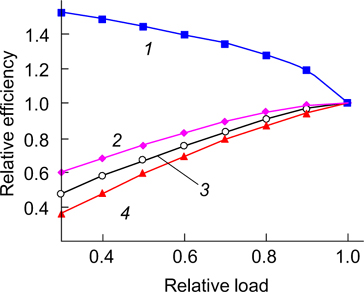
Figure 3. Theoretically calculated (maximum) thermodynamic efficiency of heat generation systems (Carnot cycle) (1), hydrogen FCs (2) and FCs based on methane conversion products (CO – hydrogen mixture) (3) versus the temperature; experimental efficiency for STPs (4), CCPs (5) and FCs (6) and expected efficiency of promising STPs (7) and CCPs (8) under development. The thermodynamic efficiency of FCs was determined as the ratio of the Gibbs free energy to the total enthalpy of the system at a given temperature; for conventional plants, as the ratio of the difference between the temperatures of heat input and output to the temperature of heat input. The Figure was drawn by the authors of the review based on thermodynamic calculations using data from Refs. 35 and 36.
Download figure:
Standard imageFigure 3 presents a comparison of the efficiency at a rated load for low-temperature and high-temperature FC power generation systems with the efficiency of the best currently used and promising steam-turbine and combined-cycle plants. 36 It is worth noting that the dependence of the electrical efficiency on the load for conventional technologies significantly differs from that for low-temperature FCs (Fig. 4). 36,37 The efficiency of conventional power plants largely decreases with decreasing load, while it increases for low-temperature FCs. This is of considerable importance taking into account that power plants operate at a partial load most of their time. Currently, the average annual load of heat power plants is 43.9%: for large gas power plants, 49.8%; for urban combined heat and power systems, 47.3%; for distributed generation plants with a power of >10 MW, 38.6%; for distributed generation plants with a power of <10 MW, 26.5%. 25 The electrical efficiency of high-temperature FC power generation plants also decreases with decreasing load but it occurs more slowly compared to conventional technologies. This is attributed to an increase in the relative heat loss with decreasing load of these plants.
Figure 4. Electric efficiency of power plants versus the load: (1) low-temperature FCs; (2) SOFCs; (3) gas-piston engine; (4) gas-turbine plant. The Figure was drawn by the authors of the review using data from Refs 36 and 37.
Download figure:
Standard imageThe second key hydrogen power technology is the hydrogen production. Hydrogen is generally produced by hydrocarbon reforming. However, pure hydrogen can be obtained only by water electrolysis. At the present time, electrolysis hydrogen is 2 – 4 times more expensive than hydrogen from natural gas. 38,39 This is because of a high cost of electrolyzers and a high cost of carbon-free electrical energy generated by RES and nuclear power plants. There is an evident progress in decreasing the cost of electricity generated by solar and wind power plants. 40 First, it is achieved due to the development of new photoelectric, electrotechnical and structural materials. 23 It is expected that the cost of solar and wind power plants will further decrease by 40 – 50% by 2040. 40 It is reasonable to expect the further improvement of nuclear power plants and a decrease in the cost of electrical energy produced by these plants. Of great importance is the development of new heat- and radiation-resistant materials, which will allow for an increase in the thermodynamic parameters of the equipment of nuclear power plants with an increase in the efficiency from 33 to 45 – 50%.
In Russia, priority is given to the development of conventional power production technologies. 41 There is a problem of the significant loss of scientific and technical competencies in many areas in the past 30 years. The development of technologies for new power production, primarily of hydrogen energy technology, is a challenge in decarbonization processes.
3. Hydrogen production
Pure hydrogen gas is scarce in Earth's atmosphere and, consequently, the hydrogen production is one of the key areas of hydrogen energy. Hydrogen can be produced in different ways, the main of which are the production from natural gas and by electrolysis. The steam reforming of hydrocarbons or coal is the cheapest method for the hydrogen production. However, both these processes are performed at high temperature and are accompanied by the formation of by-products, with CO being the major one. 42–44 The hydrogen production from methane can be carried out by the partial oxidation with air oxygen 45 or via steam reforming. 46 These processes occur at high temperatures and afford hydrogen and CO as the major products. The maximum hydrogen yield is achieved using steam reforming, with the stoichiometric H2 : CO volume ratio of 3 : 1.
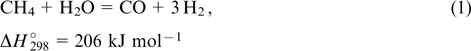
This process is generally performed at 600 – 800 °C using alumina-, silica-, ceria- or complex oxide-supported nickel or platinum-group metal catalysts. 47–50
The partial oxidation of methane can be accomplished in the presence of catalysts of similar composition in a somewhat wider temperature range (600 – 950 °C) 51–53
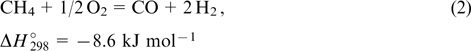
It should be noted that the reaction (1) affords hydrogen in a much higher yield than the reaction (2), but the latter is exothermic and technologically more relevant. Since the reactions (1) and (2), like all heterogeneous catalysis processes, proceed on the catalyst particle surface, nanosized materials are most efficient in these reactions. However, at high temperatures, extensive aggregation occurs. The material and morphology of the oxide support should be chosen so as to prevent these adverse processes.
