Abstract
Sapphire with different crystal planes is widely used in light-emitting diode (LED), silicon on sapphire (SOS) IC chips, hybrid microelectronic applications, and other high-tech fields. Chemical mechanical polishing (CMP) technology is widely applied to obtain super-smooth and non-damaged wafer surface on the sapphire wafer. Due to its higher hardness and brittleness, sapphire CMP processing is difficult because of its lower processing efficiency. In this paper, in order to improve the CMP performance of different plane sapphire, K2S2O8 was used as an additive in the slurry. On the basics of chemical thermodynamic theory, HSC(enthalpy (H), entropy(S), heat capacity (C)) chemistry software was used to judge whether the chemical reactions can occur spontaneously. According to the experiment results, a higher material removal rate (MRR) was obtained with the slurry containing 0.2 wt% K2S2O8 and the surface roughness Sq of different plane sapphire substrates were all less than 0.3 nm. At the same time, the reaction mechanism and process of K2S2O8 was revealed. X-ray photoelectron spectroscopy (XPS) analysis showed that there were new chemical reactions between the sapphire wafer and K2S2O8 sol and the reaction products included aluminum sulfate (Al2(SO4)3) and potassium sulfate (K2SO4), which increased the chemical effect during the polishing process and resulted in the increasing of MRR.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: permissions@ioppublishing.org.
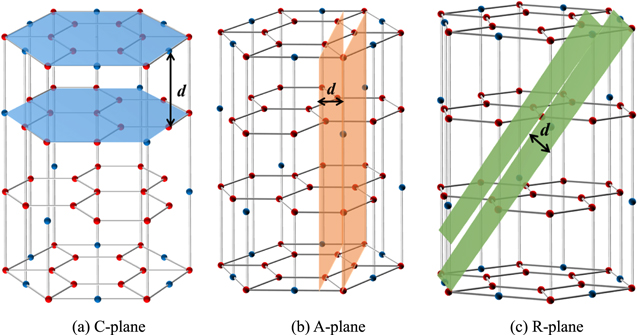
Sapphire (α-Al2O3 single crystal) has been widely applied in the semiconductor device, light-emitting diodes (LED), solid lasers, infrared window and precision optics due to its excellent optical, chemical and mechanical properties, such as high hardness, thermal stability, chemical inertness, and great electrical and dielectric properties.1 Sapphire is a brittle crystal with anisotropic and different crystal planes, and the commonly used crystal planes are C-, R- and A-planes. The physical properties of these sapphire crystals with different planes are different.2,3 Therefore, the industrial applications of them are different. For example, C-plane sapphire is usually used as the substrate for light-emitting diode (LED), R-plane sapphire is usually introduced into silicon on sapphire (SOS) IC chips, and A-plane sapphire is usually the preferred choice for hybrid microelectronic applications, which has highly insulating properties and a uniform dielectric constant.4,5 The performance and reliability of these devices critically depend on the surface quality and integrity of finished sapphire substrates.6 However, as is well-known, sapphire wafer is difficult to be polished ideally owing to its high hardness (Mohs hardness 9, second only to diamond), brittleness, and chemical inertness. Chemical mechanical polishing (CMP) is becoming one of the most important ultra-precision processing technologies because of the ability of obtaining super-smooth and non-damaged wafer surface.7 The hexagonal- scalenohedral crystal structure of sapphire (as shown in Fig. 1) results in material anisotropy along with different crystal orientation.8 So, for sapphire wafer CMP with different crystal orientations, there are still many theoretical and technical problems, especially material removal mechanism which is still poorly studied due to the complicated material characteristics. Therefore, a further investigation on polishing mechanisms of sapphire with different crystal orientations is prerequisite for improving polishing efficiency and surface quality simultaneously.
Figure 1. Crystal structure of sapphire.
Download figure:
Standard image High-resolution imageAt present, sapphire CMP of different crystal planes has been studied by some researchers. Lei et al. successively reported the influence of silica sol doped with metallic elements on C-plane sapphire CMP and some new products were found to form which were removed easily to improve polishing performance.9–11 The novel catalyst SoFeIII was reported by Xu, which played effective roles in improving the removal rate of C-plane sapphire. The results indicated that the removal rate of sapphire with containing catalyst slurry was 1.66 times than with catalyst-free slurry.12 Based on the excellent characteristics and application value of other crystal plane sapphire substrates, such as A- and R-planes, some researchers have also carried out some studies on sapphire them in recent years.