Carbon monoxide can be used to synthesize various organic compounds, but it requires large amounts of hydrogen, which, therefore, cannot be utilized for the power production. Hence, in the second step, CO can be additionally catalytically oxidized with an excess of steam by the reaction
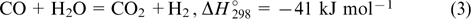
Since the reaction (3) is exothermic, the amount of CO can be diminished by cooling the product flow. 54,55 The overall reaction is described by the equation
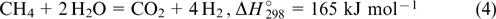
and affords hydrogen and CO2 in a volume ratio of 4 : 1.
The steam reforming of alcohols occurs under much milder conditions. Hence, this process can be performed under conditions providing the formation of hydrogen and CO2 as the major products,
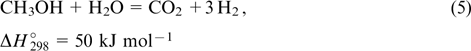
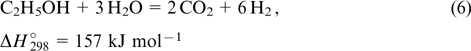
These processes are generally performed using oxide-supported catalysts based on copper, nickel or noble metals. 56–58 It is commonly assumed that the main role of the support is to absorb water vapour. 10 However, the functions of the support are much more significant. Thus, the defectiveness of the oxide and the presence of ions with variable oxidation states play an essential role, which is indicative of the involvement of oxide supports in redox transformations and ion transport processes. 59 Besides, it was shown that nanodiamonds, which proved to be good supports, have their own catalytic activity in alcohol conversion. This can be explained only by the presence, on their extensive surface, of different oxygen-containing groups, which apparently not only provide the water absorption but also can be involved in redox transformations. 60
However, even the additional treatment of the products of methanol steam reforming or the hydrogen production from alcohols does not eliminate the formation of CO impurity. The issue is that such hydrogen is unsuitable for the use in low-temperature FCs, the catalysts of which are poisoned even with CO traces. 61–63 At the present time, this type of FCs accounts for ∼90% of total sales. The utilization of hydrogen in these FCs requires its high purification, which can be performed using a wide range of polymeric, carbon and other membranes. 64–67 Meanwhile, it is more appropriate to employ palladium-based membranes for high-grade purification of hydrogen. The characteristic feature of these membranes is that they provide the selective transport of hydrogen, preventing the penetration of all other gases. The high cost of palladium and the desire to increase the rate of hydrogen transport gave impetus to the active search for alternative membrane materials, in particular based on palladium alloys. Alloys with copper and silver, which are characterized by a lower cost and high hydrogen permeation, 68–71 and alloys with ruthenium 72,73 proved to be the most successful.
To ensure satisfactory mechanical strength, palladium membranes should be rather thick (at least 25 – 30 μm), resulting in a significant decrease in the performance. Therefore, in recent years great attention was given to the development of composite membranes based of highly porous oxide supports (e.g., anodic alumina) with a thin selective palladium alloy layer 74,75 and palladium-coated tubular membranes based on cheaper metals, in particular vanadium. 76 It is worth noting that vanadium can also provide the selective hydrogen transport but, being highly active, undergoes rapid degradation. The porous support ensures satisfactory mechanical properties and does not limit gas permeability of membranes. The palladium coating provides high transport selectivity and, due to a small thickness, ensures the retention of high hydrogen permeability and, in the case of vanadium materials, chemical stability.
A promising approach is based on the application of membrane catalysis in the hydrogen production. 77–79 It is commonly employed for the hydrogenation and dehydrogenation of organic compounds. 80 Besides, it is efficient in hydrogen production from alcohols. Using palladium alloy-based membranes, the production and high purification of hydrogen can be combined. Besides, due to the shift of thermodynamic equilibrium, it is possible to increase the yield of hydrogen by removing it from the reaction zone and to significantly decrease the temperature of the synthesis. 81–85 Unfortunately, instability of palladium membranes at high temperatures makes these membranes unsuitable for the membrane-catalyzed hydrogen production from methane. Meanwhile, Tokyo Gas launched the hydrogen production from methane using such membranes for the high purification of hydrogen from associated products at 400 – 500 °C. 86 It is worthy of note that for the sake of safety, the conversion of natural gas in the presence of oxidants is often performed in membrane reactors with other types of membranes or composite membranes with a selective palladium alloy layer. 87–90 Membranes with mixed oxygen and electronic conductivity are also applied for this purpose. 91,92 In the case of methane reforming, the goal is generally to ensure the safety of the process rather than to produce pure hydrogen.
Pure hydrogen can be directly produced by water electrolysis. 93 Hydrogen is green when it is produced using RES energy. However, according to the estimates, the utilization of even such hydrogen in the most efficient FCs results in the electric power output twice as low as the energy consumed for electrolysis. Hence, it is reasonable to speak only about the accumulation of power produced, for example, during periods of the highest solar activity or periods of reduced electricity demand. A great advantage of this system is a high hydrogen storage capacity. The production of biomass followed by its reforming to hydrogen is an alternative way of solar energy storage. 94,95
At present, alkaline electrolyzers are commercially most exploited because of their low cost and energy efficiency (Table 2). 21
Table 2. Prospects for the improvement of electrolysis hydrogen production technologies. 21
| Parameter | Current status | 2030 | Beyond 2030 |
|---|---|---|---|
| Alkaline electrolyzers | |||
| Operating temperature, °C | 60–80 | 60–80 | 60–80 |
| Operating pressure, bar | 1–30 | 1–30 | 1–30 |
| Power load range (%) | 10–110 | 10–110 | 10–110 |
| Efficiency (%)
| 63–70 | 65–71 | 70–80 |
| Specific capital investment, US dollars per kW | 500–1400 | 400–800 | 200–700 |
| Ion-exchange membrane electrolyzers | |||
| Operating temperature, °C | 50–80 | 50–80 | 50–80 |
| Operating pressure, bar | 30–80 | 30–80 | 30–80 |
| Power load range (%) | 0–160 | 0–160 | 0–160 |
| Efficiency (%) (see
| 56–60 | 63–68 | 67–74 |
| Specific capital investment, US dollars per kW | 1100–1800 | 650–1500 | 200–900 |
| Solid oxide electrolyzers | |||
| Operating temperature, °C | 650–1000 | 650–1000 | 650–1000 |
| Operating pressure, bar | 1 | 1 | 1 |
| Power load range (%) | 20–100 | 20–100 | 20–100 |
| Efficiency (%) (see
| 74–81 | 77–84 | 77–90 |
| Specific capital investment, US dollars per kW | 2800–5600 | 800–2800 | 500–1000 |
Portable ion-exchange proton-conducting membrane electrolyzers have attracted attention for promising application since they can be installed at hydrogen refuelling stations, in particular in densely populated areas. These electrolyzers operate under high pressure (30 – 80 bar; in some systems, up to 100 – 200 bar), which makes the subsequent compressed hydrogen production cheaper. Besides, they well withstand overloads, which is important when using electric power produced from solar and wind plants with stochastic power output. However, these electrolyzers are more expensive than alkaline electrolyzers because they require high-cost electrode catalysts (based on platinum or iridium) and membrane materials (perfluorinated sulfonic acid) and have a shorter lifetime. The electrolyzer unit accounts for ∼60% of the total cost of electrolysis production. In alkaline electrolyzers, this cost is less than 50%. 21 Meanwhile, ion-exchange membrane electrolyzers have high potential for improvement.
High-temperature solid-oxide electrolyzers are as yet not commercially employed, 21 because they are very expensive and have a short service life due to rapid degradation of materials in the temperature range of 650 – 1000 °C. The advantages of these electrolyzers are high efficiency and the ability to operate in reverse mode. If necessary, they can convert hydrogen back into electricity, thereby allowing substantial saving when using these electrolyzers to control the operating mode, in uninterruptible power supply and distributed generation systems and in other cases when powerful high-capacity batteries are required.
The prospects of reducing the cost of electrolyzers can be related to the development of cheaper electrode and membrane materials, an increase in the power of electrolyzers, the improvement of their construction and an increase in the scale of production. It was noted 96 that, to ensure the competitiveness of the electrolysis hydrogen production with other methods, it is necessary to decrease the specific costs of electrolyzers to 400 US dollars per kW and increase their global application to 70 GW. Due to the technological progress, it would be expected that the cost of hydrogen production using electric power from RESs will decrease to 1 – 1.5 US dollars per kg of H2 by the mid-21st century, which is comparable with the current cost of hydrogen production from natural gas.
The pyrolysis of natural gas giving hydrogen and carbon is considered as a promising method 97,98
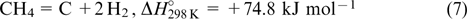
A fresh impetus was given to the further development of this commonly used approach because it provides environmentally friendly production of carbon-free hydrogen. The pyrolysis of a highly symmetric methane molecule with strong C–H bonds is highly energy-consuming and occurs only at temperatures above 1100 – 1200 °C. This temperature can be significantly decreased in the presence of catalysts. 99,100 Iron-group metals are most widely used as catalysts for this process, nickel exhibiting the highest catalytic activity. 101–103 However, nickel catalysts are rapidly passivated due to the formation of massive carbon deposits. The detrimental effects of catalyst carbonization can be diminished by the addition of copper and nickel dopants, which simultaneously promote metal reduction. 104,105 Many authors suggested the application of sintering for regeneration, which is accompanied by carbon oxidation and will play against the green character of this approach. 106 Hence, lower-activity catalysts based on iron or its oxides, which are less prone to passivation by carbon, hold more promise. 107 This phenomenon was attributed to high carbon solubility in iron, resulting in the removal of carbon from the catalyst surface, 98 although the reverse process leading to the egress of iron to the surface is apparently more important. Different supports, primarily based on refractory oxides, are used to stabilize metal catalysts and prevent their extensive sintering. 102,107–109
Carbon catalysts are much less active and can operate only at high temperatures (above 800 °C). 110 However, such materials as activated carbon, soot and so on are in high demand as catalysts due to low cost, high stability and the possibility of avoiding the regeneration. 99,111
Barriers to commercialization of this method are high energy input requirements for pyrolysis and the fact that, according to thermodynamic calculations, about one-half of the energy potentially present in hydrocarbons is not consumed upon carbon emission. It is worth noting that there is a need to utilize large amounts of carbon resulting from methane pyrolysis, while there is currently a lack of market that can accommodate carbon materials for industrial applications. 97,98
4. Hydrogen storage and transportation
Hydrogen produced by the approaches considered above is generally not directly utilized and should be delivered to consumers or stored. Transporting via pipelines is a promising hydrogen delivery option. The extensive infrastructure of natural gas pipelines can be relatively easily repurposed for hydrogen transportation. 112,113 However, there are limitations to this technology. First, hydrogen can cause explosions upon the leakage or in the presence of air in the pipeline. 114 Hence, it is suggested that hydrogen should be blended into natural gas in a volume ratio of ∼5 – 10%. Another serious problem is hydrogen embrittlement of many metals upon contact with hydrogen, resulting in the premature catastrophic failure of the pipeline. The expensive replacement of materials of the existing gas pipelines by hydrogen embrittlement resistant materials such as plastics or special grades of steel, would be required to deal with this problem.