Zhu et al. studied the CMP performance of C-, A-, and M-planes sapphire substrates with α-alumina abrasive under identical conditions.13 It indicated that the order of removal rate was C-place > A-plane > M-plane, and the reason was no hydration layer that can be easily removed on surface formed during the polishing of A- and M-planes. Zhao et al. revealed the bonds which should be broken during the polishing process of C-plane and R-plane sapphire were different. Al–Al bond with bond energy of only 186 KJ mol−1 in C-plane and Al–O bond with bond energy of 512 KJ mol−1 in R-plane should be broken, which results in the MRR of R-plane is lower than C-plane.14 Xu et al. reported a novel non-Pt catalyst (Fe-Nx/C) to improve the removal rate of C-plane sapphire. Meanwhile, the authors found pyrrole nitrogen can weaken the Al–O bond via the bonding between oxygen and nitrogen or the adjacent carbon atom, and facilitate the chemical reaction between sapphire and colloidal silica. However, there is no thorough research on this aspect in that paper.15
Besides, many researchers had also tried different ways to process sapphire to improve the removal rate. Natthaphon Bun-Athuek et al. reported that the effect of abrasive particle size on A-plane sapphire removal rate was explained by physical factors.16 Cheng et al. developed a predictive model on the grinding force in Micro-slot grinding (MSG) in three different orientations of single-crystal sapphire. During this process, the value of the variable ζ which is the number of crystal layers per volume was calculated.17 ζ in the C-plane sapphire was lower than that of other planes, so C-plane sapphire was easier to remove than the others, which is of great significance for sapphire CMP with different planes. Yin et al. used MgO-doped colloidal silica abrasive for the CMP of C-, R- and A-planes and deduced that the solid-chemical reaction between sapphire surface and MgO doped colloidal silica abrasive was occurred, which can promote chemical effects during CMP and lead to the increase of MRR.18 Xu et al. reported the MRR of sapphire was improved by using hard abrasive coated with a soft chemical layer of another abrasive type through mechanical chemical polishing (MCP).19 From the results, the main polishing process of MCP was the formation of passivation layer as a result of the solid-state reaction caused by soft abrasives, but the MRR of sapphire was still at a low level. Besides, in our previous study, different plane sapphire CMP performances were improved by trying to optimize the composition of slurry.14,20,21 Zhao et al. added a new type chelating agent in R-plane sapphire CMP slurry, and it was revealed that the chelating agent can significantly enhance the removal rate and surface quality of sapphire.14 Zhao also researched the effects of pH regulator, KNO3 concentration, and surfactant concentration on R-plane sapphire CMP and new-type alkaline slurry was obtained which can improve the removal rate.20 Cui et al. found the effect of chloride ions on the CMP efficiency of A-plane sapphire substrate and the improvement of the removal rate by KCl was verified.21 To sum up, although the increase of physical action can certainly enhance the removal rate of sapphire, it is obviously more feasible to develop the slurry based on the improvement of chemical action.
Up to now, the research on different methods to improve the polishing efficiency of sapphire substrate with different crystal planes is still going on, especially the research on the polishing mechanism of enhancing chemical action during processing is particularly important. S2O82− was added to the slurry to enhance the chemical action during the CMP process of many materials, such as copper (Cu),22 gallium nitride (GaN),23 and microcrystalline glass. So, it was also used as an additive in this paper to improve the sapphire CMP performance.
To infer the possible reaction products on sapphire substrate, the HSC(enthalpy (H), entropy(S), and heat capacity (C)) chemistry software was used to calculate the Gibbs free energy, and the chemical mechanism of the removal rate was obtained by X-ray photoelectron spectroscopy (XPS) analysis. In addition, the surface topography and roughness Sq were measured by the atomic force microscopy (AFM).
Experimental
Two-inch (diameter of 50.8 mm) single crystal commercial sapphire wafers, including C-(0001), R-(10 2), and A-(11
2), and A-(11 0), were used for comparative experiments. Self-developed alkaline sapphire slurry prepared in the lab was used and nano-SiO2 sol was selected as abrasive, whose concentration was 40 wt%. The average particle size of the abrasive was 80–90 nm. The surfactant volume fraction was 0.2 vol%. KOH was used as the pH regulator to adjust the pH value of the slurry to 10.5. K2S2O8 was added to the sapphire slurry to enhance chemical action. Each group of experiments was repeated at least three times to take the average.