All other hydrogen transportation technologies are closely related to the storage issues. Despite a high energy content of hydrogen, its storage and transportation face a problem of a very low density (less than 0.1 g L−1). For example, if hydrogen tanks are stored in the trunk of a vehicle, the latter can cover only a 4 – 5 km range. To ensure the compact hydrogen storage, hydrogen should be compressed to several hundred atmospheres, absorbed or converted to the chemically bound state.
Gaseous hydrogen is generally stored and transported in cylinders with a volume from several litres to cubic metres. However, the mass fraction of hydrogen even in modern aluminium or composite cylinders under a pressure of 500 atm is generally not higher than 7%. Besides, the cost of this compression is high.
The maximum hydrogen storage density in cryogenic tanks used in vehicles is 20 mass %. With increasing volume, this parameter may reach 86% of the total tank mass. However, the energy input for hydrogen liquefaction (the boiling point is –253 °C) is 30 – 50% of its heat capacity; besides, the cooling system during hydrogen storage requires high energy inputs. 112,115
A hydrogen molecule is non-polar. Hence, in the case of physical adsorption of hydrogen, the energy of its interaction with the adsorbent is at most 10 kJ mol−1. The total hydrogen uptake in even such good adsorbents as MOFs (metal-organic frameworks) with an extended surface area is not higher than 4.5 mass % at a temperature of about –200 °C and a pressure of 20 atm. 116 The storage capacity of some carbon structures at these temperatures can be up to 8 mass %, but it decreases to 0.5 mass % at room temperature. 117–119
Hence, chemical hydrogen storage technologies have gained more use in recent years. Ammonia and toluene (benzene) cycles are worthy of note. These cycles include reversible hydrogenation and dehydrogenation
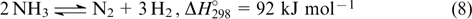
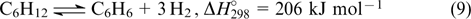
These technologies have attracted attention because they provide much easier and compact liquid transportation. Hydrogen can be extracted from hydrogenated forms directly at the point of use. The ammonia cycle theoretically affords a larger amount of hydrogen. 120,121 The ammonia decomposition and synthesis are catalytic processes. A challenge is to find catalysts for the activation of the starting compounds, primarily for the efficient nitrogen adsorption. In this aspect, iron- and ruthenium-based catalysts are catalysts of choice. 122,123 Mayenite (Ca24Al28O64), 124 which can efficiently trap hydrogen in cavities, proved to be useful for hydrogen storage. 124,125 There are barriers to the efficient application of the ammonia cycle, such as the inability to achieve the complete shift of equilibrium, the toxicity of ammonia at high concentrations, the ability of ammonia to cause irreversible degradation of FCs even at low ammonia concentrations in the fuel (a few ppm) 126 and corrosion activity.
The dehydrogenation of cyclic hydrocarbons such as cyclohexane, methylcyclohexane and decalin seems to be attractive. 127 Hydrogen is incompletely extracted from these carriers; only one molecule per two carbon atoms can be released [see Eqn (9)]. Hence, the theoretical maximum hydrogen storage capacity of these compounds is only slightly higher than 8%. Besides, the high enthalpy of dehydrogenation requires high temperatures. This temperature can be decreased by reducing the effect of kinetic factors due to the introduction of heteroatoms into the ring and using catalysts, oxide- or carbon-supported ruthenium or palladium catalysts being most generally used. 128,129 Considerable attention is paid to the development of cheaper catalysts containing no noble metals. 130,131 The advances in the use of liquid carriers are reflected in recent reviews. 132,133
The hydrogen storage in the form of metal hydrides shows great promise. These compounds are formed through the incorporation of hydrogen atoms into interstitial sites of the metal crystal lattice, which is generally accompanied by its expansion, leads to the embrittlement of the materials and complicates the construction of batteries based on these materials. Magnesium hydride, the hydrogen storage capacity of which is 7.6%, is highly promising. However, magnesium is characterized by a low rate of hydrogen adsorption and desorption, which can occur only at temperatures of about 250 – 350 °C. The kinetics of hydrogenation – dehydrogenation can be improved by using catalytic additives, in particular carbon materials. 134,135 Aluminium borohydride Al(BH4)3 has the highest volumetric hydrogen storage density, while lithium borohydride is characterized by the highest mass content (18.4 mass %). 136 However, the release of hydrogen from these compounds requires a high temperature, and the amount of desorbed hydrogen during one hydrogenation – dehydrogenation cycle is at most 9 mass % even at 600 °C. 136
Intermetallic hydrides, which are characterized by better kinetics of hydrogen uptake (release), are of great interest for hydrogen supply of portable systems, including vehicles. Hydrogen can be fed to a tank with this alloy under moderate pressure, and it can be released by opening a valve. An increased pressure is provided by slight heating. Thus, to achieve the hydrogen pressure of 150 atm, it is sufficient to heat the LaNi5 alloy to 200 °C. 116 The concentration of hydrogen atoms in these intermetallics can be eight times higher than the liquid hydrogen density. 116,137,138 It is worth noting that the use of alloys that specifically absorb hydrogen can, to some extent, solve the safety problems of hydrogen storage and extraction from gas blends.