0), were used for comparative experiments. Self-developed alkaline sapphire slurry prepared in the lab was used and nano-SiO2 sol was selected as abrasive, whose concentration was 40 wt%. The average particle size of the abrasive was 80–90 nm. The surfactant volume fraction was 0.2 vol%. KOH was used as the pH regulator to adjust the pH value of the slurry to 10.5. K2S2O8 was added to the sapphire slurry to enhance chemical action. Each group of experiments was repeated at least three times to take the average.
Material removal rate (MRR) is determined by Eq. 1:
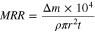
where Δm (g) is the mass loss, t is the polishing time (in the test, t = 1/2 h), ρ is the sapphire density (ρ = 3.98 g cm−3), r is the radius of sapphire substrate (r = 2.54 cm), and MRR(μm h−1) is the corresponding removal rate.
Polishing experiments were performed on X62 S82 × 305-D-S single-side CMP polisher produced by Suzhou Herriot with a Suba 600 polishing pad, and sapphire wafers were put in inlaid layer holes on the wax-free polishing template adsorption film, as shown in Fig. 2. Sapphire CMP process parameters were shown in Table I. The professional electronic balance was used to measure the weight of sapphire substrate before and after polishing, with a precision of 0.1 mg (AUY120 ASSY). Each measurement of mass was repeated at least three times to take the average. The mean particle size and zeta potential were measured by NICOMP 380ZLS laser nanoparticle size analyzer. The surface topography and roughness Sq were measured by Agilent 5600LS atomic force microscopy (AFM). Surface and subsurface products were qualitatively measured by PHI5000VersaProbe X-ray photoelectron spectroscopy (XPS). HSC chemistry software (Outokumpu Oy) was used to calculate the Gibbs free energy.
Figure 2. The wax-free polishing template.
Download figure:
Standard image High-resolution imageTable I. Sapphire CMP process parameters.
| Parameters | Conditions |
|---|---|
| Polishing time | 30 min |
| Polishing head speed | 50 rpm |
| Platen rotation speed | 50 rpm |
| Slurry flow rate | 160 ml min−1 |
| Downward pressure | 0.1 Mpa |
| Upward pressure | 0.06 Mpa |
| Temperature | 27 °C −29 °C |
Results and Discussion
The theoretical foundation of the different removal rates of different plane sapphire
Under the same conditions, the lattice structure is the decisive factor for achieving different MRR of different planes sapphire wafers. The lattice of sapphire (α-Al2O3) is composed of Al3+ and O2−. There is mainly ionic bonding for the bond in α-Al2O3, so it is the forces of electrostatic interactions that have the greatest effect on the bond energy. In the lattice of α-Al2O3 crystal, the electrostatic energy q between per pairs of ions can be defined as follows24:
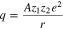
where A, a numerical quantity, is termed as the Madelung constant,  and
and  are considered as point charges, representing a pair of ions and r is the distance of per pairs of ions. Obviously, the greater the distance r, the smaller the electrostatic energy.
are considered as point charges, representing a pair of ions and r is the distance of per pairs of ions. Obviously, the greater the distance r, the smaller the electrostatic energy.
Consequently, it can be deduced that the lattice bond energy of adjacent atomic layers that belong to different crystal orientations is inversely proportional to the interplanar spacing d of different crystal planes. The distance between adjacent atomic layers of C-, A- and R-plane sapphire substrates can be obtained from the interplanar spacing d for planes (006), (220), and (036) respectively, as shown in Fig. 3.24 For the hexagonal crystal system, the interplanar spacing d for the plane (hkl) is given as follows:
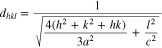
where the lattice constants of sapphire are a = 4.758 Å and c = 12.992 Å at 300 K. The calculated d values of C-, A- and R-plane sapphire plates are 2.177 Å, 1.190 Å, 1.160 Å, respectively.25 The interplanar spacing d of different crystal planes is in the order of C-plane > A- plane > R-plane. As mentioned above, the lattice bond energy of adjacent atomic layers is inversely proportional to the interplanar spacing. Therefore, the bonding energy for different crystal planes of sapphire is in the order of C-plane < A- plane < R-plane. The smaller the bonding energy is, the more easily the material to remove, thus, the material removal rate of sapphire with different planes is in the order of C-plane > A- plane > R-plane.
Figure 3. Crystal models for different orientations of sapphire.
Download figure:
Standard image High-resolution imageThe calculation of thermodynamic energy reaction
The thermodynamic reaction energy of sapphire and K2S2O8 in the slurry during the polishing processes was investigated, which can judge whether the chemical reactions can occur spontaneously or not. It was gradually discovered that the changes of entropy(S) and enthalpy(H) were not sufficient to determine whether a reaction could occur spontaneously. Afterwards, Gibbs function, G was defined as follows:
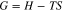
According to the principle of minimum free energy, the reaction always moves in the direction that the Gibbs free energy decreases, and the change of Gibbs free energy (∆G) becomes the criterion for determining whether the reaction can occur autonomously.26
Based on chemical thermodynamics, HSC chemistry software was used to calculate the  as a function of temperature (T) for each reaction. The standard molar Gibbs free energy change of reaction was calculated as follows:
as a function of temperature (T) for each reaction. The standard molar Gibbs free energy change of reaction was calculated as follows:

At constant temperature and pressure, chemical reaction isotherm which shows the relationship between the change of Gibbs free energy in any state and the standard molar Gibbs free energy change is as follows27:

where R is a constant, Q is termed as Reaction Quotient. If the value of  was less than zero, the chemical reactions can occur spontaneously. The possible chemical reactions are expressed in the following equations:
was less than zero, the chemical reactions can occur spontaneously. The possible chemical reactions are expressed in the following equations:
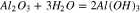
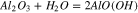

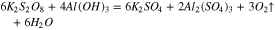
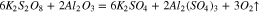
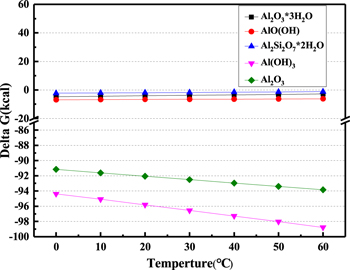
During the polishing process, these reaction products were calculated based on the Gibbs free energy, as shown in Fig. 4.
Figure 4. Relationships between delta G and temperature of reactions of Al2O3, SiO2, H2O, and K2S2O8.
Download figure:
Standard image High-resolution imageThese findings show that the polishing temperature of the thermodynamic calculation is between 0 °C and 60 °C. Figure 4 indicates the value of  is less than zero, all these chemical reactions can occur spontaneously.
is less than zero, all these chemical reactions can occur spontaneously.
Influence of K2S2O8 on CMP performance of different plane sapphire.
In order to improve the MRR and surface quality of different plane sapphire substrates, K2S2O8 was used as an additive in the sapphire slurry. The ratio of deionized water to nano-SiO2 sol was 1:1. The nonionic surfactant isobutanol polyoxyethylene ether (JFCE) was used, whose volume fraction was 0.2 vol%. K2S2O8 with different concentrations of 0.0 wt%–0.5 wt% was added to the slurry. Finally, the slurry pH value was adjusted to 10.5 with KOH.
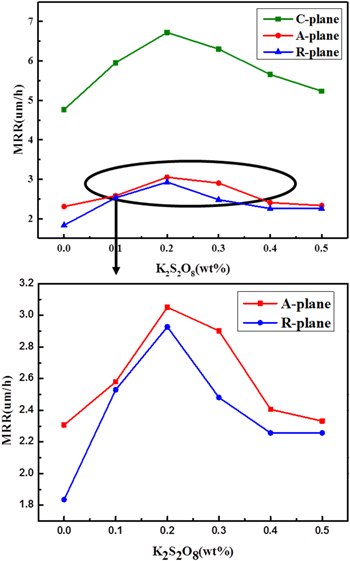
It can be seen from Fig. 5, which the MRRs of C-, A- and R-plane sapphire increase significantly with the enhancing of K2S2O8 concentration. Otherwise, the MRR of sapphire with different planes is in the order of C-plane > A-plane > R-plane, which identifies the previous theoretical foundation for different planes of sapphire. As the K2S2O8 concentration increases from 0 to 0.2 wt%, the MRRs of C-, A- and R-plane increase sharply to 6.722μm h−1, 3.051μm h−1, and 2.927μm h−1, respectively. However, with the K2S2O8 concentration continues to increase, the MRRs begin to show a downward trend. Moreover, it can be speculated that when the concentration of K2S2O8 is 0.2 wt%, the mechanical action and chemical action reach the dynamic equilibrium, optimum removal occurs. When the concentration is greater than 0.2 wt%, the chemical action is greater than the mechanical action, insufficient removal will occur, and lower removal rate and lower removal rate and surface quality will be obtained. Therefore, the optimal K2S2O8 concentration was selected at the inflection point 0.2 wt% to obtain higher MRRs.