5. Hydrogen energy production
Fuel cells convert the energy of oxidation of hydrogen-containing fuels directly to electrical energy. There are several types of FCs, which differ primarily by the type of the membrane. Low-temperature proton-conducting polymer membrane FCs and high-temperature SOFCs, in which a ceramic plate serves as an oxygen-conducting membrane, are most commonly used for this purpose. In this review, we focus on these main types of FCs, which are responsible for the progress in hydrogen energy in recent years. Apart from a membrane, FCs include catalytic and gas-diffusion layers and bipolar plates. From the point of view of chemistry and materials science, electrocatalysts and ion-conducting membranes are of primary interest.
5.1. Electrocatalysts for fuel cells
At present, low-temperature fuel cells cover ∼90% of the global fuel cell market. In these cells, oxygen or air serve as the oxidant and high-purity hydrogen serves as the fuel. The use of air as the oxidant leads to a somewhat decrease in the operating voltage, but makes it possible to significantly reduce the total mass of FCs. Hydrogen is fed through a porous gas-diffusion anode layer to the electrocatalyst, where it releases electrons,

and, due to a chemical potential gradient, protons migrate to the cathode catalyst, at which the electroreduction of oxygen takes place. 139

This environmentally friendly electric current source gives water as the only product. Besides, this device has a high efficiency, which is theoretically higher than 90%. However, the efficiency is at most 50% under reasonable current loads, a decrease of which is impractical for such expensive systems as FCs. In low-temperature proton-conducting membrane FCs, carbon monoxide impurities, which are present in hydrogen produced by the conversion of hydrocarbons or alcohols, are mostly adsorbed on platinum catalysts (which are very slowly oxidized at an operating potential of FCs), resulting in the poisoning of the catalyst surface. Hence, the current density in these FCs sharply decreases with increasing CO concentration in hydrogen. 139
Methanol is considered as a promising hydrogen-containing fuel. It can be directly oxidized in methanol FCs, avoiding the conversion to hydrogen
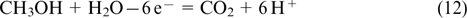
The methanol oxidation is generally performed, like with the use of hydrogen, in the presence of platinum or (more rarely) palladium catalysts. 140 The coverage of the catalyst surface with intermediates (CO molecules) reaches 90% in methanol FCs, and the oxidation with water is a slow process. This results in the low performance efficiency of methanol FCs, and, consequently, they cannot compete with hydrogen – oxygen or oxygen – air FCs. The problems during the operation of methanol FCs are associated with the adsorption and slow oxidation of intermediates (COads) 141,142 and are the same as those arising in conventional FCs when using CO-contaminated hydrogen. Hence, they are convenient examples for the consideration of the principal features of the formation of electrocatalysts used to optimize the performance of low-temperature FCs.
Platinum or its alloys, which are characterized by the maximum efficiency in electrocatalytic processes in FCs, are almost always employed as catalysts in low-temperature proton-conducting membrane FCs, despite a high cost. Attempts were made to replace platinum by cheaper transition metals, but their degradation on contact with the proton-conducting membrane leads to the rapid poisoning of the latter. The rate of electrochemical processes increases with decreasing particle size of the catalyst, thereby making it possible to decrease the consumption of platinum; however, catalysts with a small particle size undergo rapid degradation. 143 The optimal particle size of platinum for both cathodic and anodic processes is 2.5 – 4 nm. 144–146 Platinum alloys and supports having a promotory effect are most commonly used to accelerate anodic processes. 147,148 Thus, the Pt – Ru alloy causes a considerable increase in the rate of anodic processes. 149–151 This can also be achieved using cheaper platinum alloys with transition metals. 152–156
It is worth noting that the introduction of the second metal leads to a change in the Fermi level of the alloy and a weakening of the Pt–COads bond. 157–159 However, when interacting with a proton-conducting membrane having an acidic medium due to the presence of SO3H groups, active metals can be oxidized, and the ions that are released cause poisoning of the membrane. Hence, of more promise are core – shell alloys, in which surface platinum ensures stability of the catalyst, 160–163 and metals that are present in the core provide a high electron density on its atoms. In particular, such catalysts can be prepared by etching binary and even ternary alloys with acid, which removes active metals from the catalyst particle surface. 164 It was also suggested that such particles can have a more extensive surface. Similar problems arise in the preparation of catalysts for oxygen electroreduction. 165–167