Figure 5. Effect of the concentration of K2S2O8 on C-plane, A-plane, and R-plane sapphire removal rate.
Download figure:
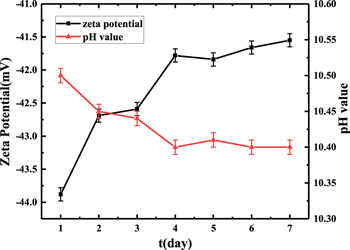
Standard image High-resolution imageMoreover, the changes of zeta potential and pH value with the standing time of the slurry containing 0.2 wt% K2S2O8 were measured, as shown in Fig. 6. Within seven days, the zeta potential and pH value slightly changed. It is generally considered that the colloid is stable when the absolute value of the zeta potential is greater than 30 mV, and the larger the absolute value, the more stable the colloid is. When the absolute value of the zeta potential is less than 30 mV, the repulsion between particles is weakened, which will cause collisions between particles to increase particle size and reduce CMP slurry stability.28 As shown in Fig. 7, the measured results show that slurry performances have fluctuated slightly. It also indicates that the slurry can be stable at least seven days, which meets the conditions for industrial production.
Figure 6. Zeta potential change and pH value with the standing time of slurry containing 0.2 wt% K2S2O8.
Download figure:
Standard image High-resolution imageFigure 7. Relationship between zeta potential and stability of silica sol.
Download figure:
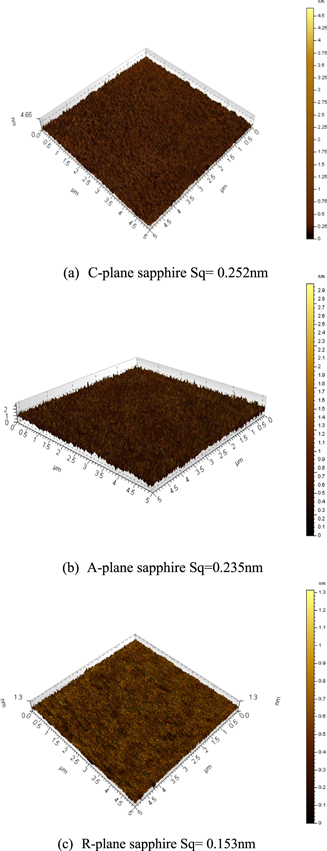
Standard image High-resolution imageMeanwhile, good surface morphology was achieved after polishing with 0.2 wt% K2S2O8 slurry, as shown in Fig. 8. When 0.2 wt% K2S2O8 was added into the slurry, MRR and Sq of sapphire substrate with different planes are shown in Table II.
Figure 8. Surface topography and roughness of sapphire polished by 0.2 wt% K2S2O8 slurry.
Download figure:
Standard image High-resolution imageTable II. Sapphire MRR and Sq.
| Sapphire | K2S2O8(wt%) | C-plane | A-plane | R-plane |
|---|---|---|---|---|
| MRR(μm h−1) | 0.0 | 4.763 | 2.307 | 1.836 |
| 0.2 | 6.722 | 3.051 | 2.927 | |
| Sq(nm) | 0.2(before) | 0.275 | 0.270 | 0.195 |
| 0.2(after) | 0.252 | 0.235 | 0.153 |
Removal mechanism analysis
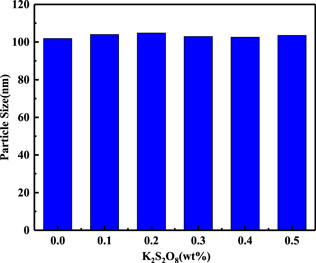
From Table II, it can be seen when adding K2S2O8 in the slurry, the removal rates of different planes all increase, which should be the enhancement of mechanical or chemical action. Firstly, the effect of K2S2O8 on abrasive was verified. The variation of mean particle size was measured under the condition of different K2S2O8 concentrations, as shown in Fig. 9. From Fig. 9, it can be found that the K2S2O8 concentration has almost no effect on the mean particle size in the slurry, which indicates there is nearly no change in mechanical action. Accordingly, it can be inferred that the addition of K2S2O8 enhanced the chemical action.