Besides, the activity of the catalyst is largely determined by the nature of the support used. In low-temperature FCs, the support is generally made of a carbon material, in particular carbon black powder, having high porosity, conductivity and specific surface area. 168,169 The electrocatalysis in FCs occurs at platinum/support/ionomer triple interfaces. In this case, the ionomer delivers protons and the support donates electrons to the site of the electrochemical reaction. Besides, water chemisorption giving rise to adsorbed OH groups is needed for the CO oxidation to occur. The electrooxidation of methanol (12) occurs through a bifunctional mechanism via the reaction between methanol and water molecules chemisorbed on the metal and the support, respectively. 147 The CO tolerance of the catalyst can be increased using oxide supports easily absorbing water. 170–173
Unfortunately, platinum catalyzes the electrooxidation of not only hydrogen but also carbon. This leads to the gradual burning out of the carbon support at the triple interfaces, resulting in the loss of catalytic activity and the detachment of platinum particles from the support. Hence, another obvious advantage of oxide supports is high stability of catalysts on their basis. 139
Thus, the methanol and CO oxidation is promoted by titanium oxide, 174–176 which also accelerates the electroreduction of oxygen. 177,178 The use of TiO2 in the form of nanoparticles, porous material, nanotubes and nanowires proved to be efficient. 179–182 The partial replacement of Ti4+ ions by pentavalent niobium or tantalum is used to improve the electronic conductivity of titania. 183,184 The electrocatalytic activity of Pt/Nb–TiO2 systems is higher than that of commercial systems supported on carbon black. 185
Tin oxide is also commonly employed as a support. Its popularity is largely due to the easy adsorption of OH groups, the formation of which during the operation of FCs, as mentioned above, promotes the electrooxidation of CO. 186,187 It is worth noting that the use of SnO2 ensures high electrochemical activity of catalysts even at a relatively low platinum content. 188 An advantage of tin oxide is a noticeable electronic component of the conductivity, which can be further increased by heterovalent doping. 189–191 Supports based on oxides of such metals as cerium, molybdenum, tungsten and niobium are applied much more rarely. 192–195 Some research groups used catalysts based on platinum supported on carbon nanotube – tungsten carbide composite powder 196 and polyaniline. 197 Iridium oxide can also be applied for electroreduction of oxygen. 198,199 It should be noted that the range of electrocatalysts for fuel cells is much wider. 200–202 It is easily seen that preference is given to materials that have own conductivity and that promote transport processes essential for the catalyst performance.
Attempts to replace platinum by cheaper catalysts in low-temperature proton-conducting membrane FCs were not very successful because the catalytic activity of other metals is generally much lower. Meanwhile, more active metals interact with the proton-conducting membrane material, which is a strong acid, resulting in a significant decrease in its conductivity. 203 It should be noted that non-platinum catalysts are often used in anion-exchange membrane FCs with much lower corrosion activity. 204–206 Non-platinum catalysts are exclusively utilized in SOFCs. Since these catalysts operate at rather high temperatures, all problems described above for low-temperature fuel cells are not essential for SOFCs. This refers to the easier occurrence of both hydrogen electrooxidation and CO oxidation, which readily proceed on complex-oxide catalysts. 207 Moreover, researchers examined the possibility of the direct oxidation of hydrocarbons in SOFCs based on materials consisting of ceramics, including yttria-stabilized zirconia and oxides of copper, cerium and other metals. 208,209 These materials are most often described as electrode (anode and cathode) ones. 210 Non-platinum catalysts are employed also in biofuel cells. 211
5.2. Proton-conducting membranes
Proton-conducting membranes are the heart of low-temperature FCs. These membranes, together with catalysts, account for most of the cost of such fuel cells. In membranes, the direct proton transport is driven by the chemical potential gradient. Another important requirement is that the material should has low electronic conductivity and gas permeability. 212 Perfluorinated sulfonic acid Nafion membranes (Nafion is a copolymer of tetrafluoroethylene and perfluorinated sulfonated vinyl ether) are of most demand for low-temperature FCs. These membranes are composed of perfluorinated carbon chains containing functional ion-exchange groups –SO3H (structures 1 and 2 ). Their obvious advantages are thermal and chemical stability, high conductivity and selectivity. 191 Hence, Nafion membranes, despite a high cost, are most commonly used in hydrogen – air and methanol FCs.
Structures 1, 2
Download figure:
Due to self-organization processes in these membranes, hydrophobic perfluorinated chains form the membrane matrix, while hydrophilic functional SO3H groups form clusters. The latter actively absorb water from the environment and form a system of pores and channels, thereby providing ion transport. 213–215 The structure of this system dictates the ionic conductivity and selectivity of transport processes in membranes. 216
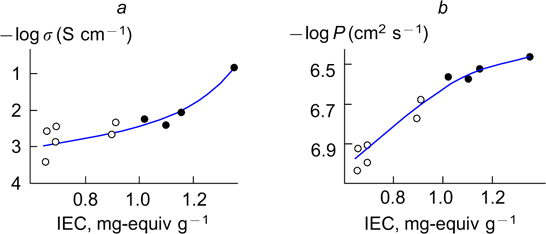
At present, great attention is paid to short-side-chain membranes (structure 2 ). Some research groups reported that such membranes have high conductivity at temperatures up to 130 °C. 217–219 Meanwhile, a decrease in the side chain length leads to an increase in the ion-exchange capacity, i.e., to an increase in the charge carrier concentration in the membrane. Hence, it is of interest to compare the conductivity and gas permeability for a series of membranes with similar chemical structures and different ion-exchange capacity. 220 This comparison was performed for a series of short- and long-side-chain sulfonic acid membranes with different m : n ratios (structures 1, 2 ). The ion-exchange capacity of these membranes varied in a rather wide range, from 0.66 to 1.35 mg-equiv g−1. 221 At high humidity, the conductivity of such membranes increased by two orders of magnitude due to an increase in the charge carrier concentration (Fig. 5 a ) and larger volumes of the pores and connecting channels. A decrease in the relative humidity to 32% led to an increase in the difference up to three orders of magnitude. This is evidence of a high conductivity of short-side-chain membranes at low humidity. Simultaneously, the diffusion permeability via anion transport increased due to an increase in the humidity and the pore size, while the anion transport numbers changed only slightly. At the same time, the gas permeability changed only by one-half of an order of magnitude (Fig. 5 b ). 221 Most probably, this is associated with the partial transport of non-polar gas molecules via diffusion across the perfluorinated matrix. 222,223
Figure 5. Proton conductivity (σ) at 95% humidity (a) and the oxygen permeability (P) (b) of perfluorinated sulfonic acid membranes versus their ion-exchange capacity (IEC) at 25 °C. Empty and solid circles represent the data for short- and long-side-chain membranes, respectively. 221
Download figure:
Standard imageIn recent years, the use of cheaper non-perfluorinated membranes in hydrogen energy has attracted attention of researchers. 224 However, heterogeneous membranes commonly applied in different electrochemical devices are characterized by the presence of a secondary large-pore system that is formed due to the imperfect packing when prepared by pressing or rolling. This leads to a significant decrease in selectivity. 225 Hence, efforts are made to prepare membranes with a composition similar to that of membranes with a much higher selectivity due to the lack of secondary porosity. This can be achieved by means of graft polymerization of polystyrene onto a hydrophobic film matrix followed by sulfonation. 226–228 Membranes prepared by a similar procedure show high selectivity of transport processes and reasonable power density in air – oxygen FCs. 229
The development of anion-exchange membrane FCs is another rapidly growing area of research. 204,230–233 These membranes have attracted attention due to the novelty and possibility of utilizing non-platinum catalysts, which should significantly reduce the cost of FCs, as well as the cost of energy production. 234–236 However, this approach has drawbacks, significantly limiting the power density of the created FCs. 204,205,237 The main disadvantages are a relatively low conductivity of anion-exchange membranes, slow kinetics of hydrogen oxidation in alkaline media, low performance stability, a non-uniform distribution of water in the membrane during the operation of FCs and carbon oxide adsorption causing a decrease in the membrane conductivity.
However, the situation has been changing rapidly in recent years. Thus, the development of new electrocatalytic materials and engineering solutions provide an approach to solving the problems of water balance and CO2 adsorption. 237–240 Extensive studies are being undertaken to develop new stable anion-exchange membranes with high conductivity, 241–245 including non-fluorinated membranes, 246 capable of operating at high temperatures. 247 This makes it possible to manufacture anion-exchange membrane FCs with a high power density of up to 2–2.1 W cm−2. 206,247,248
The performance characteristics of ion-exchange membranes can be improved by the introduction of inorganic nanoparticles. Hybrid materials have attracted great interest because of the possibility of increasing their ionic conductivity and decreasing gas and methanol permeability. 249–251 Membranes containing nanoparticles of silica, 252,253 titania, 254 zirconia 255,256 or ceria 257 are often used in FCs for this purpose. The conductivity is increased due to an increase in the size of the pores and connecting channels as a result of incorporation of nanoparticles. 258 This effect can be enhanced by introducing particles with acidic surface. 249,253,259–262 Both the ionic conductivity and selectivity of transport processes can be significantly changed by varying the nature of the nanoparticle surface. 217,260 Apparently, the hydrophilicity and acidity of the nanoparticle surface exert the largest effect. Besides, the way of introduction of nanoparticles into the ion-exchange membrane matrix also plays a role. The maximum effect was achieved by synthesizing nanoparticles directly in the pores of membranes acting as a kind of nanoreactors. 139,261
5.3. Materials for medium- and high-temperature fuel cells
The drawbacks of perfluorinated membranes are a high cost and a decrease in the conductivity at low humidity. On heating to >90 °C, perfluorinated membranes lose moisture, also resulting in a decrease in the conductivity. Hence, these membranes cannot be used in FCs at high temperatures. Meanwhile, the temperatures of >120 °C may assist in solving the problem of irreversible CO adsorption on platinum catalysts. 4 Therefore, considerable attention is paid to phosphoric acid-doped polybenzimidazole membranes, which are characterized by low methanol permeability and can operate at 160 – 200 °C. 263 For this reason, FCs based on these membranes are much less sensitive to CO poisoning and stably operate at low humidity. 264,265 However, an important issue is the leaching of phosphoric acid from these membranes when exposed to water vapour. Of interest are the studies 266–269 devoted to the modification of polybenzimidazole-based membranes with inorganic oxide nanoparticles, which can also promote the retention of phosphoric acid in the membrane matrix.