Figure 9. The variation of average particle size with K2S2O8 concentration.
Download figure:
Standard image High-resolution imageIn order to further reveal the existing chemical form of the removed Al and the forming reaction products after polishing, the sapphire wafers soaked in K2S2O8 sol were analyzed by XPS. All XPS data in the experiment were carbon calibrated.
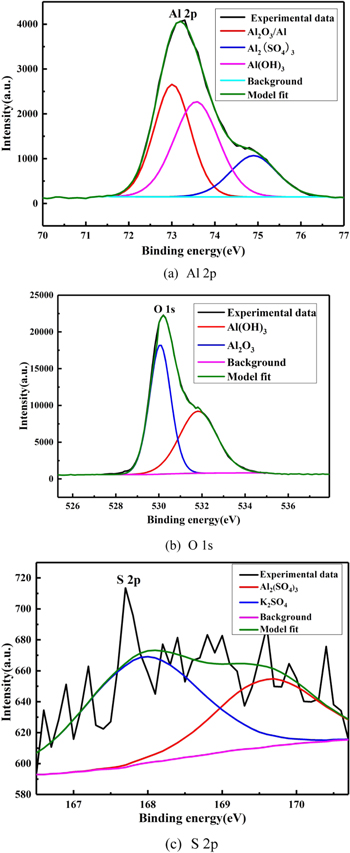
XPS spectrums of element Al 2p, O 1s, and S 2p of the sapphire surface are presented in Fig. 10. The binding energy of the different substances is shown in Tables III–V. The black line shows the real total intensity measured by XPS and the olive line shows the total intensity after curve fitting using the Casa XPS software. The closer between the real result (black line) and the fitting result (olive line) means more accurate measurement results.
Figure 10. XPS spectrums of element Al, O, and S of sapphire substrate soaked in K2S2O8 sol.
Download figure:
Standard image High-resolution imageTable III. The binding energy of Al 2p.
| Chemical state | Measurement Binding energy(eV) | Theoretical Binding energy(eV) |
|---|---|---|
| Al2O3/Al29 | 73.01 | 73.02 |
| Al2(SO4)330 | 74.89 | 74.90 |
| Al(OH)331 | 73.60 | 73.90 |
Table IV. The binding energy of O 1s.
| Chemical state | Measurement Binding energy(eV) | Theoretical Binding energy(eV) |
|---|---|---|
| Al2O332 | 530.06 | 530.30 |
| Al(OH)331 | 531.82 | 531.53 |
Table V. The binding energy of S 2p.
| Chemical state | Measurement Binding energy(eV) | Theoretical Binding energy(eV) |
|---|---|---|
| Al2(SO4)330 | 169.60 | 169.50 |
| K2SO433 | 167.93 | 168.10 |
It can be observed from the fitting drawings of Fig. 10a, that there are three peaks of Al(2p) and binding energies of different peaks are 73.01 eV, 74.89 eV and 73.60 eV corresponding to Al(2p) in Al2O3/Al29 state, Al2(SO4)330 state and Al(OH)331 state respectively. Seeing from fitting drawings of Fig. 10b, the binding energy at 530.06 eV and 531.82 eV in the XPS spectra of O(1s) is consistent with Al2O332 state and Al(OH)331 state. From Fig. 10c, the peaks at the binding energy of 169.60 eV consisted with S 2p in Al2(SO4)330 and the peak at the binding energy of 167.93 eV corresponds to S 2p in K2SO4.33
Therefore, the chemical reactions did occur between sapphire and K2S2O8 sol, and the equations are deduced as follows:
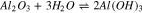
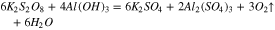
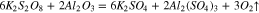
At the same time, the Gibbs free energy ( ) of the reaction 13 and 14 are −95.814 kcal and −92.062 kcal at 20 °C in the previous part, so that the reaction 13 occurs easier than 14 when the reactants are enough, that is to say, K2S2O8 will react with Al(OH)3 at first precedence when Al(OH)3 and Al2O3 exist simultaneously. However, Al(OH)3 was an absence in the beginning, hence the reactions may occur in the following sequence. Firstly, reaction 14 occurred. At the same time, Al(OH)3 was generated in reaction 12. Afterward, K2S2O8 reacted with Al(OH)3, which caused the reaction 12 to go forward to the direction of positive reaction.
) of the reaction 13 and 14 are −95.814 kcal and −92.062 kcal at 20 °C in the previous part, so that the reaction 13 occurs easier than 14 when the reactants are enough, that is to say, K2S2O8 will react with Al(OH)3 at first precedence when Al(OH)3 and Al2O3 exist simultaneously. However, Al(OH)3 was an absence in the beginning, hence the reactions may occur in the following sequence. Firstly, reaction 14 occurred. At the same time, Al(OH)3 was generated in reaction 12. Afterward, K2S2O8 reacted with Al(OH)3, which caused the reaction 12 to go forward to the direction of positive reaction.
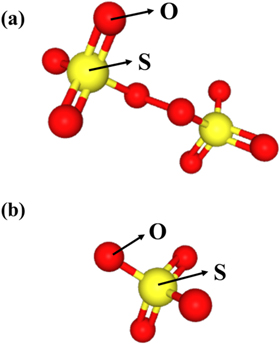
From reaction 13 and 14, it can be well perceived the transformation from S2O82− to SO42− occurred. It can be observed from Fig. 11a that the valence of S and O can be inferred, which contributes to speculate the mechanism of the reactions. One O–O bond, four S=O bonds, four S–O bonds exist in the structural formula of S2O82−. O shows −1 valence in the O–O bond, and in the S=O and S–O bonds, O shows −2 valence, hence S shows +6 valence. Thus, during the process of the transformation from S2O82− to SO42−, there was no change in the valence of S element, instead of the O element in the O–O bond changed to 0 and −2 valence and according to this, O2 and SO42− are produced. What is more, it can be seen from Fig. 12, the O–O bond in S2O82− is broken to generate SO42−, which further indicates the accuracy of the analysis above.
Figure 11. Structural formulas of (a) S2O82− and (b) SO42−.
Download figure:
Standard image High-resolution imageFigure 12. The transformation from S2O82− to SO42−.
Download figure:
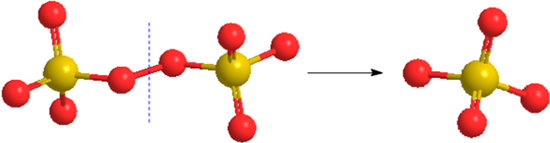
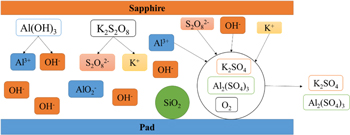
Standard image High-resolution imageBased on these results, it can be deduced that the improvement of MRR in sapphire substrate is attributed to the concurrently chemical reactions between sapphire surface and slurry during polishing, and the products are Al2(SO4)3 and K2SO4. Meanwhile, the reaction products Al2(SO4)3 and K2SO4 are soluble and easy to be removed. Otherwise, Fig. 13 shows the schematic illustration of the chemical reaction model in the CMP process. The reaction between K2S2O8 and Al(OH)3 increases the consumption of OH−, which causes the reaction 11 to go in the direction of positive reaction, ensuring the continuous production of Al2(SO4)3 and K2SO4.
Figure 13. Schematic illustration of the chemical reaction model in the CMP process.
Download figure:
Standard image High-resolution imageConclusions
The CMP of C-, A- and R-plane sapphire wafers was investigated by using slurry with adding K2S2O8. Higher MRR and good surface morphology after polishing with containing 0.2 wt% K2S2O8 slurry were obtained, which was due to the new chemical reaction between K2S2O8 and sapphire surface. Meanwhile, the reaction product Al2(SO4)3 and K2SO4 were easy to be removed. Furthermore, the process of the reaction products was also revealed. As a result, adding K2S2O8 to sapphire slurry was conductive to the improvements of sapphire CMP efficiency and quality, which has guiding significance to practical production.
Acknowledgments
This work is supported by the Major National Science and Technology Special Projects (No. 2016ZX02301003-004-007), Natural Science Foundation of Tianjin China (16JCYBJC16100, 18JCTPJC57000), and the Key Laboratory of Electronic Materials and Devices of Tianjin, China. The authors also thank the teachers and classmates for their helpful suggestions.