Alkali metal and ammonium hydrogen salts with tetrahedral anions Mn Hm (XO4)p are also materials capable of operating at high temperatures with a conductivity of up to 10−1 S cm−1 at 150 – 200 °C. 270 However, the conductivity of the low-temperature phases of these materials, which are stable below the phase transition temperature, is several orders of magnitude lower. Composite materials doped with nanosized oxides to stabilize the high-temperature phase 271,272 can be used to start up fuel cells based on these materials at low temperatures.
The problem of working with CO-contaminated hydrogen is completely eliminated for SOFCs operating at much higher temperatures compared to the low-temperature FCs described above. Oxygen-conducting membranes, in particular yttria-stabilized zirconia membranes are characterized by a higher activation energy of conductivity and are commonly used in such SOFCs. A reasonable conductivity of these materials is achieved only at >700 °C. 273 However, external heating to these temperatures for a few hours is required to start up SOFCs, thereby significantly limiting their application in mobile systems and the transport sector. Hence, attempts are made to reduce the operating temperature of SOFCs and increase their efficiency. 274–276 For instance, thin-film materials allowed the reduction of ohmic losses in SOFCs. 277–279
Another promising line of research is the development of proton-conducting materials capable of operating at much lower temperatures compared to oxygen-conducting materials. Complex oxides based on barium cerate or zirconate are the most well-known proton-conducting materials. 280,281 To increase the conductivity, these materials are often doped with trivalent cations incorporated in zirconium sites. Under a dry atmosphere, these materials have oxygen conductivity due to O2– ion migration over vacancies. However, under a humid atmosphere, these vacancies are occupied by water oxygen atoms, and the released hydrogen ions ensure proton conductivity. The drawback of these materials is their low resistance to moisture and CO2 because of the presence of alkaline earth metals. Hence, a challenge is to find materials with a low alkaline earth cation concentration or those containing no these cations. However, currently available electrolytes of this type are characterized by a relatively low conductivity. 282–284
Solid oxide fuel cell electrodes are generally produced using highly porous conducting materials: 285–287 oxides generally serve as cathodes; porous metal ceramics (e.g., nickel composites with yttria-stabilized zirconia), as anodes. As in low-temperature FCs, hydrogen electrooxidation and oxygen reduction occur at three-phase interfaces. 288–290
6. Conclusion
The development of hydrogen energy poses a number of challenges in the field of materials science. The solution of these issues will contribute to the progress in this area, including the hydrogen production, purification, storage and conversion to energy. Significant research and engineering advances have been made, but many problems remain to be addressed. A number of approaches are available to produce hydrogen, including such important methods as the water electrolysis allowing the pure hydrogen production and the catalytic conversion of natural gas, alcohols, etc. Composite materials such as oxide-supported metal particles or alloys are generally applied as catalysts in conversion processes. However, these processes afford carbon oxides as associated products. Apparently, carbon dioxide emissions will be kept under strict regulations, while carbon monoxide is a serious poison for catalysts in the most commonly used low-temperature FCs.
Palladium alloy-based membranes proved to be efficient in hydrogen purification. The major drawbacks of these membranes are a high cost and low performance. The main ways to tackle this issue are apparently the design of porous composite materials with a thin selective layer and the direct production of high-purity hydrogen using membrane catalysis. Membrane technologies may also be useful on a large scale for hydrogen extraction from blends with natural gas in the case of pipeline transportation of hydrogen – natural gas blends. Attention is paid to high-temperature pyrolysis of natural gas. Important challenges in this process are the production of pure hydrogen and environmental protection.
Several hydrogen storage technologies are available, the high-pressure compression and liquefaction being most commonly used at the present time. However, both methods require high energy input. Therefore, hydrogen adsorption on alloys is considered as a promising technology. The hydrogen storage density in alloys may be several times higher than that in liquid hydrogen. The hydrogenation – dehydrogenation of nitrogen or cyclic organic compounds can be considered as an alternative to this method.
The key technology of hydrogen utilization will be fuel cell power systems. Unlike conventional engineering technologies, the latter have unique properties: high efficiency of low-power installations, the possibility of increasing the efficiency with decreasing temperature of the process, an increase in the efficiency of the plant at low operating loads. The main challenges in the development of FCs are the design of highly efficient catalysts for oxygen electroreduction and the construction of CO-tolerant FC electrodes. To address these issues, attempts are made to prepare binary platinum alloys with cheaper metals or core – shell structures and design carbon- or oxide-based supports. Among modern ion-exchange membranes, homogeneous persulfonic acid-based materials are characterized by high conductivity and selectivity and exhibit the best transport properties. Short-side-chain membranes are extensively developed. It should be noted that perfluorinated membranes are expensive and efficiently operate only at temperatures below 100 °C and at high humidity. A serious problem is catalyst poisoning with carbon monoxide, adsorption of which at temperatures below 120 °C is almost irreversible. Therefore, there is a need to design alternative membrane materials, polybenzimiazole materials being of great interest. The modification of membranes by the introduction of nanoparticles can lead to a significant improvement of proton conductivity and selectivity of transport processes.
By contrast, the major problems for SOFCs are the reduction of the operating temperature and the decrease of ohmic losses. The main ways of addressing these problems are the design of thin-film materials and the replacement of oxygen-conducting systems by proton-conducting ones.
This review was prepared with the financial support of the Ministry of Science and Higher Education of the Russian Federation within the framework of the state assignments to the IGIC RAS and the ERI RAS.